DOI:10.32604/jrm.2021.011868

| Journal of Renewable Materials DOI:10.32604/jrm.2021.011868 |  |
| Article |
Water Repellency of Cellulosic Fibrous Mats Impregnated with Organic Solutions Based on Recycled Polystyrene
1Department of Forestry and Natural Environment, Laboratory of Forest Utilization, Aristotle University of Thessaloniki, Thessaloniki, Greece
2Department of Forestry and Wood Technology, Linnaeus University, Växjo, Sweden
*Corresponding Author: Stergios Adamopoulos. Email: stergios.adamopoulos@lnu.se
Received: 02 June 2020; Accepted: 12 August 2020
Abstract: Recycled polystyrene in combination with paraffin wax, alkyd resin, and gum rosin were used as components in formulations to investigate their water repellency when applied to cellulosic filter paper substrates. Polystyrene was used in concentration of 5, 10, 15 and 20%, alkyd resin and gum rosin of 5% each and paraffin wax of 0.5%. Totally, twenty four water repellent solutions were prepared. Water repellency was evaluated in terms of water absorption of the cellulosic fibrous mats. The relations between retention of solid substances of the formulations and grammage and water absorption of filter paper samples were also determined. The results showed that all the water repellent formulations exhibited a degree of water repellency. Water absorption decreased by increasing the polystyrene concentration in the solution and polystyrene retention by the impregnated filter paper samples. The incorporation of 0.5% paraffin wax improved the hydrophobicity of treated samples. The best of the three water repellent formulations including paraffin wax was found to be the “polystyrene + gum rosin + paraffin wax” solution followed by the “polystyrene + alkyd resin + paraffin wax” and “polystyrene + paraffin wax” solution. The inclusion of 5% gum rosin in polystyrene solutions compared to that of 0.5% paraffin wax was found more effective in almost all cases.
Keywords: Water repellents; polystyrene; gum rosin; alkyd resin; paraffin wax; water absorption
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
Commercial water repellents are penetrating wood finishes that are used extensively for water uptake protection of timber constructions exposed to outdoor conditions [1,2]. The main solid substances of water repellents diluted in organic solvents or water include a water-repelling substance (e.g., wax) and a synthetic resin (e.g., alkyd resin) to act as a binder of the water repellent to the wood [3]. Water repellent preservatives contain, also, a small amount of fungicides and insecticides to enhance further the effectiveness of the finishes against biological attack [4–7]. The water repellent solutions are applied on millwork and joinery (e.g., window frames, doors, fences) by dipping or brushing. After solvent evaporation, a continuous thin solid film is deposited firmly on the external and internal surface of wood [8]. The closed capillaries and the properties of this film are adequate to repel liquid water from the wood surfaces while wood can dry faster after rain, and thus a reduction in moisture content slows down the rate of fungal attack [9]. In addition, the slower water uptake and the decreased dimensional changes of wood lead to less cracking and warping [10]. However, the properties of the cell walls are not changed permanently by water repellent treatments as in the case of wood modification [11–13], and with time, the exposed wood will swell to the same extent as non-treated wood.
Most of the materials used in water repellents are petroleum based products, such as organic solvents, oils, waxes and synthetic resins, with relatively high cost, and therefore, it is desirable to develop low cost as well as effective and more sustainable substitutes [14,15]. Already during the 1980s, concerns about solvent evaporation in urban areas and air quality have triggered changes in the water repellent formulations [1,16]. Today, stingiest environmental regulations, and associated environmental and health and safety issues favor the use of low or no emission volatile organic compounds (VOC) as solvents and co-solvents in both solvent- and waterborne finishes [17,18]. Although there have been considerable research efforts on the use of environmentally compatible compounds in wood protection formulations, industrial uptake is still limited.
Oleoresin (pine gum), the naturally occurring exudates, collected by tapping from pine trees, is an important renewable forest product. Rosin, the solid component of natural resin, is produced by various technologies, i.e., gum rosin by distillation of oleoresin, wood resin by extraction of resinous wood, tall oil rosin as byproduct in the sulfate pulping process of pine wood [19,20]. Gum rosin, the major solid component from distillation of oleoresin, is a mixture of resin acids (abietic, levopimaric, palustric, neoabietic, isopimaric, etc.) in various proportions depended on Pinus species [21,22]. Gum rosin and the derivatives are used in the plastic industry during vulcanization process of rubber vehicle tires and for manufacturing of varnishes lacquers, printing inks and adhesives [22–24].
Polystyrene is a synthetic, aromatic, thermoplastic polymer made from the monomer styrene. It is soluble in organic solvents, clear, hard and rather brittle, poor barrier to oxygen and water vapor and with relatively high melting point 212°C. It is an inexpensive synthetic resin and one of the most widely used plastics. Polystyrene in rigid form is mainly used for production of low cost rigid plastics like CD cases, model assembly kits, food and beverage containers (cups, plates, egg cartons) as well as for production of other disposable plastics such as laboratory petri dishes and containers [25]. Polystyrene in foamed form (expanded polystyrene-EPS) is used for packaging and as thermal and insulating barrier in building construction [26]. Polystyrene is resistant to biodegradation, so that it is increasingly abundant scrap material in the outdoor environment especially in its foam form [27]. In industry, reclaimed polystyrene can be added in proportion up to 20% with virgin polystyrene [28,29].
In literature, the information on the use of oleoresin, gum rosin and reclaimed polystyrene as basic constituents in water repellent formulations for wood protection are limited. Water repellent formulations based on oleoresin and gum rosin from Aleppo pine trees provided significant protection of oak, poplar, beech, fir and pine sapwood specimens against liquid water uptake [30,31]. Gum rosin in experimental particleboards was found to be at least equally effective to commercial paraffin wax concerning hydrophobicity and internal bond [32,33]. Comparison between polystyrene based treatments and commercial water repellent treatments showed that the former were comparable to the latter in the initial protection of small Scotch pine and beech sapwood specimens [34]. Reclaimed polystyrene organic solutions have been investigated for improving particleboard properties [35], conservation of old wood [36] and improving the bonding strength in glued wood products [37]. Reclaimed polysterene has recently been used as a bonding material for wood composites [38,39].
The aim of the present work was to investigate the hydrophobic properties of recycled polystyrene diluted in organic solvents when applied alone or in combination with gum rosin, alkyd resin and paraffin wax on cellulosic filter paper substrates. The inclusion of polystyrene in water repellent formulations is expected to perform both as water repellent and binding substance.
The composition of experimental water repellent solutions was based on recycled polystyrene (styrofoam), alkyd resin (alkydal FSOW/63% in butyl glycol; Bayer, Germany), paraffin wax (with melting point 55°C) and gum rosin (quality ww) after distillation of Aleppo pine (Pinus halepensis Mill.) oleoresin diluted in proper proportions of commercial Nitro and toluene solvents (Pansil Industry of Chemical Products, Attica, Greece). Totally, 24 water repellent formulations were prepared (Tab. 1).
Table 1: Water-repellent formulations based on polystyrene, alkyd resin, gum rosin and paraffin in organic solvents

The experimental formulations were applied on cellulose laboratory crepe filter paper of grammage 62 g/m2 (Coleman, Bristol, UK). Filter paper is a high hydrophilic and porous material used for filtering liquids and, hence, it is suitable as a substrate for fast screening tests concerning to the initial water uptake effectiveness [40].
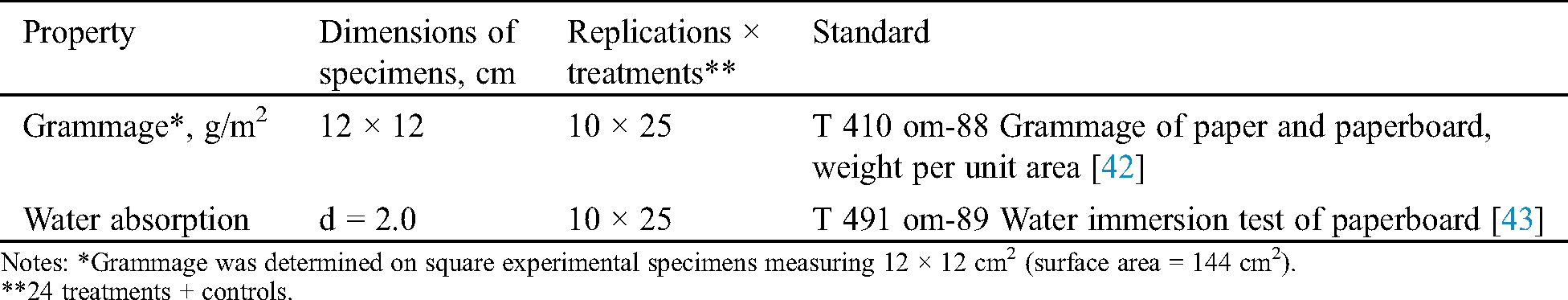
Filter paper square samples, 12 × 12 cm2 in dimensions, were impregnated by simple immersion for 3 min in the pre-mentioned water repellent formulations. After the immersion, the filter samples were air-dried for evaporation of solvents in a horizontal position. Ten samples were impregnated for each treatment. After air-drying and conditioning at 23 ± 1°C and 50 ± 2% RH [41], the impregnated filter paper samples were used for determination of grammage and water uptake after immersion in water. The determined properties, the number and dimensions of filter paper samples for each test and the corresponding standards used are shown in Tab. 2. A schematic illustration for the preparation of the water repellents, the impregnation and testing of cellulose filter papers, and associated analysis of results is shown in Fig. 1.

Figure 1: Schematic illustration of the experimental set up for preparing water-repellent formulations and testing of impregnated cellulose filter papers. RP: recycled polystyrene; PW: paraffin wax; AR: Alkyd resin; GR: gum rosin (all expressed as solids, g)
Table 2: Dimensions, number of specimens and standards applied for determination of grammage and water absorption
The grammage of non-impregnated and impregnated samples with the 24 water repellent formulations was determined by formula (1) (g/m2):

where, W1 is the weight before impregnation (g);
W2 is the weight after impregnation (g);
S is the surface area of samples (144 cm2).
The weights W1 and W2 were determined after conditioning of the samples at 23 ± 1°C and 50 ± 2% RH.
The retention of solid substances of the formulations by the filter paper samples was calculated with formula (2) (%):

For determination of water absorption, no standard exists for filter paper samples. The methodology used in this work is the modified standard method TAPPI T 491 OM-80 [43] as has been used also by others [40]. According to this standard, samples of dimensions 15 × 15 cm2, are fully immersed in water at depth of 7.5 cm for 10 min and the excess of water is suggested to be removed by applying rolling weight of 13 kg. Specifically in this study, round samples of diameter 2 cm were cut with a proper knife (10 samples for each treatment) from filter paper square samples and weighted after conditioning at 23 ± 1°C and 50 ± 2% RH until they reached a constant weight (W3). After this stage, the samples were fully immersed in water at a horizontal position and at depth of 2 cm for 15 min and then they were re-weighted (W4). For control samples, 3 min and 30 min immersion times were used in order to determine the time needed to reach the maximum water absorption by the filter paper (see Tab. 4). Before re-weighting, the excess of water was removed by placing the wet samples between two filter papers and applying a pressure of 200 g for 30 sec.
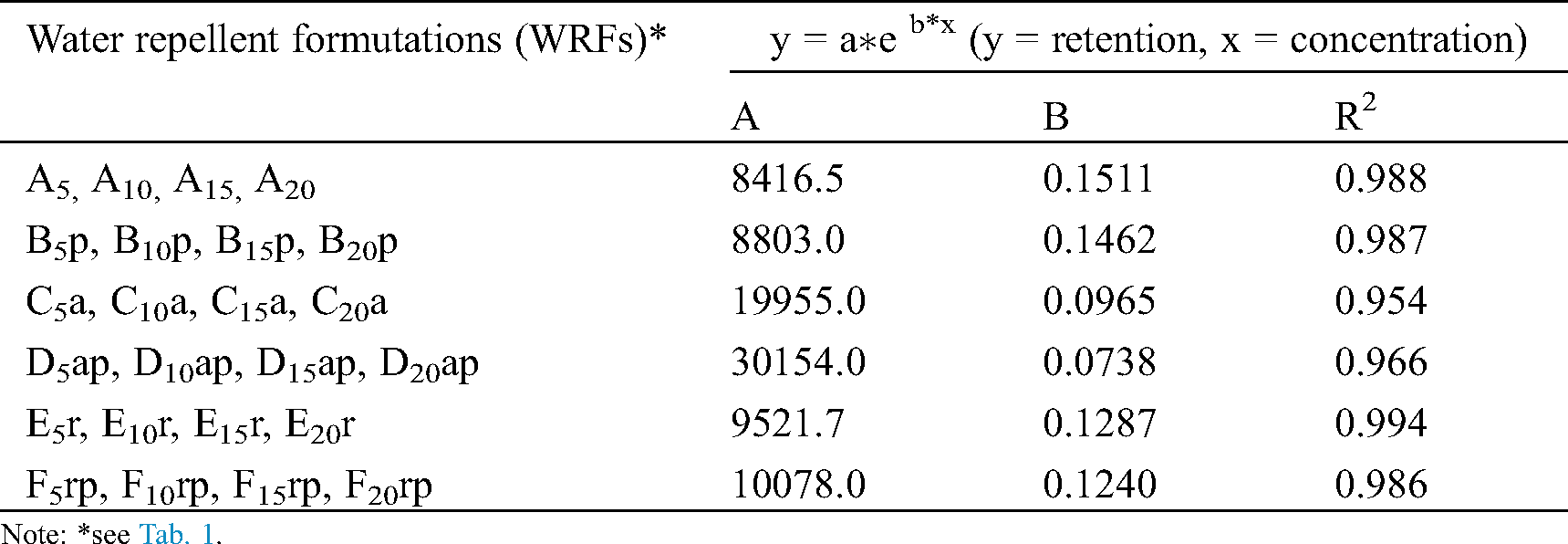
Table 4: Relationships between the concentration of formulations tested and the retention of their solid substances by the filter table samples
The water absorption by the filter paper samples was determined by formula (3) (%):

The statistical differences between the mean water absorption values were assessed by ANOVA and one sample t-test using a p-value of under 0.05 as the threshold of statistical difference.
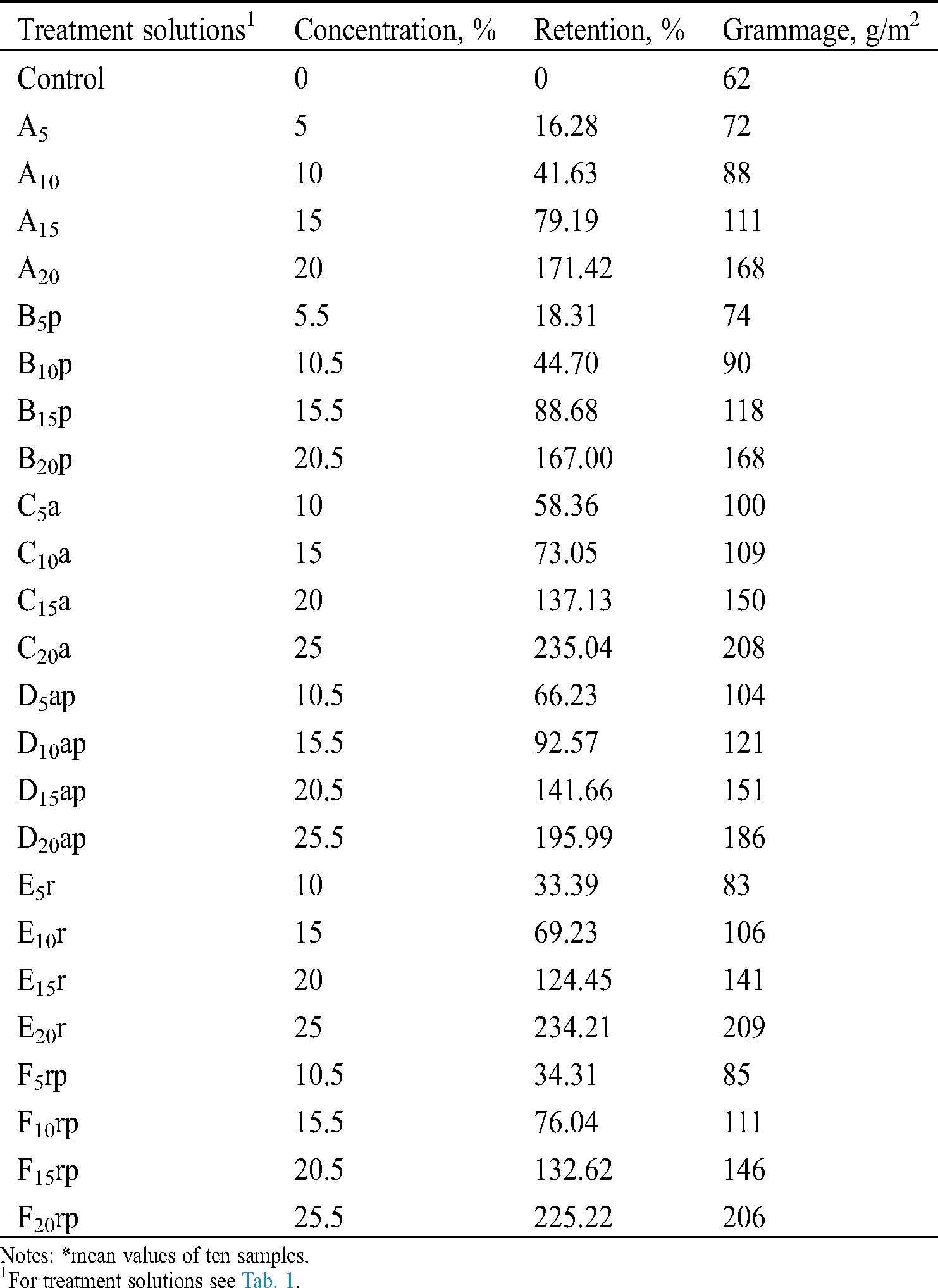
The concentration of solid substances of the water repellent formulations, the retention by the filter paper samples and the grammage of impregnated samples are shown in Tab. 3.
Table 3: Retention of solid substances of water repellent formulations and grammage of impregnated filter paper samples*
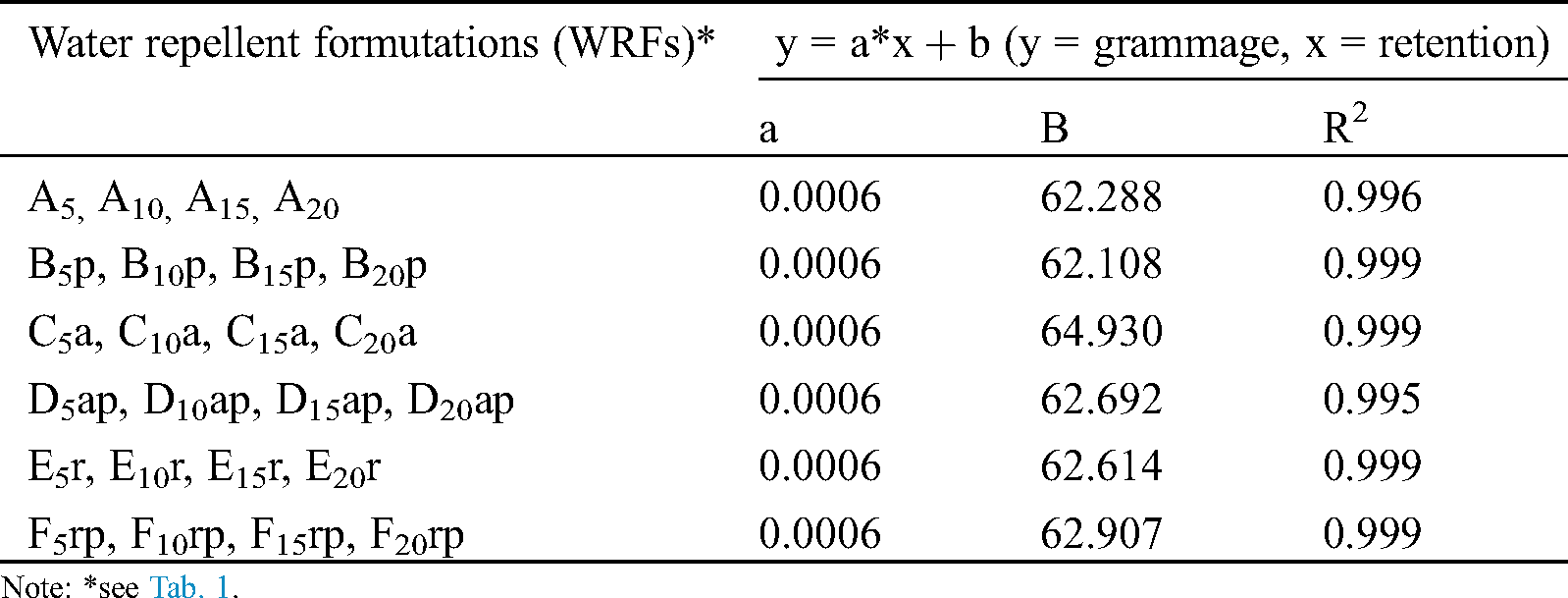
The relation between the concentration of formulations and the retention of their solid substances by the filter paper samples is presented in Fig. 2a and Tab. 4 while the relation between the retention and grammage in Fig. 2b and Tab. 5. As shown in Fig. 2a, the retention increased with increasing the concentration for all experimental solutions. As a result of this increased retention, the grammage of impregnated filter paper samples was found to increase (see Fig. 2b). As Tab. 3 and Fig. 2a show, retention is related to concentration but in different way in various formulations (see Fig. 2a). These differences may be due to different viscosities of the formulations tested. The relation between retention and grammage is linear for all formulations (see Fig. 2b) since grammage depends on retention. Exponential and linear correlations between concentration-retention and grammage-retention respectively, were found to be high, as shown in Tabs. 4 and 5.
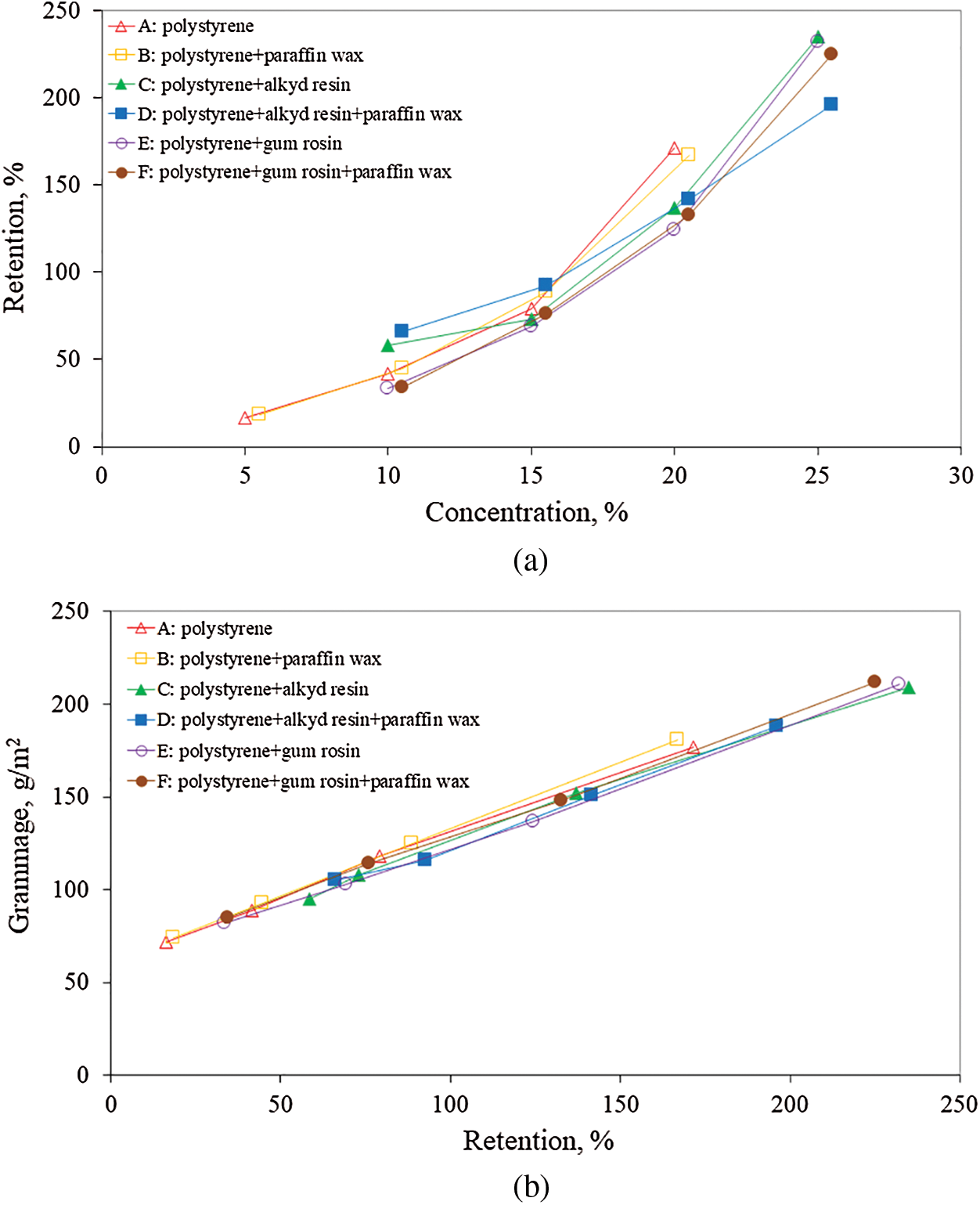
Figure 2: (a) Relation between concentration of water repellent formulations and their retention by the filter paper samples, (b) Relation between retention of solid substances of water repellent formulations and grammage of filter paper samples. The abbreviations A, B, C, D, E and F correspond to 24 formulations as explained thoroughly in Tab. 1
Table 5: Relationships between the grammage of impregnated filter paper samples and the retention of solid substances of WRFs by the samples
Water absorption by the impregnated filter paper samples after 15 minutes immersion in water is presented in Tab. 6 for all the formulations tested. In control samples a small difference of water absorption between the 3 min and 30 min immersion time has been observed. Due to high absortptivity of filter paper samples, the maximum water absorption was almost achieved after 3 min immersion in water (Tab. 6).
Table 6: Water absorption by the impregnated filter paper samples
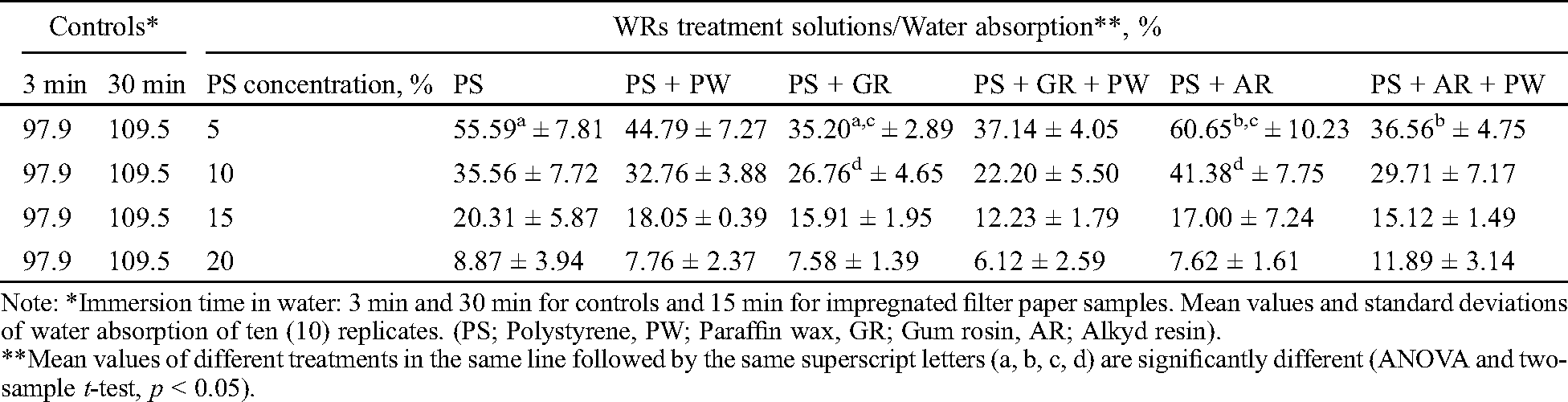
The addition of 0.5% paraffin wax in polystyrene, “polystyrene + gum rosin” and “polystyrene + alkyd resin” water repellent formulations improved the hydrophobicity in all cases except in the solutions “5% polystyrene + 5% gum rosin” and “20% polystyrene + 5% alkyd resin”. Amongst the three water repellent formulations including paraffin wax, the best formulation from the point of hydrophobicity was the “polystyrene + gum rosin + paraffin wax” solution followed by “polystyrene + alkyd resin + paraffin wax” and “polystyrene + paraffin wax” (see Tab. 6). Comparing the first two formulations, it seems that gum rosin increases the water repellency better than alkyd resin due probably to the double role of gum rosin as repelling and binding agent. The third formulation “polystyrene + paraffin wax” does not include alkyd resin or gum rosin to act as binding substance, thus resulting to lower effectiveness.
All experimental water repellent formulations exhibited a good degree of hydrophobicity reducing the water absorption compared to untreated control specimens (see Tab. 6). Statistically significant differences in water absorption were observed in a few cases and only in low PS concentrations 5% and 10%. More specifically these differences occurred between the treatments “5% polystyrene” and “5% polystyrene + 5% gum rosin”, “5% polystyrene + 5% gum rosin” and “5% polystyrene + 5% alkyd resin” and “5% polystyrene + 5% alkyd resin” and “5% polystyrene + 5% alkyd resin + 0.5% paraffin wax” as well as between “10% polystyrene + 5% gum rosin” and “10% polystyrene + 5% alkyd resin”. Between the treatments “5–20% polystyrene + 0.5% paraffin wax” (PS + PW) and “5–20% polystyrene + 5% gum rosin” (PS + GR) it was observed that the “polystyrene + gum rosin” treatment was more effective than “polystyrene + paraffin wax”, although the paraffin wax is more hydrophobic. This is probably due that the film created by the “polystyrene + gum resin” treatment in the capillaries of filter papers were more continuous and resistant to water penetration. Moreover, the incorporation of 0.5% paraffin wax to “polystyrene + gum rosin” treatments improved a little the effectiveness in formulations where polystyrene concentration was 10%, 15% and 20% but no imrovement of effectiveness was observed in the case of 5% polystyrene concentration. The incorporation of 0.5% paraffin wax to “polystyrene + alkyd resin” treatments led to an improvement of effectiveness in formulations where polystyrene concentration was 5%, 10% and 15% but it was decreasing with increased concentration. That trend resulted to the decrease of effectiveness in “20% polystyrene + alkyd resin + paraffin wax” formulation. The explanation for the above mentioned reversal behaviour of the two formulations “5% polystyrene + gum rosin + paraffin wax” and “20% polystyrene + alkyd resin + paraffin wax” may be associated with solubility and compatibility of the type of resin with polystyrene that may affect the quality of the film created (see Tab. 6).
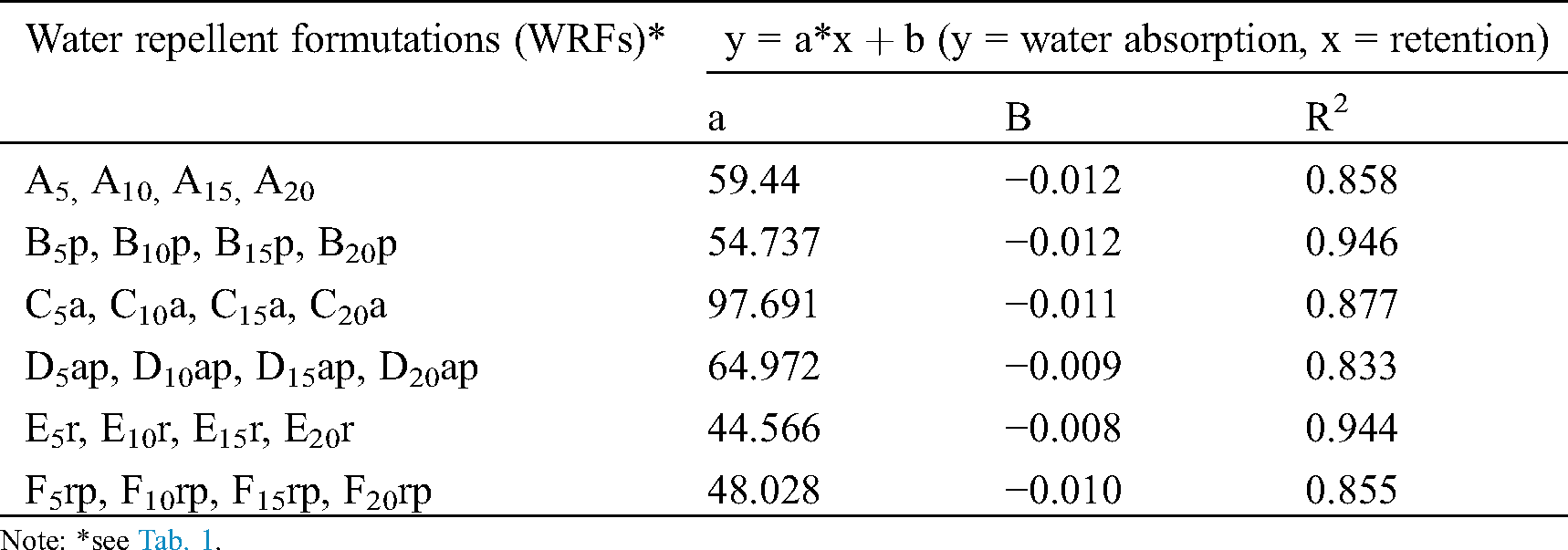
The increase in retention, especially up to 125%, due to PS increased concentration, led to a decrease of water absorption by the impregnated filter paper samples (Fig. 3). Correlations between retention and the exponential water absorption were found to be high (Tab. 7).

Figure 3: Relation between retention of water repellent formulations and water absorption by the filter paper samples. The abbreviations A, B, C, D, E and F correspond to 24 formulations as explained thoroughly in Tab. 1
Table 7: Relationships between retention and water absorption by the impregnated filter paper samples
Previous work investigated oleoresin, gum rosin and recycled polystyrene separately for their water repellency in sapwood specimens of various woods [30,31,34] and particleboards [32,33,35]. In all cases, the above materials exhibited a comparable water repellent effectiveness when compared to commercial water repellents. Literature on investigation of formulations including recycled polystyrene as basic water repellent material combined with the pre-mentioned materials such as gum rosin, synthetic resin and paraffin wax, as used in the present work, are not available. Such formulations compine hydrophobation and blocking mechanisms against water entry and as such can be more effective and sustainable solutions in the protection of fiber based materials from liquid water penetration. Gum rosin, alkyd resin, paraffin as well as the organic solvents used in this study are commonly used in water repellent and paint industry without any special restrictions. Polystyrene is, also, an inert material as the above mentioned water repellents and no chemical reactions were reported when they diluted in organic solvents. In addition, polystyrene is widely used in food packaging industry [44].
The conclusions of the present work are summarized as follows:
1. All water repellent formulations based on recycled polystyrene, gum rosin, alkyd resin and paraffin wax applied to filter papers exhibited more or less a degree of water repellency.
2. Water repellent formulations with polystyrene concentration in the range between 5%–20% resulted gradually to increased retention ranging from 16.28% up to 235.04%.
3. Increased retention led to increased grammage and to considerable reduction of absorption ranging between 60.65% and 6.12%.
4. The incorporation of 0.5% paraffin wax improved the hydrophobicity of water repellent formulations tested except two cases (“5% polystyrene + 5% gum rosin + 0.5% paraffin wax” and “20% polystyrene + 5% alkyd resin + 0.5% paraffin wax”).
5. The inclusion of 5% gum rosin in polystyrene solutions compared to that of 0.5% paraffin wax was found to be more effective in all cases, especially in treatments based on 5% and 10% polystyrene concentration.
6. The addition of 0.5% paraffin wax to “polystyrene + gum rosin” and to “polystyrene + alkyd resin” generally improved only to a small degree the effectiveness except in two cases (“5% polystyrene + 5% gum rosin” and “20% polystyrene + 5% alkyd resin”.
7. The best of the three water repellent formulations including paraffin wax was found to be the “polystyrene + gum rosin + paraffin wax” solution followed by the “polystyrene + alkyd resin + paraffin wax” and “polystyrene + paraffin wax”.
Funding Statement: Financial support has been provided by the Swedish Research Council Formas (Project Grant No. 942-2016-2, 2017-21).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Williams, R. S., Feist, W. C. (1999). Water repellents and water-repellent preservatives for wood. Madison, WI, USA. USDA Forest Service Agriculture: Gen. Tech. Rep. FPL–GTR–109. [Google Scholar]
2. Can, A., Sivrikaya, H. (2019). Surface characterization of wood treated with boron compounds combined with water repellents. Color Research & Application, 44(3), 462–472. DOI 10.1002/col.22357. [Google Scholar] [CrossRef]
3. Rowell, R. M., Banks, W. B. (1985). Water repellency and dimensional stability of wood. Madison, WI, USA. USDA Forest Service Agriculture: Gen Tech Rep FPL-50. [Google Scholar]
4. Panov, D., Terziev, N. (2009). Study on some alkoxysilanes used for hydrophobation and protection of wood against decay. International Biodeterioration & Biodegradation, 63(4), 456–461. DOI 10.1016/j.ibiod.2008.12.003. [Google Scholar] [CrossRef]
5. Preston, A. F. (2000). Wood preservation: Trends of today that will influence the industry tomorrow. Forest Products Journal, 50(9), 12–19.
6. Schultz, T. P., Nicholas, D. D., Preston, A. (2007). A brief review of the past, present and future of wood preservation. Pest Management Science, 63(8), 784–788. DOI 10.1002/ps.1386.
7. Sivrikaya, H., Can, A., Tümen, I., Aydem, D. (2017). Weathering performance of wood treated with copper azde and water repellents. Wood Research, 62(3), 437–450. [Google Scholar]
8. Voulgaridis, E. V., Banks, W. B. (1983). Laboratory evaluation of the performance of water repellents applied to long wood specimens. Holzforschung, 37(5), 261–266. DOI 10.1515/hfsg.1983.37.5.261. [Google Scholar] [CrossRef]
9. Banks, W. B., Voulgaridis, E. V. (1980). The performance of water repellents in the control of moisture absorption by wood exposed to the weather. In Annual Convention of the British Wood Preserving Association. Cambridge, London, UK: Grange Press, pp. 43–53. [Google Scholar]
10. Williams, R. S. (1999). Effect of water repellents on long-term durability of millwork treated with water-repellent preservatives. Forest Products Journal, 49(2), 52–58. [Google Scholar]
11. Donath, S., Militz, H., Mai, C. (2006). Creating water-repellent effects on wood by treatment with silanes. Holzforschung, 60(1), 40–46. DOI 10.1515/HF.2006.008. [Google Scholar] [CrossRef]
12. Mohammed-Ziegler, I., Tánczos, I., Hórvölgyi, Z., Agoston, B. (2008). Water-repellent acylated and silylated wood samples and their surface analytical characterization. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 319(1–3), 204–212. DOI 10.1016/j.colsurfa.2007.06.063.
13. Kielmann, B. C., Butter, K., Mai, C. (2018). Modification of wood with formulations of phenolic resin and iron-tannin-complexes to improve material properties and expand colour variety. European Journal of Wood and Wood Products, 76(1), 259–267. DOI 10.1007/s00107-017-1180-0. [Google Scholar] [CrossRef]
14. Singh, T., Singh, A. P. (2012). A review on natural products as wood protectant. Wood Science and Technology, 46(5), 851–870. DOI 10.1007/s00226-011-0448-5. [Google Scholar] [CrossRef]
15. Voulgaridis, E. (1980). Physical factors affecting the performance of water repellents applied to wood (Ph.D. Thesis). University of North Carolina Wilmington, Bangor, U.K. [Google Scholar]
16. William, K. R. (1991). The new clean air act: An environmental milestone. Environmental Protection Agency (EPA) Journal, 17(1), 2. [Google Scholar]
17. Donatello, S., Moons, H., Wolf, O. (2017). Revision of EU Ecolabel criteria for furniture products. Final Technical Report. 28443 EN. [Google Scholar]
18. European Commission (EC). (2007). Reference document on best available techniques on surface treatment using organic solvents. Brussels, Belgium, Okopol GmbH. [Google Scholar]
19. Hocking, M. B. (1985). Production of pulp and paper. In: Britt, K.W. (eds.Modern chemical technology and emission control. 2nd edition, pp. 300–337. Berlin, Heidelberg, Germany: Springer. [Google Scholar]
20. Sushland, O., Woodson, G. E. (1986). Fiberboard manufacturing practices in the United States. 1st edition. Madison, WI, U.S.: USDA Forest Service Agriculture. [Google Scholar]
21. Maiti, S., Ray, S. S., Kundu, A. K. (1989). Rosin: A renewable resource for polymers and polymer chemicals. Progress in Polymer Science, 14(3), 297–338. DOI 10.1016/0079-6700(89)90005-1. [Google Scholar] [CrossRef]
22. Valkanas, G. (1972). The composition of commercial rosins in general and the Greek rosin with its precursors in particular. Athens, Greece: Annals of Technical University of Athens. [Google Scholar]
23. Abdel-Raouf, M. E., Abdul-Raheim, A. M. (2018). Rosin: Chemistry, derivatives, and applications: A review. BAOJ Chemistry, 4, 039.
24. Arrieta, M. P., Samper, M. D., Jiménez-López, M., Aldas, M., López, J. (2017). Combined effect of linseed oil and gum rosin as natural additives for PVC. Industrial Crops and Products, 99, 196–204. DOI 10.1016/j.indcrop.2017.02.009. [Google Scholar] [CrossRef]
25. Harper, C. A. (2002). Handbook of plastics, elastomers and composites. 4thedition. Lutherville, Maryland: McGraw-Hill Handbooks. [Google Scholar]
26. EPS Industry Alliance. (2016). Properties, performance and design fundamentals of expanded polystyrene packaging. Crofton, Maryland, U.K: EPS Recycling Advancements & Technology Innovations, Technical Bulletin, EPS Industry Alliance. [Google Scholar]
27. Thakur, S., Verma, A., Sharma, B., Chaudhary, J., Tamulevicius, S. et al. (2018). Recent developments in recycling of polystyrene based plastics. Current Opinion in Green and Sustainable Chemistry, 13, 32–38. DOI 10.1016/j.cogsc.2018.03.011. [Google Scholar] [CrossRef]
28. Maharana, T., Negi, Y. S., Mohanty, B. (2007). Review article: recycling of polystyrene. Polymer-Plastics Technology and Engineering, 46(7), 729–736. DOI 10.1080/03602550701273963. [Google Scholar] [CrossRef]
29. Samper, M. D., Garcia-Sanoguera, D., Parres, F., López, J. (2010). Recycling of expanded polystyrene from packaging. Progress in Rubber, Plastics and Recycling Technology, 26(2), 51–60. DOI 10.1177/147776061002600202. [Google Scholar] [CrossRef]
30. Voulgaridis, E. (1988). Protection of oak wood (Quercus conferta Kit.) from liquid water uptake with water reppelents. Wood and Fiber Science, 20(1), 68–73. [Google Scholar]
31. Voulgaridis, E. (1993). Oleoresin and gum rosin from Pinus halepensis Mill. as basic constituents in water repellent formulations applied to wood. Holz als Roh- und Werkstoff, 51(5), 324–328. DOI 10.1007/BF02663803. [Google Scholar] [CrossRef]
32. Grigoriou, A., Passialis, C. (1990). Gum rosin as water-repellent additive for particleboards. Holzforschung und Holzverwertung, 42(5), 93–94. [Google Scholar]
33. Passialis, C., Grigoriou, A. (1992). Effect of application method of gum rosin as water-repellent additive on hydrophobicity and internal bond of particleboards. Holzforschung und Holzverwertung, 44, 88–89. [Google Scholar]
34. Voulgaridis, E., Passialis, C. (1982). Preliminary studies on water repellent properties of reclaimed polystyrene applied to small wood specimens. Holzforschung und Holzverwertung, 34(5), 66–69. [Google Scholar]
35. Passialis, C. (1986). Improving the properties of particleboards by impregnation with a toluene-based polystyrene solution. Holz als Roh- und Werkstoff, 44(5), 193–195. DOI 10.1007/BF02611293. [Google Scholar] [CrossRef]
36. Passialis, C. (1980). Conservation of old wood. Aristotle University, Thessaloniki, Greece. Annals Schools Agric. For. 23 Annes. pp. 199–207. [Google Scholar]
37. Passialis, C. N., Philippou, J. L. (2002). Investigation of bonding strength in glued wood and wood products with reclaimed polystyrene. Dassiki Erevna (Forest Research), 15, 81–90. [Google Scholar]
38. Adefisan, O. O. (2018). Evaluation of strength and sorption properties of polystyrene bonded composites of mahogany (Khaya ivorensis) and teak (Tectona grandis) woods. Arid Zone Journal of Engineering, Technology and Environment, 14(2), 201–207. [Google Scholar]
39. Adefisan, O. O., Oyelola, D. J. (2019). Strength, sorption and chemical properties of Ceiba pentandra and Tectona grandis wood composite. Arid Zone Journal of Engineering, Technology and Environment, 15(2), 91–99. [Google Scholar]
40. Hosseinpourpia, R., Adamopoulos, S., Parsland, C. (2019). Utilization of different tall oils for improving the water resistance of cellulosic fibers. Journal of Applied Polymer Science, 136(13), 47303. DOI 10.1002/app.47303. [Google Scholar] [CrossRef]
41. SCAN-P 2 (2002). 75 paper and board conditioning of test samples. Drotting Kristinas Vag 61, Stockholm, Sweden: Paper and Board Testing Committee. [Google Scholar]
42. TAPPI T 410 om-88. (1991). Grammage of paper and paper board (weight per unit area). TAPPI test methods. Vol. 1. Atlanda, USA: Technology Park.
43. TAPPI T 491 om-89. (1991). Water immersion test of paperboard. TAPPI test methods. Vol. 1. Atlanta, USA: Technology Park. [Google Scholar]
44. Genualdi, S., Nyman, P., Begley, T. (2014). Updated evaluation of the migration of styrene monomer and oligomers from polystyrene food contact materials to foods and food simulants. Food Additives & Contaminants: Part A, 31(4), 723–733. DOI 10.1080/19440049.2013.878040. [Google Scholar] [CrossRef]