
Materials

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2021.015027
ARTICLE
Study on Improvement of Hygroscopicity of Magnesite-Bonded Wood Wool Panel
Nanjing Forestry University, Nanjing, 210037, China
*Corresponding Authors: Li Luo. Email: luoli0044@163.com; Bin Na. Email: nabin8691@126.com
Received: 17 November 2020; Accepted: 09 February 2021
Abstract: Magnesite-bonded wood wool panel (MWWP) is an inorganic-bonded panel product in which wood excelsior is bonded with magnesite. Lowering the hygroscopicity is one of the key measures to improve the quality of the panel. In this study, moisture absorption mechanism of MWWP and measures generally applied to lower its hygroscopicity were reviewed. Three methods were then experimented to improve the dimensional stability of the panel, including adjusting the molar ratio of raw materials, adding additives and optimizing the conditioning process. The results showed that satisfying dimensional stability could be achieved when the molar ratio of MgO to MgCl2 was 5:1, the No.2 composite additive (aluminum powder + NH4H2PO4 + ferric alum) was adopted and a constant temperature and humidity treatment was applied in the first stage of conditioning.
Keywords: Magnesite-bonded wood wool panel (MWWP); hygroscopicity; steam pressing; additive
With the development of social economy and the improvement of people’s living standard, people’s environmental concern is gradually increasing and the requirement of new building material is also continuously getting higher every year [1]. In recent years, the interest in the environmental-friendly new building material is growing, possessing favourable thermal insulation, acoustics resistant properties and modern decorative appearance simultaneously. Nowadays, wood-cement composites are widely applied as construction materials for interior and exterior applications, mostly in school buildings, gyms, parking garages [2–4]. This is attributed to their both excellent strength properties for building materials and acoustic properties for sound barriers. Low density magnesite-bonded wood wool panel (MWWP) is a panel product made of wood excelsior bonded with magnesite. In recently, wood wool panel is mostly utilized as a decorative and sound absorbing material in many countries for its good sounding absorbing and thermal insulation properties [5–7]. However, the incompatibility between wood and cement is the main factor leading to the quality of the wood-cement composites and it could compromise the final stability of panels. This is due to the presence of some soluble chemicals in the wood. Its presence might have a negative impact on the composites by hindering the hydration of cement in the alkaline environment and scattering into the cement paste. Therefore, the mechanical performance of wood-cement composites lower than the neat cement.
Magnesia (main chemical component: MgO) is the ground product of calcined magnesite. It can react with MgCl2 to form magnesium oxychloride cement. Magnesium oxychloride cement is mainly composed of two alternate salt crystals of 5Mg (OH)2 • MgCl2 • 8H2O and 3Mg (OH)2 • MgCl2 • 8H2O produced by the reaction of magnesium oxide and magnesium chloride, which is characterized by quick hardening, high strengths, abrasion resistance and easy forming. However, changes of temperature and humidity will greatly affect the surface stability of magnesium oxychloride cement, causing excessive moisture absorption, back to halogen, efflorescence and so on, and deteriorating physical and mechanical properties of magnesium oxychloride cement products. When excessive MgCl2, a highly hydrophilic material, exists, it exhibits poor water resistance, limiting its application as an inorganic binder [8,9].
The fundamental reason for moisture absorption of MWWP is the existence of free MgCl2 or NaCl, both of which are highly hydrophilic, in the board. Industrial-class MgCl2 inevitably contains some impurities. That’s where NaCl comes from. NaCl does not participate in the reaction, causing its contribution to the moisture absorption. As regards to the free MgCl2, two factors are considered the main causes of its existence. The first is the improper molar ratio of raw materials. Studies on the formation of magnesium oxychloride cement can be summarized as follows: It was first believed that both 5-phase crystal and 3-phase crystal were formed by reaction between Mg (OH)2 and MgCl2 solution. It has been found that the molar ratio of MgO to MgCl2 should be at least 5:1 to form the stable reaction product of 5Mg (OH)2·MgCl2·8H2O (hydrated phases: 5.1.8) and avoid excessive MgCl2 [10]. But some experimental studies suggested that the hydrated phases of the magnesium oxychloride cement were rather complex, with even unknown phases existing. So, the molar ratio of more than 5 cannot guarantee the exact reaction product of 5Mg (OH)2·MgCl2·8H2O, making some unreacted MgCl2 left. Second, to guarantee complete mix of wood excelsior with magnesia, proper liquid to solid ratio should be obtained, i.e., enough MgCl2 solution should be used. With its concentration unchanged (Otherwise, incomplete reaction will occur, followed by low strengths of the board.), this quantity requirement makes MgCl2 excessive in the reaction system.
Another reason for moisture absorption of MWWP is due to the property of 5Mg (OH)2·MgCl2·8H2O. Although it exhibits high stability and strengths in the air [11,12], it is unstable in the water and will be decomposed to MgCl2. That means, when the board is long exposed in high humidity environment or water, free MgCl2 will be formed.
To reduce the MgCl2 content in the board, the following methods have been tried [13,14]: First, optimize the molar ratio of MgO to MgCl2. The molar ratio was adjusted so that less MgCl2 was used while the strengths of the boards were not affected. Second, add suitable additives. Several additives, such as ferric alum and phosphate, were added in to react with free MgCl2 to form precipitates. They would stay on the face of the panel and reduce the hygroscopicity of the panel. Third, condition the panel properly. The hydration reaction is an intensive exothermic process. Stress due to thermal expansion and crystal growth will concentrate in the panel when proper control is absent. The stress concentration can damage the internal structure of the panel and induce the moisture absorption. It was recommended that the panel should be sealed and placed in an environment with the temperature of 18~35°C and relative humidity of 60~70% for 28 days.
Low density magnesite-bonded wood wool panel has been utilized widely in industrial and residential buildings as soundproofing and thermal insulating material. However, its poor performance of the moisture resistance has limited application as an inorganic binder because of excessive MgCl2. Therefore, the aim of this study is how to improve the Hygroscopicity of Magnesite-bonded Wood Wool Panel. Thus, this experimental study was conducted to identify the improvement of Hygroscopicity of Magnesite-bonded Wood Wool Panel by adjusting the molar ratio of raw materials, adding additives and optimizing the conditioning process. This paper could provide a theoretical basis for improving the hygroscopicity of MWWP.
Magnesia with MgO (120 mesh) content of 60% was supplied by Shandong Laizhou Hongda Building Material Factory. Wood excelsior was prepared from poplar veneer, and the dimension was 200 × 2 × 0.5–1 mm (length × width × thickness). MgCl2 solution was prepared from analytically pure MgCl2 crystal. The additives chosen were talc, aluminum powder, NH4H2PO4, ferric alum (NH4Fe (SO4)2·12H2O) (FeSO4) and milky-white glue [15–22]. Talc was marked as additive No.1. Aluminum powder + NH4H2PO4 + ferric alum was marked as additive No.2. Additive No.3 was the mixture of all the 4 additives, and additive No.4 was milky-white glue.
Magnesia, wood excelsior, MgCl2 and additives were weighted and mixed together. They were then moved in a wooden mould with the dimension of 350 × 350 × 16 mm. The mould was put in a hot press and pressed for 30 min. The heat was generated by a steam generator another 5 min. Then, it was conditioned in a climate chamber (Shanghai Minyi, SPX-150C) at a temperature of 25°C and relative humidity of 60% for 3 days, after which, it was sealed and placed in a room for 28 days.
The density of the final product was 0.45–0.55 g.cm−3 g/cm2, and its dimension was 350 × 350 × 16 mm.
Mechanical properties were tested on a universal test machine (SANS, SMT6104). Modulus of rupture (MOR) of the samples was tested carefully.
Samples were cut to the dimension of 50 × 50 × 16 mm and vertically immersed into pure water with their tops 20 mm below the surface of the water. After 24 h, they were taken out and wiped. The thickness of every sample was measured before and after the test. For each sample formulation, five replicated samples were tested. The TS was calculated according to Eq. (1):
where: e0 and e1 were the thickness of the sample before and after the test, respectively.
The samples were dried at 85°C first, and then they were conditioned at 20 ± 2°C and 95% relative humidity for 28 days. Weights of the samples were measured on the 3rd, 5th, 7th, 14th, 21st and 28th day before and after the moisture absorption test. For each sample formulation, five replicated samples were tested. The moisture absorption rate (W) was calculated according to Eq. (2):
where: G0 and G1 were the mass of the sample before and after the test, respectively.
The experimental design consisted of three methods (including adjusting the molar ratio of raw materials, adding additives and optimizing the conditioning process) and their single effect on the hygroscopicity of MWWP. Data for each treatment were statistically studied by analysis of standard deviation. When standard deviation was more than 10% in the case, the significant difference among these formulations could be considered.
3.1 Influence of Molar Ratio of MgO to MgCl2
From Fig. 1, it could be found that, with the increase of molar ratio of MgO to MgCl2, the TS of the samples continued to improve with a standard deviation of 1.06%. Higher molar ratio means relatively low MgCl2 content, which results in uncompleted reaction of MgO. Mg (OH)2 cannot be totally converted to crystal. With the evaporation of water, some crystals/particles will move to the panel face as white precipitate. Such panels have low hardness and strengths and are easy to swell when immersed in water. However, if the molar ratio is too low, i.e., the MgCl2 content is high, intensive reaction will occur, leaving some MgCl2 unreacted. This part of MgCl2 will be transferred to the panel face and absorb moisture during the hardening, making the panel face covered with drops of moisture. So, when the proportion of MgO and MgCl2 is appropriate, the 5 phases and Mg (OH)2 with high content and stability, magnesia-bonded wood-wool panel will have a better performance of the moisture resistance accordingly. Therefore, proper molar ratio of MgO to MgCl2 should be chosen to guarantee good dimensional stability of the board. According to the experimental data in this study, the ratio of 5:1 is the optimal value.
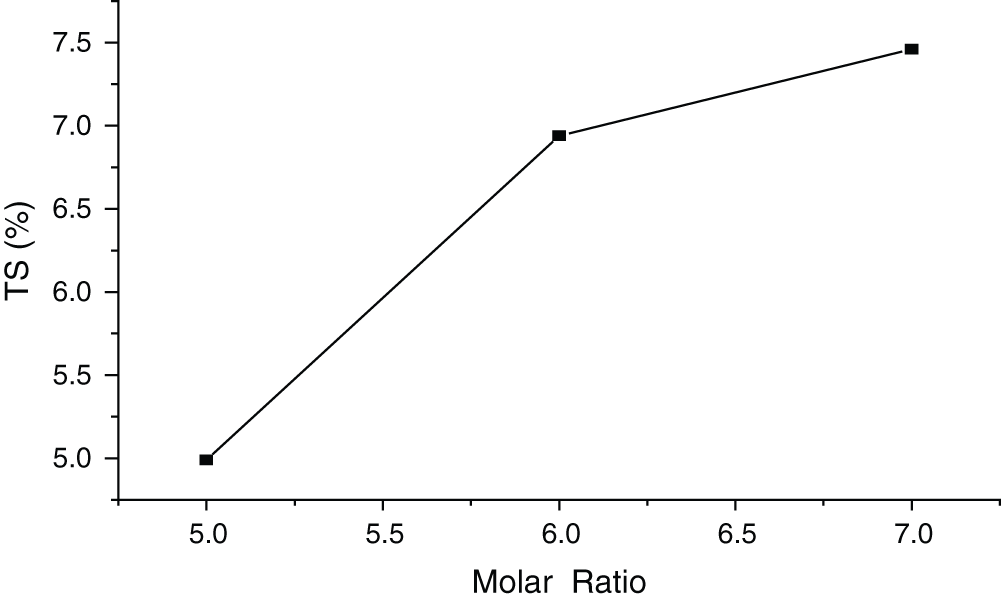
Figure 1: Influence of molar ratio of MgO to MgCl2 on TS of MWWP (24 h)
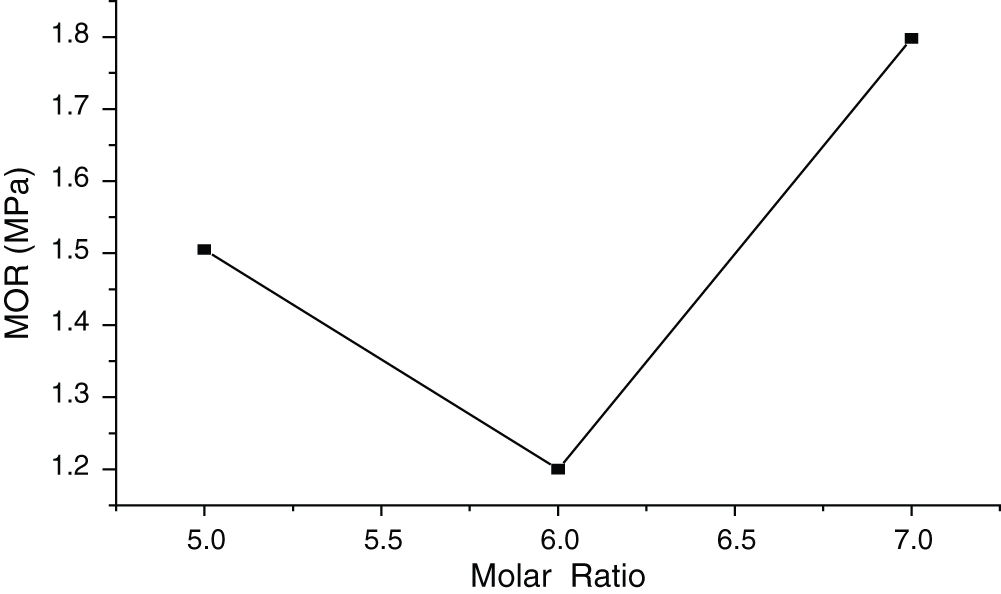
Figure 2: Influence of molar ratio of MgO to MgCl2 on MOR of MWWP
Fig. 2 shows the influence of molar ratio on MOR (standard deviation of 0.244 Mpa) of the panels. The lowest MOR could be observed when the molar ratio was 6:1. Magnesium oxychloride cement is mainly composed of two alternate salt crystals of 5Mg (OH)2 • MgCl2 • 8H2O (phase 5) and 3Mg (OH)2 • MgCl2 • 8H2O (phase 3) produced by the reaction of magnesium oxide and magnesium chloride. Compared with phase 3, phase 5 is preferred because of its more excellent physical and mechanical properties [17]. As for MgO-MgCl2-H2O system, the composition of hydration product is determined by MgCl2 concentration and the relative amount of MgO to MgCl2. That is most probably due to the low chemical activity of MgO. When the molar ratio reaches 5:1, the 5-phase crystal becomes the main reaction product. But it is not stable. With the increase of the molar ratio and the MgCl2 concentration more than 2.25 mol·kg−1 [18], it turns from 5-phase crystal to 3-phase crystal, lowering the MOR of the board. Mg (OH)2 is hydration product at low MgCl2 concentration. When the molar ratio becomes even higher, the excessive MgO turns to Mg (OH)2, which to some extent increases the strength of the board. Therefore, the improvement of the panel’s water resistance heavily depended on the molar ratio of MgO to MgCl2.That may explain the highest MOR at the molar ratio of 7:1.
The additives chosen were talc, aluminum powder, NH4H2PO4, ferric alum (FeSO4) and milky-white glue. Fig. 3 shows the influence of additives on the TS of the panel (standard deviation of 2.64%). It was found that panels with additive No.2 exhibited the lowest TS, while those with additive No.4 had the highest. The ferric alum, aluminum powder and NH4H2PO4 in additive No.2 are water-resistant agents. They can react with MgCl2 to form precipitates, preventing the penetration of water. On the contrary, MgO was wrapped by talc and milky-white glue in additive No.1 and No.4 and it cannot react with MgCl2 to form a protective membrane, making the dimensions of the panel less stable.
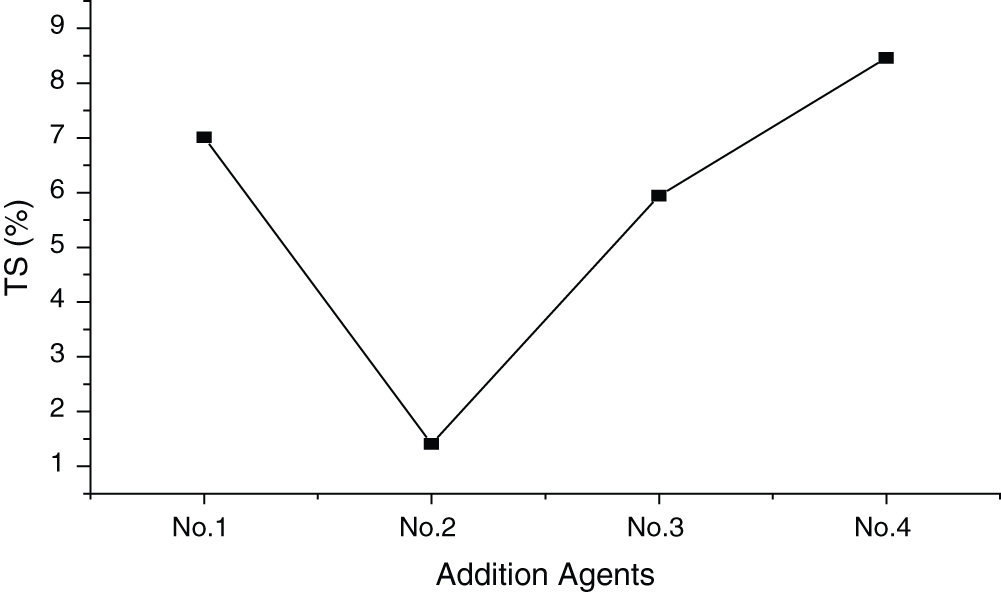
Figure 3: Influence of additives on TS of MWWP
According to Fig. 4, further analyze showed that panels with additive No.1 have the highest MOR, while those with additive No.4 have the lowest (standard deviation of 0.28 Mpa). Additive No.2 and No.3 exhibit similar influence on MOR. The talc in additive No.1 blocks the capillary channels in the wood, preventing free MgCl2 from absorbing moisture in the air. Meanwhile, it can also act as adhesive. Both of the above significantly improve the MOR of the panel. Similarly, ferric alum, aluminum powder and NH4H2PO4 in additive No.2 and No.3 can react with MgCl2 to form precipitates, which can partly act as adhesive and improve the value of MOR. Milky-white glue in additive No.4 has no influence on MOR because it only has the function of water resistance and can’t cannot glue wood excelsior. Thus, the impact of additives on the hygroscopicity of magnesite-bonded wood wool panel came mainly from the blocking of the capillary channels in the wood and adhesion.
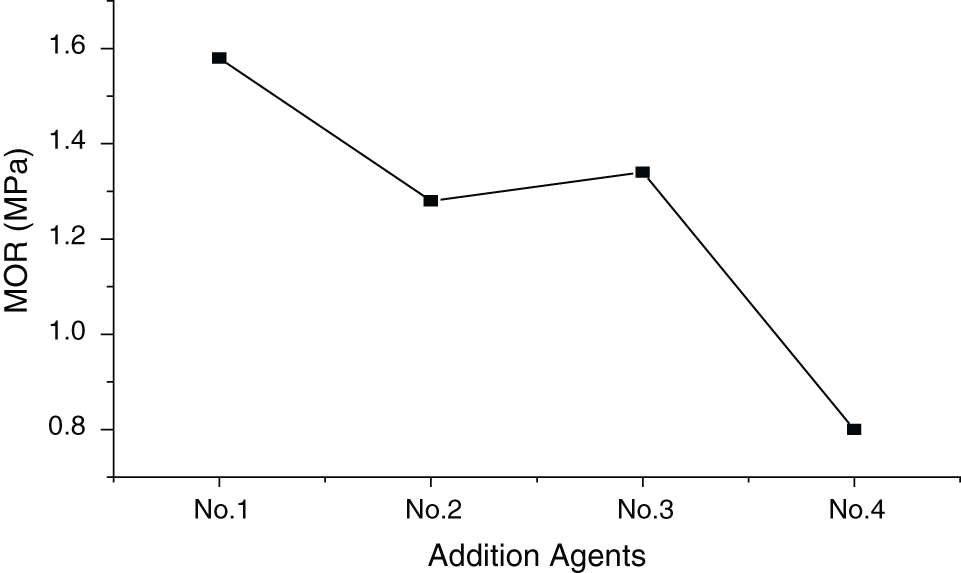
Figure 4: Influence of additives on MOR of MWWP
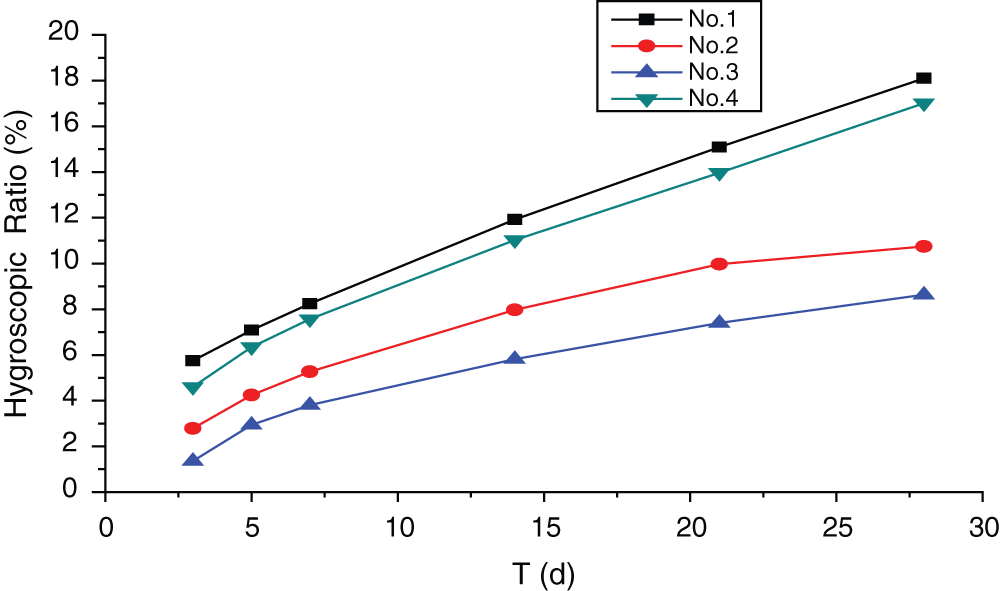
Figure 5: Influences of additives on hygroscopicity of MWWP
According to Fig. 5, panels with additive No.3 exhibit the lowest hygroscopicity, while panels with additive No.1 show the highest. The hygroscopicity difference caused by additive No.3 and additive No.2 is modest. During the experiment, the faces of the panels with additive No.2 and No.3 were dry, while those with additive No.1 and No.4 were wet. That demonstrated good moist resistance of boards with additive No.3 and No.2. The main reason is that, as mentioned above, the talc (particle size = 325 mesh) in the additive shows small particle size and span factor. The micron-sized talc may fill voids within the wood. It implied that the performance of the moisture resistance can be improved for the hydrophobicity and the high adsorption of talc [19]. Therefore, the talc in the additive can block the capillary channels in the wood and prevent free MgCl2 from absorbing moisture in the air. Another reason is that the ferric alum, aluminum powder and (NH4) H2PO4 in the additives reacted with MgCl2, forming minor amount of unknown substances and preventing moisture absorption.
Conditioning environment influences the hygroscopicity of MWWP significantly. That is because the hydration reaction between magnesia (main content: MgO) and MgCl2 solution is a very complex physicochemical process and its products are the binder of the panel, which is an intensive exothermic process. Stress concentration may occur due to thermal swelling and crystal growth, and damage the internal structure of the panel. Besides, incomplete hydration of MgO and changes of internal structure may also cause dehalogenation and efflorescence. The conditioning temperature is commonly between 20~40°C [20]. So, the hydration reaction between magnesia (main content: MgO) and MgCl2 directly influences the performance of the product, and is therefore the key factor to the improvement of MWWP quality. It is of great importance to choose an appropriate conditioning environment.
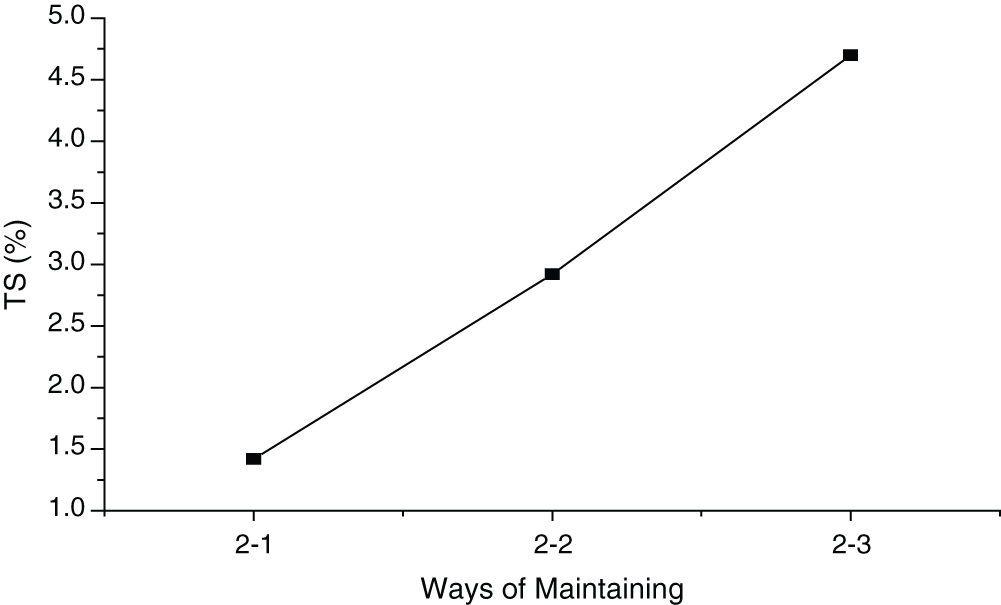
Figure 6: Influence of conditioning on TS of MWWP
Fig. 6 illustrates thickness swelling rate (TSR) of the samples in different conditioning environment (standard deviation of 1.34%). The lowest TSR was found when the samples were conditioned in climate chamber at the temperature of 20 ± 2°C and relative humidity of 60% for 3 days, and then sealed for 26 days (condition 2-1). Higher TSR was observed when the samples were conditioned in sealed condition (2-2). The highest TSR occurred when the samples were conditioned in the air (2-3). Climate chamber created a condition favoring complete hydration, making it hard for moisture to penetrate into the board. That’s why lowest TSR was found in condition 2-1.
According to Fig. 7, the highest MOR was found when the samples were conditioned in sealed condition, while the lowest was observed when climate chamber conditioning was adopted (standard deviation of 0.25 Mpa). Sealed condition kept the moisture content unchanged, which minimized the phase transition and was beneficial to higher MOR. In climate chamber condition, however, higher moisture content made the phase transition easier and also resulted in lower MOR. With respect to conditioning in the air, it can be concluded that water in the samples evaporated in this case, which made the hydration incomplete, causing relatively low MOR.
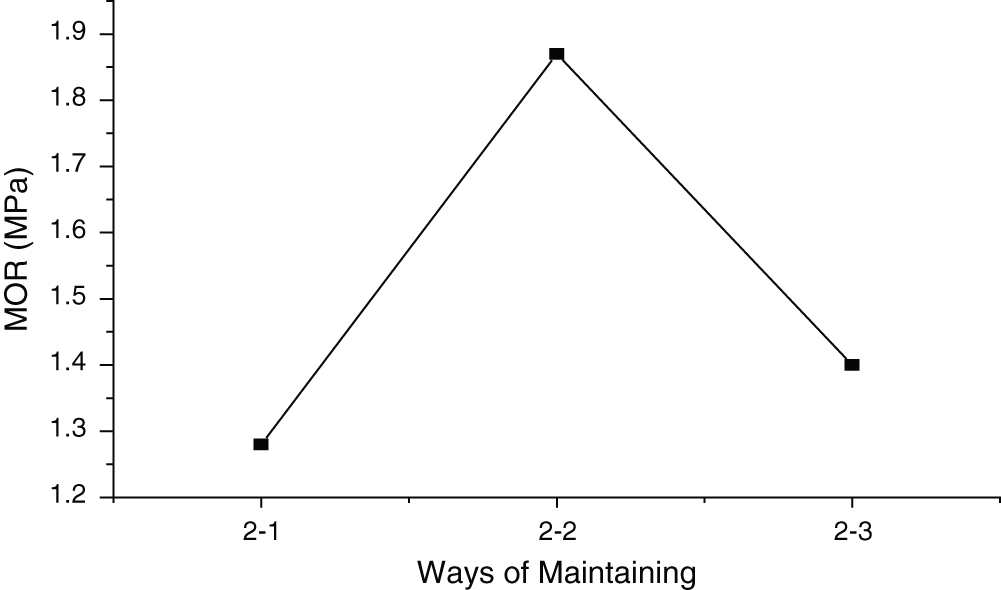
Figure 7: Influence of conditioning on MOR of MWWP
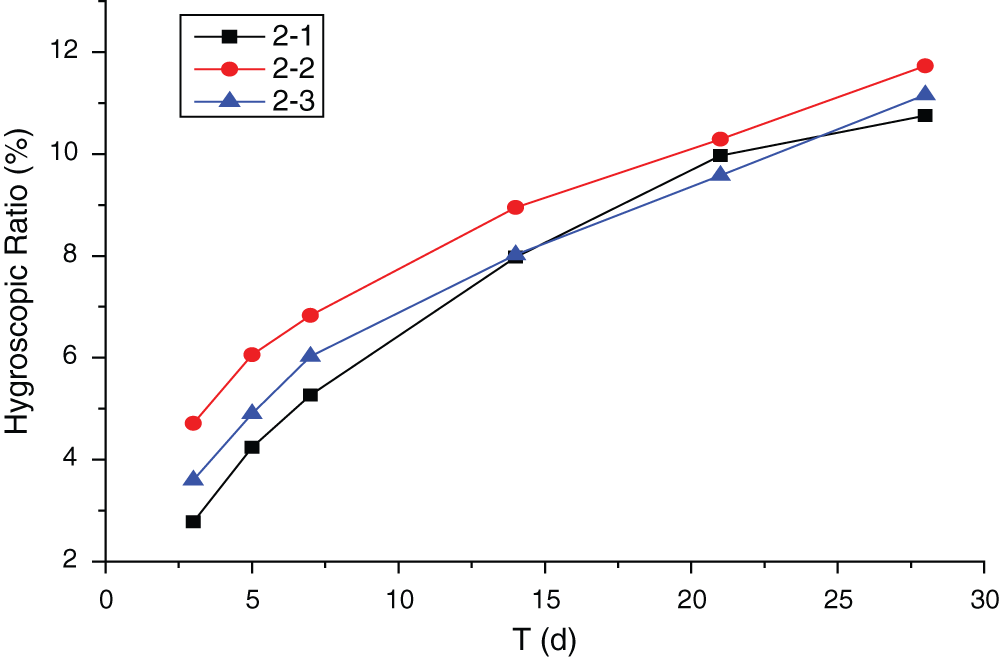
Figure 8: Influence of conditioning on hygroscopicity of MWWP
According to Fig. 8, the results showed that hygroscopicity of the panels was the lowest when conditioned in condition 2-1. The highest hygroscopicity was found in air condition. In the early stages of conditioning, sample hygroscopicity in each condition was quite different. But the differences were reduced later and standard deviation was less than 5%.
The reason is that with increased conditioning time, free MgCl2 was formed as a result of moisture absorption, causing dehalogenation. Higher MgCl2 content also resulted in lower heat release. That is because higher MgCl2 content caused higher MgCl2-to-MgO ratio, which in turn resulted in unstable reaction products and retarded the hydration process. Both the climate chamber and air condition provided enough moisture to boost the hydration reaction, while the sealed condition prevented moisture absorption and resulted in incomplete hydration. Consequently, it was condition 2-1 that brought the best dimensional stability.
When the molar ratio of MgO to MgCl2 was 5:1, thickness swelling rate of MWWP could be reduced significantly. The corresponding MOR was not as high as the one when the molar ratio was 7:1, but it could still meet the demand of the application.
Both additive No.2 and No.3 showed significant influence on hygroscopicity and MOR of MWWP. Additive No.2 is superior to additive No.3 given that it can make the thickness swelling rate of the panel even lower, only 1.41% in our experiment.
All the 3 conditioning environments guaranteed adequate MOR of the panel (all above 1.2 MPa). The best dimensional stability was obtained when the samples were conditioned in a climate chamber for 3 days and then subjected to a sealed condition. The hygroscopic rate was only 1.42% according to the experiment, the lowest in all the 3 conditioning environments.
Acknowledgement: The author of this paper would like to thank the scholars who have put forward suggestions for revising this paper.
Funding Statement: This study is funded by National Natural Science Foundation (31070502) and Public Farewell Project of National Forestry Bureau (201004006-6). The authors would also like to thank the support of the Priority Academic Program Development of Jiangsu Higher Education Institutions and Co-innovation Center of Efficient Processing and Utilization of Forest Resources, Nanjing Forestry University, Nanjing 210037, China.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. China Building Material Academy (2003). Green building material and technologies reaching it. China: Chemical Industry Press. [Google Scholar]
2. Zhang, S., Shui, B., Wang, H. (2002). Manufacture and application of chemical building material. China: Chemical Industry Press. [Google Scholar]
3. Yang, S. (1999). Thermal insulation and soundproofing building material. China: China Planning Press. [Google Scholar]
4. Ma, M. (1997). Essential measures to solve efflorescence of magnesium oxychloride cement products. Guangxi Sciences, 4(3), 238–240. [Google Scholar]
5. An, G. (2006). A review on cement-bonded wood panel. Liaoning Building Material, 3, 33–37. [Google Scholar]
6. Tu, P. (2007). Cement excelsior board and its manufacture technology. China Forest Products Industry, 34(2), 34–37. [Google Scholar]
7. Guo, X., Zhu, Z., Wang, J., Lin, Y., Chen, G. (2020). Dimensional stability of glass fiber reinforced poplar scrimber. Materialwissenschaft und Werkstofftechnik, 51(10), 1364–1371. DOI 10.1002/mawe.202000001. [Google Scholar] [CrossRef]
8. Wang, H., Xu, Q., Zhang, M. (1994). Analysis on water resistance methods for magnesia-bonded products. Shandong Build Material, 64(5), 14–15. [Google Scholar]
9. Chen, X., Cao, J., Liu, Y. (1996). Looking into the ways of solving water tolerant behavior of magnesite materials. Hunan Chemical Industry, 26(3), 34–37. [Google Scholar]
10. Feng, Q., Cui, C., Gao, D., Hou, L., Tong, G. (2002). Analysis of frosting on magnesia product surface and its depressing measures. Multipurpose Utilization of Mineral Resources, 5(10), 17–20. [Google Scholar]
11. Matkovic, B., Young, J. F. (1976). Microstructure of magnesium oxychloride cements. Nature Physical Science, 246(153), 79–80. DOI 10.1038/physci246079a0. [Google Scholar] [CrossRef]
12. Rogic, V., Matkovic, B. (1972). Phase in magnesium oxychloride cements. Cement, 16, 61. [Google Scholar]
13. Deng, D., Zhang, C. (1996). Theoretical mechanism of hygroscopicity of magnesium oxychloride cement and technical methods to lower it. New Building Materials, 33(10), 24–27. [Google Scholar]
14. Na, B., Wang, Z. P., Wang, Z. Q., Ding, T., Huang, R. et al. (2014). Study on hydration mechanism of low density magnesia-bonded wood wool panel. Wood Research, 59(1), 137–148. [Google Scholar]
15. Xia, S., Wang, J., Huang, J., Chen, R. (1994). Influence of additives on dimensional stability of magnesium oxychloride cement. Journal of Salt and Chemical Industry, 23(6), 18–22. [Google Scholar]
16. Deng, D. (2003). The mechanism for soluble phosphates to improve the water resistance of magnesium oxychloride cement. Cement and Concrete Research, 33(9), 1311–1317. DOI 10.1016/S0008-8846(03)00043-7. [Google Scholar] [CrossRef]
17. Li, Z. J., Chau, C. K. (2007). Influence of molar ratios on properties of magnesium oxychloride cement. Cement and Concrete Research, 37(6), 866–870. DOI 10.1016/j.cemconres.2007.03.015. [Google Scholar] [CrossRef]
18. Zhou, Z., Chen, H., Li, Z., Li, H. (2015). Simulation of the properties of Mgo-MgfCl2-H2O system by thermodynamic method. Cement and Concrete Research, 68(4), 105–111. DOI 10.1016/j.cemconres.2014.11.006. [Google Scholar] [CrossRef]
19. Huang, R., Zhang, X., Chen, Z., Wan, M., Wu, Q. (2020). Thermal stability and flame resistance of the coextruded wood-plastic composites containing talc-filled plastic shells. International Journal of Polymer Science, 1–9. [Google Scholar]
20. Ji, Y., Wu, Z., Zhang, F. (1995). Influence of the additive on the microstructure and performance of the new water-resisting magnesium cement. Journal of Inorganic Material, 10(2), 241–247. [Google Scholar]
21. Yao, C. (1994). Study on dehalogenation and efflorescence of magnesia products. Silicate Building Products, (3), 11–12. [Google Scholar]
22. Jiang, J., Luo, Y., Lan, X. (2002). Novel thermal insulation and soundproofing building material. China: Chemical Industry Press. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |