DOI:10.32604/jrm.2021.013366

| Journal of Renewable Materials DOI:10.32604/jrm.2021.013366 |  |
| Article |
Characterization of Extracts from the Bark of the Gabon Hazel Tree (Coula edulis baill) for Antioxidant, Antifungal and Anti-termite Products
1Université de Lorraine, Institut National de Recherche pour l’Agriculture, l’Alimentation et l’Environnement, Laboratoire d’Etudes et de Recherche sur le Matériau Bois, Nancy, France
2Université de Montpellier, Centre de Coopération Internationale en Recherche Agronomique pour le Développement-Research Unit Biomass, Wood, Energy, Bioproducts, Montpellier, France
3Multidisciplinary Laboratory of Sciences of Libreville, Normal Superior School, Libreville, Gabon
*Corresponding Author: Philippe Gérardin. Email: philippe.gerardin@univ-lorraine.fr
Received: 04 August 2020; Accepted: 09 September 2020
Abstract: Chemical composition of the bark extracts of Coula edulis was investigated to find potential antioxidant, anti-termite and antifungal compounds which can find useful applications in the fields of food, nutraceuticals, cosmetics or agrochemical. Phytochemical screening revealed the presence of several groups of active molecules such as alkaloids, polyphenols, flavonoids, saponins and sterols and/or terpenes in the different extracts. Total phenols, condensed tannins and flavonoids contents corroborated phytochemical screening. Gas chromatography-mass spectrometry (GC-MS) analysis revealed compounds in dichloromethane extract different from those obtained with all the other solvents. Hexadecanoic and trans-9-octadecenoic acids, as well as stigmasterol and β-sitosterol have been identified as the major compounds in the dichloromethane extract. Extracts obtained with acetone and toluene/ethanol mixture (2/1, v/v) indicated the presence of few amounts of fatty acids and sugars, catechin in small amount and huge amounts of phenolic acids like gallic and ellagic acids. The radical 2,2-diphenyl-1-picrylhydrazyle (DPPH) and the cationic radical 2,2’-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS+) were used for evaluation of antioxidant properties of the different extracts. The dichloromethane extracts had a very low antioxidant activity, while acetone and toluene/ethanol extracts presented EC50 values similar to those of catechin and BHT used as reference antioxidant compounds. Effect of the different extracts of the bark of C. edulis on fungal growth inhibition indicated better inhibition of the mycelium growth of brown rot fungi compared to white rot fungi. Low anti-termite activities were recorded with the aqueous extracts, while stronger activities were recorded with dichloromethane, acetone and toluene/ethanol extracts.
Keywords: Antifungal; antioxidant; anti-termite; Coula edulis baill; extracts; valorisation
Nature and its huge chemical diversity constitute an immense source of bio-inspiration for chemists. According to Newman et al. [1], nearly 52% of current drugs are made up or developed from natural products. Wood is an important natural resource containing numerous molecules of interest corresponding to secondary metabolites accumulated during the life of the trees. The Gabonese forest belonging to the forests of the Congo Basin is one of the largest forest in Africa after that of the Democratic Republic of Congo and Cameroon. According to the Gabonese Agency for Space Studies and Observations [2], it represents nearly 80% of the national territory with nearly 400 wood species, which may contain valuables biomolecules. Even if chemical composition of wood or bark extracts have been studied for some of these species, a majority of them have not been investigated up to now. This is the case of the hazel of Gabon (Coula edulis) considered by local populations as a multi-purpose species. Very durable and resistant to attack by fungi and insects, and more particularly those of termites, the wood of C. edulis is appreciated by the populations for its longevity [3]. Bark extracts are used in traditional pharmacopoeia like medicinal plant for the treatment of many diseases. In Ivory Coast, for example, bark decoction is used for purging or as an enema and for lumbar or kidney pain [4]. According to literature, bark is reported to contains high amounts of polyphenols among which tannin leading to many application [5,6]. Polyphenols are also reported to exhibit different biological activities, such as antifungal or antioxidant activities, which are responsible for their protective function in the tree. In addition to these activities numerous biological properties like antibacterial, antidiabetic or anticancer activities have been reported for bark extracts of different wood species [7–10]. In this context, it seemed interesting to investigate the chemical composition of C. edulis bark extracts for potential further applications. For this purposes, extractive contents, phytochemical screening, total phenols, tannins and flavonoids contents, GC-MS characterization and antioxidant, antifungal and anti-termite properties of these extracts were evaluated.
All chemicals, reagents, malt extract for microbiology, agar and solvents were purchased from Sigma-Aldrich. Ultrapure water was produced by a PURELAB Option-Q equipment (Elga). Mayer’s reagent (potassium mercuric iodide solution), iron perchloride 10% solution in water and Shinoda reagent (magnesium turn in hydrochloric acid) were prepared from reagent-grade chemicals according to well established procedures [11,12].
Two types of basidiomycete fungi were used to carry out this work: Two white (or fibrous) rot fungi Trametes versicolor (TV) ((Linneus) L. Quélet strain CTB 863 A) and Pycnoporus Sanguineus (PS) and two brown (or cubic) rot fungi Rhodonia Placenta (RP) ((Fries) Cooke sensu J. Eriksson, strain FPRL 280), Coniophora Puteana (CP) ((Schumacher ex Fries) Karsten, strain BAM Ebw.15) have been used for growth inhibition tests.
Effect of extractives on termites was evaluated using Reticulitermes flavipes (ex.santonensis de Feytaud) collected from Soulac-sur-Mer (France). Termites are kept in breeding tanks in a dark, well-ventilated climatic enclosure set at a temperature of 27 ± 1°C and minimum relative humidity of 75%.
The bark was collected at breath height (1.3 m) from the log of a hazel Gabonese tree (Coula edulis) cut from a Gabonese primary forest in the area of Ayémé located in the province of Estuaire. Tree age was estimated to be between 50 and 75 years old. After air drying, the bark was ground into a fine powder using a ball mill (Retsch SM 100), sieved to a grain size (Ø = 0.160 mm) to optimize the extraction yields and then stored in glass jars after drying 24 hours in an oven at 70°C for future extractions.
10 g of sawdust from the bark of C. edulis was successively extracted with solvents of increasing polarity, dichloromethane first followed by acetone, then toluene/ethanol mixture (2/1, v/v) followed by water. Two extraction methods using either Soxhlet or Dionex ASE 350 were investigated. With the Soxhlet method, extractions were performed for, 24 h before solvent evaporation under vacuum using a rotary vacuum evaporator, except for the aqueous extracts, which were lyophilized. For extraction with Dionex ASE 350, the same amount of sawdust was introduced into 60 mL cells. The extraction was carried out automatically during 15 minutes under to the combined effect of temperature and pressure (100°C and 100 bar). Once the extractions finished, the extracts were transferred into previously weighed flasks and the solvents removed using a rotary vacuum evaporator or lyophilized in the case of water. The extracts were then dried under vacuum in a desiccator in the presence of P2O5 and weighed regularly until constant mass. Each extraction was carried out in triplicate and the extractive content was determined using Eq. (1):
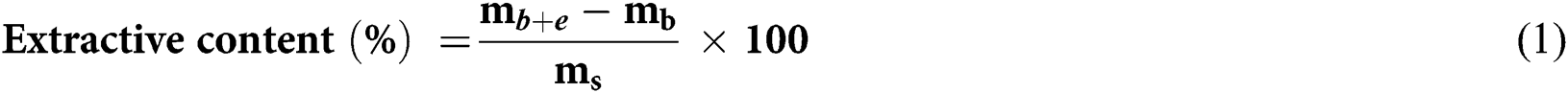
where, mb is the mass of the empty flask, mb+e is the mass of the flask with the extractives after removal of the solvent and ms the dry mass of sawdust before extraction.
Every needed reagent was prepared and used according to the protocols described in the literature [12,13]. All the different tests were carried out in triplicate.
Alkaloids were detected as follow: 20 mg of extracts and 10 mL of a dilute 10% sulfuric acid solution were poured into a test tube. The mixture was strong stirred for two minutes and some drops of Mayer’s reagent were then added. The appearance of a yellowish precipitate was characteristic of the presence of alkaloids.
Flavonoids presence determination is obtained by dissolution of 2 mg of extracts in 2 mL of 95% ethanol with a few drops of hydrochloric acid and 0.5 g of magnesium ribbon, leading to a cherry pink color taken by the solution.
The detection of polyphenols was achieved by adding a drop of 10% aqueous iron perchloride solution in 2 mL of extracts solution(1 g/L) involving an intense blackish color appearance.
The saponins were identified by mixing 50 mg of extracts with 30 mL of distilled water in a water bath at 30°C for 5 minutes. After cooling, 10 mL of this solution were introduced into a test tube and vigorously vortex-shaken for 10 seconds. The presence of a 1 cm thick persistent foam indicates the presence of saponins.
Sterols and terpenes detection was carried out by mixing 20 mg of extracts, 2 drops of oleum, 10 drops of acetic anhydride into 3 mL of chloroform causing the appearance of a purple ring, turning blue and then green in the test tube.
The determination of total phenols was carried out using the Folin-Ciocalteu colorimetric method [14] with a slight modification. First, a six point gallic acid calibration curve (0, 10, 30, 50, 80 and 100 ppm) was performed. The different extracts were dissolved in methanol. To carry out the assay, 0.5 mL of solution of extract dissolved in methanol and 2.5 mL of Folin-Ciocalteu reagent (diluted 10 times in distilled water) were successively introduced into a test tube. Then, 30 seconds to 8 minutes after adding the Folin-Ciocalteu reagent, 2 mL of sodium carbonate Na2CO3 (0.7 M) were added. A reaction blank containing no phenolic compounds is also produced. The reaction mixtures are stirred and incubated for 5 min at 50°C in a water bath. After this reaction time, all the samples were transferred to a cold water bath. the reaction mixtures are centrifuged (Eppendorf® Centrifuge 5702) at 4.4 rpm for 10 minutes. Then using a UV-Visible spectrophotometer (UV-2550, SHIMADZU) the absorbance is measured at 760 nm. The test was carried out in triplicate and the content of total phenols determined by means of the gallic acid calibration curve (y = 0.0096x + 0.0058; r² = 0.9997) by calculating the average concentration polyphenols present in extracts in mg equivalent of gallic acid/g of dry extract.
The determination of condensed tannins or proanthocyanidins was carried out using the colorimetric method of acid condensation of vanillin [15] with a slight modification. First, a six point catechin calibration curve (0, 10, 30, 50, 80 and 100 ppm) was performed. The different extracts were then dissolved in methanol. To carry out the assay, a 0.5 mL solution of extract solution dissolved in methanol were mixed in a test tube with 3 mL of vanillin reagent also dissolved in methanol (4%, weight/volume). Then 1.5 mL of concentrated HCl (37%) were added and the mixture was kept in the dark at room temperature for 15 minutes. The absorbance of all the reaction mixtures corresponding to each point in the range and the samples is read at 500 nm using a UV-Visible spectrophotometer (UV-2550, SHIMADZU). The test was carried out in triplicate and the content of condensed tannin determined by means of the catechin calibration curve (y = 0.0063x + 0.0759; r² = 0.9987) by calculating the average concentration of flavonoids present in extracts in mg equivalent of gallic acid/g of dry extract.
The flavonoid assay was carried out using the aluminum chloride colorimetric method described by [16] with a slight modification. First, a six points catechin calibration curve (0, 10, 30, 50, 80 and 100 ppm) was performed. The different extracts were then diluted in methanol. To carry out the assay, 0.5 mL of the extract solution diluted in methanol, 2 mL of distilled water and 1 mL of aluminum chloride (10%) are successively introduced into a test tube, and leaved for 6 minutes. Then, 1 mL of 1 M potassium acetate is added. After incubation at room temperature for 30 minutes, the absorbance of all the reaction mixtures corresponding to each concentration in the calibration curve and the samples were measured at 415 nm using a UV-Visible spectrophotometer (UV-2550, SHIMADZU). The test was carried out in triplicate and the flavonoid content determined using the catechin calibration curve (y = 0.0088x + 0.0818; r² = 0.9989) by calculating the average concentration of flavonoids present in extracts in mg equivalent of gallic acid/g of dry extract.
The GC-MS analysis was carried out with a 95% dimethyl/5% diphenylpolysiloxane column (30 m × 0.25 mm × 0.25 μm) on a Perkin Elmer Clarus 680 chromatograph coupled to a SQ8 mass spectrometer, controlled by Turbomass v6.1 software and having the 2011 edition NIST database. The samples were derivatized to facilitate the detection of all the present compounds by pouring 50 μL of N,O-bis(trimethylsilyl) trifluoroacetamide containing 1% of trimethylchlorosilane on 1 to 2 mg of dry extracts in a 2 mL vial. This preparation was incubated for 2 hours at 70°C, closed vial in order to perform the silylation reaction. The vial was then opened to allow BSTFA evaporation and the remaining silylated extracts were dissolved in 1 mL of ethyl acetate. 1 μL of this solution was injected in splitless mode into the gas chromatograph at an inlet temperature of 250°C. A typical oven program was: 80°C for 2 minutes, heating to 10°C min−1 to 190°C, followed by 15°C min−1 to 280°C and maintained at this temperature for 10 minutes, heated again at 10°C min−1 at 300°C and maintained at this temperature for 14 minutes. If necessary, the heating program was slightly modified to improve the resolution of certain samples, leading to different retention times for one compound. A mobile phase of 1 mL min−1 of helium was used. After the separation step, the compounds were transferred by a transfer line heated to 250°C to the mass spectrometer and ionized by electronic impact with an ionization energy of 70 eV. The compounds were then identified by comparison of their mass spectrum with the NIST library via NIST MS Search 2.0 (2011). The identification was considered relevant for matching and reverse matching when the values of the coefficients were greater than 900.
Antioxidant activity of the different extracts was evaluated using (2,2-diphenyl-1-picrylhydrazyl) radical (DPPH) or 2,2’-azinobis (3-ethylbenzothiazoline-6-sulfonic) cationic radical (ABTS+). Catechin and butylated hydroxytoluene (BHT) were used as reference antioxidant compounds.
2.11.1 DPPH Free Radical Method
The antioxidant activity was estimated using the radical (2,2-diphenyl-1-picrylhydrazyl) or DPPH inspired by the method described by Brand-William et al. [17]. DPPH was prepared by mixing 39.4 mg of solid DPPH in 100 mL of pure methanol. Then 20 mL of this solution was taken and diluted in a 100 mL flask. Extracts at different concentrations were dissolved in methanol. Then, 1 mL of DPPH and 1 mL of extract dissolved in methanol were introduced in a test tank and the mixture stored in the dark at room temperature for 30 minutes. After this incubation time, the absorbance is measured at 517 nm using a UV-Visible spectrophotometer (UV-2550, SHIMADZU). The test was carried out in triplicate and the antioxidant activity of extracts (AA) was calculated in percentage relative to the control (DPPH alone in methanol without extract) according to the following Eq. (2):

where A0 is the absorbance of the control (DPPH alone in methanol without extract) and A the absorbance of the DPPH solution in the presence of extracts. The effective concentration for consuming 50% DPPH (EC50) is determined from the equation of the curve representing the percentage of inhibition as a function of the concentrations of the samples. The low EC50 concentrations correspond to high antioxidant activities.
2.11.2 ABTS+ Free Cationic Radical Method
The antioxidant activity was also estimated using ABTS+ inspired by the protocol of Simona et al. [18] with a slight modification. ABTS+ (7 mM) was prepared by mixing 384 mg of solid ABTS in 100 mL of potassium persulfate (2.45 mM). The solution was then diluted in a sodium phosphate buffer solution (5 mM) of pH = 7 in order to have an absorbance at 1.4 at 734 nm. Extracts at different concentrations are dissolved in methanol. In test tubes, 1 mL of ABTS+ and 1 mL of extract dissolved in methanol are introduced. The whole is stored in the dark at room temperature for 30 minutes. After this incubation time, the absorbance is recorded at 734 nm using a UV-Visible spectrophotometer (UV-2550, SHIMADZU). The test is carried out in triplicate and the antioxidant activity (AA) for each concentration was calculated according to Eq. (2). Where A0 is the absorbance of the control (ABTS+ alone in methanol without extract) and A the absorbance of the solution of ABTS+ in the presence of extracts. The effective concentration for consuming 50% of ABTS+ (EC50) is determined as previously from the curve representing the percentage inhibition as a function of the concentrations of the samples.
2.12 Fungal Growth Inhibition Tests
Effect of extracts on fungal growth inhibition was determinate according to the method described by Chang et al. [19] with a slight modification. The different extracts were dissolved in a minimum of ethanol (1 mL maximum). The agar media were prepared in 100 mL Erlenmeyer flasks by adding 4 g of extracted malt, 2 g of agar and 96 g of water. The agar solutions are sterilized in an autoclave at 120°C for 25 minutes. Then, 50 mg or 100 mg of the different extracts dissolved in the minimum amount of ethanol (1 mL maximum) were added to the sterilized agar media, so that to obtain a final extract concentration of 500 ppm or 1000 ppm, respectively. The mixtures were prepared near a flame in a laminar flow hood in sterile condition, then homogenized and left to stand. After cooling (to around 40°C), each Erlenmeyer flask containing the agar media at different extract concentrations was well homogenized and about 20 mL of sterilized agar media were distributed in Petri dishes under sterile conditions. After solidification, the media were inoculated with a freshly cultivated mycelium disc of the various fungal strains. For each fungus and each concentration, three Petri dishes with extract and three Petri dishes with ethanol only as a control were prepared. The dishes were incubated at 22°C and 70% Relative Humidity (RH) in a Memmert climate chamber. The growth of the fungi was measured daily, until the control Petri dishes were completely covered with mycelium (10 to 12 days). The tests were then stopped and the evaluation of the fungal growth are presented in the form of growth curves as a function of incubation time.
70 μL of extract solution (500 ppm and 1000 ppm in ethanol) were impregnated on Whatman filter papers previously conditioned at 20°C, 65% RH were weighed (m0) before being exposed to termites (Reticulitermes flavipes). The papers impregnated with the various solutions were air dried (20°C–65% RH for 2 hours) and weighed (m1). The tests were carried out in 9 cm Petri dishes, in which 40 g of wet Fontainebleau sand (1 volume of water for 4 volumes of sand) were placed on the periphery. The impregnated Whatman papers were placed on a plastic grid (to avoid moisture saturation) in the middle of the Petri dish and 20 termite workers were added to each Petri dish. The Petri dishes were then stored in the dark at 27°C, 75% RH. Papersoaked in ethanol or water only were also tested as controls and Petri disheswithout filter paper were used as diet control. Petri dishes were monitored regularly and the test stopped when all the worker termites in the diet control Petri dishes died (21 days approximately). At the end of the tests, the alive termites are counted and the survival rate of the termites was determined as indicated in Eq. (3). The samples were cleaned and dried in the open air then weighed (m2) to determine the mass losses due to termite attacks (MLter) according to Eq. (4)


where m0 was the initial stabilized mass of Whatman paper before impregnation, m1 the mass of Whatman paper after impregnation and air drying before exposure to termites, m2 the mass of Whatman papers after impregnation and air drying after exposure to termites.
XLSTAT 2019 was used and the results were known as the main values representing the mean of the repetitions ± the standard deviations. The Spearman correlation test was carried out between the EC50, the total content of phenols, condensed tannins and flavonoids. The values are statistically significant at ρ < 0.05.
Table 1: Extractive content in C. edulis bark sawdust
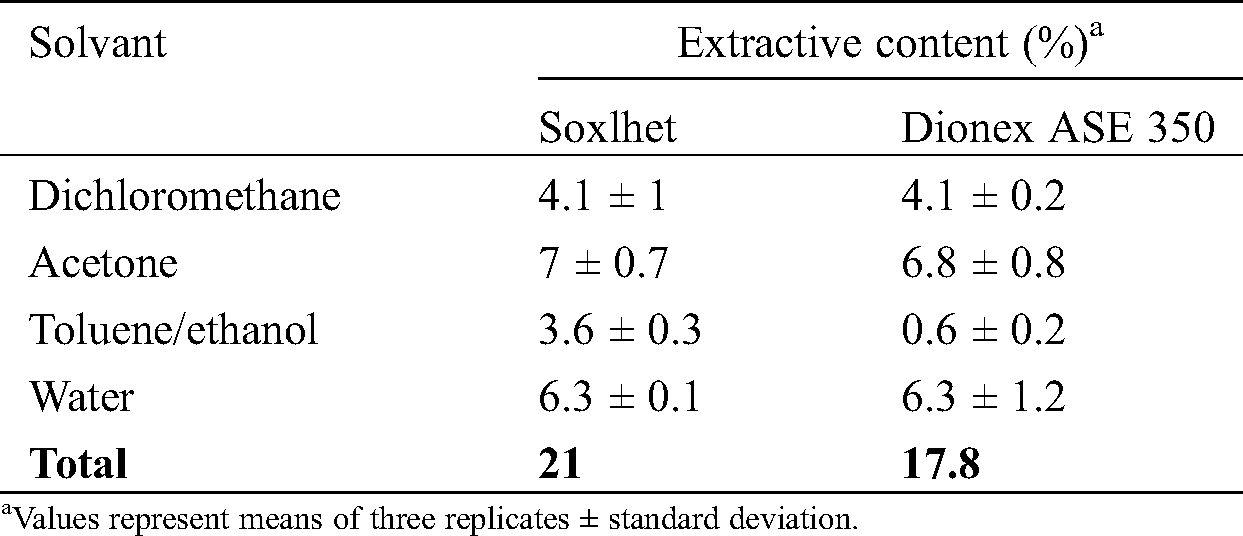
Extractives content (Tab. 1) depends of the solvent used and to a lesser extent from the method used for extraction. Amounts of extracts are, like very often for tropical wood species described to content high amounts of extractives, in agreement with bibliographic data [20–22]. The extractives contents obtained with dichloromethane are practically similar independently of the extraction method used indicating non negligible amounts of apolar compounds. The highest extractives contents were obtained with acetone and water indicating probably the presence of the high amounts of polar compounds like phenolic compounds and sugar. Results obtained with toluene/ethanol mixture indicated different amounts of extractives depending of the method used, Soxhlet extraction leading to higher yields of extracts, which may result from ethanolysis during extraction. Indeed, shorter extraction time used during solvent pressurised extraction with Dionex are reported to limit by-products formation. The total bark extractives contents obtained with Soxlhet (21%) are higher than those obtained with Dionex (17.8%). Numerous studies carried out on several tropical species reported quite similar high amounts of bark extractives [23–28].
Table 2: Phytochemical analysis of the main groups in C. edulis bark
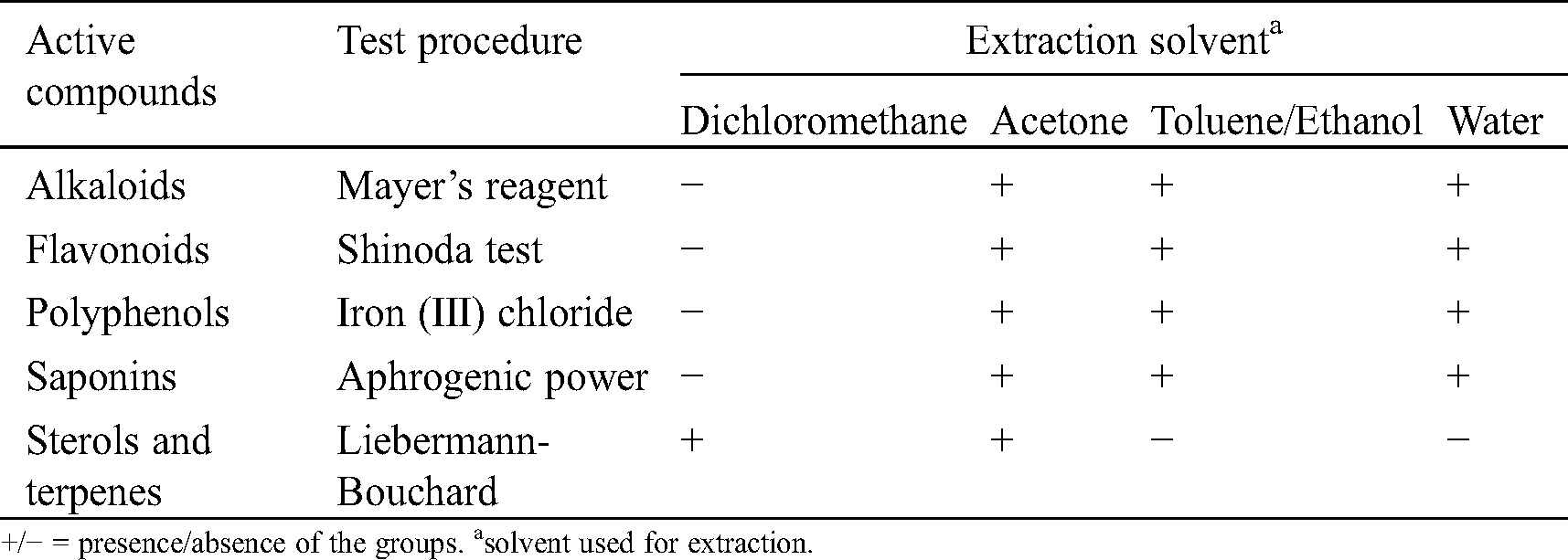
Sterols and terpenes were detected in dichloromethane extracts and acetone. Such groups of compounds have been described in the literature to possess antifungal and anti-termite properties [25,29–37]. Alkaloids, polyphenols, flavonoids and saponins were detected in all extracts except those obtained with dichloromethane. Such groups are known in the literature as potential antioxidant, antibacterial, antifungal, antimicrobial and termicidal compounds [22,38–41]. Phytochemical screening is reported in Tab. 2.
Table 3: Total phenols, condensed tannins and flavonoids contents in the different extracts of C. edulis bark
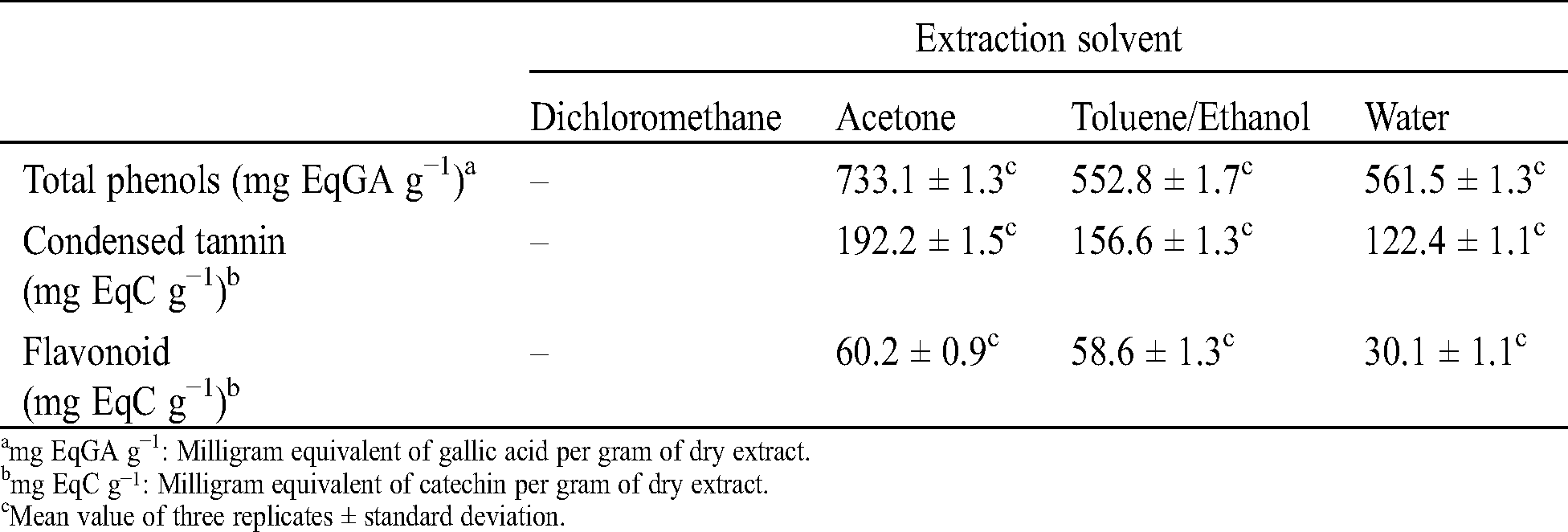
Total phenols, condensed tannins and flavonoids contents vary from one solvent to another (Tab. 3). The total phenol content varies between 733.1 and 552.8 mg EqGA per gram of extract, while condensed tannins content varies between 192.2 and 122.4 mg EqC per gram of extract and flavonoids content between 60.2 and 30.1 mg EqC per gram of extract. Independently of the test performed, the highest contents of phenolic compounds were obtained with the acetone extracts and the lowest with the aqueous extracts. This is easily explained by the polarity of the solvent used during our successive extractions, the higher polarity of water allowing to extract higher polarity compounds such sugar, while acetone is known to extract mainly phenolic compounds [42]. These contents in total phenols. condensed tannin and flavonoids are similar to those found by Saha Tchinda et al. [20] during their study on antioxidant activity, total phenol content and chemical composition of extracts from four Cameroonian woods: Padouk (Pterocarpus soyauxii Taubb). tali (Erythrophleum suaveolens). moabi (Baillonella toxisperma) and movingui (Distemonanthus benthamianus).
Chromatograms obtained with the different extracts excepted water are presented in Fig. 1.
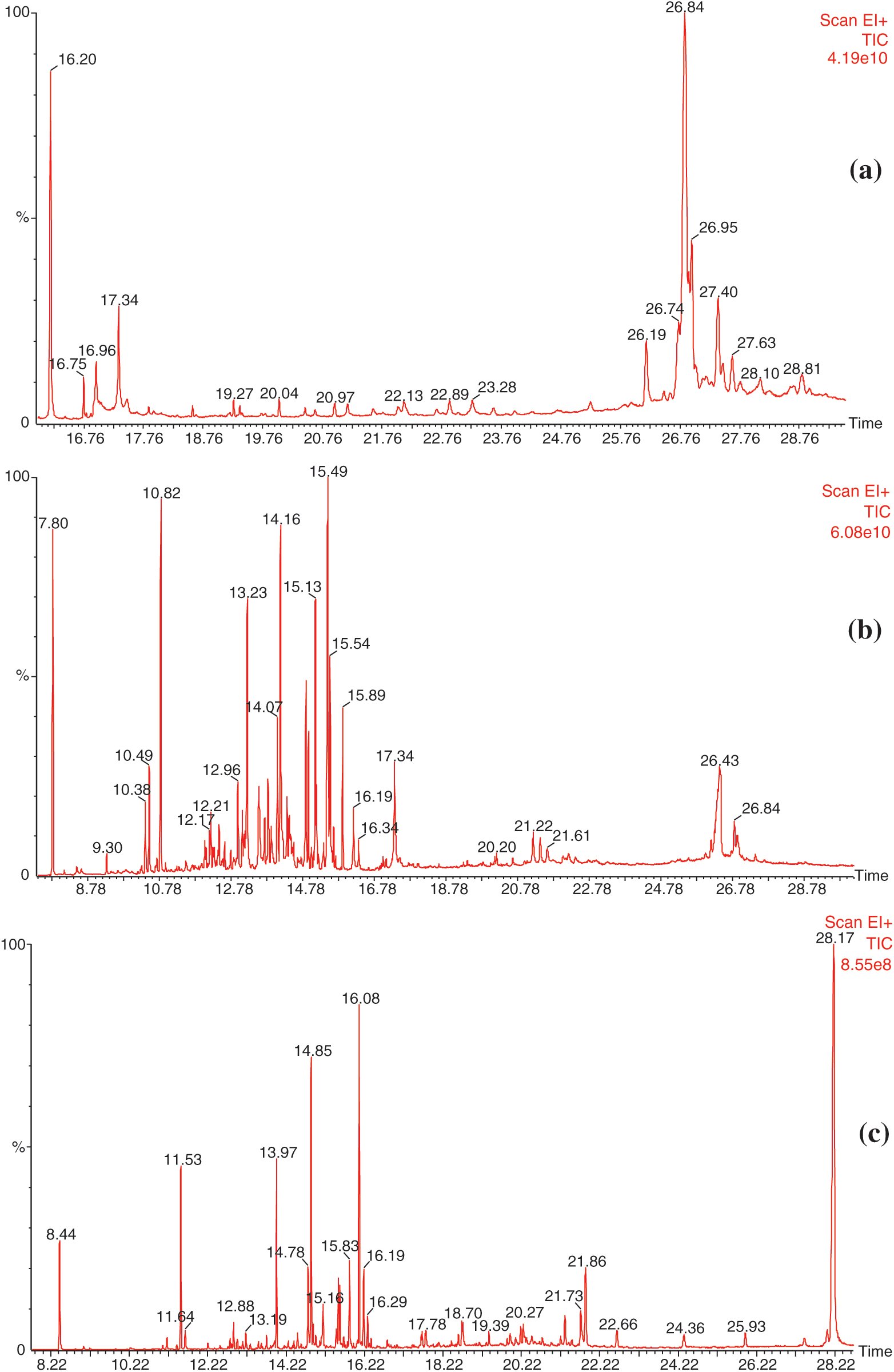
Figure 1: Chromatograms of the different extracts from the bark of C. edulis. a) dichloromethane extract. b) acetone extract. c) toluene/ethanol extract
Identification of the chemicals present in the different extracts using the NIST database is presented in Tab. 4.
Table 4: Compounds identified by GC-MS and their abundance relative to TICa in extracts from the bark of C. edulis
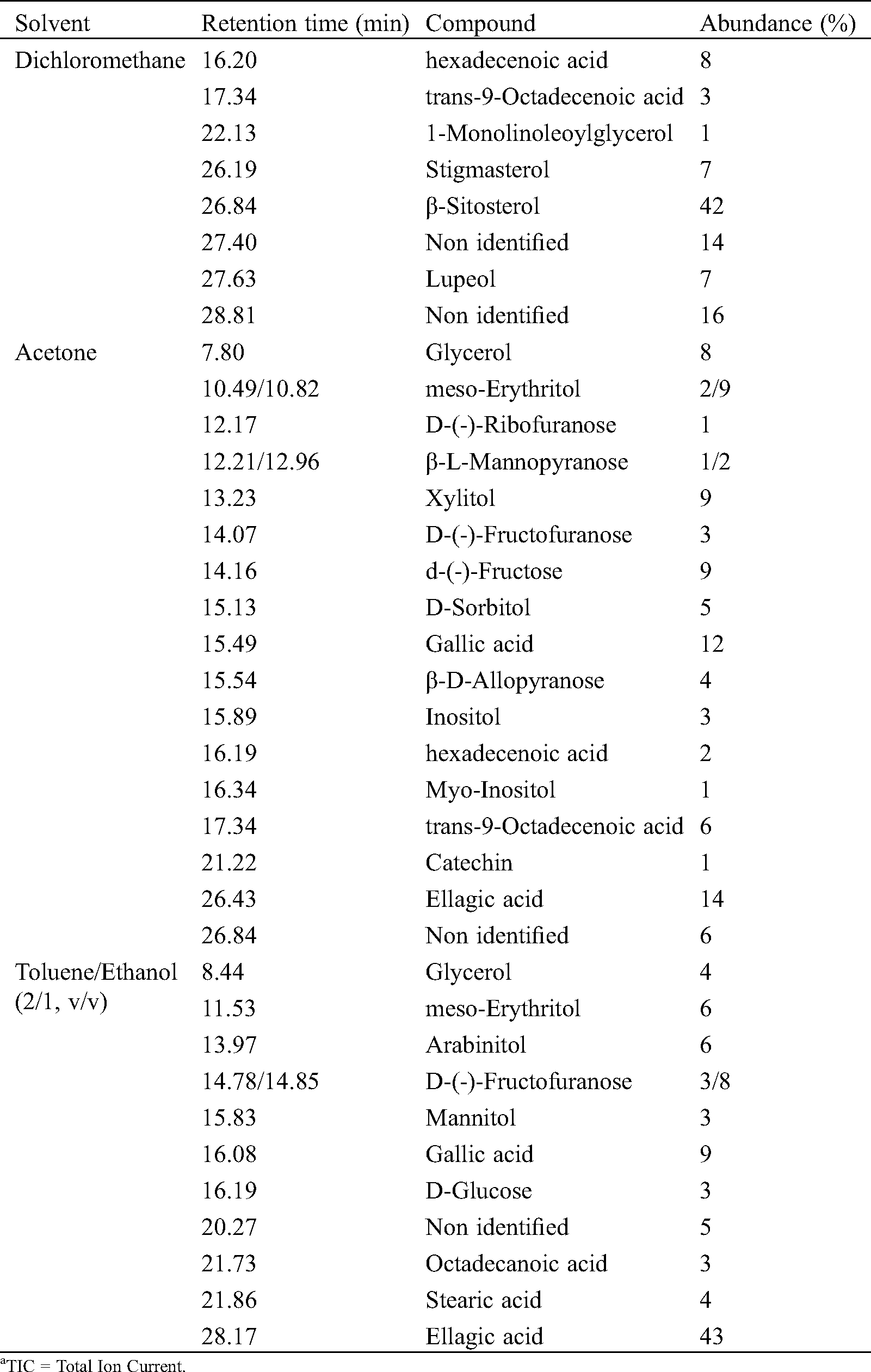
The results obtained from the GC-MS analysis of the different extracts of the bark of C. edulis are in agreement with those of phytochemical screening and those obtained during the assays of phenolic compounds. Indeed, the analysis of dichloromethane extracts indicated the presence of fatty acids and triterpenes like stigmasterol, β-Sitosterol and lupeol, which may react with the Liebermann-Bouchard test, but can also be part of saponins structures involving hydrophobic terpenoid moiety and hydrophilic sugar moiety. Different phenolic compounds like gallic and ellagic acids or catechin have been identified in acetone and toluene/ethanol extracts corroborating the different tests indicating the presence of phenolic compounds. The presence of high amounts of gallic and ellagic acids, as well as numerous sugar compounds, in the last extraction using toluene/ethanol reflects probably the presence of hydrolysable tannins whose ethanolysis or hydrolysis leads to a significant presence of these compounds, even after prior extraction with acetone. Such phenomena may also explain the presence of fatty acid from the hydrolysis of monoglyceride or suberin.
Table 5: Antioxidant activity from the different extracts from the bark of C. edulis
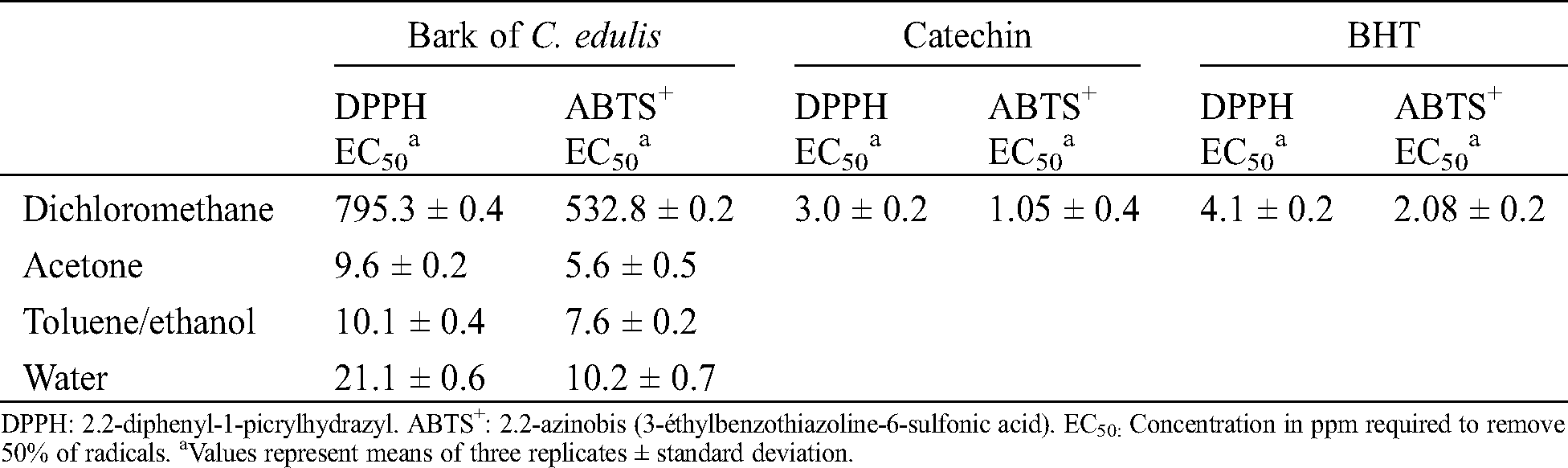
The antioxidant activity of the different bark extracts using DPPH or ABTS+ methods were evaluated by measuring their EC50 values, corresponding to the concentration of extract necessary for to trap 50% of the free radical used (Tab. 5). The lowest EC50 value is, the higher the antioxidant activity is. The ability of the extracts of the bark of C. edulis to trap free radicals varies depending on the extraction solvent. Dichloromethane extracts present very low trapping capacity of free radicals as evidenced by the high values of EC50 recorded, 795.3 ppm with DPPH and 532.8 ppm with ABTS+. In contrast, all the other extracts have shown an interesting capacity to trap free radicals. The highest activities were recorded with the acetone extracts followed by toluene/ethanol extracts, whose EC50 values approximate the values of the antioxidant activities of catechin and BHT used as representative antioxidants used in industry. Water extracts presented slightly lower antioxidant activities compared to activities of acetone and toluene/ethanol extracts, but much better than that of dichloromethane extract. These results matched perfectly with chemicals analysis and phytochemical screening indicating the presence of phenolic compounds in acetone, toluene/ethanol and water extracts. Similar studies already described in the literature correlated antioxidant properties of extracts to their phenolic compounds contents [43–45].
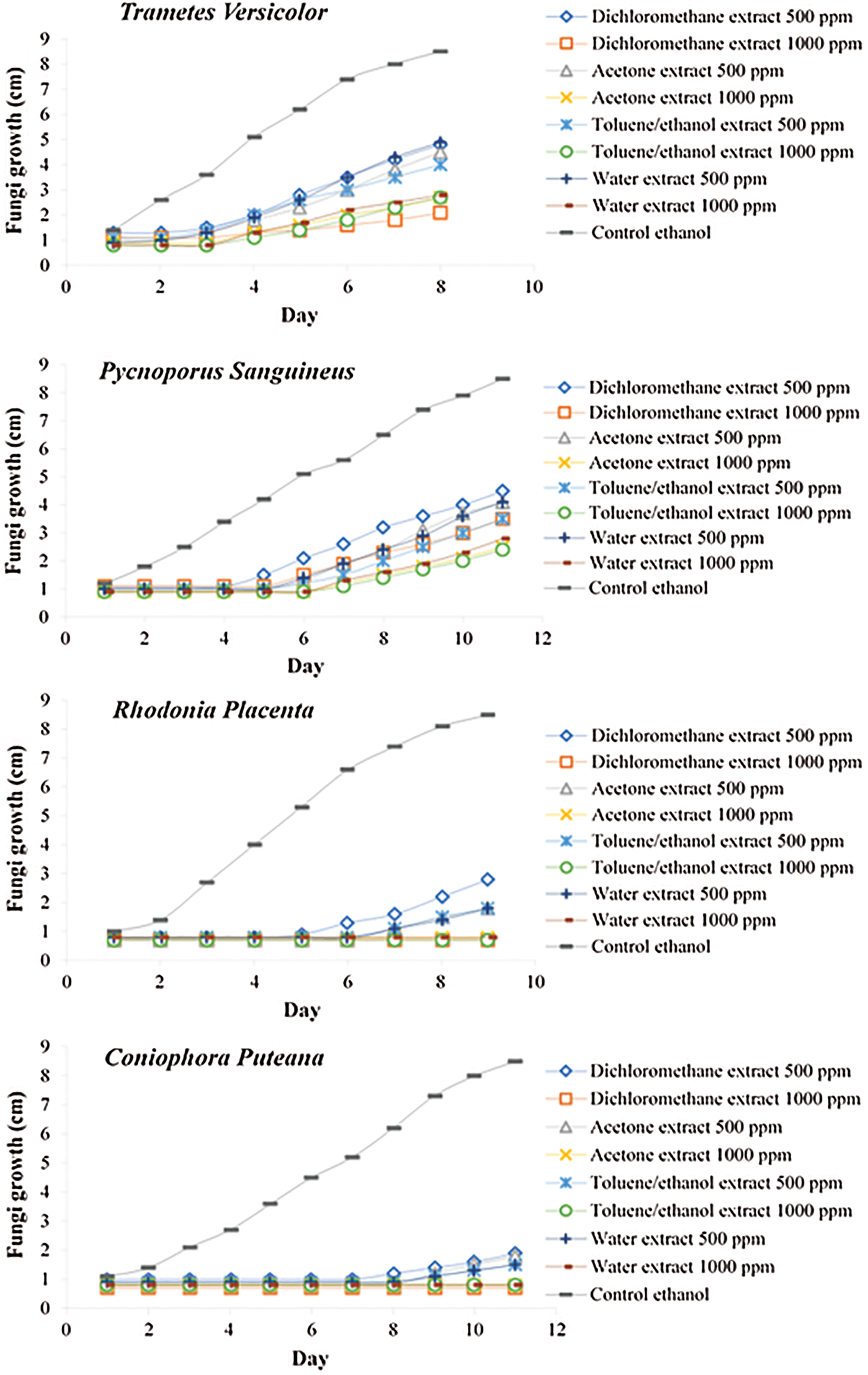
Figure 2: Growth inhibition effect of the extracts on different fungi
In all cases, extracts present a retarding effect on the growth of the different fungal strain tested, which was more pronounced in the case of brown rot fungi than white rot (Fig. 2). At 1000 ppm, all extracts were able to inhibit totally the growth of brown rot fungi R. placenta and C. puteana, while only partial inhibition of the fungal development was observed for white rot fungi P. sanguineus and T. versicolor. At 500 ppm, all extracts lead only to a partial inhibition of the fungal growth independently of the fungal strain tested. In all cases, effect of extractives present in the different extracts seems more fungistatic than fungicidal. Difference in behaviour of the extracts between white rot and brown rot fungi may be due to their difference of mechanisms involved in wood polymer degradation. White rot secretes oxidative enzymes such as laccases and peroxidases, which may be able to degrade phenolic compounds by oxidation. These enzymes are capable of metabolizing phenolic compounds limiting their toxicity and therefore the effectiveness of the extracts to inhibit the growth of the mycelium [46]. Brown rot were, on the contrary, less effective to degrade phenolic compounds, resulting in a lower ability to detoxify the medium. These results are in agreement with those obtained described in the literature [47–50,28,22]. Phenolic compounds are also known to affect the membranes of microorganisms causing leakage of the cytoplasmic content [51]. In this last case, the mechanism of inhibition depends on the ability of phenolic functions to affect the membranes of cellular lipoproteins, causing the weakening of ionic cell homeostasis which destroys the entire cellular structure [52]. Analysis of the dichloromethane extract of the bark have highlighted the presence of triterpenes such as stigmasterol or β-Sitosterol known in the literature to present antifungal properties [30,33,37], which can be at the origin of the activity of this extract.
Table 6: Anti-termite activity tested on Whatman paper filters impregnated with different concentrations of C. edulis bark extracts
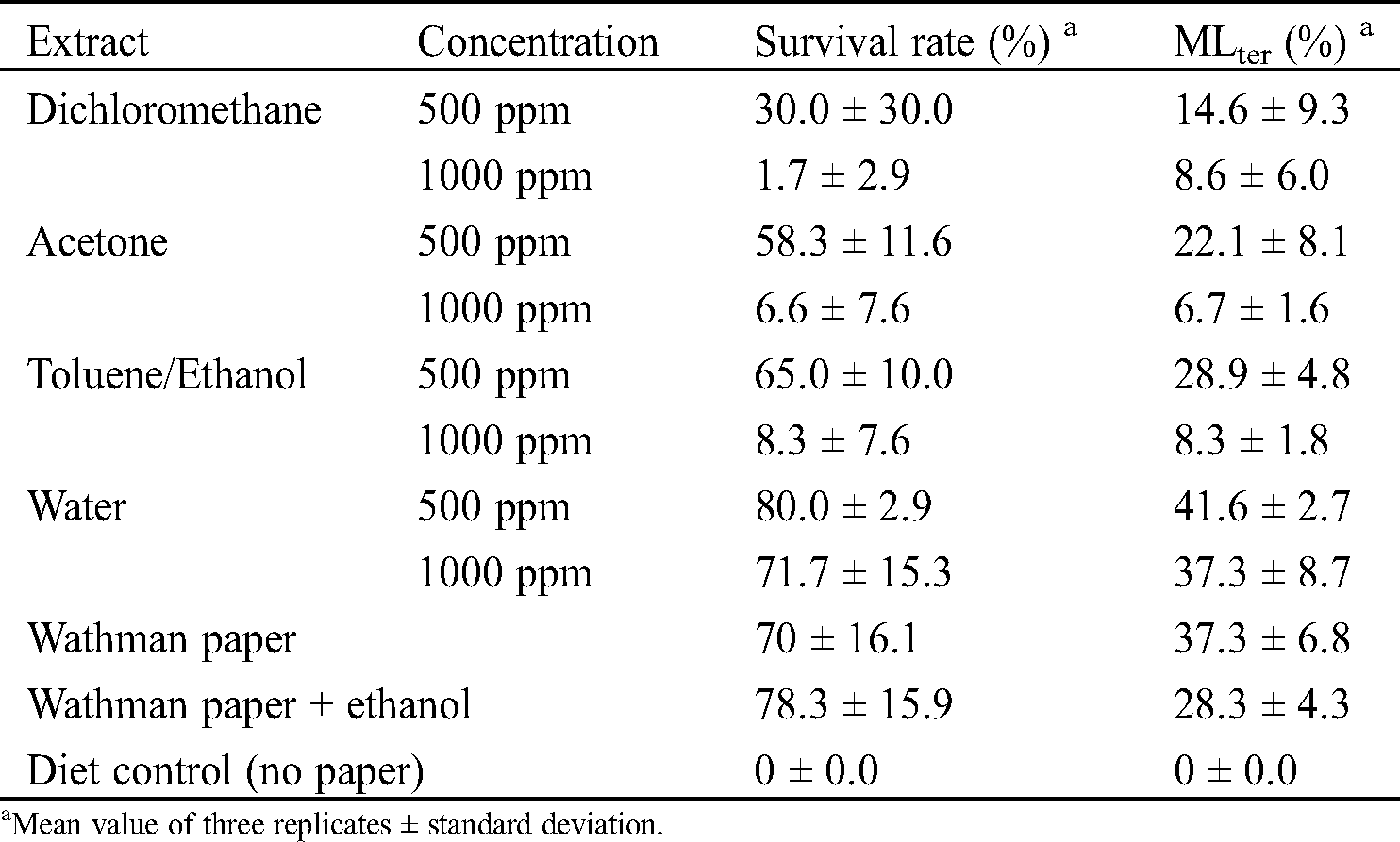
Water extracts of the bark of C. edulis presented low anti-termite activities with termite’s survival rates of 80 and 71.7% and mass losses of 41.6 and 37.3% at 500 ppm and 1000 ppm, respectively (Tab. 6). Dichloromethane, acetone and toluene/ethanol presented more interesting anti-termite activities with survival rates of 30, 58.3 and 65% and mass losses of 14.6, 22.1 and 28.9% at 500 ppm and survival rates of 1.7, 6.6 and 8.3% and mass losses of 8.6, 6.7 and 8.3% at 1000 ppm. Stigmasterol and β-sitosterol present in the dichloromethane extract could explain this activity. Indeed, according to literature, terpenes and terpenoids have been reported to present toxic, antifeeding and repellent properties against termites and other insects [29,31–32,34–35,37]. For the acetone extracts and with the toluene/ethanol mixture. The phytochemical screening and the GC-MS analysis made it possible to demonstrate the presence of phenolic compounds such as gallic acid and ellagic acid which are generally different fragments of the structure of the tannins and which could explain the good anti-termite activity of these extracts vis-à-vis the termites. These results are corroborated by the literature which explains that tannins, due to their protein binding properties and the inhibition of several digestive enzymes, are known to be strongly astringent [53].
Chemical composition of C. edulis bark was investigated and elucidated for the first time. Phytochemical screening indicated the presence of terpenes and sterols in dichloromethane and acetone extracts, while alkaloids, flavonoids, polyphenols and saponins were detected in acetone, toluene/ethanol and water extracts. Determination of total phenols, condensed tannins and flavonoids contents corroborated results issued from phytochemical analysis. Further investigations carried out using GC-MS analysis confirmed the presence of terpenes in dichloromethane extracts with stigmasterol, lupeol and β-sitosterol as the major compounds and fatty acids. Characterization of more polar fractions indicated the presence of gallic and ellagic acid, catechine and different sugars. High amounts of gallic and ellagic acids in the toluene/ethanol extracts suggested the presence of hydrolysable tannins, which may be degraded during extraction leading to phenolic acid. Characterization of antioxidant properties of acetone and toluene/ethanol extracts indicated quite similar antioxidant activities than BHT or catechin used as antioxidant reference compounds. Aqueous extract presented also interesting antioxidant value, while dichloromethane extract presented no activity. Growth inhibition tests performed on different brown and white rot fungi indicated fungistatic effects resulting in a delay of the mycelium growth, brown rots being more susceptible than white rots. Anti-termite activity measured after impregnation of the different extracts on Whatman filter paper revealed weak activities for aqueous extracts, while other extracts presented more interesting anti-termite, efficacy increasing with the solution concentration. Extracts obtained from the bark of C. edulis could therefore be interesting compounds for the formulation of bio-pesticides with potential lower effect on the environment due to their natural origin. Moreover, antioxidant properties may be of valuable interest for food and cosmetic industries and other sector, where such properties are required.
Funding Statement: The authors first thank the National Agency of Grants and Internship of Gabon for the financial support through his chemistry thesis grant (Specialty Sciences of Wood and Fibers) on the person of Bopenga Bopenga Christ Stone Arnaud and LERMAB is supported by a grant overseen by the French National Research Agency (ANR) as part of the “Investissements d’Avenir” program (ANR-11-LABX-0002-01. Lab of Excellence ARBRE). The authors also gratefully acknowledge the GDR 3544 “Science du Bois” for its financial support for the STSM attributed to Bopenga Bopenga Christ Stone Arnaud to perform termite tests at the Research Unit BioWooEB, CIRAD.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Newman, D. J., Cragg, G. M. (2007). Natural products as sources of new drugs over the last 25 years. Journal of Natural Products, 70(3), 461–477. DOI 10.1021/np068054v. [Google Scholar] [CrossRef]
2. Ageos. (2016). Report on the state of satellite coverage of the Gabonese forest heritage. [Google Scholar]
3. Tchatat, M., Ndoye, O., Nasi, R. (1999). Produits forestiers Autres que le bois d’œuvre (PFABplace dans l’aménagement durable des forêts denses humides d’Afrique Centrale. Série Forafri, 18, 102. [Google Scholar]
4. Téké, H., Boesh, H. (2005). Le savoir de nos anciens. Paroles de forêt. 4.4. [Google Scholar]
5. Fengel, D., Wegener, G. (1984). Wood chemistry, ultrastructure, reactions, pp. 27–65. Berlin: De Gruyter. [Google Scholar]
6. Hergert, H. L. (1983). The tannin extraction industry in the United States. Forest & Conservation History, 27(2), 92–93. DOI 10.2307/4004985. [Google Scholar] [CrossRef]
7. Rajkumar, V., Guha, G., Kumar, R. A. (2012). Isolation and bioactivity evaluation of two metabolites from the methanolic extract of Oroxylum indicum stem bark. Asian Pacific Journal of Tropical Biomedicine, 2(1), S7–S11. DOI 10.1016/S2221-1691(12)60120-8. [Google Scholar] [CrossRef]
8. Miranda, I., Lima, L., Quilho, T., Knapic, S., Pereira, H. (2016). The bark of Eucalyptus sideroxylon as a source of phenolic extracts with antioxidant properties. Industrial Crops and Products, 82, 81–87. DOI 10.1016/j.indcrop.2015.12.003.
9. Wang, C., You, C., Yang, K., Guo, S., Geng, Z. et al. (2015). Antifeedant activities of methanol extracts of four Zanthoxylum species and benzophenanthridines from stem bark of Zanthoxylum schinifolium against Tribolium castaneum. Industrial Crops and Products, 74, 407–411. DOI 10.1016/j.indcrop.2015.05.045.
10. Yessoufou, K., Elansary, H. O., Mahmoud, E. A., Woznaik, K. S. (2015). Antifungal: Antibacterial and anticancer activities of Ficus drupacea L. stem bark extract and biologically active isolated compounds. Industrial Crops and Products, 74, 752–758. DOI 10.1016/j.indcrop.2015.06.011. [Google Scholar] [CrossRef]
11. Vigor, C., Vercauteren, J., Montels, J. (2009). Les substances naturelles dans «la chaîne du médicament», première partie initiation à la pharmacognosie. Université de Montpellier, I, 49. [Google Scholar]
12. Akinjogunla, O. J., Yah, C. S., Eghafona, N. O., Ogbemudia, F. O. (2010). Antibacterial activity of leave extracts of Nymphaea lotus (Nymphaeaceae) on Methicillin resistant Staphylococcus aureus (MRSA) and Vancomycin resistant Staphylococcus aureus (VRSA) isolated from clinical samples. Annals of Biological Research, 1, 174–184. [Google Scholar]
13. Houghton, P., Raman, A. (1998). Laboratory handbook for the fractionation of natural extracts. US: Springer. [Google Scholar]
14. Scalbert, A., Monties, B., Janin, G. (1989). Tannins in wood: Comparison of different estimation methods. Journal of Agricultural and Wood Chemistry, 35(5), 1324–1329. DOI 10.1021/jf00089a026. [Google Scholar] [CrossRef]
15. Broadhurst, R. B., Jones, W. T. (1978). Analysis of condensed tannins using acidified vanillin. Journal of the Science of Food and Agriculture, 29(9), 788–794. DOI 10.1002/jsfa.2740290908. [Google Scholar] [CrossRef]
16. Fagbohoun, L. (2014). Chemical study of natural dyes and traditional resinous materials from Benin in the craft sector. https://tel.archives-ouvertes.fr/tel-01168469. [Google Scholar]
17. Brand-Williams, W., Cuvelier, M. E., Berset, C. (1995). Use of free radical method to evaluate antioxidant activity. Lebensmittel-Wissenschaft und-Technologie, 28(1), 25–30. DOI 10.1016/S0023-6438(95)80008-5. [Google Scholar] [CrossRef]
18. Simona, P., Antonio, F., Severina, P., Brigida, A., Piera, U. et al. (2008). Antioxidant properties of sour cherries (Prunus cerasus L.Rôle of coloeless phytochemicals from the methanolic extract of ripe fruits. Journal of Agricultural Food and Chemistry, 56(6), 1928–1935. DOI 10.1021/jf0734727. [Google Scholar] [CrossRef]
19. Chang, S. T., Wang, S. Y., Wu, C. L., Su, Y. C., Kuo, Y. H. (1999). Antifungal compounds in the ethyl acetate soluble fraction of the extractives of Taiwania (Taiwania cryptomerioides hayata) heartwood. Holzforschung, 53(5), 487–490. DOI 10.1515/HF.1999.080. [Google Scholar] [CrossRef]
20. Saha Tchinda, J. B., Abia, D., Dumarçay, S., Ndikontar, K. M., Gérardin, P. et al. (2013). Antioxidant activities, total phenolic contents and chemical compositions of extracts from four Cameroonian woods: Padouk (Pterocarpus soyauxii Taubbtali (Erythrophleum suaveolensmoabi (Baillonella toxispermaand movingui (Distemonanthus benthamianus). Industrial Crops and Products, 41, 71–77. DOI 10.1016/j.indcrop.2012.04.012. [Google Scholar] [CrossRef]
21. Mounguengui, S., Saha Tchinda, J. B., Kor Ndikontar, M., Dumarçay, S., Attéké, C. et al. (2016). Total phenolic and lignin contents, phytochemical screening, antioxidant and fungal inhibition properties of the heartwood extractives of ten Congo Basin tree species. Annals of Forest Science, 73(2), 287–296. DOI 10.1007/s13595-015-0514-5.
22. Bopenga Bopenga, C. S. A., Dumarçay, S., Edou Engonga, P., Gérardin, P. (2020). Relationships between chemical composition and decay durability of Coula edulis Baill as an alternative wood species in Gabon. Wood Science and Technology, 54(2), 329–348. DOI 10.1007/s00226-020-01158-5. [Google Scholar] [CrossRef]
23. Huang, Z., Hashadi, K., Makino, R., Kawamura, F., Kuniyoshi, S. et al. (2009). Evaluation of biological activities of extracts from 22 African tropical wood. Journal of Wood Science, 55(3), 225–229. DOI 10.1007/s10086-008-1024-y. [Google Scholar] [CrossRef]
24. Sirmah, P., Dumarçay, S., Gérardin, P. (2009). Effect of unusual amount of (-)-mesquitol of from the heartwood of Prosopis juliflora. Natural Product Research, 23(2), 183–189. DOI 10.1080/14786410801940968.
25. Mburu, F., Dumarçay, S., Gérardin, P. (2007). Evidence of fungicidal and termicidal properties of Prunus africana heartwood extractives. Holzforschung, 61(3), 323–325. DOI 10.1515/HF.2007.043. [Google Scholar] [CrossRef]
26. Neya, B., Hakkou, M., Pétrissans, M., Gérardin, P. (2004). On the durability of Burkea africana heartwood: Evidence of biocidal and hydrophobic properties responsible for durability. Annals of Forest Science, 6(3), 277–282. DOI 10.1051/forest:2004020.
27. Cheumani, Y. A. M. (2009). Study of the microstructure of wood/cement composites by proton NMR relaxometry. Thesis from the University of Bordeaux 1 (France171.
28. Rosdiana, N. A., Dumarçay, S., Gérardin, C., Chapuis, H., Santiago-Medina, F. J. et al. (2017). Characterization of bark extractives of different industrial Indonesian wood samples for potential valorization. Industrial Crops and Products, 108, 121–127. DOI 10.1016/j.indcrop.2017.06.034. [Google Scholar] [CrossRef]
29. Lajide, L., Escoubas, P., Mizutani, J. (1995). Termite antifeedant activity in Xylopia aethiopica. Phytochemistry, 40(4), 1105–1112. DOI 10.1016/0031-9422(95)92653-P. [Google Scholar] [CrossRef]
30. Escalente, M. A., Santecchia, C. B., Lopez, S. N., Gattuso, M. A., Ravelo, A. G. et al. (2002). Isolation of antifungal saponins from Phytolacca tetra-mera, an Argentinean species in citric risk. Journal of Ethnopharmacology, 82(1), 29–34. DOI 10.1016/S0378-8741(02)00145-9. [Google Scholar] [CrossRef]
31. Viegas, J. C. (2003). Terpenos com atividade inseticida: Uma alternativa para o controle quimico de insetos. Quimica Nova, 26(3), 390–400. DOI 10.1590/S0100-40422003000300017. [Google Scholar] [CrossRef]
32. Blaske, V. U., Hertel, H., Forschler, B. T. (2003). Repellent effects of isoborneol on subterranean termites (Isoptera: Rhinotermitidae) in soils of different composition. Household and Structural Insects, 96, 1267–1274. [Google Scholar]
33. Becker, H., Scher, J. M., Speakman, J. B., Zapp, J. (2005). Bio-activity guided isolation of antimicrobial compounds from Lythrum salicaria. Fitoterapia, 76(6), 580–584. DOI 10.1016/j.fitote.2005.04.011. [Google Scholar] [CrossRef]
34. Park, I. K., Shin, S. C. (2005). Fumigant activity of plant essential oils and components from garlic (Allium sativum) and clove bud (Eugenia caryophyllata) oils against the Japanese termite (Reticulitermes speratus Kolbe). Journal of Agricultural and Food Chemistry, 53, 4388–4392. [Google Scholar]
35. Watanabe, Y., Mihara, R., Mitsunaga, T., Yoshimura, T. (2005). Termite repellent sesquiterpenoids from Callitris glaucophylla heartwood. Journal of Wood Science, 51(5), 514–519. DOI 10.1007/s10086-004-0683-6. [Google Scholar] [CrossRef]
36. Andrea, L. B. D., Claudia Maranhao, A. C., Santos, J. M., Cunha, F. M., Conceiçao, G. W. et al. (2009). Antitermitic activity of extractives from three Brazilian hardwoods against Nasutitermes corniger. International Biodeterioration & Biodegradation, 64, 7–12.
37. Bedounguindzi, W. F., Candelier, K., Edou Engonga, P., Dumarçay, S., Thévenon, M. F. et al. (2020). Anti-termite and anti-fungal bio-sourced wood preservation ingredients from Dacryodes edulis (G. Don) H. J. Lam resin. Holzforschung, 74(8), 745–753. DOI 10.1515/hf-2019-0106. [Google Scholar] [CrossRef]
38. Ekhlil, B., Ghanmi, M., Satrani, B., Alaoui, M. S. B., Rhafouri, R. et al. (2016). Chemical quality, antibacterial and antifungal activities of Cotula cinerea essential oil from South Morocco. Environmental Science-An Indian Journal, 12(5), 209–216. [Google Scholar]
39. Ohmura, W., Doi, S., Aoyama, M. (2000). Antifeedant activity of flavonoids and related compounds against the subterranean termite Coptotermes formosanus Shiraki. Journal of Wood Science, 46(2), 149–153. DOI 10.1007/BF00777362.
40. Wu, T. S., Lin, F. W. (2001). Alkaloids of the wood of Cryptocarya chinensis. Journal of Natural Products, 64(11), 1404–1407. DOI 10.1021/np010258i.
41. Oliveira, L. S., Santana, A. L. B. D., Maranhão, C. A., Miranda, R. C. M., Galvão de Lima, V. L. A. et al. (2010). Natural resistance of five woods to Phanerochaete chrysosporium degradation. International Biodeterioration & Biodegradation, 64(8), 711–715. DOI 10.1016/j.ibiod.2010.08.001. [Google Scholar] [CrossRef]
42. Brennan, M., Fritsch, C., Cosgun, S., Dumarcay, S., Colin, F. et al. (2020). Quantitative and qualitative composition of bark polyphenols changes longitudinally with bark maturity in Abies alba Mill. Annals of Forest Science, 77, 9. DOI 10.1007/s13595-019-0916-x. [Google Scholar] [CrossRef]
43. Mardisadora, O. (2010). Identification and antioxidant potential of flavonoids mahogany bark (Swietenia Macrophylla King). Undergraduate Theses Bogor Agricultural University, Bogor. [Google Scholar]
44. Zhang, L. L., Chen, J. H., Wang, Y. M., Wu, D. M., Xu, M. (2010). Phenolic extracts from Acacia mangium bark and their antioxidant activities. Molecules, 15(5), 3567–3577. DOI 10.3390/molecules15053567.
45. Fahrizal, M. D. (2014). Total flavonoid. phenolic. and antioxidant activity of Paraserianthes Falcataria (L.) bark extract. Undergraduate Theses Bogor Agricultural University, Bogor. [Google Scholar]
46. Gochev, V. K., Krastanov, A. I. (2007). Fungal laccases. Bulgarian Journal of Agricultural Science, 13(1), 75–83. [Google Scholar]
47. Mori, M., Aoyama, M., Doi, S., Kanetoshi, A., Hayashi, T. (1997). Antifungal activity of bark extract of deciduous trees. Holz als Roh und Werkstoff, 55, 130–132. DOI 10.1007/BF02990531. [Google Scholar] [CrossRef]
48. Novriyanti, E., Santosa, E., Syafii, W., Turjaman, M., Sitepu, I. R. (2010). Antifungal activity of wood extract of Aquilaria crassna Pierre ex Lecomte against agarwood-inducing fungi, Fusarium solani. Journal of Forest Research, 2, 155–165.
49. Martínez-Sotres, C., López-Albarrán, P., Cruz-de-León, J., García-Moreno, T., Rutiaga Quiñones, G. J. et al. (2012). Medicarpin, an antifungal compound identified in hexane extract of Dalbergia congestiflora Pittier heartwood. International Biodeterioration & Biodegradation, 69, 38–40. DOI 10.1016/j.ibiod.2011.11.016.
50. Saha Tchinda, J. B., Ndikontar, K. M., Fouda Belinga, A. D., Mounguengui, S., Njankouo, J. M. et al. (2018). Inhibition of fungi with wood extractives and natural durability of five Cameroonian wood species. Industrial Crops and Products, 123, 183–191. DOI 10.1016/j.indcrop.2018.06.078. [Google Scholar] [CrossRef]
51. Borges, A., Ferreira, C., Saavedra, M. J., Simoes, M. (2013). Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microbial Drug Resistance, 19(4), 256–265. DOI 10.1089/mdr.2012.0244. [Google Scholar] [CrossRef]
52. Zabka, M., Pavela, R. (2013). Antifungal efficacy of some natural phenolic compounds against significant pathogenic and toxinogenic filamentous fungi. Chemosphere, 93(6), 1051–1056. DOI 10.1016/j.chemosphere.2013.05.076. [Google Scholar] [CrossRef]
53. Monteiro, J. M., Albuquerque, U. P., Araujo, E. L., Amorim, E. L. C. (2005). Tannins: from chemistry to ecology. Quimica Nova, 28(5), 892–896. DOI 10.1590/S0100-40422005000500029. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |