DOI:10.32604/jrm.2021.013626

| Journal of Renewable Materials DOI:10.32604/jrm.2021.013626 |  |
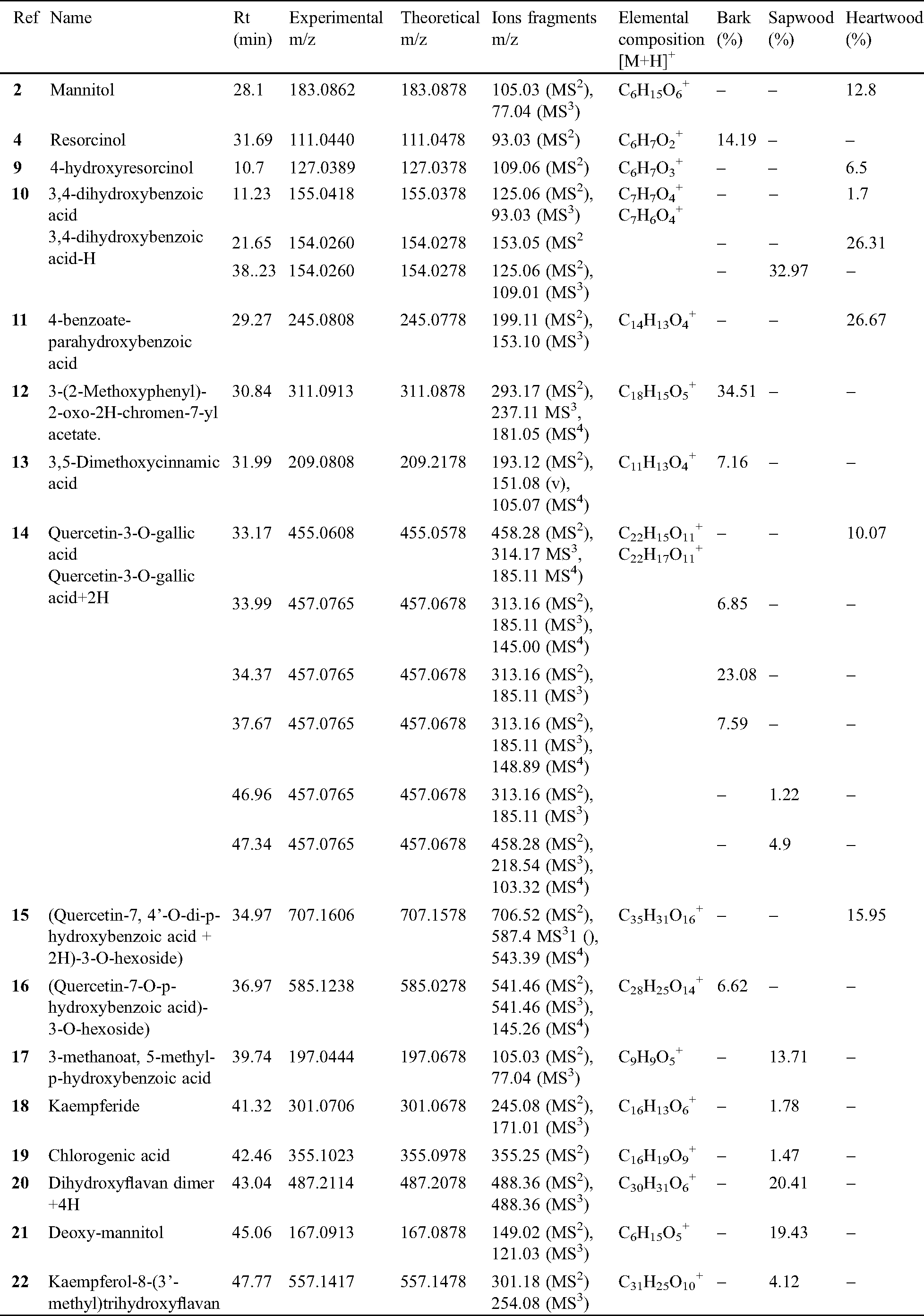
| Article |
Identification of Phenolic Compounds from K. ivorensis by Selected Chromatographic and Spectrometric Techniques
1CNRS, Université de Pau et des Pays de l’Adour, Institut des Sciences Analytiques et de Physico-Chimie pour l’Environnement et les Matériaux-Xylomat, UMR5254, Mont de Marsan, France
2Laboratoire de Recherche et de Valorisation du Matériau Bois (LaReVa Bois), Ecole Normale Supérieure d’Enseignement Technique (ENSET), Libreville, Gabon
3Laboratoire des Substances Naturelles et de Synthèses Organométalliques (LASNSOM), Unité de Recherche en Chimie, Université des Sciences et Techniques de Masuku, Franceville, Gabon
4Institut Clément Ader (ICA), Université de Toulouse, CNRSUMR 5312-INSA-ISAE-Mines Albi-UPS, Tarbes, France
5CNRS, Univ Pau & Pays de l’Adour/ E2S UPPA, Institut des Sciences Analytiques et de Physicochimie pour l’Environnement et les Matériaux, UMR5254, Pau, France
*Corresponding Author: B. Charrier. Email: bertrand.charrier@univ-pau.fr
Received: 14 August 2020; Accepted: 19 October 2020
Abstract: A complementary approach using Liquid Chromatographic-Mass Spectrometric analysis was proposed to characterize phenolic compounds from the methanol-water extracts of K. ivorensis A. Chev. Two High Performance Liquid Chromatography (HPLC) complementary methods were used for the determination of phenolic compounds from the bark, sapwood and heartwood of K. ivorensis. Methods employed involved direct analysis after filtration at 0.20 µm, using a RP C18 column and UV-VIS/ESI-FTMS detection. The methods used were different by their elution gradient and allowed analyzing the chemical composition of three parts of African mahogany extracts. In this study, 22 phenolic compounds and derivatives from K. ivorensis were separated, determined or tentatively characterized for the first time based on their methanol/water/formic acid extract mass spectra. The difference of gradients eluted various compounds, 8 were obtained with the first method and 14 with the second one. The main products were hydroxybenzoic acid and derivatives, resorcinol, esterified compounds, mannitol, quercetin and derivatives, dihydroxyflavan, and trihydroxyflavan.
Keywords: Bark; RP_HPLC; ESI-FTMS; sapwood; heartwood; K. ivorensis; phenolic compounds
Wood is one of the main sources of biomass in the world. Energy sectors such as carbonization, methanization, wood pellets and bioenergy have been developed in Europe, America and Asia; giving added value to wood wastes. Wood is also a source of natural molecules that attracts large companies not only for its lignin, cellulose and hemicellulose but also for its extractives compounds, which possess virtues for cosmetic, pharmaceutical or agro-alimentary industries.
In underdeveloped countries of the third world, wood is mostly used as source of energy for cooking. A large amount of wood wastes rising up to 50% of the initial biomass in some industrial transformation of logs in sub-Saharan countries are mainly focused on slicing and rotary cutting for plywood mainly exported to developed countries. However, the main part of wood wastes is let in forest or just burned. Therefore, new insight for a suitable valorization of these African countries wood wastes as source of raw materials for wood based composites or molecules of interest is now investigated by various authors. In this order, various tropical wood wastes were already used in composites [1], their lignin and cellulose extracted and characterized [2] and the thermal stability of their tannins were investigated for future green adhesives [3]. Wood wastes from K. ivorensis slicing industry were not in rest. So, the chemical structure of condensed tannins extracted from that African mahogany was determined for the first time by Bikoro Bi Athomo et al. [4]. The hygrothermal stability and the potential of the mahogany wastes for new wood plastic composites were recently reported [5]. Although various phenolic compounds have been identified in the bark, sapwood and heartwood of many tropical woods, comprehensive studies on phenolic compound structures or tannins identification from tropical woods are scarcest [4–6] although some phenolic compounds like condensed tannins or related phenols are generally analyzed by MALDI-TOF and Fourier Transformed Infrared Spectroscopy (FTIR) or LC-MS [7]. To our knowledge, any African mahogany’s species have not been extensively studied for this purpose. Nevertheless, due their structural variability and complexity, it is difficult to do the mass analysis of polyphenols compounds [8].
Thus, the identification of K. ivorensis phenolic compounds profile is still lacking. High-resolution/accurate mass measurement mass spectrometry like Matrix Assisted Laser Desorption/Ionization coupled to Time of Flight (MALDI-TOF) or Electrospray-Fourier Transform Mass Spectrometry (ESI-FTMS) is a good technique for elucidating structure of unknown compounds. Linear ion trap quadrupole-Orbitrap-mass spectrometry (LTQ-Orbitrap-MS) provides single-stage mass analysis that supplies molecular weight information. Two-stage mass analysis (MS/MS) and multi-stage mass analysis (MSn) are usefull for structural information [9]. Exact mass measurements and molecular formula assignment are necessary for the characterization of phenolic compounds [10]. Moreover, accurate mass measurement of the product ions obtained after fragmentation facilitates the elucidation of unknown compounds.
Furthermore, difference in the structure of phenolic compounds is linked to their solubility in solvents of different polarity. Some studies such as that Zlotek et al. [11] have tried to give best conditions for extraction of phenolic compounds. Indeed, phenolic compounds present in biomass have received attention in recent years due to the different properties they have. It is in that case that the aim of this study was to analyze the methanol/water extracts [12] from K. ivorensis’s bark, sapwood and heartwood based on a new method of separation and isolation of that tropical wood’s phenolic compounds which have many health benefits [13].
All the chemicals used in this study were purchased from Fisher Scientific, Acros Organic (France) and Sigma Aldrich (France). Acetone (99.98%, Fisher Scientific), phosphoric acid (H3PO4, Fisher Scientific), formic acid (98.0%–100%, Fisher Scientific), methanol-HPLC (99.98%, Fisher Scientific). Ultrapure water (18 m Ω cm) was produced using a mQ system (Millipore, France).
The bark, sapwood and heartwood were collected from three K. ivorensis or African mahogany (AMG) trees, sampled from a disk section of 10 cm of thickness and 85 (AMG1 = tree 1) cm, 80 (AMG2 = tree 2) cm and 75 (AMG3 = tree 3) cm of diameter. Woods were harvested at Mitzic natural forest in the North of Gabon by the SNBG (Société Nationale des Bois du Gabon) in 2016 and 2017. The fresh samples were placed in sterilized bags, air-dried for one week in the laboratory and oven-dried (105°C) for 48 h. The dried samples were grinded to pass through 60 mesh (≈ 250 µm diameter) with a rotative knife grinder (Retsch SK1).
2.2.1 Extraction of Polyphenols at Room Temperature
350 mg of dried wood powder from each sample was mixed separately at room temperature with 30 ml of methanol/water/formic acid (4/0.9/0.1, v/v/v). The mixtures were stirred for 3 h. The obtained extracts were then filtrated on Coaster Nylon 0.20 µm before analysis.
2.2.2 High Performance Liquid Chromatography (HPLC) Analysis
Liquid chromatography analysis was performed using a Dionex Ultimate 3000 (Thermo Scientific, AcclamTM Polar Advantage II) equipped with a quaternary pump, a photodiode array detector (PDA) and a thermostated autosampler. Chromatographic separation was accomplished with a AcclamTM C18 column 150  3.0 mm i.d., 3 µm. Gradient elution of analysis was carried out with methanol/0.1% formic acid (solvent A) and water/0.1% formic acid (Solvent B) at a constant flow rate 0.5 mL/min and the injection volume was 20 µL. Two multi gradient systems methods were applied and pressure limit was 500 bar at 20°C. Relative abundance was calculated by making the ratio of peak area to the total area of the identified peaks.
3.0 mm i.d., 3 µm. Gradient elution of analysis was carried out with methanol/0.1% formic acid (solvent A) and water/0.1% formic acid (Solvent B) at a constant flow rate 0.5 mL/min and the injection volume was 20 µL. Two multi gradient systems methods were applied and pressure limit was 500 bar at 20°C. Relative abundance was calculated by making the ratio of peak area to the total area of the identified peaks.
Method 1 : 0 min, 0% A; 0–2 min, 0% A; 2–30 min, 10% A; 30–50 min, 100% A; 50–55 min, 100% A; 55–70 min, 0% A; flow rate : 0.5 ml/min. At the end, column was equilibrated to 70–73 min at 0% A to return to initial conditions.
Method 2 : 0 min, 5% A; 0–2 min, 5% A; 2–9 min, 25% A; 9–16 min, 40% A; 16–25 min, 50% A; 25–27 min, 70% A; 27–32 min 70% A; 32–35 min, 90% A; 35–38 min, 92% A; 38–45 min, 100% A; 45–50 min, 100% A; 50–52 min, 15% A; 52–60 min, 5% A; flow rate: 0.5 ml/min. At the end, column was equilibrated for 2 min at 5% A to return to initial conditions.
2.2.3 Mass Spectrometry (MS) Analysis
An LTQ Orbitrap Velos mass spectrometer (Thermo-Fisher Scientific, Bremen, Germany) equipped with an HESI source working in positive mode was used for accurate mass measurements. Mass spectra were acquired in profile mode with a setting of 30,000 resolution at m/z 400. Operation parameters were as follows: source voltage, 4 kV; sheath gas, 50; auxiliary gas, 15; sweep gas, 5 (arbitrary units); heat temperature, 250°C; and capillary temperature, 350°C. Default values were used for most other acquisition parameters (FT Automatic gain control (AGC) target 5.106 for MS mode and 5.105 for MSn mode). Methanol extracts were analyzed in full scan mode (m/z range 80.000-1500.000) at a resolving power of 30,000 at m/z 400 and data dependent MS/MS events acquired at a resolving power of 15,000. The most intense ions detected during full scan MS triggered data dependent scanning. Data dependent scanning was carried out without the use of a parent ion list. An isolation width of 2 amu was used and precursors were fragmented by collision-induced dissociation (CID) with a normalized collision energy of 35 V and an activation time of 10 ms. The data analysis was achieved using XCalibur software (Thermo Fisher Scientific). An external calibration for mass accuracy was carried out before the analysis to assure a precision < 5 ppm.
HPLC-MS analysis performed in this study were adapted from previous published methods [9,10] and many methods were tested before to considere these both comparatives methods. Positive ionization was used for MS assays, and compound absorbance were between 205 nm and 310 nm (λmax). All samples were analyzed in triplicate. This kind of investigation, which was probed for chemical identification in various natural substances, was performed for the first time on the bark, sapwood and heartwood of K. ivorensis extracts. The phenolic compounds with accurate retention time were discussed according to the mobile phases used.
3.1 Chemical Analysis of K. ivorensis According to Method 1
All the chromatogram obtained from Method 1 are presented in Fig. 1 and the corresponding molecular structures were listed in Tab. 1. All the relative abundances were calculated based on peak area obtained in UV. The Method 1 was based on a mobile phase which leads molecules of m/z < 300 Da in [M+H]+ mode, the solvent of elution was mixture of methanol/water/formic acid [14,15].
Typical fingerprints of flavanons, flavanols with a strong presence of p-hydroxybenzoic acid derivatives were identified in the extracts (Fig. 1). The major compounds eluted by Method 1 were the same for the three parts of the wood; the cumulated areas of mannitol (m/z 182.0784 Da, C6H14O6) showed that the heartwood should be more abundant in this sugar of interest (26.41%) for renal blood flow than the bark and sapwood which displayed close mannitol content (Tab. 1). Evidence of free methyl resorcinol (m/z 123.0440 Da, C7H8O2) was identified. This compound should be found in higher extent in K. ivorensis’s bark and sapwood where they showed respective relative abundances of 23.22% and 22.31% compared to the heartwood (Tab. 1). However, the three wood extracts eluted by Method 1 unveiled free resorcinol units (m/z 110.0362 Da, C6H6O2) which were slightly higher in the heartwood (6.61%) with regard to the bark and sapwood (Tab. 1). The presence of free resorcinol and methyl resorcinol moieties in K. ivorensis should be supported by their respective signals at m/z 112.7 and 124.7 Da in that African mahogany’s bark MALDI-TOF spectra [4]. These phenolic units of strong reactivity with formaldehyde would explain at least the high Stiasny number (~ 90%) observed in K. ivorensis condensed tannins [5]. Similar results previously reported by Pizzi et al. [16] for phenol-resorcinol-formaldehyde wood resin agreed with the presence of free resorcinol units in wood extracts. Ester compounds belonging to p-hydroxybenzoate-3H-resorcinol and p-hydroxybenzoate-4H-resorcinol (m/z 234.0886 Da and 235.0964 Da, C13H13O4 and C13H14O4) series were identified in K. ivorensis’s bark, sapwood and heartwood.
Table 1: Relative abundance expressed in percent (Relative abundance was calculated by making the ratio of peak area to the total area of the identified peaks) of phenolic compounds tentatively identified in K. ivroensis extracts by Method 1
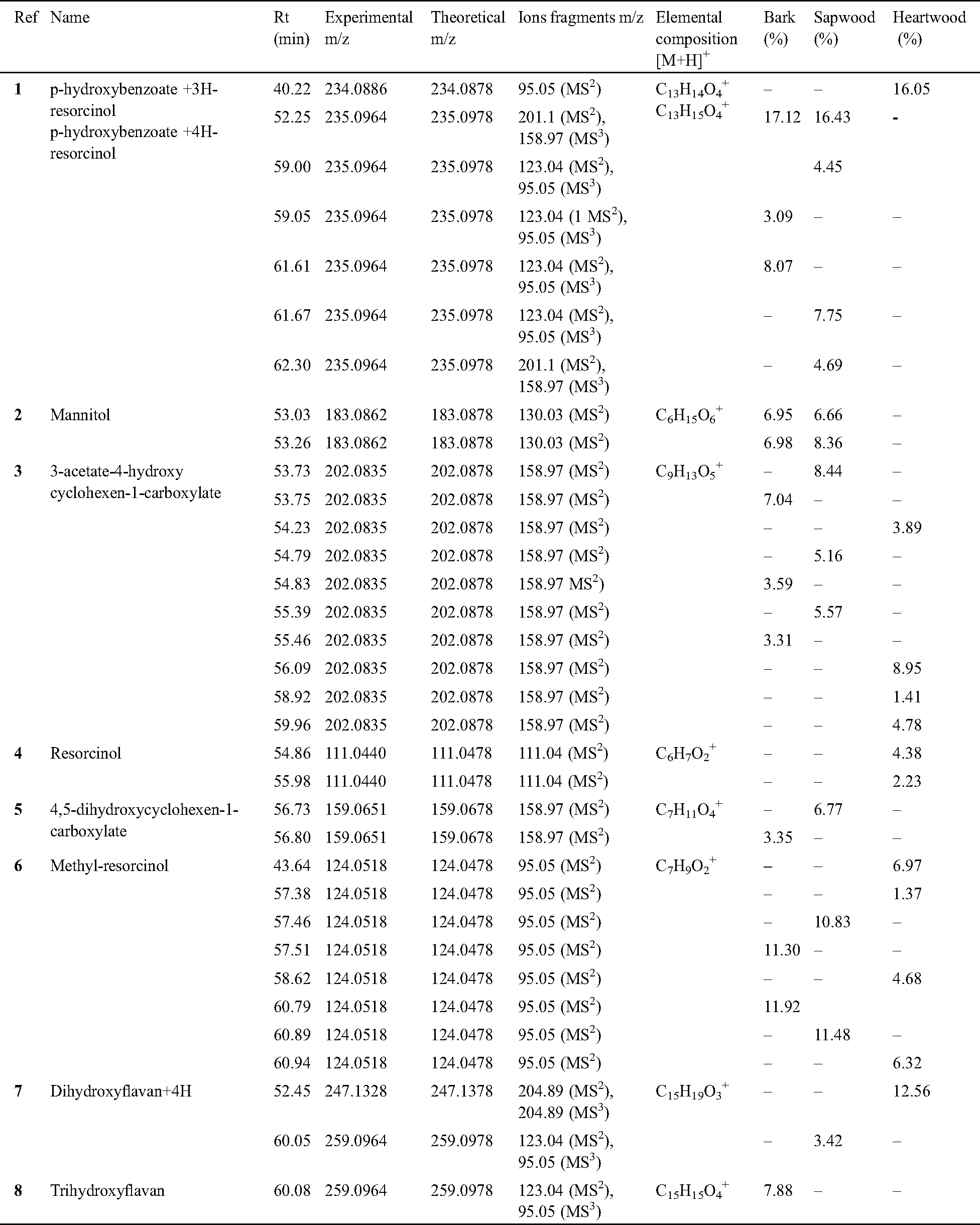
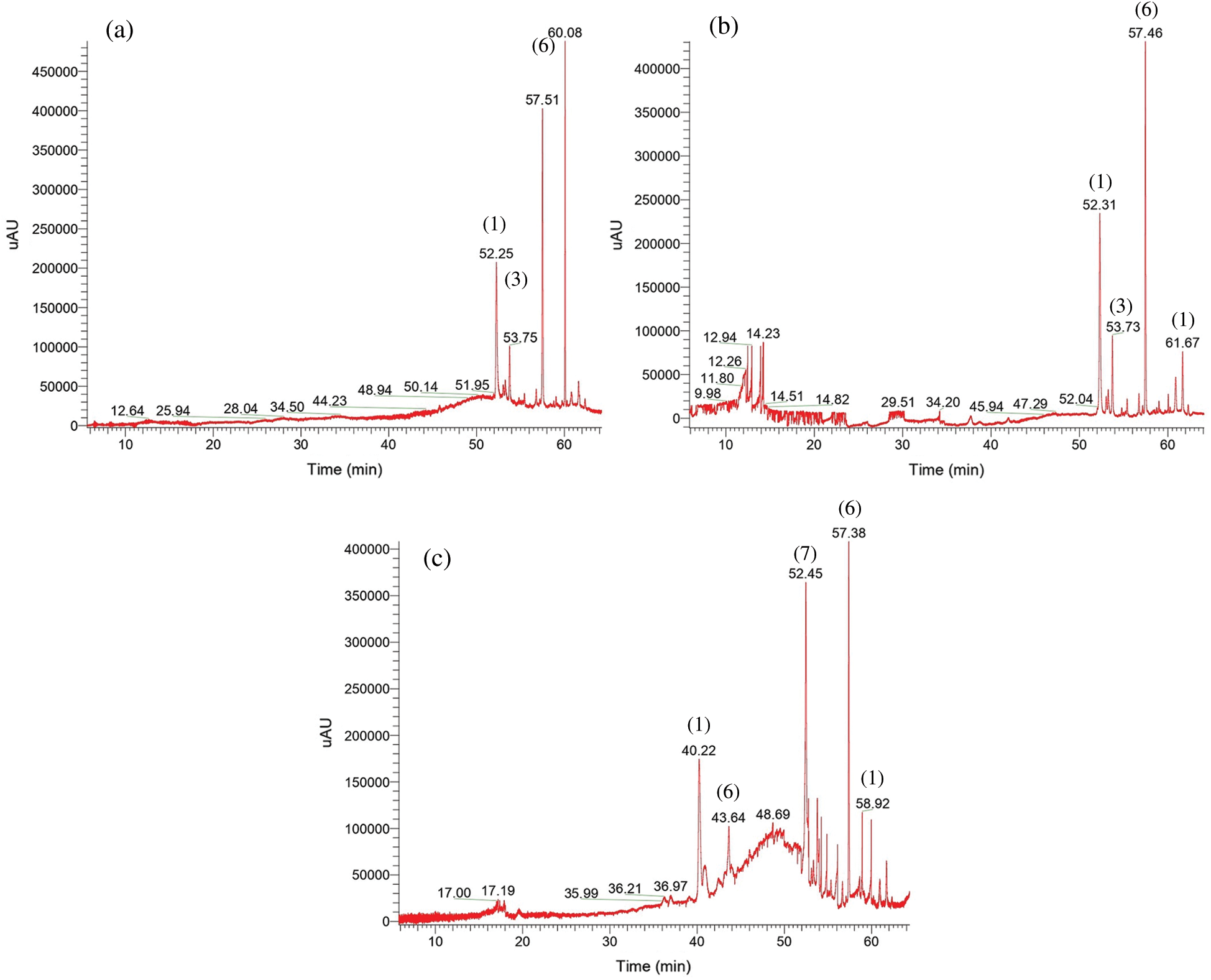
Figure 1: UV chromatogram at 280 nm obtained by Method 1 of bark (a), sapwood (b) and heartwood (c) extract. For the identification of compounds, see Tab. 1
All the wood extracts pointed out the same p-hydroxybenzoate-xH-resorcinol content, but p-hydroxybenzoate-4H-resorcinol (m/z 235.0964, [C13H15O4+H]+) released at Rt 52.25; 52.31 and 40.22 with fragmentation patterns 201.1 MS2, 158.97 MS3 accounted for the most abundant p-hydroxybenzoate-resorcinol like compounds (Fig. 1 and Tab. 1). The cumulated integrated areas of these p-hydroxybenzoate-resorcinol esters in the bark (28.28%), sapwood (33.32%) and heartwood (16.05%) revealed the predominance of this benzoic-resorcinol type ester with Method 1.
It was noteworthy that 3-acetate-4-hydroxycyclohexen-1-carboxylate (3) (m/z 202.0835, [C9H13O5+H]+) was also found in all parts of K. ivorensis wood (Tab. 1). The relative abundances of Tab. 1 showed that 3-acetate-4-hydroxycyclohex-1-ene-carboxylate did not have significant different among K. ivorensis’s bark (10.63 ± 2.43%), sapwood (19.17%) and heartwood (19.03%). This molecule was listed as teratogen compound causing birth defects [17]. That should explain in some extent the stillbirths usually observed among African women subject to traditional treatments with African mahogany’s bark [18–20].
LC-MS analysis with Method 1 pointed out the occurrence of 4,5-dihydroxycyclohex-1-ene carboxylate (m/z 159.0651, [C7H11O4+H]+) at Rt 56.80 and 56.73 in the bark, sapwood respectively (Fig. 1). This compound which shows structural similarities to chorismic acid was tested as possible inhibitor of chorismate mutase enzymatic activity [21]. It has high commercial value which should open opportunities for African mahogany wood wastes valorization despite the weak yield of 4,5-dihydroxycyclohen-1-ene carboxylate in the bark (3.35%) compared to the sapwood (6.77%) in Tab. 1.
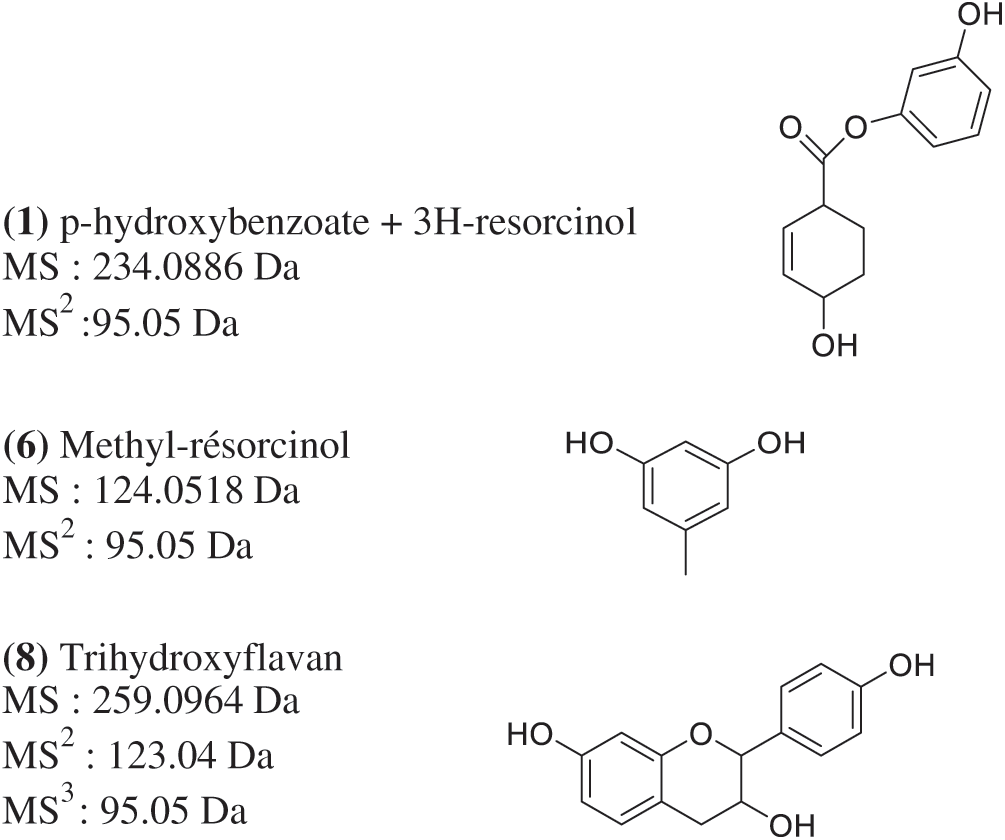
Figure 2: Some identified compounds proposal with Method 1
An extent of flavan-3-ol compounds was found in K. ivorensis methanol/water extracts. Trihydroxyflavan (m/z 259.0964, [C15H15O4+H]+) (Fig. 2) was more abundant in the bark which displayed stronger peak at Rt 60.08 than the sapwood and heartwood ones indeed (Fig. 1 and Tab. 1). This result emphasized a better extraction of trihydroxyflavan by the methanol/water mixture while the Maldi-ToF spectra of this compound showed a weak 258.8 Da signal strength [4] which suggested a low content of free trihydroxyflavan units in K. ivorensis acetone/water extracts. The trend of methanol/water solvent system to exact such a compound was reinforced by the presence of high content (12.56%) of dihydroxyflavan+4H (m/z 247.1328, [C15H19O3+H]+) (7) released only by the heartwood at Rt 52.45 (Fig. 1c).
3.2 Chemical Analysis of K. ivorensis According Method 2
The chromatogram obtained by Method 2 which used methanol/water as mobile phase in the LC/MS [M+H]+ mode was depicted in Fig. 3 and the related compounds were listed in Tab. 2. Method 2 eluted molecules of high m/z which remained inferior to 800 Da in [M+H]+ mode. Fig. 4. 14 phenolic compounds were identified with this method, i.e., 4 supplementary compounds than Method 1. This can be explained by the slow rate of gradient 2 which has a better separation and ionization of these additional compounds. Method 2 focused more on polar compounds which reveal themselves, and the heaviest compounds shall be eluted at 92% < 100% of methanol. Method 2 shall therefore improve the separation of heavier compounds probably present in Method 1, but eluted simultaneously, which rendered their separation difficult.
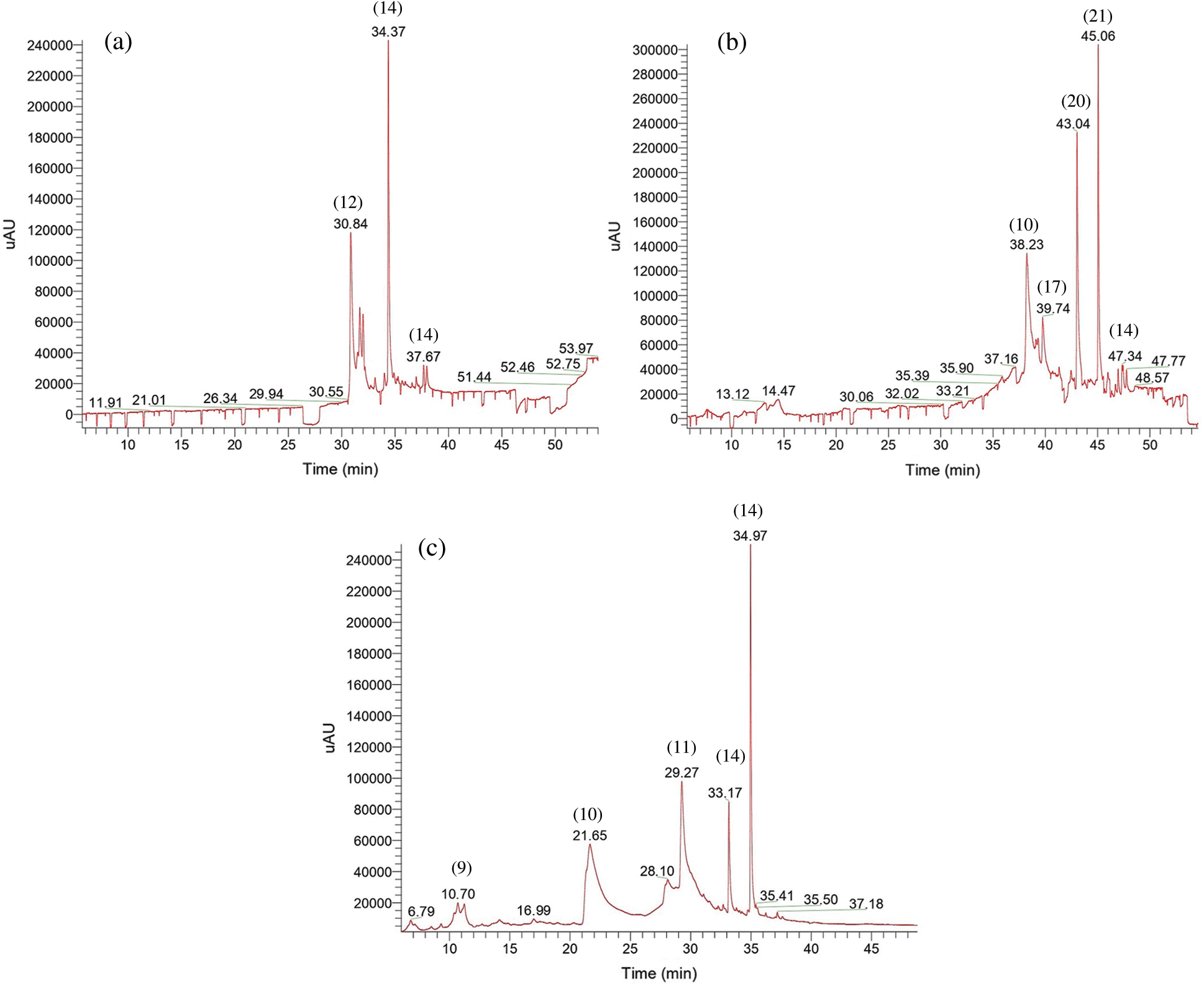
Figure 3: UV chromatogram at 280 nm obtained with Method 2 of bark (a), sapwood (b) and heartwood (c) extract. For the identification of compounds, see Tab. 2
With the exception of mannitol (2) and resorcinol (4) which were eluted by the two methods (Tabs. 1 and 2), all the compounds released by Method 2 were very different from Method 1 ones. Mannitol which appeared in the three wood extracts eluted by Method 1 (Tab. 1) was found only in the heartwood extracts when Method 2 was applied. The same trend was observed for resorcinol (4) which was found only in the bark extracts whereas it was exhibited in the three K. ivorensis wood extracts for Method 1. These results pointed out that the gradient used in the mobile phase of Method 2 was more susceptible to the chemical environment of the wood extracts than the gradient used in Method 1. This observation highlighted accordingly strong differences on the eluting power of these two methods.
Therefore, contrarily to Method 1, a strong chemical variability was found between K. ivorensis bark, sapwood and heartwood. Tab. 2 and Fig. 3 showed that only quercetin-3-O-gallic acid + xH (m/z 455.0578, [C22H15O11+H]+; m/z 457.0765, [C22H17O11+H]+) was found in the bark, sapwood and heartwood of K. ivorensis. The cumulated integrated areas of that quercetin-gallate (Tab. 2) pointed out its highest content in the bark (37.52%) compared to the sapwood (6.12%) and the heartwood (10.07%). On the other hand, the highest quercetin-3-O-gallic acid isomer was eluted by the bark at Rt 34.37 with MS2 = 313.16 and MS3 = 185.11 fragments. In addition, 3,4-dihydroxybenzoic acid derivate (m/z 155.0418, and m/z 154.0260 [C7H6O4+H]+) (10) was found also in higher extent in K. ivorensis sapwood (32.97%) than the heartwood (28.01%) while it lacked in the bark extracts (Tab. 2). However, the absence of that free galloyl moiety in the bark agreed with MALDI-TOF analysis where typical signal of gallic acid (m/z 170 Da) were not detected [4] in the sapwood and heartwood. Nevertheless, signals depicted at m/z 155.7 Da in the MALDI-TOF spectra of the bark, sapwood and heartwood acetone/water and assigned to free 3,4-benzoic acid [4] would support the presence of that galloyl type compound in the present extracts. In addition, 3,4-benzoic acid unit should also result from the loss of 1xOH from gallic acid (170-1x16) released by H2CO2 acid hydrolysis of ester like quercetin-3-O-gallic acid structures.
With the exception of quercetin-3-O-gallic acid and 3,4-benzoic acid, all the compounds released by Method 2 were different between the bark, sapwood and heartwood of the studied African mahogany. That emphasized a strong intra tree chemical variability, so that 3-(2-methoxyphenyl)-2-oxo-2H-chromen-7-yl acetate (m/z 311.0913, [C18H15O5+H]+) (12) accounted for the second major compound of the bark (34.51%). The occurrence of 3,5-dimethoxycinnamic acid (m/z 209.0808, [C11H13O4+H]+) (13) like ferulic compound usually linked with arabinose in hemicelluloses side chain units or pectin structures should indicate a difference on hemicelluloses’ side chain structures of K. ivorensis bark and xylem. This cinnamic acid is a plant second metabolite exclusively released by the bark which displayed repellent properties against deter feeding in herbivores [22]. Hence, K. ivorensis’s bark could be a fruitful source of chemical repellents. The last compound exclusively identified in the bark of K. ivorensis extracts eluted in low content by Method 2 was isoquercitrin-7-O-p-hydroxybenzoic acid or isoquercitrin-3-O-p-hydroxybenzoic acid (m/585.1238, [C28H25O14+H]+) (16) (Tab. 2) linked to glycosyl unit. Similar compound previously reported for antiradical activity of isorquercitrin esters with aromatic acids and their homologues [23] put forward the presence of glycosylated flavone or flavonoid like compounds in K. ivorensis bark, which supported therefore the presence of glycosylated tannins like isoquercetin-gallate formerly identified in the acetone/water bark extracts of the studied African mahogany [4].
Table 2: Relative abundance expressed in percent (%) of phenolic compounds tentatively identified in K. ivroensis extracts by Method 2
The LC-UV chromatogram of Fig. 3 and the compounds listed in Tab. 2 revealed the presence of molecules only present in the sapwood. Among the major compounds (abundance > 10%), 3-methanoate-5-methyl-p-hydroxybenzoic acid (m/z 197.0444, [C9H9O5+H]+) (17) supported the presence of benzoic acid linked condensed tannins units observed in MALDI-TOF spectra of the K. ivorensis sapwood acetone/water extracts [5]. The dominating signal at Rt 43.04 (Fig. 3c) assigned to C4-C8 dihydroxyflavan dimer + 4H (m/z 487.2114, [C30H31O6+H]+) (20) highlighted the existence of such condensed tannins that the integrated area (20.41%) suggested a high content in K. ivorensis sapwood (Tab. 2). This compound (20), which lacked in the acetone/water extracts characterized by MALDI-TOF [4], was the most abundant in the methanol/water extracts eluted by Method 2. With a relative abundance of 19.43% (Tab. 2), deoxy-mannitol (m/z 167.0913, [C6H15O5+H]+) (21) which is one of the hexitols principally observed in angiosperms and found in high content in Agave sisalana [24] accounted also among the most abundant compounds in K. ivorensis sapwood (Tab. 2).
Other minor compounds released by the sapwood were listed in Tab. 2. Traces of kaempferide (m/z 301.0706, C16H13O6+) (18), an 4’-O-methylated flavonol previously reported in wine [25,26] as well as in aromatic ginger Kaempferia galanga inhibits pancreatic cancer growth and migration [27] should be found in K. ivorensis sapwood at Rt 41.32 (Fig. 3b). However, an analogue of kaempferide methylated at the 3’ position known as kaempferol should appear as kaempferol-8-(3’-methyl) trihydroxyflavan (m/557.1417, [C31H25O10+H]+) dimer probably condensed in C4-C8 as shown in Tab. 2. Traces of chlorogenic acid (m/z 355.1023, [C16H19O9+H]+) (19) were found in K. ivorensis sapwood methanol/water extracts (Tab. 2). This compound taken as a dietary supplement or in coffee would reduce pressure blood [28,29] and displayed anti-inflammatory effects [30]. Kaempferol-8-(3’-methyl) trihydroxyflavan (22) should be only found in K. ivorensis sapwood (Tab. 2). That compound which has never been reported would be found for the first time in K. ivorensis, it showed the following fragmentation patterns: MS2 = 301.18 assigned to 3’-methyl-kaempferol in [M+H]+ ion and MS2 = 254.08 related to trihydroxyflavan with a loss of 4xH [5]. Therefore, these two fragments could be condensed by C4-C8 bond as suggested in Tab. 2. Concerning the heartwood, 4-hydroxyresocinol (m/z 126.0362 Da, C6H6O3) (9) which accounted for low content among the compounds eluted from the heartwood by Method 2 (6.5%) (Tab. 2) did not differ significantly from its counterpart free resorcinol (2) (8.27%) released by the heartwood extracts eluted with the Method 1 (Tab. 1). On the other hand, 4-benzoate-p-hydroxybenzoic acid (m/z 245.0808, [C14H13O4+H]+) (11) was found in a higher extent in the heartwood (26.67%). Additional isoquercitin like compound such as isoquercitin-7,4’-O-di-p-hydroxybenzoic acid+2H (m/z 707.1606, [C35H31O16+H]+) bearing a supplementary p-hydroxybenzoic acid at the 4’ position of flavone unit (15) accounted among the major compounds eluted from the heartwood extracts (Tab. 2). That underlined the potential of K. ivorensis heartwood wastes as source of quercetin or isoquercitin derivatives of anti-free-radical, antioxidant, antifungal properties [31].
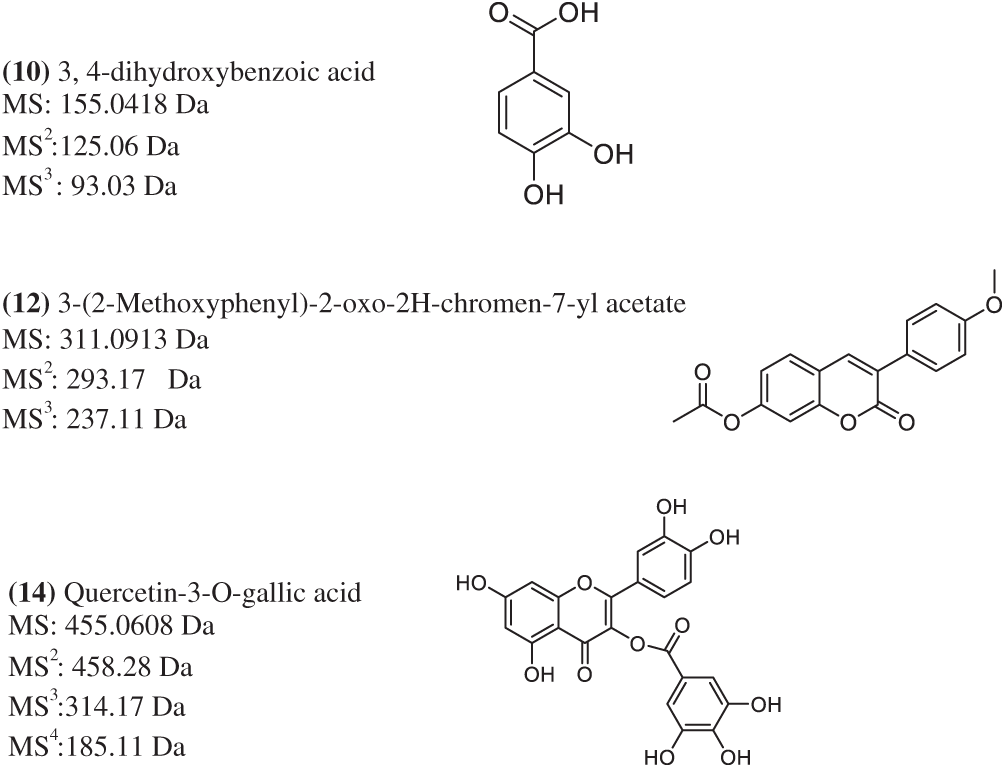
Figure 4: Some identified compounds proposal with Method 2
It was noteworthy that the cumulated areas of benzoic type acid series of Tab. 2 supported the richest of the heartwood extracts (70.63%) in this galloyl type unit compared to the sapwood (40.68%) and bark (6.62%). Even if benzoic type acid compounds strongly dominated in K. ivorensis heartwood extracts eluted by Method 2, no significant difference was found between benzoate type ester structures entirely released by the bark (37.67%), sapwood (37.81%) and heartwood (36.04%) extracts eluted by Method 1 (Tab. 1). The unique ester obtained by Method 2 was 4-benzoate-parahydroxybenzoic acid (11) eluted from the heartwood extracts (Tab. 2). Therefore, LC-MS results from Method 2 showed a clear variability regarding K. ivorensis acid type units, which would be globally dominated by benzoic type acid rather than gallic acid. These acids’ distribution varied according to the wood extracts as follows: Gallic acid (37.52%) was more abundant than benzoic acid (6.62%) in the bark while the sapwood was more abundant in benzoic acid (46.62%) than gallic acid (6.12%), and the strongest abundance of the heartwood in benzoic acid (70.63%) than gallic acid (10.06%) should be ascertained (Tab. 2). These results which supported a high content of carbonyl groups (C=O) from the richest heartwood in ester and acid structures compared to the bark and sapwood agreed with the strongest signal of C=O raising at 1731.8 cm-1 in the FTIR spectra of tannins extracted from K. ivorensis’s heartwood as reported in previous work [4].
The methanol/water extracts from Khaya ivorensis bark, sapwood and heartwood eluted with two different gradients in liquid chromatography associated with mass spectrometry were studied. The first method did not show significant difference regarding the compounds released by K. ivorensis bark, sapwood and heartwood. Nevertheless, some compounds eluted by Method 1 (e.g., p-hydroxybenzoate +3H-resorcinol: 16.05%) were specific to certain parts of the wood. Hence, trihydroxyflavan was only found in the bark, and a protonated dihydroxyflavan type molecule was found in high extent in the heartwood. The second gradient allowed a better separation of the heavier compounds. Different gradient of MeOH on HPLC induced important variation on compounds separation. Both Methods 1 and 2 showed that mannitol content was not different in the three part of K. ivorensis wood while free resorcinol unit appeared only in the African mahogany bark and heartwood. This would explain in some extent the better reactivity of their tannins with formaldehyde or hexamine compared to the sapwood ones. Nevertheless, LC/MS analyses of methanol/water extracts eluted by Method 2 have shown the richest of K. ivorensis heartwood in benzoic acid moiety whereas gallic acid was more abundant in the bark. Hexoside derivatives such as (quercetin-7-O-p-hydroxybenzoic acid)-3-O-hexoside and (quercetin-7, 4’-O-di-p-hydroxybenzoic acid + 2H)-3-O-hexoside were released respectively in the bark and the heartwood. These compounds are known for their anti-free-radical, antioxidant or antifungal activities without excluding the presence of traces of kaempferide in the bark of K. ivorensis. This compound inhibits pancreatic cancer growth and migration. Despite these promising results, further investigation based on semi-quantitative extraction and biological or microbiological essays of the extracts are necessary to ascertain the potential of K. ivorensis industrial wood wastes from Gabon to be source of molecules of interest for medicine, fine chemistry, adhesives or wood protection.
Acknowledgement: Authors’ contribution: Dr. Arsène Bikoro Bi Athomo was the principal investigator of the project. Dr. Carine Arnaudguilhem helped in chemical analysis. Dr. Péguy Starlin Engozogho participated as a worker in a related field of tropical wood chemistry. Dr. Rodrigue Safou Tchiama contributed actively as wood scientist and PhD committee’s member; he criticized and corrected the final manuscript. Pr. Florent Eyma and Pr. Bertrand Charrier acted as scientific directors. Université de Pau et des Pays de l’Adour are thanked for the material support and facilities offered by the ANR-10-EQPX-16 Xyloforest (Xylomat, Mont de Marsan).
Funding Statement: We gratefully acknowledge the financial support of the Agence Nationale des Bourses du Gabon (ANBG).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Safou-Tchiama, R., de Jéso, B., Akagah, A. G., Sèbe, G., Pétraud., M. (2007). A preliminary survey of the interfacial bonding of some tropical hardwoods towards succinic anhydride and 2-octen-1-yl succinic anhydride molecules: Impact of lignin and carbohydrate polymers structure on the chemical reactivity. Industrial Crops and Products, 26(2), 173–184. DOI 10.1016/j.indcrop.2007.03.001. [Google Scholar] [CrossRef]
2. Safou-Tchiama, R., Bikoro Bi Athomo, A., Engozogho Anris, S. P., Akagah, A. G., De Jeso, B. (2019). Characterization of some African tropical heartwood lignins by 1D 13C and 1H-NMR: molecular structure and hydroxyl groups’ distribution. Journal of the Indian Academy of Wood Science, 16, 73–86. DOI 10.1007/s13196-019-00239-8. [Google Scholar] [CrossRef]
3. Engozogho Anris, S. P., Arsene, B. B. A., Marcia, V., Louis, D., Rodrigue, S. T., Bertrand, C. (2019). Extraction and Characterization of Aucoumea klaineana Pierre (Okoume) Extractives. Journal of Renewable Materials, 7, 518–522. DOI 10.32604/jrm.2019.04051. [Google Scholar] [CrossRef]
4. Bikoro Bi Athomo, A., Engozogho Anris, S. P., Safou-Tchiama, R., Santiago-Medina, F. J., Cabaret, T. et al. (2018). Chemical composition of African mahogany (K. ivorensis A. Chev) extractive and tannin structures of the bark by MALDI-TOF. Industrial Crops and Products, 113, 167–178. DOI 10.1016/j.indcrop.2018.01.013. [Google Scholar] [CrossRef]
5. Bikoro Bi Athomo, A., Engozogho, S. P., Safou-Tchiama, R., Leroyer, L., Pizzi, A. et al. (2019). Chemical analysis and thermal stability of African mahogany (Khaya ivorensis A. Chev) condensed tannins. Holzforschung, 74(7), 683–701. DOI 10.1515/hf-2019-0113. [Google Scholar] [CrossRef]
6. Safou-Tchiama, R., Anzi Barhé, T., Soulounganga, P., Akagah, A. G., De Jeso, B. (2017). A comparative study of the syringyl, guaiacyl and hydroxyl groups units distribution in some African tropical hardwoods’ lignin by Py-GC/MS and spectroscopic techniques. Journal of Materials and Environmental Science, 8(7), 2530–2540. [Google Scholar]
7. Zhao, H. Y., Sun, J. H., Fan, M. X., Fan, L., Zhou, L. (2008). Analysis of phenolic compounds in Epimedium plants using liquid chromatography coupled with electrospray ionization mass spectrometry. Journal of Chromatography A, 1190(1), 157–181. DOI 10.1016/j.chroma.2008.02.109. [Google Scholar] [CrossRef]
8. Cerrato, A., Cannazza, G., Capriotti, A., Citti, C., La Barbera, G. et al. (2020). A new software-assisted analytical workflow based on high-resolution mass spectrometry for the systematic study of phenolic compounds in complex matrices. Talanta, 209, 120573. DOI 10.1016/j.talanta.2019.120573. [Google Scholar] [CrossRef]
9. Quifer-Rada, P., Vallverdu-Queralt, A., Martinez-Huelamo, M., Chiva-Blanch, G., Jauregui, O. et al. (2015). A comprehensive characterisation of beer polyphenols by high resolution mass spectrometry (LC-ESI-LTQ-Orbitrap-MS). Food Chemistry, 169, 336–343. DOI 10.1016/j.foodchem.2014.07.154. [Google Scholar] [CrossRef]
10. Zhang, X., Zhang, S., Gao, B., Qian, Z., Liu, J. et al. (2019). Identification and quantitative analysis of phenolic glycosides with antioxidant activity in methanolic extract of Dendrobium catenatum flowers and selection of quality control herb-markers. Food Research International, 123, 732–745. DOI 10.1016/j.foodres.2019.05.040. [Google Scholar] [CrossRef]
11. Złotek, U., Mikulska, S., Nagajek, M., Świeca, M. (2016). The effect of different solvents and number of extraction steps on the polyphenol content and antioxidant capacity of basil leaves (Ocimum basilicum L.) extracts. Saudi Journal of Biological Science, 23(5), 628–633. DOI 10.1016/j.sjbs.2015.08.002. [Google Scholar] [CrossRef]
12. Barnaba, C., Nardin, T., Pierotti, A., Malacarne, M., Larcher, R. (2017). Targeted and untargeted characterisation of free and glycosylated simple phenols in cocoa beans using high resolution-tandem mass spectrometry (Q–Orbitrap). Journal of Chromatography A, 1480, 4149. DOI 10.1016/j.chroma.2016.12.022. [Google Scholar] [CrossRef]
13. Liu, R., Zhao, Z., Dai, S., Che, X., Liu, W. (2019). Identification and quantification of bioactive compounds in Diaphragma juglandis Fructus by UHPLC-Q-Orbitrap HRMS and UHPLC-MS/MS. Journal of Agricultural and Food Chemistry, 67(13), 3811–3825. DOI 10.1021/acs.jafc.8b06890. [Google Scholar] [CrossRef]
14. Charrier, B., Janin, G., Haluk, J. P., Mosedale, J. R. (1995). Colour and chemical characteristics of moon rings in Oakwood. Holzforschung, 49(4), 287–292. DOI 10.1515/hfsg.1995.49.4.287. [Google Scholar] [CrossRef]
15. Morais, S. A. L., Nascimento, E. A., Queiroz, C. R. A. A., Piló-Veloso, D., Drumond, M. G. (1999). Studies on polyphenols and lignin of Astronium urundeuva wood. Journal of Brazilian Chemical Society, 10(6), 447–452. DOI 10.1590/S0103-50531999000600005. [Google Scholar] [CrossRef]
16. Pizzi, A., Pasch, H., Simon, C., Rode, K. (2004). Structure of resorcinol, phenol, and furan resins by MALDI-TOF mass spectrometry and 13 C NMR. Journal of Applied Polymer Science, 92(4), 2665–2674. DOI 10.1002/app.20297. [Google Scholar] [CrossRef]
17. Meyers, V. (1983). Chemicals which cause birth defects—teratogens: A special concern of research chemists. The Science of the Total Environment, 32(1), 1–12. DOI 10.1016/0048-9697(83)90128-6. [Google Scholar] [CrossRef]
18. Takin, M. C., Attindehoua, S., Sezan, A., Attakpa, S. E., Baba-Moussa, L. (2013). Bioactivity, therapeutic utility and toxicological risks of Khaya senegalensis. Indian Journal of Pharmaceutical and Biological Research Earch, 1(4), 22–129. [Google Scholar]
19. Iwu, M. M., Kiayman, D. L. (1992). Evaluation of the in vitro antimalarial activity of Picralima nitida extracts. Journal of Ethnopharmacology, 36(2), 133–135. DOI 10.1016/0378-8741(92)90012-G. [Google Scholar] [CrossRef]
20. Belewu, M. A., Olatunde, O. A., Giwa, T. A. (2009). Underutilized medicinal plants and spices: Chemical composition and phytochemical properties. Journal of Medicinal Plants Research, 3(12), 1099–1103. [Google Scholar]
21. Gorischi, J., Lingens, F. (2009). Chorismate Mutase from Streptomyces aureofaciens: A heat stable enzyme. Journal of Bacteriology, 114(2), 645–651. DOI 10.1128/JB.114.2.645-651.1973. [Google Scholar] [CrossRef]
22. Crocker, D. R., Perry, S. M. (1990). Plant chemistry and bird repellents. Ibis, 132(2), 300–308. DOI 10.1111/j.1474-919X.1990.tb01047.x. [Google Scholar] [CrossRef]
23. HeřmánkováVavříková, E., Křenková, A., Petrásková, L., Chambers, C. S., Zápal, J. (2017). Synthesis and antiradical activity of isoquercitrin esters with aromatic acids and their homologues. International Journal of Molecular Sciences, 18(5), 1074. DOI 10.3390/ijms18051074. [Google Scholar] [CrossRef]
24. Branco, A., Santos, J. D. G., Pimentel, M. M. A. M., Osuna, J. T. A., Lima, L. S. et al. (2010). d-Mannitol from Agave sisalana biomass waste. Industrial Crops and Products, 32(3), 507–510. DOI 10.1016/j.indcrop.2010.06.025. [Google Scholar] [CrossRef]
25. García-Villalba, R., Espín, J. C., Tomás-Barberán, F. A., Rocha-Guzmán, N. E. (2017). Comprehensive characterization by LC-DAD-MS/MS of the phenolic composition of seven Quercus leaf teas. Journal of Food Composition and Analysis, 63, 38–46. DOI 10.1016/j.jfca.2017.07.034. [Google Scholar] [CrossRef]
26. Magalhães, P. J., Vieira, J. S., Gonçalves., L. M., Pacheco, J. G., Guido, L. F. et al. (2010). Isolation of phenolic compounds from hop extracts using polyvinylpolypyrrolidone: Characterization by high-performance liquid chromatography–diode array detection–electrospray tandem mass spectrometry. Journal of Chromatography A, 1217(19), 3258–3268. DOI 10.1016/j.chroma.2009.10.068. [Google Scholar] [CrossRef]
27. Lee, J., Kim, J. H. (2016). Kaempferol inhibits pancreatic cancer cell growth and migration through the blockade of EGFR-related pathway In Vitro. PLoS One, 11(5), e0155264. DOI 10.1371/journal.pone.0155264. [Google Scholar] [CrossRef]
28. Zhao, Y., Wang, J., Ballevre, O., Luo, H., Zhang, W. (2012). Antihypertensive effects and mechanisms of chlorogenic acids. Hypertension Research, 35(4), 370–374. DOI 10.1038/hr.2011.195. [Google Scholar] [CrossRef]
29. Onakpoya, I. J., Spencer, E. A., Thompson, M. J., Heneghan, C. J. (2015). The effect of chlorogenic acid on blood pressure: A systematic review and meta-analysis of randomized clinical trials. Journal of Human Hypertension, 29(2), 77–81. DOI 10.1038/jhh.2014.46. [Google Scholar] [CrossRef]
30. Tajik, N., Tajik, M., Mack, I., Enck, P. (2017). The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. European Journal of Nutrition, 56(7), 2215–2244. DOI 10.1007/s00394-017-1379-1. [Google Scholar] [CrossRef]
31. Valentová, K., Vrba, J., Bancířová, M., Ulrichová, J., Křen, V. (2014). Isoquercitrin: Pharmacology toxicology, and metabolism. Food and Chemical Toxicology, 68, 267–282. DOI 10.1016/j.fct.2014.03.018. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |