
Materials

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2021.014904
ARTICLE
Biodegradable Behavior of Waste Wool and Their Recycled Polyester Preforms in Aqueous and Soil Conditions
1Nanostructured and Advanced Materials, Chemicals Cluster, CSIR, Pretoria, 0001, South Africa
2Department of Chemistry, Nelson Mandela University, Port Elizabeth, 6001, South Africa
3Department of Clothing and Textile Technology, Faculty of Engineering and the Built Environment, Cape Peninsula University of Technology, Bellville Campus, Cape Town, 7535, South Africa
*Corresponding Authors: Sudhakar Muniyasamy. Email: smuniyasamy@csir.co.za; Asis Patnaik. Email: patnaika@cput.ac.za
Received: 06 November 2020; Accepted: 19 February 2021
Abstract: Present study deals with the biodegradable behavior of individual components and their preforms of nonwoven biocomposites developed from waste wool fibers including coring wool (CW), dorper wool (DW) and recycled polyester fibers (RPET). A respirometric technique was employed to estimate the production of CO2 during the biodegradation experiments under soil and aqueous media conditions. Functional groups of test samples before and after biodegradation were analyzed using Fourier transform infrared spectroscopy (FTIR). Leaching chemicals such as formaldehyde (hydrolyzed) and Chromium VI (Cr VI) was also measured. The CO2 emission in wool fibers CW and DW indicated 90% and 60% biodegradation in soil burial and aqueous media conditions respectively, for 100 days incubation. RPET fibers, 20% and 10% biodegradation in soil burial and aqueous media conditions was measured respectively while the preforms of waste wool and RPET reflected 30% and 25% biodegradation in soil burial and aqueous media conditions, respectively. The degradation of end functional groups such as carbonyl (keto and ester), aldehyde and hydroxyl were also confirmed by FTIR. The DW and CW wool fibers showed higher Cr(VI) concentration as compared to the RPET. The released formaldehyde results showed higher concentration for RPET preforms as compared to waste wool preforms. These results suggest that waste wool preforms are extremely environment friendly as compared to RPET preforms. Thus, waste wool preforms it can be potentially utilized for preparing biocomposite materials and associated biobased products.
Keywords: Biocomposites; biodegradation; leaching chemicals; synthetic polyesters; waste wool fibers
Biobased materials and its composite products made from natural fibers and polymers have attracted considerable attention because of their ability to reduce environmental pollution by allowing degradation of materials in soil and compost conditions. The plant based natural fibers are easily biodegradable, have minimum effect on the environment and they have been explored as new sources of raw materials to produce composite materials [1–4]. The main advantages of these materials are low cost, abundantly available, renewable, environmental friendly and low carbon footprint. However, the hydrophilic characteristics of these natural fibers make low adhesion between hydrophobic polymer matrix, which cause rapid deterioration, and loss of strength of the composite material [5,6]. Natural fibers from animal sources like waste wool obtained from sheep consisting of short fibers which are not suitable to make apparel fabrics. Waste wool is considered to have a lower environmental impact than other natural fibers as it requires less energy to process and dispose [7]. In nature, microorganisms like dermatophytic fungi, Bacillus and Streptomyces bacteria degrade wool slowly and the degradation mainly depends on abiotic (pH, temperature, sunlight) and biotic (soil, compost and aqueous) environmental conditions. About 70% wool fibers are degraded in 100 days under controlled composting conditions [7]. The wool rate of biodegradation is influenced by the soil composition, pH, oxygen and temperature. During degradation, wool nutrients become available to the plants. Waste wool can be mixed with other building materials to make affordable composite building materials such as mats. Wool geotextiles are used to cover soil to minimize moisture evaporation and provide thermal protection for plants [8]. Waste wool can also be used as a filtration material as it is able to react with volatile organic compounds and formaldehyde. It can also be used as a fertilizer when it degrades [9].
In recent years, many research papers have been published dedicated on the biodegradation studies of polymeric materials derived from natural and synthetic origin. In literature, one of the most commonly used technique is weight loss of the polymeric samples, however, the weight loss measurement of the samples are not directly related with true biodegradation, i.e., measuring polymeric carbon conversion into CO2. Weight loss of polymeric materials is a primary degradation step breaking the long chain molecular into smaller oligomers and monomers influenced by various environmental abiotic (heat, UV, humidity) or biotic factors (enzymatic), and both. Monitoring CO2 release from polymeric materials action by microorganisms is direct evidence of ultimate biodegradation (mineralization) in contrast to just deterioration or disintegration and weight loss [10]. This CO2 biodegradation test method is globally accepted by established standards such as ISO, ASTM and European normative for claiming environmental friendly polymeric materials. Therefore, the present study CO2 biodegradation techniques was followed for studying biodegradation of wool fibers in soil and aqueous media conditions.
Synthetic polymers containing structural units of typical terephthalic polyesters (PET) are being widely used in man-made fibers for apparel, technical textiles and composite materials [11]. The biodegradability of natural and synthetic polymers depends on various physical and chemical configuration including internal structure, hydrophilic characteristics, thickness and also environmental factors where these are disposed such as land fill, compost, soil and aqueous media conditions [12]. The biodegradability of materials and products cannot be predicted based on the raw materials whether it is bio-derived or synthetic origin. To claim biodegradable materials and products, it requires CO2 measurement to validate the complete biodegradation that leaves no toxic residues in a defined period as per the relevant standard test methods [13–15]. For this reason, every new product is necessary to be verified in their biodegradability in natural environmental conditions [13–15].
The aim of this study was to investigate the potential biodegradation behaviors of raw materials (fibers) and preforms developed from these fibers (waste wool and recycled polyester (RPET) by measuring the CO2 evolution from these test samples under soil and aqueous media conditions. Fourier transform infrared spectroscopy (FTIR) functional groups characterization of test samples before and after biodegradation were analyzed for identifying the degradation mechanisms. Also, formaldehyde (free and hydrolyzed) and Chromium VI content were analyzed to determine any carcinogenic compounds leached from the test samples.
In this study, two different sheep breed waste wool fibres coring wool (CW) and dorper wool (DW) were obtained from Eastern Cape Province, South Africa. Recycled polyethylene terephthalate (RPET) was obtained from PETCO, South Africa. The chemicals 1, 5-diphenylcarbazide, chromium hexavalent (CrVI), potassium hydroxide (KOH), hydrochloric acid (HCl), barium chloride (BaCl2), phenolphthalein and other synthetic media used in this work were analytical grade obtained from Sigma-Aldrich, South Africa.
Various preforms in the form of nonwoven mats were prepared from waste wool and RPET fibers. For preparing the nonwoven mat, each individual fibres was prepared using needle punching equipment (Tab. 1). The following specification of fibres were used in this study: CW-diameter 20.7 μm, staple length 22 mm; DW-diameter 28.6 μm, staple length 38 mm; and RPET-a linear density of 6.7 denier per filament, staple length 32 mm. Nonwoven mat of 100% pure CW and DW were prepared without any RPET. Similarly, 100% pure RPET fibre non-woven mat was also prepared. In another set, nonwoven mats of CW/RPET (50/50%) and DW/RPET (50/50%) were also prepared. All these mats are nominal area weight of 1000 g/m2 and having thickness ranges from between 15–17 mm (Tab. 1). All the test samples were conditioned for 24 h prior to testing in a standard testing atmosphere maintained at 65 ± 5% humidity and 20 ± 2°C temperature.
Table 1: Nonwoven sample compositions and its thickness. Adapted with permission from Ref 7, copyright 2021, Elsivier
The biodegradation of nonwoven test samples CW, DW, RPET and DWP and CWP was performed in soil and aqueous media conditions. The test samples microbial assimilation conversion to carbon dioxide (CO2) were evaluated by respirometric technique.
For soil burial biodegradation test, a fresh agricultural field surface soil was collected at CSIR Port Elizabeth campus, South Africa. Tab. 2 illustrates the physical-chemical analysis of the soil used in this study.
Table 2: Physical and Chemical analysis of the soil material
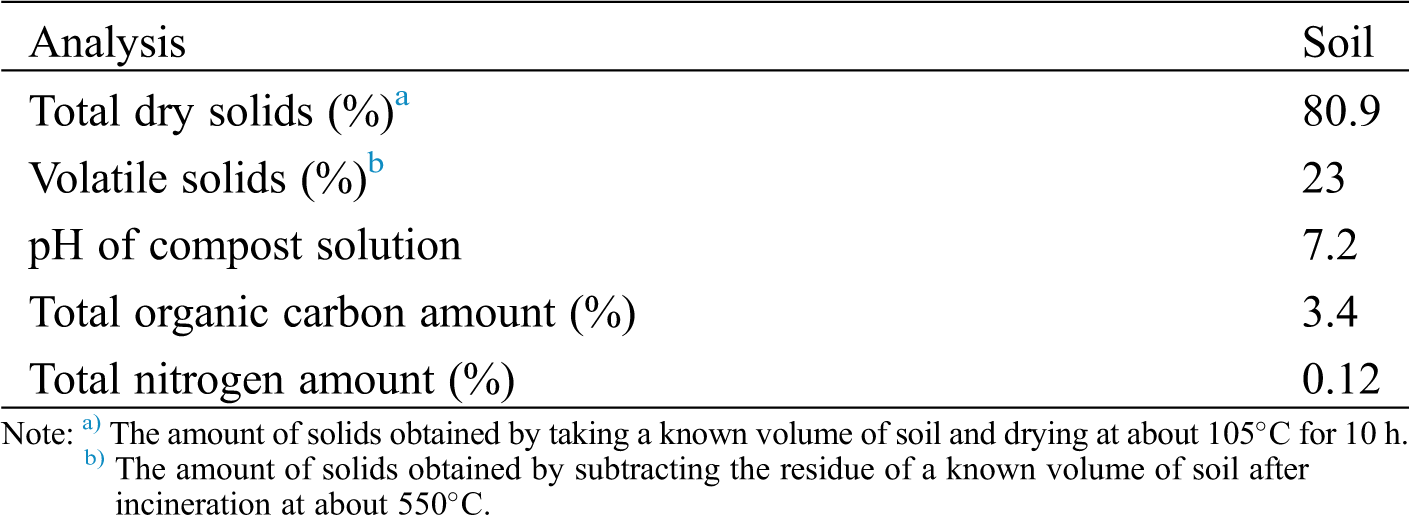
The fresh soil collected was sieved into ≤ 1 mm. Further, it was mixed with finely powdered perlite in 1:1 dry weight ratio to maintain the humidity and to keep the biometer flask in aerobic conditions, as well as to eliminate noise variations. Fig. 1 shows the schematic diagram of soil biodegradation set up and 1 L capacity biometer flask with air tight seal setup used in this study.
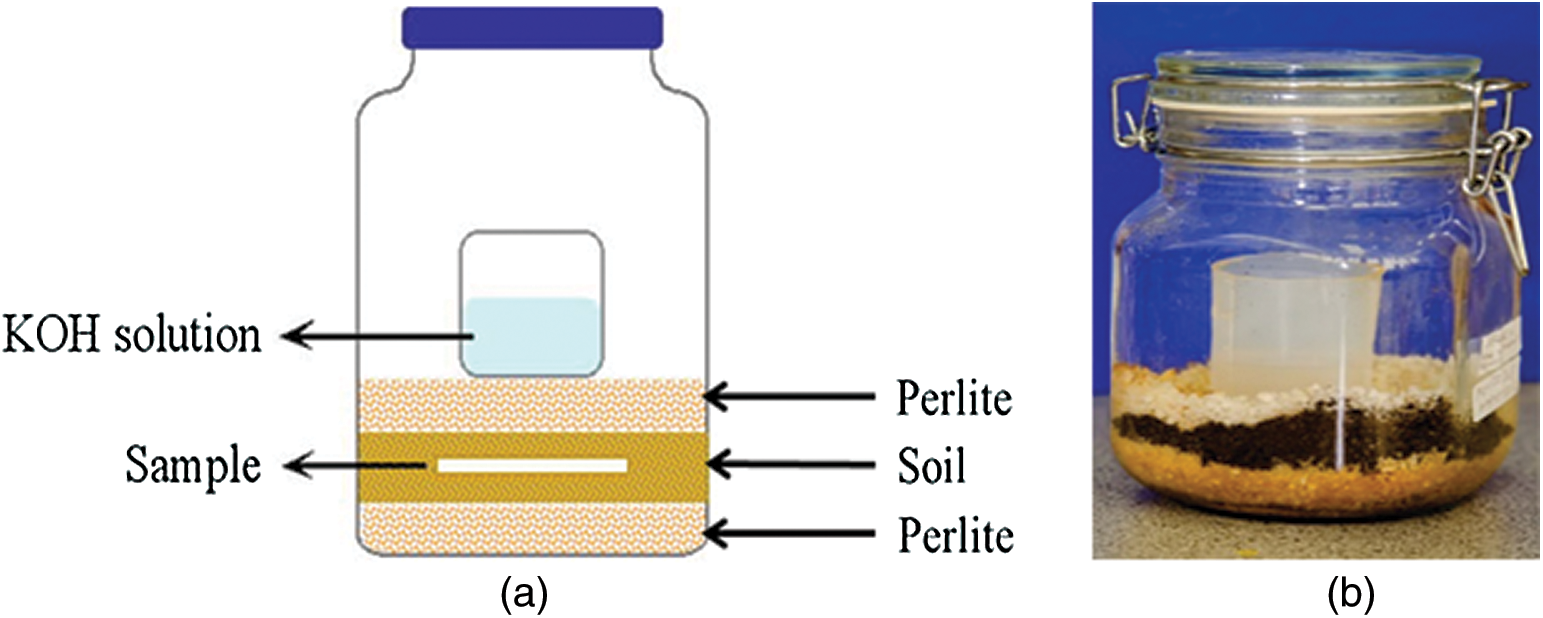
Figure 1: a) Schematic diagram of biodegradation set up and b) a real biometer flask respirometric system used for studying the biodegradation of test samples in soil condition
In this biodegradation set up, 15 g perlite wetted with 15 ml distilled water was placed in the bottom layer, followed by 100 gram of soil/perlite in the middle layer. Approximately 16 mg of test sample to 1 gram dry soil ratio was buried in the middle layer. Thereafter, another 15 gram of perlite wetted with 15 ml of distilled water was placed on the upper layer. A glass beaker filled with 40 ml 0.1 M KOH was placed on the top of upper layer of reactor to trap the CO2 evolved from test samples. Three replicates for each test sample including blank (soil without test sample) and a known biodegradable material microcrystalline cellulose was also tested as positive reference. These biometer flasks were kept in room temperature at 23–25°C in dark condition, which simulates the conditions of real soil environment. At every 1–3 day interval, the KOH solution was removed from reactor with adding 1 mL of 1 N BaCl2 solution to covert soluble K2CO3 into insoluble BaCO3. The solution was back titrated with 0.1 N HCl by adding a drop of phenolphthalein as an indicator. After titrations, each beaker washed and refilled with fresh standard 0.1 N KOH solution.
2.3.2 Aqueous Biodegradation Test
The aqueous biodegradation of test materials was conducted in a synthetic mineral salt aqueous medium at pH 7.4 ± 0.2 using 250 mL bioreactor (Fig. 3). The mineral salt aqueous medium contains the following composition in per litre distilled water: Na2HPO4 (334 mg), K2HPO4 (218 mg), CaCl2 (72 mg), KH2PO4 (35 mg), MgSO4 7H2O (23 mg), (NH4)2 SO4 (10 mg), NH4 NO3 (10 mg) and FeCl6 H2O (0.3 mg). For the aqueous biodegradation test, microbial inoculum derived from activated sludge of wastewater treatment plant was collected at Port Elizabeth, South Africa. 1 ml of microbial inoculum was filtered using whatman filter paper and then inoculated into each 100 ml sterilized mineral salt medium bioreactor. After adding microbial inoculum to the bioreactor, a plastic vial with series of holes containing 20 ml of 0.1 M KOH was used to trap the CO2 evolved from the biodegradation process as shown in Fig. 2a. Each test sample (mineral salt medium + microbial inoculum + test sample), blank control (mineral salt medium + microbial inoculum) and a known biodegradable water soluble polymer aniline was also tested as positive reference. All test samples were tested in three replicates and these bioreactor flasks were kept in room temperature at 23–25°C in the dark condition, which simulates the conditions of real aqueous environment. At every 1–3 day intervals, the KOH solution containing trapped CO2 was back titrated with 0.1 N HCl by adding 1 mL of 1 N BaCl2 solution to covert soluble K2CO3 into insoluble BaCO3 by adding a drop of phenolphthalein as an indicator. After titrations, each beaker washed and refilled with fresh standard 0.1 N KOH solution.
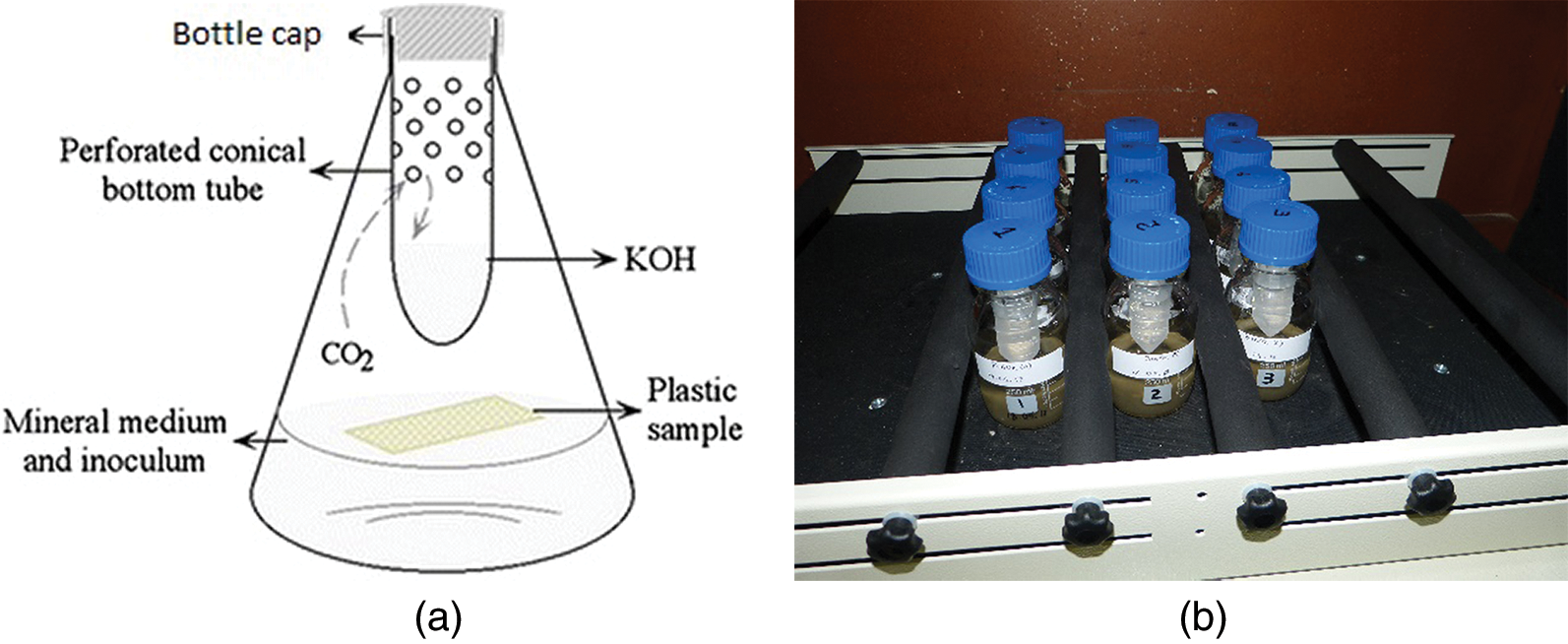
Figure 2: (a) Schematic diagram of biodegradation set up and (b) a real bioreactor respirometric system used for studying the biodegradation of test samples in aqueous medium
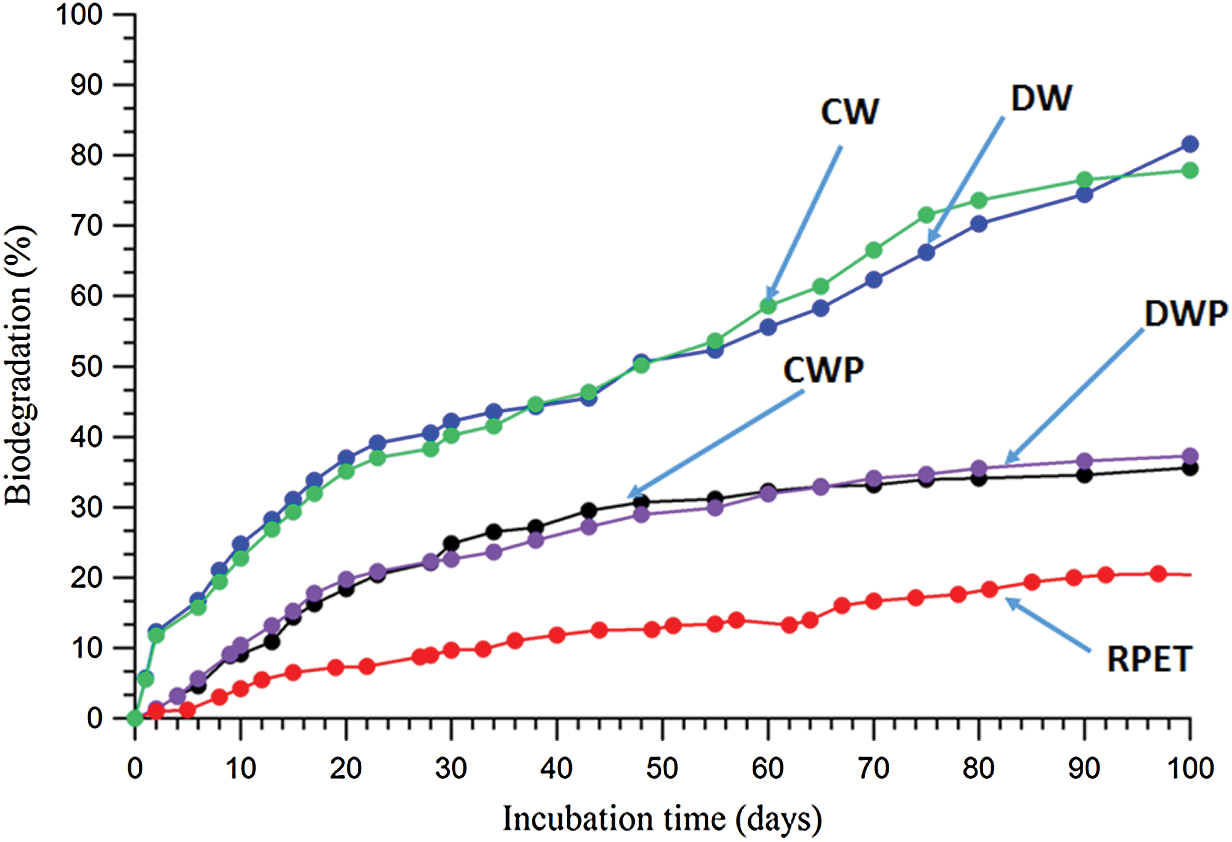
Figure 3: CO2 biodegradation results of test samples (CW, DW, RPET, CWP and DWP) in soil burial conditions
2.3.3 Determination of CO2 and Evaluation of Percentage Biodegradation
The following chemical reactions and equations were followed to measure the percentage biodegradation (Eqs. (1)–(5)).
Tc is total of carbon (g) present in the test sample analysed by elemental analysis.
Tw is the weight (g) of test sample used for the biodegradation test.
ThCO2 is the theoretical CO2 that test sample contains.
The percentage biodegradation was calculated based on the below Eq. (5):
2.4 Fourier Transform Infrared Spectroscopy (FTIR) Analysis
FTIR technique was utilized to collect IR spectra of the test samples before and after biodegradation. The total reflectance of the fabric test samples was analyzed on a PerkinElmer IR spectrometer with 32 scans and IR information for the sample was collected and processed with OMNIC software. After 1 month soil biodegradation, a small amount of test samples were taken and carefully washed with distilled water to remove soil and dried at 40°C for 8 h for measuring the spectra.
2.5 Determination of Leach Chemicals from Fibers
In this work, soluble chromium (Cr) (VI) and formaldehyde (hydrolyzed) were analyzed in the test samples if any carcinogenic compounds leached from the test samples. Soluble Cr(VI) leached from the test sample in aqueous solution at pH 5.5 was analyzed by solid phase extraction method as per the BS EN ISO 17075 test method. Perkin Elmer Spectrometer (UV, visible and NIR) was used to measure the Cr(VI) concentration in solution that oxidizes 1, 5-diphenylcarbazide to 1, 5-diphenylcarbazone to give a red/violet complex with chromium which can be quantified spectrophotometer absorbance at 540 nm. Similarly, the amount of formaldehyde was measured by extracting the fiber samples using a mild detergent solution and a known aliquots solution was measured at absorbance 412 nm using spectrometric techniques.
In this work, the biodegradation behaviors of raw materials (fibers) and preform of nonwoven biocomposites made from waste wool fibers such as CW and DW and RPET fibers were tested under aerobic soil burial and aqueous media conditions using respirometric CO2 evolution methods. Figs. 3 and 4 show the percent biodegradation of test samples in aerobic soil burial and aqueous media conditions and conversion of carbon to evolved CO2 has been studied.
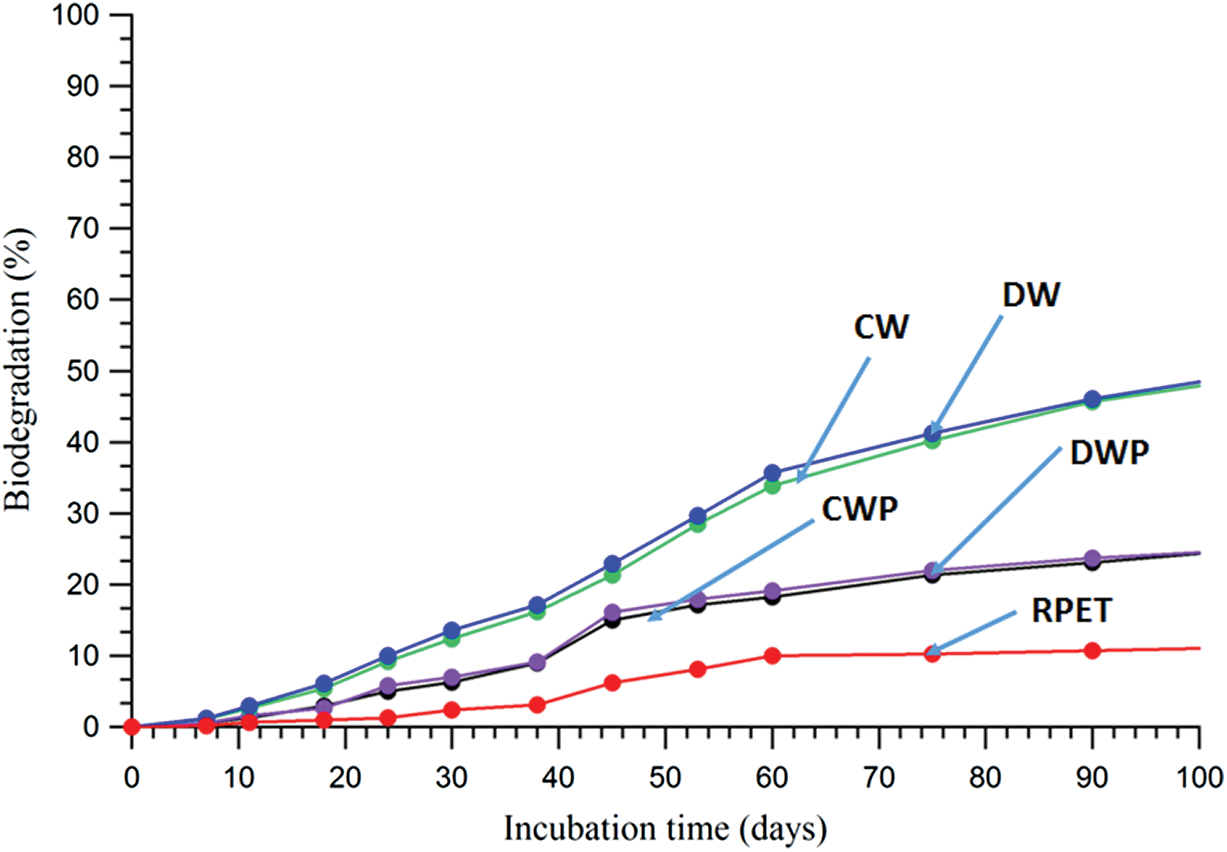
Figure 4: CO2 biodegradation results of test samples (CW, DW, RPET, CWP and DWP) in aqueous media conditions
The obtained test results showed that waste wool fibers CW and DW undergo readily degraded by the action of microorganisms present in the soil (Fig. 3). There was an accelerated biodegradation in the first 30 days reaching 40% biodegradation, thereafter a slow exponential phase degradation occurred reaching 90% within 100 days incubation under soil medium conditions. The first acceleration phase could be due to the soil microorganisms degrades the waste wool fibers low molecular weight compounds and simultaneously secretes enzymes to breakdown the remaining hydrophilic fibers into low molecular weight compounds for the second step microbial assimilation process. Also, it is important to mention that the rate of waste wool degradation which was mainly influenced by its chemical compositions made from keratin. The enzymatic degradation of proteins may occur readily for fatty acids and delay of degradation which could be due to the cross linked structure of keratin, which has high concentration of sulphur crosslinks [8]. In contrast, the man-made RPET fibers showed a lag phase in the first 8–9 days incubation where there was no microbial activities but after that there was a slight increase in the CO2 production reaching 20% biodegradation in 100 days. It is well known that conventional polyester is not readily biodegradable due to the physical and chemical properties such as high molecular weight, hydrophobicity and crystallinity. These physical-chemical factors severely influence the rate of biodegradation in the adopted soil and aqueous medium conditions. On the other hand, the preforms of CWP and DWP showed a lag phase in the 5–7 days incubation where the soil microorganisms were acclimatized to the conditions to degrade and assimilate to final products. A slight exponential phase reached 30% biodegradation in 100 days. The obtained CWP and DWP results were mainly due to the degradation of waste wool fibers and not from other man made RPET fibers (Fig. 3).
Fig. 4 shows the aqueous biodegradation results of test samples CW, DW, RPET, CWP and DWP. The test samples of waste wool fibers CW and DW showed a lag phase in 11 days where there was no microbial degradation in the adopted aqueous conditions. This could be due to the microorganism acclimatization stage to the environment. After 11 days, there was a slight exponential phase reaching 40% in 60 days, followed by a slow CO2 production where it reached 55% within 100 days incubation. The man-made RPET fibers showed a lag phase in the first 30 days, thereafter a slow biodegradation reaching 10% for 60 days and approached a stationary phase where no CO2 emissions. However, an exponential phase was observed for the preforms CWP and DWP test samples approaching 20% biodegradation in 100 days incubation. The obtained results suggested that soil conditions is more vulnerable for the biodegradation of waste wool fiber based materials as compared to aqueous condition. Volova et al. [16] reported that the rate of biodegradation in compost and soil is higher, which mainly due to abiotic conditions temperature is influenced for degradative reactions as compared to costal tropical water. Moreover, the concentration and diversity of microbial communities are higher in soil as compared to aqueous media. Therefore, the present study the slow rate of biodegradation under aqueous media could be mainly due to low concentration and microbial species presence.
Fig. 5 shows the FTIR results of test samples before and after 30 days soil burial biodegradation condition. The FTIR characterization of waste wool fibers CW and DW showed a prominent changes in the spectra in the intensities range of 3000 cm−1–3600 cm−1 corresponding to amide and hydroxyl groups and 1000 cm−1 to 1650 cm−1 corresponding to carbonyl groups (Tab. 3). The FTIR of CW wool fibers before biodegradation showed higher intensities in the regions of 3100–3500 cm−1 and 1600–1800 cm−1 which is mainly due to amino acids present corresponding to amide hydrogen and hydroxyl groups. The FTIR of CW fibers after biodegradation showed significant reduction of peaks intensities. These results represent that the initial functional groups present in the waste wool were attacked by soil microorganisms through hydrolytic enzymatic processes into water soluble compounds where it can be easily assimilated by the microorganisms for converting them into new biomass (Fig. 5a). The FTIR of DW wool fibers after biodegradation showed significant increase in the regions of 3100–3500 cm−1 and 1600–1800 cm−1 which could be mainly due to primary degradation of longer chain molecules into smaller water soluble compounds by enzymatic hydrolysis process (Fig. 5b). On the other hand, man-made RPET was not severely affected in the functional groups, however, there was slight increases in the absorbance of 1650 cm−1 carbonyl keto and ester groups formation. These increases in carbonyl functional groups represent the absorption of water molecules, which may easily attacked by abiotic and biotic, factors (Fig. 5c). Moreover, the increased absorbance also represent the crystalline structural molecules change into amorphous phase, which means that test materials were degraded. The same phenomena was shown in preforms of CWP and DWP, major degradation in the waste wool fibers CW and DW components, but no significant changes in the RPET fibers (Figs. 5d and 5e).
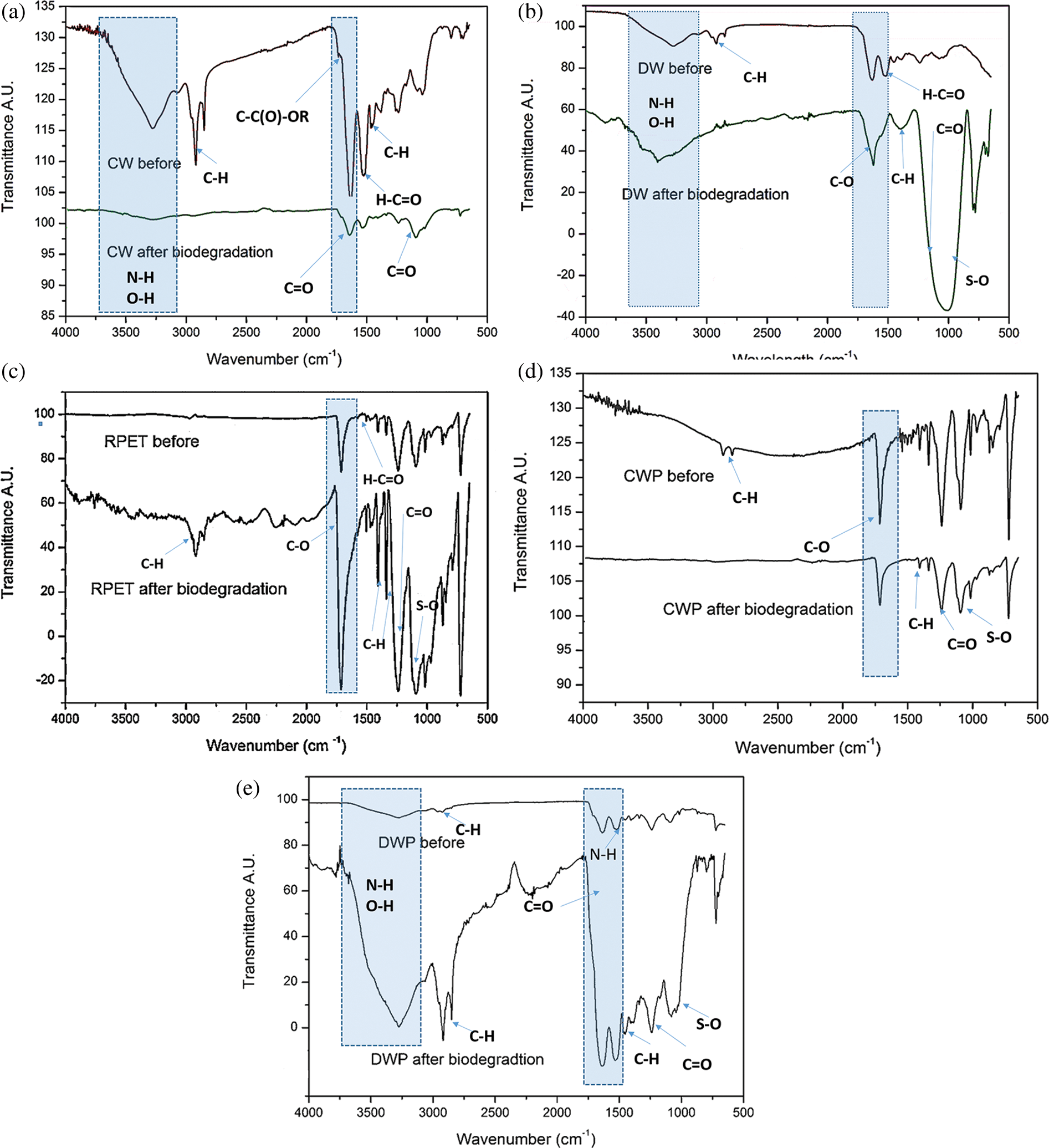
Figure 5: FTIR results of before and after biodegradation–waste wool fibers a) CW, b) DW; man-made fiber c) RPET; preforms d) CWP and e) DWP
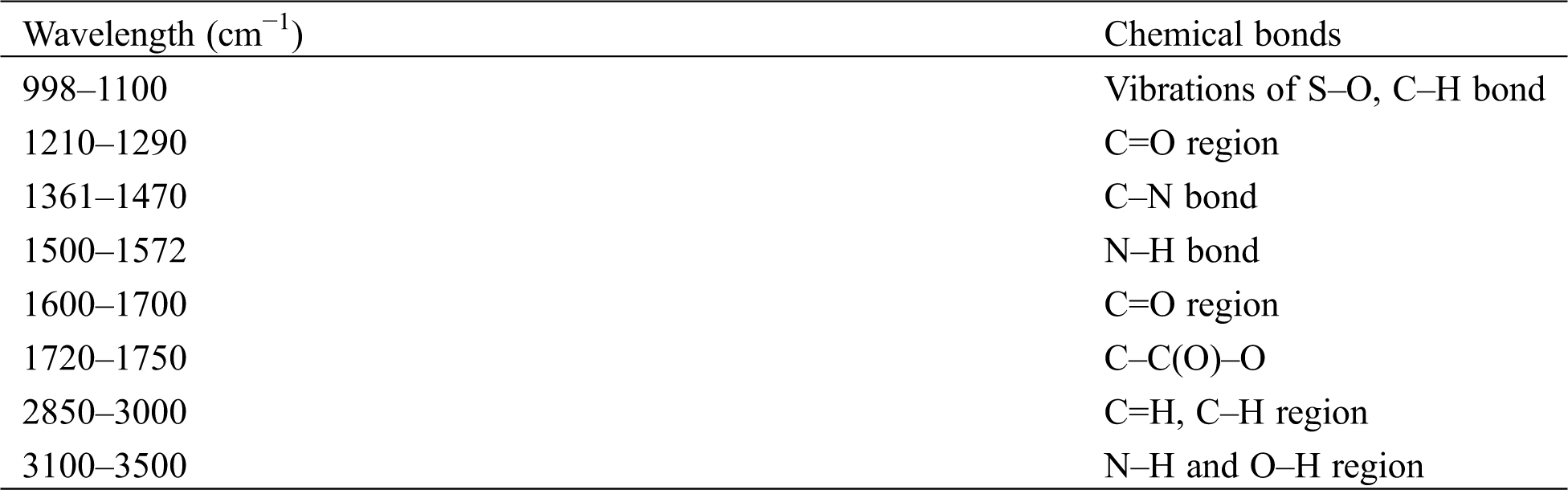
Table 3: FTIR results of functional groups present in wool fibers and synthetic fibers [17]
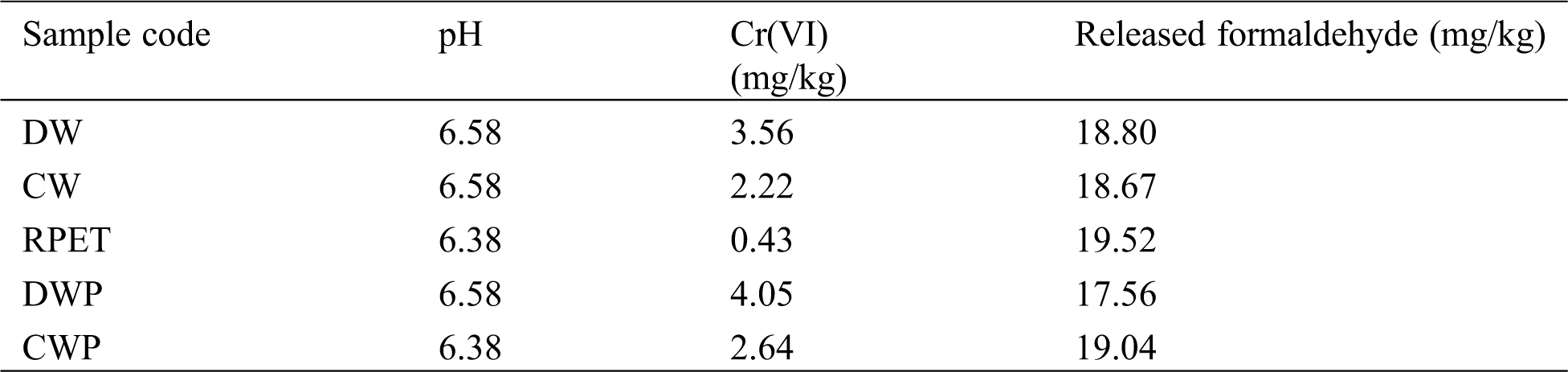
Harmful chemical substances such as Cr(VI) and formaldehyde content in the textile and nonwoven products are necessary to be evaluated in order to determine harmful substances. Tab. 4 shows leaching chemicals Cr(VI) and hydrolyzed formaldehyde present in these test samples. The Cr(VI) is being used in textile industry as pigments in dyes, as anticorrosive agents for surface coatings, protective coating and other applications. The Cr oxidizing groups are toxic as well as carcinogenic and the use of hexavalent chromium in textiles prohibited by the restriction of hazardous substances directive. With these aspects, the test carried out on individual fiber components and preforms showed that, Cr(VI) component was slightly higher for DW (3.56 mg/kg) as compared to CW and RPET fibers (Tab. 4). Similarly, released formaldehyde from RPET showed highest (19.52 mg/kg), whereas for waste wool fibers (CW), it was 18.67 mg/kg. The released formaldehyde from waste wool preform was 17.56 mg/kg, which is lowest among the samples. These results suggest that waste wool preforms are more environment friendly as compared to the RPET preforms where it can be potentially used for design and preparation of non-woven biocomposites materials and biobased products.
Table 4: Leaching chemicals present in the test samples
The biodegradation behaviors of RPET and waste wool preforms were reported in this paper. Wool fibers (DW and CW), synthetic recycled polyester fibers and their preforms (DWP and CWP) were studied in soil and aqueous conditions by measuring the CO2 evolution. The functional groups of these materials before and after biodegradation were evaluated by FTIR spectroscopy. The leaching chemicals were evaluated in forms of released formaldehyde (mg/kg) and Cr(VI) (mg/kg) as per standard test methods. The waste wool fibers showed 90% biodegradation behaviors in soil and 60% biodegradation in aqueous media conditions for 100 days but no signification degradation in the RPET fibers. The slow rate of degradation of RPET could be due to its physical-chemical composition as well as treatment involved during recycling of fibers. The released formaldehyde content of RPET showed highest (19.52 mg/kg) as compared to waste wool preforms (17.56 mg/kg). The results suggested that waste wool fibers based preforms (DWP and CWP) are identical candidates to develop eco-friendly biocomposites materials and products.
Funding Statement: The author disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is based on the research supported in part by the National Research Foundation of South Africa (Grant-specific unique reference numbers (UID) 104840.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Dixit, S., Goel, R., Dubey, A., Shivhare, P. R., Bhalavi, T. (2017). Natural fiber reinforced polymer composite materials-A review. Polymers from Renewable Resources, 8(2), 71–78. DOI 10.1177/204124791700800203. [Google Scholar] [CrossRef]
2. Muniyasamy, S., Ofosu, O., Thulasinathan, B., Rajan, A. S. T., Ramu, S. M. et al. (2019). Thermal-chemical and biodegradation behaviour of alginic acid treated flax fibers/poly (hydroxybutyrate-co-valerate) PHBV green composites in compost medium. Biocatalysis and Agricultural Biotechnology, 22, 101394. DOI 10.1016/j.bcab.2019.101394. [Google Scholar] [CrossRef]
3. Bhagwat, G., Gray, K., Wilson, P. S., Muniyasamy, S., Vincent, S. G. T. et al. (2020). Benchmarking bioplastics: A natural step towards a sustainable future. Journal of Polymers and the Environment, 28, 3055–3075. DOI 10.1007/s10924-020-01830-8. [Google Scholar] [CrossRef]
4. Vivekanandhan, S., Pin, J. M., Misra., M. (2018). Composites from renewable and sustainable resources: Challenges and innovations. Science, 362(6414), 536–542. DOI 10.1126/science.aat9072. [Google Scholar] [CrossRef]
5. Abdullah, N. M., Ahmad, I. (2013). Potential of using polyester reinforced coconut fiber composites derived from recycling polyethylene terephthalate (PET) waste. Fibers and Polymers, 14(4), 584–590. DOI 10.1007/s12221-013-0584-7. [Google Scholar] [CrossRef]
6. Pothan, L. A., Thomas, S. (2003). Polarity parameters and dynamic mechanical behaviour of chemically modified banana fiber reinforced polyester composites. Composites Science and Technology, 63(9), 1231–1240. DOI 10.1016/S0266-3538(03)00092-7. [Google Scholar] [CrossRef]
7. Patnaik, A., Mvubu, M., Muniyasamy, S., Botha, A., Anandjiwala, D. R. (2015). Thermal and sound insulation materials from waste wool and recycled polyester fibers and their biodegradation studies. Energy and Buildings, 92, 161–169. DOI 10.1016/j.enbuild.2015.01.056. [Google Scholar] [CrossRef]
8. Przybyło, S., Kobiela-Mendrek, K., Biniaś, D., Rom, M., Grzybowska-Pietras, J. et al. (2016). Biodegradation of sheep wool geotextiles. International Biodeterioration & Biodegradation, 115, 31–38. DOI 10.1016/j.ibiod.2016.07.012. [Google Scholar] [CrossRef]
9. Zoccola, M., Montarsolo, A., Mossotti, R., Patrucco, A., Tonin, C. (2015). Green hydrolysis as an emerging technology to turn wool waste into organic nitrogen fertilizer. Waste and Biomass Valorization, 6(5), 891–897. DOI 10.1007/s12649-015-9393-0. [Google Scholar] [CrossRef]
10. Narayan, R. (2006). Biobased and biodegradable polymer materials: Rationale, drivers, and technology exemplars. Degradable Polymers and MaterialsUSA: ACS Symposium Series. [Google Scholar]
11. Rawal, A., Anandjiwala, R. (2007). Comparative study between needlepunched nonwoven geotextile structures made from flax and polyester fibers. Geotextiles and Geomembranes, 25(1), 61–65. DOI 10.1016/j.geotexmem.2006.08.001. [Google Scholar] [CrossRef]
12. Mohanrasu, K., Gada, A., Mokhena, T. C., Mtibe, A., Boobalan, T. et al. (2019). Biobased biodegradable polymers for ecological applications: A move towards manufacturing sustainable biodegradable plastic products. Integrating Green Chemistry and Sustainable Engineering, USA: Scrivener Publishing LLC. [Google Scholar]
13. Zumstein, M. T., Narayan, R., Kohler, H. P. E., McNeill, K., Sander, M. (2019). Dos and do nots when assessing the biodegradation of plastics. Environmental Science and Technology, 53(17), 9967–9969 DOI 10.1021/acs.est.9b04513. [Google Scholar] [CrossRef]
14. Mayekar, P. C., Aguirre, E. C., Auras, R., Selke, S., Narayan, R. (2020). Effect of nano-clay and surfactant on the biodegradation of poly(lactic acid) films. Polymers, 12(2), 311. DOI 10.3390/polym12020311. [Google Scholar] [CrossRef]
15. Muniyasamy, S., Ofosu, O., John, M. J., Anandjiwala, R. D. (2016). Mineralization of poly(lactic acid) (PLApoly(3-hydroxybutyrate-co-valerate) (PHBV) and PLA/PHBV blend in compost and soil environments. Journal of Renewable Materials, 4(2), 133–145. DOI 10.7569/JRM.2016.634104. [Google Scholar] [CrossRef]
16. Volova, T. G., Boyandin, A. N., Vasiliev, A. D., Karpov, V. A., Prudnikova, S. V. et al. (2010). Biodegradation of polyhydroxyalkanoates (PHAs) in tropical coastal waters and identification of PHA-degrading bacteria. Polymer Degradation and Stability, 95, 1–10. DOI 10.1016/j.polymdegradstab.2010.08.023. [Google Scholar] [CrossRef]
17. McGregor, B., Liu, X., Wang, X. (2018). Comparisons of the Fourier transform infrared spectra of cashmere, guard hair, wool and other animal fibers. The Journal of the Textile Institute, 109(6), 813–822. DOI 10.1080/00405000.2017.1372057. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |