 Open Access
Open Access
REVIEW
Techno-Functional Properties and Potential Applications of Peptides from Agro-Industrial Residues
1 Department of Food, Agriculture and Bioresources, Asian Institute of Technology, Klong Luang, 12120, Thailand
2 National Center for Genetic Engineering and Biotechnology (BIOTEC), National Science and Technology Development Agency (NSTDA), Khlong Luang, 12120, Thailand
* Corresponding Author: Anil Kumar Anal. Email:
(This article belongs to the Special Issue: Recent Advances on Renewable Materials)
Journal of Renewable Materials 2025, 13(3), 553-582. https://doi.org/10.32604/jrm.2025.058857
Received 23 September 2024; Accepted 17 December 2024; Issue published 20 March 2025
Abstract
The growing population and industrialization have led to significant production in agro-industrial sectors, resulting in large amounts of agro-industrial residues often left untreated, posing potential environmental issues. Therefore, finding effective ways to utilize these bio-based residues is crucial. One promising approach is to use these low- or no-value agro-industrial wastes as raw materials for producing renewable biomaterials, including proteins and peptides. Research has extensively explored peptide extraction using plant and animal-based agro-industrial residue. Due to lower processing costs and beneficial bioactive properties, peptides derived from waste could replace synthetic peptides and those extracted from food sources. The isolation, purification, and analysis processes of these peptides are thoroughly examined to optimize their extraction and ensure their purity and efficacy. These peptides’ bioactive properties and mechanisms are being analyzed for their potential applications in the biomedical field. Additionally, the applications of bioactive peptides in medical fields, such as drug delivery systems, tissue engineering, and bioprinting, are discussed.Keywords
Nomenclature
| CE | Circular economy |
| ACE | Angiotensin-converting enzyme |
| MIC | Minimum inhibitory concentration |
| IC50 | Half maximal inhibitory concentration |
| MALDI-TOF | Matrix-assisted laser desorption/ionization time-of-flight |
| LC-MS | Liquid chromatography-mass spectrometry |
| ESI-MS | Electrospray ionization–mass spectrometry |
| FAB-MS | Fast atom bombardment mass spectrometry |
| MALDI-MS | Matrix-assisted laser desorption/ionization mass spectrometry |
| EGFR | Epidermal growth factor cells |
The surge in waste generated by both agricultural and industrial sectors worldwide has pushed linear economic models to their limits. Reports estimate that global waste production will reach 2.59 billion tons by 2030, and this number will continue to rise [1]. Particularly in the food and agricultural sectors, about 33 percent of food produced for human consumption is wasted across the supply chain, totaling around 1.6 billion tons annually. The food and agriculture sectors are the largest producers of industrial waste. By 2025, they are expected to account for approximately 44 percent of global waste and by-products [2]. Tremendous amounts of agro-industrial waste end up in landfills or are incinerated. Additionally, the rising population exacerbates waste issues [3]. Improper waste management leads to significant environmental consequences, including severe water and land pollution. Substantial investment is required for treatment and pollution control, and inadequate waste management can result in financial losses and additional expenses. In response, the circular economy (CE) has been emphasized and implemented in several countries to replace the linear economic model. CE focuses on regenerative actions, minimizing resource and waste utilization, emissions, and energy usage through long-lasting design, maintenance, repair, reuse, remanufacturing, refurbishing, and recycling [4].
Achieving a circular economy requires implementing zero waste practices. However, in reality, waste cannot be eliminated but can be converted, recycled, or dissipated within the environmental system [5]. Significant challenges in CE implementation include constraints on material properties and manufacturing and reprocessing technologies. During the initial stages of CE implementation, aspects related to economic feasibility should have been considered. Effective and environmentally friendly methods to reuse, convert, and improve waste must be developed alongside CE to enhance the value of waste. Consequently, waste valorization processes, which convert low- or non-value waste into high-value products, are essential in implementing CE practices.
To recycle and develop high-value products from agro-industrial residues, several aspects were observed to examine the potential of the waste, which ultimately led to a better understanding of the organic structure of the agro-based residues. Most agricultural wastes contain rich organic compounds such as proteins, carbohydrates, fats, fibers, and several bioactive ingredients. Specifically, agro-industrial residues with high protein content are suitable for food and medical applications. Consequently, extensive research on using these wastes to produce bioactive compounds and functional foods is conducted through waste valorization processes. An exciting approach involves synthesizing bioactive peptides using bio-based wastes as substrates. An exciting approach involves synthesizing bioactive peptides using bio-based wastes as substrates. For example, antioxidant peptides have been extracted from chicken feathers, a major bio-waste from animal farming and processing [6]. Other examples of seafood-based wastes used include shrimp shells [7], fish skin [8], and fishbone [9]. Plant-based wastes also provide sufficient protein for producing bioactive peptides [10]. For instance, bioactive peptides have been extracted from brewer’s spent grain, a significant by-product of the beverage industry [11].
2 Bioactive Peptides from the Agro-Industrial Wastes
2.1 Brief Overview of Peptides
The advancement of wellness has encouraged trends that theoretically improve the concept of food. Beyond serving as a source of energy or satisfying hunger, an enhanced diet can significantly improve health and prevent nutrition-related diseases [12]. This concept has led to the development of functional foods, which are diets consisting of active ingredients that benefit consumers [13]. As a result, several bioactive compounds, such as peptides and polyphenolic compounds, have been extensively explored and used as active ingredients [14]. A tremendous amount of protein-based waste is generated daily, generally underutilized and traditionally sold as low-value animal feed or buried in soil, potentially leading to environmental issues. Utilizing low- or non-value protein-based wastes and by-products as protein sources for peptide production presents a promising solution [15]. The categorization of peptide production from agro-industrial wastes, illustrated in Fig. 1, identifies many waste sources.

Figure 1: Categorization of bioactive peptide production from the agro-industrial wastes
Due to their unique bioactive properties, peptides are among the most investigated functional ingredients. Peptides are defined as short-chain proteins composed of 2–20 amino acids linked by peptide bonds. These oligopeptides are inactive within complex food proteins. However, hydrolysis through biological, chemical, or enzymatic reactions releases the oligopeptides from complex protein structures, activating them. Oligopeptides exhibit various biologically active properties, such as angiotensin-converting enzyme (ACE) inhibition, antimicrobial, anti-inflammatory, antithrombotic, antioxidative, antidiabetic, anticancer, antiadhesive, dipeptidyl-peptidase IV-inhibitory, opioid, immunomodulatory, and mineral-binding activities, depending on their amino acid compositions, number of molecules, and sequences [16]. Generally, peptides are inactive in complex protein structures. However, enzymatic hydrolysis occurs in gastrointestinal tracts, potentially breaking down protein structures and releasing active peptides [17]. Producing bioactive peptides from the in vivo hydrolyzation process is impractical and inefficient. Therefore, fermentation using microorganisms and in vitro enzymatic hydrolysis are the primary production methods for acquiring bioactive peptides [18].
Animal-based waste is one of the most promising sources of peptides. This waste is generated at all levels of food production, from farming to food processing. The meat, seafood, and dairy industries produce significant amounts of protein waste annually. The meat industry, a crucial industrial sector, produces approximately 330 million tons of meat worldwide [19]. However, over half of a live animal’s weight is not suitable for human consumption and is considered waste. The meat processing industry generates by-products such as bone [20], blood [21], shredded meats and muscle, and feathers [6]. These high-protein wastes have potential as sources of bioactive peptides. For example, the poultry industry generates various wastes, including poultry feathers [22], eggshell membranes [23], poultry blood [24], and poultry bone [25]. Several bioactive peptides have been identified and extracted from these materials.
Processing raw fish and seafood also produces substantial waste and by-products with high protein content, making them potential sources of peptides. By-products from fish processing, such as fishbone [26], fish skin [27], fish viscera [28], and fish wastewater [29], have been tested as protein sources for bioactive peptide production.
The seafood industry generates significant waste and by-products. For instance, by-products (heads and hard carapaces) from crustacean processing account for 45%–60% of the original materials [30]. Shrimp waste, which contains abundant organic components, particularly protein, includes shrimp heads and shells with protein contents of 54.4% and 47.43%, respectively [31]. Due to their high production volume and protein content, shrimp shell wastes have been explored as sources of bioactive ingredients, especially peptides. Peptide production from shrimp shell wastes has yielded peptides with various bioactive properties, such as antioxidant [32], antimicrobial [33], and ACE-I inhibitory peptides [7]. The hydrolysis of shell wastes and shrimp cooking juices also produces bioactive peptides [34].
The dairy industry is a leading sector in the food industry, generating vast amounts of by-products and waste that significantly impact the environment. Annually, 4–11 million tons of fat and protein-rich dairy waste are released into the environment, causing severe environmental issues [35]. Therefore, appropriate valorization of dairy waste is essential. These protein-rich wastes include whey protein [36] and skim milk [37]. The production of bioactive peptides from animal-based wastes is illustrated in Table 1.
Due to changing consumer consumption behavior and habits from animal-based diets toward plant-based diets, the production of plant-based foods has been steadily growing. This shift has led to the generation of a tremendous amount of by-products from plant sources. Among these, cereal and legume by-products are the most explored due to their high protein content and essential amino acids [49]. For example, the worldwide production of brewers’ spent grain, a by-product of beer production, is approximately 1900 million tons annually. This waste, with an adequate protein content of 18.6%, has potential as a protein source for peptide production [50]. Researchers have extensively studied this waste as a primary ingredient for plant-based peptide production [11]. The waste has been hydrolyzed to produce antioxidant and ACE-inhibitory peptides. Additionally, peptides from brewers’ spent grain, exhibiting significant antioxidant properties, have been obtained through bacterial fermentation using Bacillus cereus and Bacillus lentus [51].
Similarly, in the oil-producing industries, most of the seeds, cereals, beans, nuts, and fruits such as rape seed, soybean, peanut, sunflower seed, olive, rice, corn, and palm, utilized in the oil extraction process, resulting in oil cake or meal considered as by-products or waste [52,53]. For instance, soybean meal, a by-product of oil production, is produced globally at approximately 243 million tons annually. With a protein content of 30.58%, soybean meal is suitable for peptide production [54]. Consequently, soybean meal has been widely explored and subjected to fermentation processes to develop bioactive peptides [55,56]. The antioxidant peptide production from rapeseed, another by-product of oil production, has also been studied [57]. Rapeseed waste, generated from mechanical pressing, contains 30%–40% protein, making it highly suitable for peptide production [58]. The antioxidant mechanism of the peptide WDHHAPQLR, derived from rapeseed protein, was tested in animal models, revealing its ability to inhibit the apoptosis of oxidative stress cells, key to its antioxidant mechanism [57].
Lastly, agro-industrial sources of plant-based protein wastes are linked to juice and beverage processing. During juice extraction from fruits, the by-products generated are primarily seeds, which are protein-rich and can be potential materials for peptide production. For example, tomato seeds, a by-product of tomato processing, contain around 28% protein [59]. These protein-rich wastes were fermented using Bacillus subtilis A14h strains, resulting in a mixture of peptides exhibiting both antioxidant and angiotensin-converting enzyme (ACE)-inhibitory activities [60]. Another example is watermelon seeds, which contain adequate protein. Five different peptides were extracted from watermelon seed protein through enzymatic hydrolysis, followed by separation and purification. The extracted peptide RDPEER exhibits high antioxidant capacity and can significantly inhibit reactive oxygen species (ROS), minimizing oxidative damage to HepG2 cells [61]. Peptides extracted from orange seeds have shown various bioactive properties, including antioxidant activities, gastrointestinal resistance, and ACE inhibitory activities [62]. The production of bioactive peptides from plant-based wastes is illustrated in Table 2.
3 Isolation, Purification, and Analysis of Peptides from Agro-Industrial Wastes
Native proteins are inactive and present in an encrypted form, preventing the release of bioactive peptides from complex protein structures. Thus, an extraction process that breaks down the protein structure into small chains of amino acids is required. Several hydrolysis methods, such as enzymatic hydrolysis, microbial fermentation, and acid-alkaline solvent extraction, are generally utilized depending on the desired bioactive properties. However, current practices mainly favor green-based methods to avoid the environmental consequences of chemical residues.
The enzymatic hydrolysis process typically involves using commercial enzymes to break peptide bonds in proteins, thereby releasing active peptides. Traditionally, this process was performed under batch conditions, often leading to lower yields and the production of undesirable secondary metabolites [18]. To address these issues, advanced enzymatic hydrolysis techniques, such as enzyme immobilization and ultrafiltration membranes, have become more popular. Enzyme immobilization creates a two-phase system, simplifying the purification process, while membrane filtration separates small hydrolyzed fractions and recycles larger molecules back into the hydrolysis chamber for further processing [73]. Enzymatic hydrolysis offers several advantages, including faster reaction times compared to uncatalyzed processes and operation under mild conditions, leading to lower operational costs and energy consumption [74]. Additionally, enzyme specificity enhances the selectivity of desired peptides and controls amino acid structures. Despite its potential in laboratory settings, the industrial application of enzymatic hydrolysis is often limited by enzyme costs and low yields [75]. For example, enzymatic hydrolysis of fish viscera using a protamex enzyme produced peptides with antibacterial and antioxidant activities [76]. Similarly, enzymatic hydrolysis of leg shrimp (Litopenaeus vannamei) with an alcalase enzyme yielded peptides with strong antibacterial properties [33]. Anticancer peptides were also extracted from tuna by-products using protease XXIII and papain [77]. Enzymatic hydrolysis of salmon bone residues with trypsin was conducted and produced peptides with high ACE inhibitory effects [78], and shortfin scad wastes hydrolyzed with alcalase resulted in potent ACE inhibition peptides [79].
Several auxiliary methods have been developed to enhance extraction efficiency and promote greener technologies, including ultrasonic-assisted extraction, pulsed electric fields, and high-pressure processing. Ultrasonic-assisted extraction uses high-frequency ultrasound to induce chemical and physical changes in proteins, such as cavitation and thermal reactions, increasing reaction rates and yields [80]. Combining ultrasonic technology with enzymatic hydrolysis has improved peptide production efficiency. For example, ultrasonic-assisted enzymatic hydrolysis of peanut protein significantly enhanced hydrolysis efficiency and antioxidant activities [81]. Pulsed electric fields apply low-intensity voltage to cells, creating tiny pores in cell membranes (electroporation), allowing enzymes to permeate cells more rapidly and cleave intracellular proteins [82]. This method increases hydrolysis rates while consuming less energy. High-pressure processing, another nonthermal method, uses high pressure to denature proteins and improve enzyme binding [83]. While these innovative methods can boost yield and reduce production time, the additional costs of these pre-treatment processes must be considered.
Microbial fermentation is another prominent method for producing proteins and peptides. This process utilizes living microorganisms that secrete proteolytic enzymes to cleave peptide bonds, releasing short amino acid chains. Fermentation involves controlling environmental factors like pH, temperature, and oxygen levels to optimize microbial activity. High protease activity during fermentation can lead to shorter production times compared to enzymatic hydrolysis, depending on the microorganisms and conditions used [84]. For example, brewer’s spent grain waste fermented with Bacillus cereus and Bacillus lentus produced antioxidant peptides [51]. Soybean meal fermented with Bacillus subtilis E20 yielded peptides with high antibacterial properties [63]. Tomato seed fermentation using Bacillus subtilis A14h strains produced peptides with both antioxidant effects and ACE inhibition [59]. For rapeseed by-product fermentation, a mixed solid-state process using Bacillus subtilis and A. elegans resulted in peptides with antiproliferative activities against cancer cells [85]. Fermentation also has cost advantages, as microbial cells and substrates are generally cheaper than commercial enzymes. Additionally, it is an environmentally friendly process with no chemical residues. However, maintaining consistent product quality requires careful environmental control and monitoring.
The purification and analytical process of bioactive peptides is essential for separating them from protein mixtures or impurities and determining their structure and sequence. Purification involves processes that classify peptides based on chemical properties, physical properties, structure, size, amino acid residues, charge, and solubility [86]. Several analytical processes, such as membrane filtration systems, chromatography techniques, and capillary electrophoresis, are used to separate bioactive peptides while retaining their functionality [87]. The key challenge in peptide purification is the structural similarity between desired peptides and impurities. Therefore, understanding peptide properties, such as charge, sequence composition, and hydrophobicity, is essential for selecting the suitable separation method. For instance, ion exchange chromatography is effective for purifying charged peptides. The stationary phase of chromatography is coated with opposite charge groups compared to the active charge on desired peptides, resulting in the binding and separation of peptides from impurities [88]. Membrane filtration systems separate peptides based on specific molecular weight cut-offs, using permeation and retention in a membrane system driven by pressure differences [89]. To improve peptide selection specificity, multimodal or mixed-mode chromatography combines selective ligands and multiple measures, such as size exclusion, ion exchange, and hydrophobic reactions, carefully separating desired peptides based on several chemical and physical properties. Mixed-mode chromatography provides better separation output and can separate polar species not typically separated by standard reversed-phase liquid chromatography [90]. Mixed-mode chromatography exhibits better separation output and also separates polar species that cannot be normally separated with standard reversed-phase liquid chromatography [91]. For analyzing peptides to determine their sequences and purification degree, several analytical methods are used, including matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF), liquid chromatography-mass spectrometry (LC-MS), electrospray ionization–mass spectrometry (ESI-MS), fast atom bombardment mass spectrometry (FAB-MS), and matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS). The accuracy of these techniques depends on the ratio, chemical structure, sensitivity, and mass-to-charge ratio of peptides [92].
4 Bioactive Properties of Peptides from Protein-Based Wastes
Antibacterial peptides are protective compounds secreted by the animal immune system to control and prevent foreign compounds that induce inflammation. These peptides can be constitutively active or induced by contaminants. Antibacterial peptides function in two ways: directly affecting and disrupting bacteria by damaging their cell walls and enhancing the immune system’s response to bacterial infiltration. Antibacterial peptides are short-chained molecules composed of 12 to 50 amino acids. Generally, around seven to eight amino acids are required in the peptide structure to maintain surface activity [93]. However, approximately 15–20 amino acids are necessary for membrane-spanning in helical peptides. The molecular weight of antibacterial peptides is usually lower than 10 kDa, ranging from 1 to 5 kDa [94]. Antibacterial properties of the peptides are related to a cationic nature, which interacts with bacterial cytoplasmic membranes [95].
The interaction mechanisms with bacterial membranes involve net positive charge, hydrophobicity, and flexibility. The first mechanism involves the peptide’s positive charge and its binding ability to anionic lipids on the bacterial membrane. The second mechanism affects the membrane insertion of peptides. The third mechanism involves the peptide-protein flexibility to conform to the membrane [96]. These characteristics vary depending on the peptide and its amino acid sequence. The relationship between peptide charge and antimicrobial activity is complex. Increasing the positive charge in the peptide chain above a certain threshold does not necessarily enhance antibacterial properties due to reduced hydrophobicity [97]. High hydrophobicity of α-helical peptides is required to adsorb and penetrate the hydrophobic membrane of bacterial cells, increasing antibacterial properties [98]. Antibacterial peptides require over 30% hydrophobic residues, and the ratio between hydrophobic and uncharged amino acid residues to charged residues can vary between 1:1 and 2:1 [99]. Initially, the permeabilization of bacterial cell membranes was considered the sole mechanism of antibacterial action. However, evidence shows that peptides have other functions to destroy bacterial cells. In all modes of action, the interaction between the peptide and the microbial cell occurs at the bacterial cell membrane. The ionic interaction between the positive charge peptide and anionic charge residues on the bacterial lipopolysaccharide of gram-negative bacteria and lipoteichoic acids of gram-positive bacteria attracts the peptide to the bacterial membrane. The peptide insertion into the bacterial membrane disrupts membrane structure due to hydrophobic interactions [95]. Defensins and cathelicidins are significant compounds secreted for membrane-disrupting peptides in vertebrates. Antibacterial properties result from electrostatic interactions between positive charge residues on defensin structures and anionic phospholipid groups on bacterial membranes, forming pores that destroy membrane integrity and promote cell lysis [98]. Human cathelicidin peptide LL37 reacts with bacterial membranes through electrostatic interactions, peptide insertion, and membrane disruption. Besides electrostatic interactions, some antibacterial peptides disrupt bacterial membranes through enzymatic hydrolysis. For instance, the lysozyme enzyme hydrolyzes the beta-glycosidic linkage between N-acetylmuramic acid and N-acetylglucosamine in bacterial cell walls [100]. The phospholipase A2 enzyme, secreted by human platelets, can terminate bacterial cells. The disruption of bacterial cell walls also enhances the permeability of other peptides to target cells [101]. Some peptides translocate into cells without disturbing membranes and act as inhibitors to prevent essential cellular processes in bacterial cells.
As mentioned in the previous sections, agro-industrial wastes possess properties such as adequate protein content, cost-effectiveness, and availability, making them suitable for the production of antibacterial protein hydrolysates and peptides. Various animal-based and plant-based wastes have been utilized as ingredients for peptide production. For instance, fish viscera (yellowfin tuna) was hydrolyzed using the protamex enzyme, further fractionated using ultrafiltration, and tested for antibacterial and antioxidant activities [76]. The lowest molecular fraction (less than three kDa) exhibited the highest antibacterial properties against Gram-positive (Listeria and Staphylococcus) and Gram-negative (E. coli and Pseudomonas) pathogenic and fish spoilage-associated microorganisms. Antibacterial peptides were extracted from white leg shrimp (Litopenaeus vannamei) using alcalase enzyme, ultrafiltration, and purification methods [33]. The antimicrobial results showed higher inhibition zones with protein hydrolysate smaller than 10 kDa, effectively working as an antimicrobial agent against S. iniae and Y. ruckeri at concentrations of 1, 2, and 3 mg/mL. Chicken feathers were used for antibacterial peptide production using the catapult steam explosion (ICSE) process at 1.5 MPa for 150 s, resulting in small soluble peptides (<3 kDa) [102]. Antibacterial tests with E. coli showed that peptides with high hydrophobic ratios had the highest antibacterial properties, with a minimum inhibitory concentration (MIC) value of 50 mg/mL. The ICSE method effectively breaks down chicken feathers into soluble keratin quickly and without chemicals, enhancing enzymatic hydrolysis efficiency and resulting in antibacterial peptides. By-products of the dairy industry, such as whey, are promising ingredients for antibacterial peptide production. Protein hydrolysate production was conducted using goat whey with Alcalase enzyme; the extracted protein hydrolysate was further fractionated using size exclusion chromatography and tested with pathogenic bacteria to determine antibacterial properties [103]. The peptide showed high antibacterial activities against Escherichia coli and Bacillus cereus, with MIC values of 0.09 and 0.03 mg/mL, respectively. Plant-based proteins have also been explored for peptide production. Walnut (Juglans regia L.) cake meal, a by-product of walnut oil production, was used as a protein source for antibacterial peptide production [104]. Antibacterial peptides were extracted using pepsin enzyme and purified through ultrafiltration and reversed-phase liquid chromatography. The purified peptides exhibited high antibacterial properties against both gram-positive and gram-negative bacteria, including Escherichia coli (MIC = 1.33 mg/mL), Staphylococcus aureus (MIC = 0.33 mg/mL), and Bacillus subtilis (MIC = 0.66 mg/mL). The amino acid sequences of extracted antibacterial peptides were analyzed, indicating a high proportion of hydrophobic amino acid residues correlating with their antibacterial properties.
Immunomodulatory substances can effectively enhance, reduce, or modify immune responses by adjusting any part of the innate and adaptive immune systems [105]. The immune system is essential for human survival, providing a defensive barrier against various pathogens. However, several factors, such as pathogens, antigens, improper lifestyles, and accumulated stress, can influence the system. Consequently, several drugs such as phytol, cyclosporine, glucocorticoids, and plumbagin have been developed to control and adjust the human immune response. However, these drugs often come with issues such as toxic effects and high costs, limiting their use in patients. Most immunomodulatory drugs are unsuitable for chronic and protective use [106]. Adapting immune activities through dietary compounds is a valid strategy. Novel immunomodulatory compounds from food-based proteins, known as immunomodulatory peptides, have been developed and offer advantages over traditional dietary treatments [105]. Thus, developing immunomodulatory peptides from food or waste is key to controlling the immune system without adverse health effects.
Recent studies indicate that food-derived peptides can induce both innate and adaptive immune responses, such as cytokine and antibody production, improvement of killer cell function, enhancement of the immune system’s defense against pathogens, and inhibition of pro-inflammatory reactions [107]. Immunomodulatory peptides typically do not react or bind directly with pathogenic cells but bind with receptors on the surface of immune-related cells [108]. These peptides are short (2–10 residues) and hydrophobic. Interestingly, the amino acid residues in immunomodulatory peptides are primarily hydrophobic amino acids such as phenylalanine (Phe), proline (Pro), leucine (Leu), glycine (Gly), and valine (Val). Additionally, anion-based amino acids such as tyrosine (Tyr) and glutamic acid (Glu) also exhibit strong immune responses. These amino acid residues are responsible for the immunomodulatory effects of food-based peptides. Immunomodulatory peptides target immune cells such as NK cells, monocytes, T cells, lymphocytes, and mast cells. Although the mechanisms of immunomodulatory effects are not fully understood, several mechanisms have been identified, such as macrophage activation, induction of immune modulators (cytokines, NK cells, immunoglobulins), inhibition of pro-inflammatory mediators, stimulatory effects on splenocytes, and increased leukocyte count. The composition of amino acids, sequences, and length of the peptides contribute to their immunomodulatory effects, resulting in controlled innate and adaptive immune responses [109].
Immunomodulatory peptides have been widely explored and extracted from various food-based matrices. Dairy products with high protein content, such as milk, have been extensively studied as ingredients for immunomodulatory peptide production. Several extractions of immunomodulatory peptides from the enzymatic process of milk have been reported [110]. For instance, the peptide Val-Glu-Pro-Ile-Pro-Tyr extracted from human casein exhibits the phagocytosis of sheep red blood cells. Whey protein, a dairy waste product, was hydrolyzed using a combination of trypsin and chymotrypsin, resulting in an immunomodulatory peptide capable of proliferating splenocytes [111]. Immunomodulatory peptides have also been extracted from various food matrices, including soybean, rice, fish, marine species, and eggs. For example, hexapeptides (His-Cys-Gln-Arg-Pro-Arg) extracted from the tryptic digest of soybean protein can induce the production of tumor necrosis factor in mice and stimulate the phagocytic ability of human polymorphonuclear leukocytes [112]. Similarly, soybean protein hydrolyzed using pepsin enzyme and further isolated resulted in three different immunostimulatory peptides (Ala-Glu-Ile-Asn-Met-Pro-Asp-Tyr, Ile-Gln-Gln-Gly-Asn, and Ser-Gly-Phe-Ala-Pro), which significantly improve the proliferation of mice splenocytes [113]. A novel immunomodulatory peptide was isolated from the tryptic digestion of rice soluble protein, resulting in Gly-Tyr-Pro-Met-Tyr-Pro-Leu-Pro-Arg peptides, which effectively enhance the phagocytosis activity of polymorphonuclear leukocytes [114]. The extraction of immunomodulatory peptides was also conducted using Alaska pollock protein, resulting in Gly-Met-Thr-Tyr, Asn-Gly-Leu-Ala-Pro, and Trp-Thr peptides, which exhibit high lymphocyte proliferation function [115]. In marine species, Paphia undulate clam was utilized for peptide production, yielding six different peptides (Pro-His-Thr-Cys, Val-Gly-Tyr-Thr, Glu-Phe, Leu-Phe, Glu-Gly-Ala-Lys, and Trp-Ile or Trp-Leu) that enhance lymphocyte cell proliferation [116]. Eggs are another widely explored food matrix for immunomodulatory peptides. Peptides derived from egg yolk can induce the humoral immune response, increasing IgA+ cells in the lamina propria of mice [117] Protein hydrolysates from the enzymatic hydrolysis of whole egg whites, ovalbumin, lysozyme, and ovomucin decrease stimulated lymphocyte proliferation and secretion of Th2-biased cytokines (IL-13 and IL-10) while reducing the production of the Th1 cytokine TNF-α [118].
The exploration and discovery of new anticancer compounds from natural sources, such as food proteins, are considered superior alternatives for cancer treatment and prevention. Recently, peptides and protein hydrolysates extracted from food-based proteins have been widely explored and used in the food, pharmaceutical, and nutraceutical sectors. Anticancer tests conducted to measure the potential of these peptides have been performed both in vitro and in vivo. Several food-based peptides from sources such as sea cucumber, crabs, milk, rice, corn, and rapeseed have shown promising anticancer properties. Additionally, protein-based wastes are also being explored as ingredients for anticancer peptide production.
The mechanism of anticancer peptides, which inhibit the growth of cancer cells, has been studied to understand their cytotoxic effects. Several peptides extracted from food matrices exhibit antiproliferative and cytotoxic effects against human cancer cells. Generally, anticancer peptides consist of short chains of amino acids ranging from 3 to 25 residues. The anticancer properties depend on the composition, length, sequences, hydrophobicity, and overall charge of the peptide structure. Notably, peptides with a high ratio of hydrophobic amino acids, such as glycine, alanine, tyrosine, leucine, proline, and lysine, exhibit high anticancer properties. This is due to interactions between hydrophobic amino acids on the peptide structure and the outer leaflets of tumor cell membrane bilayers, resulting in selective and intensive cytotoxicity against target cancer cells [119]. The composition of cell membrane bilayers and the arrangement of phospholipids potentially determine the cell’s susceptibility to lysis by anticancer peptides [120]. For example, an anticancer peptide extracted from half-fin anchovy, with a 50% hydrophobic ratio, can effectively induce antiproliferative activity against cancer cells [121]. Additionally, the presence of heterocyclic amino acids such as proline and charged glutamic acid heavily influences the anticancer ability of peptides. The strength of the positive charge in the structure correlates with and enhances cytotoxic activity [119]. Lower molecular weight peptides with higher hydrophobic amino acid groups on their surface can effectively induce high anticancer activities. Short-chain anticancer peptides exhibit efficient molecular diffusivity and mobility compared to larger molecules, resulting in fast reactions with cancer cell structures and high anticancer activity. Thus, molecular weight, charge, hydrophobic residue content, and the number of cationic residues are critical aspects that significantly affect anticancer activities.
Due to the presence of anticancer-related residues, anticancer peptides have been extracted from various food matrices, especially plant sources. For instance, a hydrolyzed peptide (Met-Leu-Pro-Ser-Tyr-Tyr) from soybean protein exhibits high cytotoxicity against a macrophage-like cell line from a mouse with lymphoma, hindering cell cycle development by arresting cells at the G2/M phase [122]. The enzymatic hydrolysate of soy protein isolates using pepsin and pancreatin resulted in three peptides (Phe-Glu-Ile-Thr-Pro-Glu-Lys-Asn-Pro-Gln, Ile-Glu-Thr-Trp-Asn-Pro-Asn-Asn-Lys-Pro, and Val-Phe-Asp-Gly-Glu-Leu), which exhibit repressive effects on human topoisomerase II, leading to inhibition of cell proliferation in carcinogenesis [123]. In another investigation, purified pentapeptides (Glu-Gln-Arg-Pro-Arg) isolated from rice bran effectively inhibited the growth of colon cancer cells, breast cancer cells, and liver cancer cells by 84%, 80%, and 84%, respectively, at 600–700 μg/mL doses [124]. Anticancer peptides isolated from gastrointestinal digestion of common beans resulted in five short-chain peptides composed of 5–7 amino acids, inducing antiproliferative effects on HCT116 and RKO human colorectal cancer cells by amplifying the expression of p-p53 Ser392 and p21 and reducing the expression of cyclin-B1 [125]. Recently, an anticancer peptide (Trp-Thr-Pro) from enzymatic hydrolysis and fermentation of rapeseed effectively inhibited the growth of HepG2 cells through up-regulation of p53 and Bax and down-regulation of Bcl-2 [126]. Marine products and fish are also excellent sources of anticancer peptide production. For example, antiproliferative peptides extracted from the tuna dark muscle by-product using papain and protease XXIII inhibited the growth of human breast cancer cells with IC50 values of 8.1 and 8.8 μM, respectively [77]. Another study reported that peptides derived from Ruditapes philippinarum proteins exhibit cytotoxic activities against PC-3 (prostate), A549 (lung), and MDA-MB-231 (breast) cancer cells without inducing cytotoxicity in normal liver cells [127]. While most research on anticancer peptide production has used food matrices, some studies have focused on agro-industrial wastes. For example, two anticancer peptides (Lys-Pro-Glu-Gly-Met-Asp-Pro-Pro-Leu-Ser-Glu-Pro-Glu-Asp-Arg-Arg-Asp-Gly-Ala-Ala-Gly-Pro-Lys and Lys-Leu-Pro-Pro-Leu-Leu-Leu-Ala-Lys-Leu-Leu-Met-Ser-Gly-Lys-Leu-Leu-Ala-Glu-Pro-Cys-Thr-Gly-Arg) were fractionated from tuna cooking juice protein hydrolysate, exhibiting inhibitory effects against the breast cancer cell line MCF-7 [128]. These peptides stimulated cell cycle arrest in the S phase by increasing p21 and p27 and reducing cyclin A expression. Algae protein wastes hydrolyzed with pepsin resulted in highly effective anticancer peptides that induced antiproliferation of AGS cells by stimulating post-G1 cell cycle arrest [72]. These peptides showed no cytotoxicity when tested with WI-38 lung fibroblast cells in vitro. Among the extracted peptides, the anticancer peptide with the amino acid sequence VECYGPNRPQF induced both antioxidant activities and antiproliferation. Another study explored shrimp shells as ingredients for anticancer peptide production. The shrimp waste was hydrolyzed with cryotin enzyme and fractionated to acquire gastrointestinal-resistant peptides [129]. The extracted peptides from shrimp shell whites and langoustine shells effectively prevented and decreased colon and liver cancer cell growth by 60%.
Hypertension is a chronic disease generally induced by high blood pressure within the human system, often related to the insufficient relaxation of blood vessels, which leads to reduced blood flow. Consequently, inadequate blood flow to vital organs can induce stroke and death. The renin-angiotensin system (RAS) routinely controls human blood pressure, maintained by renin and angiotensin-converting enzyme (ACE). When hypertension develops, the activity of renin or ACE in the blood increases, resulting in high levels of angiotensin II and an increase in superoxide radical production, which leads to increased nitric oxide degradation. This results in excessive blood vessel contraction and insufficient relaxation.
In most cases, the exact cause of systematic hypertension is unidentified. However, excessive RAS enzyme activities can still be recognized as a marker of the disease. ACE inhibition peptides are bioactive peptides that exhibit antihypertensive effects by specifically inhibiting RAS enzyme activities. Bioactive properties that enhance nitric oxide production are also related. For instance, several researchers have found that antihypertensive protein hydrolysates can reduce and regulate ACE activities and downregulate angiotensin II levels [130,131]. Egg protein-derived tripeptides also induce ACE inhibition while upregulating the NO production pathway [132].
Bioactive peptides and protein hydrolysates exhibit ACE inhibition activities in both in vivo and in vitro experiments. However, inhibition activity and efficiency depend on several factors, such as the type of enzyme used during hydrolysis and the chemical structure of protein-based ingredients [133]. For instance, whey protein concentrate hydrolyzed using three different enzymes to produce ACE inhibition peptides showed that peptides extracted using alcalase induced the highest ACE activities compared to other enzymes [134]. The compatibility between the material and enzyme potentially induces different products depending on the hydrolysis sites and reaction. Additionally, hydrolysis conditions, such as hydrolysis time, enzyme-to-substrate ratio, and enzyme concentration, significantly contribute to ACE-inhibitory properties. Higher enzyme concentration and enzyme-to-substrate ratio enhance ACE activity due to the immense amounts of enzyme presence in the solution. However, the correlation between the ratio and ACE-inhibitory properties is not precisely linear; an excess amount of enzyme does not necessarily enhance the production of ACE-inhibitory peptides. Thus, the optimized level of enzyme usage should be determined for the hydrolysis process.
The mechanism by which ACE inhibition peptides regulate ACE activities can vary depending on the peptide structure and amino acid residues. Inhibition can occur through competitive, noncompetitive, uncompetitive, or mixed-type peptide-enzyme interactions. For instance, peptides extracted from collagen hydrolysate effectively induce ACE-inhibitory properties in a competitive reaction by blocking proteolysis [135]. In contrast, peptides extracted from egg white lysozyme exhibit noncompetitive ACE inhibitory activities by binding at sites other than the enzyme’s active sites [136]. The binding between peptides and ACE molecules potentially reduces α-helix content, resulting in an increase in disordered fractions and a reduction in the catalytic ability of ACE residues.
Apart from hydrolysis conditions, the structural or amino acid residues in the peptide structure are heavily correlated with ACE-inhibitory properties. Peptides containing aromatic amino acid side chains potentially have high ACE-inhibitory activities [137]. Enzymes with specific active sites, such as pepsin, chymotrypsin, and alcalase, are prominent in creating aromatic-based peptides with high ACE-inhibitory properties. Additionally, the C-terminal sequence in peptide structures can also induce a high ACE effect. For strong ACE-inhibitory activities in tetrapeptides, these amino acid residues are required: C1 (tyrosine, proline, phenylalanine), C2 (phenylalanine), C3 (arginine, histidine, tryptophan, phenylalanine), and C4 (valine, isoleucine, methionine). For pentapeptides and longer amino acid chains, these residues are required at the four C-terminal positions: C1 (tyrosine, cysteine), C2 (histidine, tryptophan, methionine), C3 (valine, leucine, isoleucine, methionine), and C4 (tryptophan).
The extraction of ACE inhibition peptides using protein-based wastes has been widely researched with both animal and plant-based materials to explore the potential of low-value by-products as ingredients for peptide production. For example, ACE inhibition peptides derived from shortfin scad waste were produced using alcalase enzyme and a series of purification steps [79]. The extracted ACE inhibition peptide with the amino acid sequence of RGVGPVPAA functioned as a competitive ACE inhibitor. ACE inhibitory peptides from salmon bone waste hydrolyzed with trypsin enzyme yielded peptides (Phe-Cys-Leu-Tyr-Glu-Leu-Ala-Arg) that possess ACE inhibitory properties [78]. This peptide acts as an uncompetitive ACE inhibitor through hydrogen bond interactions between ACE compounds and the peptide. In the case of plant-based wastes, several studies have reported the extraction of ACE inhibition peptides from plant by-products, especially from fruit processing by-products such as skin and seeds, which are valuable yet underutilized [138]. For instance, tomato seeds, a by-product of tomato juice production, were hydrolyzed through fermentation using Bacillus subtilis A14h. The protein hydrolysate was fractionated and purified, yielding both antioxidant and ACE inhibition peptides [60]. The extracted hexapeptide (DGVVYY), composed of aromatic and hydrophobic residues, exhibits prominent ACE inhibition properties with an IC50 value of 2 µM. Peptides from protein-rich cherry seed wastes were extracted using enzymes such as alcalase, thermolysin, and flavourzyme [138]. The peptide extracted using thermolysin exhibited the highest antihypertensive properties. Further fractionation yielded high antioxidant and antihypertensive peptides, with the presence of proline contributing to the antihypertensive activity. Similar procedures have focused on seeds from fruits like plums [139] and olives [65] for ACE inhibition peptide production. Olive seeds, a by-product of olive oil production, contain high protein content suitable for peptide production. Enzymatic hydrolysis of the wastes using five different enzymes resulted in antihypertensive peptides with IC50 values ranging from 29 to 350 µg/mL [65]. Purification through fractionation and ultrafiltration isolated ACE inhibitory and antioxidant peptides. The isolated peptides, composed of several hydrophobic residues such as alanine, valine, leucine/isoleucine, proline, methionine, phenylalanine, and tryptophan, promote ACE inhibitory activities. In another study, ACE inhibition and antioxidant peptides were derived from defatted rapeseed meal by-product using several proteolytic enzymes. The protein hydrolysate obtained using alcalase enzyme exhibited the highest ACE inhibitory effects with an IC50 value of 0.02 mg/mL [140]. The rapeseed hydrolysate was further fractionated to acquire ACE peptides; the purified ACE inhibitory peptide functions as an uncompetitive ACE inhibitor and exhibits high stability in an in vitro digestion model using human gastric and duodenal fluids.
5 Application of Bioactive Peptides in Medical Fields
Biocomposite materials are synthesized by combining biological substances with synthetic polymeric materials. These materials are fundamental building blocks supporting medical and biological engineering fields. They can actively function at the biological system interface by interacting with proteins, cells, and biological tissues. Due to their biocompatibility, biocomposite materials are applied in several medical applications, such as artificial organs, drug delivery, and tissue engineering [141]. Bioactive peptides, a group of short-chain amino acids with biocompatibility and bioactive properties, are suitable for synthesizing biocomposite materials. These biodegradable peptides have side chains that can be modified with other chemical or biological compounds. Moreover, the primary structure of amino acid chains contains binding sites that can effectively react with polymeric compounds. Therefore, bioactive peptides are incorporated as building blocks in medical applications, such as scaffolds for tissue engineering [142], drug delivery systems [143], and bioprinting [144]. Fig. 2 illustrates the schematic diagram of biomedical applications of peptides, 3 main applications were mentioned. The functionality of peptides is the key factor in determining their specific applications, while other factors such as size, length, type of amino acid sequences, and hydrophobicity also play important roles. Apart from the mentioned applications, peptides can also be applied in diagnostic tools, hormone therapy, and wound healing.
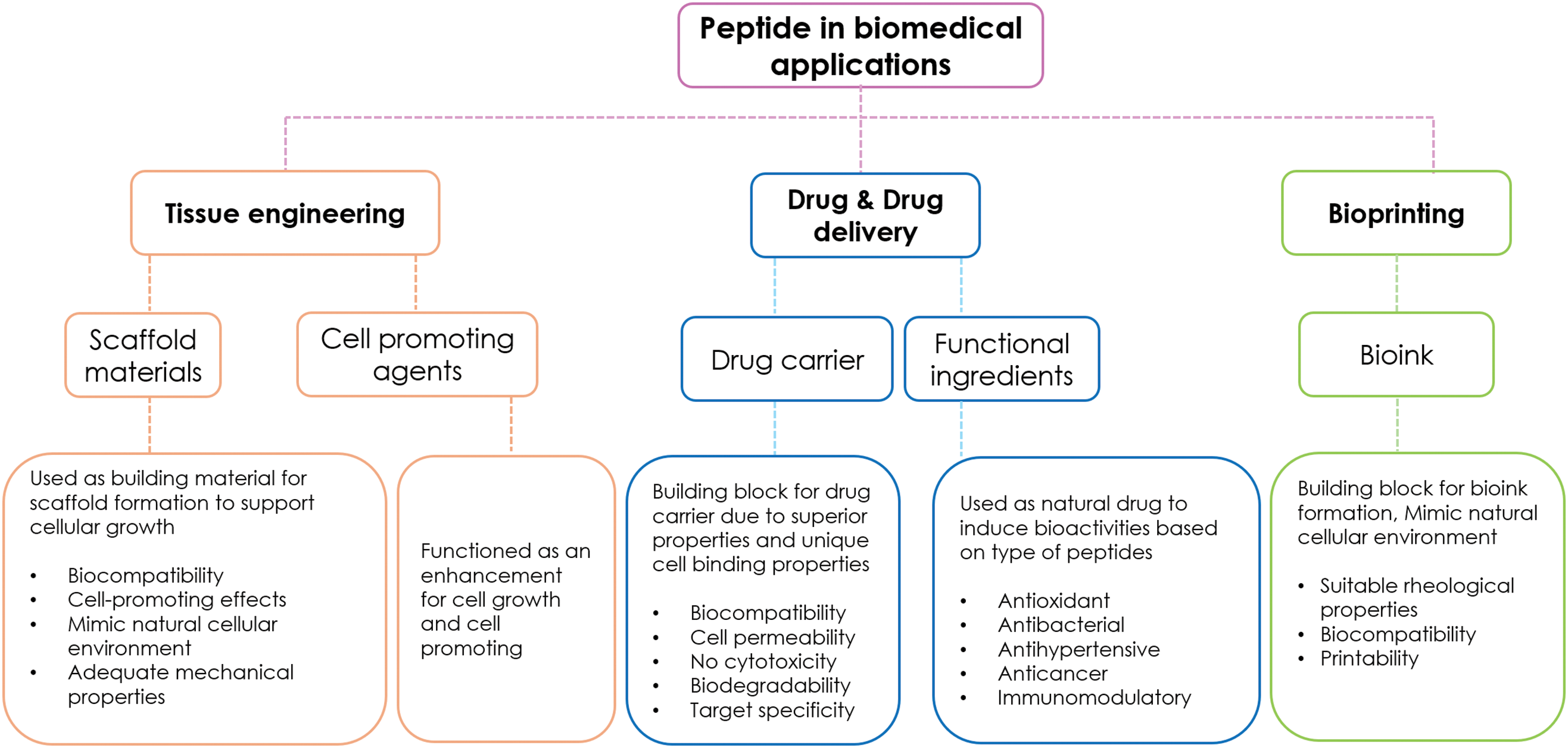
Figure 2: Application of Bioactive peptides in biomedical fields
5.1 Peptide-Based Biomaterials Extracted from Agro-Industrial for Tissue Engineering
To develop the 3D geometry of tissue cultivation, scaffolds are required to support cells and organize tissues. Scaffold materials are developed under strict selection criteria, including in vivo degradation rate, cytotoxicity, immune response or inflammation, cell-promoting properties, and sufficient mechanical properties to induce tissue remodeling. Multi-dimensional scaffolds are required for proper cellular differentiation and tissue development. The lack of these qualities can reduce the functionality of the developed material in the medical field. Extensive research has been conducted to explore bio-based materials for scaffold formation. However, issues such as lack of adhesion sites, cell toxicity of degraded residues, and high batch-to-batch variations hinder the use of several materials [145]. Therefore, protein- and peptide-based scaffolds, composed of several adhesion sites, high biocompatibility, and adaptable structures, have become prominent materials for scaffold synthesis [146]. As illustrated in Fig. 3, three main applications of bioactive peptides in tissue engineering are listed. Peptides can be directly incorporated into scaffold matrices as primary building materials or added as supplementary compounds depending on the desired outcome. However, peptide-based scaffolds often suffer from weak mechanical properties and rapid biodegradability [147]. To address these issues, a combination of peptides and polymeric materials was developed to mitigate the shortcomings and enhance overall properties. The mechanical strength was increased due to the presence of polymeric materials, while cell adhesion and tissue growth were enhanced by the active peptide functions.

Figure 3: Applications of peptides in tissues engineering and scaffold formation
The development of self-assembling peptides, designed from the natural conformation of protein molecules, creates β-sheet and α-helix structures. These structures are formed using stimulation from external factors such as pH, temperature, and ionic strength to induce supramolecular nanostructures [73]. Self-assembling peptide hydrogels are generally used as scaffolds due to their 3-dimensional structure, which supports cell adherence, migration, and growth. For instance, using self-assembled RADA16-I nanofiber hydrogels to cultivate human dermal fibroblasts can effectively increase proteoglycan aggrecan expression [74]. The self-assembling of FKFE-II hydrogel was engineered to adjust and optimize the mechanical properties of gels to replicate the native nucleus pulposus [75]. The developed scaffold can cultivate pulposus cells and increase cell viability. Results indicate that cells are more prone to expand into RADA scaffold structures compared to KLDL and FKFE hydrogels [148]. The α-helix peptide hydrogel developed using RGD peptide significantly improves cell proliferation of murine embryonic neural stem cells, indicating potential for nerve tissue repair.
Functional peptides with bioactive properties can be added to self-assembling peptide scaffolds to enhance functional and physiological properties. For example, the LLVFGAKMLPHHGA peptide was inserted into a structure to mimic the nature of hydroxyapatite. The scaffold then exhibited mineralization functions and can be applied to bone tissue cultivation [80]. In another study, RAD-SNV, RAD-KPS, and RAD-KAI peptides were introduced into RADA16-I peptides for the regeneration of the nucleus pulposus [149].
Although peptides exhibit several bioactive functions suitable for tissue engineering applications, they can be degraded by protease enzymes and renal clearance, affecting their bioavailability in vivo [81]. The polysaccharide-peptide conjugation method was developed to mitigate these issues and improve the overall properties of peptides. Polymer materials are conjugated with peptides to develop synergistic properties and reduce undesired functions. Tissue engineering aims to repair and regenerate damaged tissues using cell cultivation and scaffold biomaterials. Although polysaccharides exhibit biocompatibility, high water retention, and mechanical properties, they generally lack cell adhesion properties and controllable degradation, hindering their use in the field [82]. Adding peptides mitigates these shortcomings by providing bioactive properties, cell stimulation, and cell adhesion properties. As a result, biocomposite materials combining peptides and polysaccharides are utilized to develop scaffolds and hydrogels for tissue regeneration. RGD peptides, extracted from laminin and fibronectin, are considered cell-binding peptides used in scaffold formation to enhance cell adhesion and proliferation. The addition of RGD peptide crosslinked to the chitosan scaffold structure effectively enhances the growth of mesenchymal stem cells [83]. In the presence of RGD peptide, cell density increased after 10 days of cultivation. The development of RGD peptide-dextran hydrogel with different concentrations of RGD peptides indicated that 0.1% RGD peptides added to the dextran hydrogel structure effectively enhance proliferation and cell viability of human umbilical vein endothelial cells [150]. Additionally, scaffolds were developed from the conjugation of hyaluronic acid and carboxymethyl chitosan with several short and long RGD peptides [151]. Results from preosteoblast cell cultivation indicated that cells spread in a peptide density-based manner and induced a bell-shaped response of preosteoblast spreading. The Schiff-base reaction is also utilized to develop polysaccharide-peptide scaffolds, where crosslinking is developed by oxidizing hydroxyl groups on the polysaccharide structure to create aldehyde branches on the polymer chain. Then, the dialdehyde groups on the oxidized polymeric chain can be conjugated with the N-terminal amino acid groups on the peptide chain [152]. These linkages exhibit strong mechanical strength and stability relative to environmental factors such as pH, suitable for tissue regeneration [153]. Several conjugation processes, such as noncovalent interactions, grafting methods, and the copper-catalyzed azide-alkyne cycloaddition reaction, are used to conjugate peptide-polysaccharides.
Most bioactive peptides used in forming peptide-based scaffolds are synthetic due to specific requirements for the self-assembly process. Peptides must be precisely synthesized with all functional groups on the structure. Currently, there is very little to no research incorporating natural peptides from food, animal, or plant sources as ingredients for scaffold formulation. Moreover, incorporating bioactive peptides extracted from agro-industrial sources into scaffold formation is still unexplored. Compared to synthetic peptides, peptides from natural sources are potentially more biocompatible with cells and require lower production costs. However, contamination with diseases and foreign pathogens from natural protein sources must be carefully managed when applying peptides in tissue culture.
5.2 Peptides from Agro-Industrial Waste for Drug Delivery
Due to their diverse bioactive properties, bioactive peptides have a wide range of applications in various fields, especially in pharmaceutical, cosmetic, and functional food sectors, depending on their structure, size, and amino acid composition. Bioactive peptides are widely used in pharmaceutical applications for their unique properties such as anticancer, anti-inflammatory, antidiabetic, antihypertensive, and immunomodulatory effects [16]. Depending on these properties, peptides extracted mainly from food and synthetic sources are applied as bioactive ingredients. However, due to processing costs, peptides extracted from agro-industrial wastes have become preferred options. For instance, bioactive peptides extracted from whey waste from mozzarella cheese production can effectively decrease the growth of human colorectal cancer cells [154]. Opioid peptides are also extracted from casein using enzymatic reactions to produce peptides with opioid activity, which are extremely valuable and marketable [93]. Moreover, effective hypertensive peptides have been extracted from several plant-based proteins [94]. For example, peptides from rapeseed exhibit antihypertensive properties by reducing blood pressure in hypertensive rats [155]. Incorporating these antihypertensive peptides into pharmaceutical and nutraceutical products can effectively prevent hypertension. Peptides with antidiabetic properties are used in several nutraceutical products to maintain and prevent type II diabetes and remain active during digestion [156].
One of the most common applications of peptides in the biomedical field is related to drug delivery systems. To develop a drug, compounds must overcome several biological obstacles, such as insolubility, biodegradability, metabolism, cell permeability, and excretion through the kidneys. Additionally, the drug must have no cytotoxicity and a high pharmacokinetic rate. Many potential compounds fail to pass through preclinical and clinical trials during studies. However, due to their superior biocompatibility and biodegradability, peptides are potential candidates for biomedical applications [157]. Peptides can be modified into supramolecular nanostructures, which are utilized as carriers to entrap and deliver drugs to target locations. Peptides possess properties such as high cell membrane permeability, high loading capacity, and target specificity [158]. As illustrated in Table 3, peptides are utilized in various medical applications, particularly in cancer treatment. The mechanism of peptide toward drug delivery system can be categorized into 3 type which are cell penetrating, cell targeting, and stimulation responded [157]. Cell-penetrating peptides are peptides capable of entering cells without damaging the cell membrane, utilizing mechanisms such as endocytosis and direct translocation [159]. Cell-targeting peptides specifically bind to cell structures, providing selectivity towards the desired target cells. In contrast, stimulation peptides respond to the cellular microenvironment, such as pH, temperature, and enzymes within cells. These types of peptides are used as guides to directly transfer drugs to specific target sites and also serve as mechanisms for controlled drug release. Additionally, the self-assembly nature of peptides can be used as a drug-releasing mechanism. For example, a nano hydrogel developed from RADA16 peptides aimed to transport TGF-beta 1 to the target location, constantly releasing growth factors and increasing cell proliferation [160]. The developed peptide carrier constantly releases growth factors, increasing cell proliferation. Modified peptides are extensively researched and tested to develop superior drug delivery systems due to their target specificity and low side effects. Drug delivery systems and peptide applications also relate to cell delivery, vaccine adjuvants, soluble fibrils, and antimicrobial compounds.
5.3 Peptides from Agro-Industrial Waste for Bioprinting
While 3D structure scaffolds offer several benefits for enhancing cell growth and proliferation, challenges such as a lack of homogeneous cell distributions and inability to imitate the natural cellular environment persist [172]. The combination of hydrated polymers and bioprinting technologies is widely explored to address these issues. The bioprinting process requires bioinks that effectively enhance cellular growth and maintain the 3D structure, while possessing suitable mechanical properties such as viscosity, shear-thinning, and yield stress [173]. Peptide hydrogels are promising building blocks due to their biocompatibility and innate bioactive properties. Additionally, these gels exhibit suitable rheological properties required for extrusion and reversible physicochemical properties [172]. However, the primary issue preventing the development of peptides as bioinks remains a lack of knowledge about design principles for creating printable peptides. Recent studies have been conducted to observe the printability of peptides.
Two gelation processes, non-covalent and covalent crosslinking, are used to prepare peptide hydrogels as bioinks for 3D printing applications. Short-chain peptides are synthesized using solution-phase peptide synthesis and amino acid N-carboxy anhydride ring-opening polymerization, depending on the desired molecular weight and structure [174]. In the case of a non-covalent reaction, the peptide hydrogel formation strategy relates to modulating the amphiphilic balance of the backbone sequence. Balancing hydrophobic and hydrophilic residues effectively induces physical gelation [174]. Additionally, innate protein folding mechanisms, such as hydrogen bonding and charge-to-charge interactions, naturally change the peptide into a secondary structure. Peptide hydrogels developed through non-covalent reactions exhibit favorable traits, such as biomimetic stiffness, high porosity, biodegradability, and deformation under stress [175]. Physical gelation can be induced by physiological environments such as temperature and pH. For example, the gelation process of glucono-δ-lactone hydrogel is induced by an acidic environment [176]. Several reactions, such as light irradiation, enzymatic reactions, and chemical ligation, have been tested to crosslink peptides and develop hydrogels for covalent crosslinking [177].
One of the strong points of supramolecular systems based on peptide gelation is the customization of hydrogel. Functional groups, bioactive properties, and chain length can be adjusted according to the desired outcome, resulting in new 3D printable bioinks [178]. For example, hydrogel peptides developed from peptide chains (Ac-Ile-Ile-Phe-Lys-NH2, Ac-Ile-Ile-Cha-Lys-NH2, and Ac-Ile-Cha-Cha-Lys-NH2) exhibit stable rheological properties suitable for printing applications [179]. Peptide hydrogel loaded with human bone marrow stem cells resulted in high cell growth and suitable conditions for chondrogenesis. Similarly, 3D structure peptide amphiphiles hydrogel, developed through alkyl-chain conjugation, was printed and stabilized using salt and pH [178]. To enhance printability, charged polymers were added to peptide chains to enhance hydrogel’s mechanical properties. For instance, adding methylcellulose to peptide solution increased bioink viscosity and printed gel stability [180]. Additionally, print resolution of bioinks improved by combining oppositely charged keratin protein and peptide amphiphiles (C15H31CONH-V3A3H2K), resulting in complex sheet structures with high printing resolution [181].
6 Conclusion and Future Perspective
Due to the rise in population and the increasing demand for resources, the agro-industrial sector produces a tremendous amount of waste. Improper waste management potentially leads to environmental issues. Hence, the waste valorization process is essential, maximizing resource usage and converting low-value products into high-value ones. Bioactive peptides extracted from agricultural and agro-industrial wastes become one of the solutions and play a crucial role in CE implementation. Due to processing costs, bioactive peptides extracted from waste could replace synthetic peptides and those derived from food sources. These bioactive peptides, sourced from agro-industrial residues, exhibit several bioactive properties that can be effectively implemented in the biomedical field. However, certain aspects related to the safety of bioactive peptides from wastes still require extensive research to ensure they do not pose any health risks. Additionally, in vivo research is necessary to improve credibility and evaluate the efficiency of peptides extracted from waste sources.
Acknowledgement: The authors would like to thank the Asian Institute of Technology and the Thailand National Center for Genetic Engineering and Biotechnology for providing support.
Funding Statement: This research was funded by the Thailand Graduate Institute of Science and Technology (TGIST) (Grant No. TG-BT-AIT-63-002D).
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Chaichawin Chavapradit, Anil Kumar Anal; data collection: Chaichawin Chavapradit; analysis and interpretation of results: Chaichawin Chavapradit, Wonnop Visessanguan, Suwan Panjanapongchai; draft manuscript preparation: Chaichawin Chavapradit, Suwan Panjanapongchai; review and editing: Anil Kumar Anal, Wonnop Visessanguan. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The authors confirm that the data supporting the findings of this study are available within the article.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Suchek N, Fernandes CI, Kraus S, Filser M, Sjögrén H. Innovation and the circular economy: a systematic literature review. Bus Strategy Environ. 2021;30(8):3686–702. doi:10.1002/bse.v30.8. [Google Scholar] [CrossRef]
2. Singh R, Das R, Sangwan S, Rohatgi B, Khanam R, Peera SPG, et al. Utilisation of agro-industrial waste for sustainable green production: a review. Environ Sustain. 2021;4(4):619–36. doi:10.1007/s42398-021-00200-x. [Google Scholar] [CrossRef]
3. Gaur VK, Sharma P, Sirohi R, Awasthi MK, Dussap C-G, Pandey A. Assessing the impact of industrial waste on environment and mitigation strategies: a comprehensive review. J Hazard Mater. 2020;398:123019. doi:10.1016/j.jhazmat.2020.123019. [Google Scholar] [PubMed] [CrossRef]
4. Kirchherr J, Yang N-HN, Schulze-Spüntrup F, Heerink MJ, Hartley K. Conceptualizing the circular economy (revisitedan analysis of 221 definitions. Resourc Conservat Recycl. 2023;194:107001. doi:10.1016/j.resconrec.2023.107001. [Google Scholar] [CrossRef]
5. Giampietro M, Funtowicz SO. From elite folk science to the policy legend of the circular economy. Environ Sci Policy. 2020;109:64–72. doi:10.1016/j.envsci.2020.04.012. [Google Scholar] [CrossRef]
6. Bhari R, Kaur M, Sarup Singh R. Chicken feather waste hydrolysate as a superior biofertilizer in agroindustry. Curr Microbiol. 2021;78(6):2212–30. doi:10.1007/s00284-021-02491-z. [Google Scholar] [PubMed] [CrossRef]
7. Singh A, Kadam D, Gautam AR, Rengasamy KR, Aluko RE, Benjakul S. Angiotensin-I-converting enzyme and renin inhibitions by antioxidant shrimp shell protein hydrolysate and ultrafiltration peptide fractions. Food Biosci. 2024;60(12):104524. doi:10.1016/j.fbio.2024.104524. [Google Scholar] [CrossRef]
8. Deng Z, Cui C, Wang Y, Ni J, Zheng L, Wei H-K, et al. FSGHF3 and peptides, prepared from fish skin gelatin, exert a protective effect on DSS-induced colitis via the Nrf2 pathway. Food Fun. 2020;11(1):414–23. doi:10.1039/C9FO02165E. [Google Scholar] [PubMed] [CrossRef]
9. Luo J, Yao X, Soladoye OP, Zhang Y, Fu Y. Phosphorylation modification of collagen peptides from fish bone enhances their calcium-chelating and antioxidant activity. LWT. 2022;155(9):112978. doi:10.1016/j.lwt.2021.112978. [Google Scholar] [CrossRef]
10. Peydayesh M, Bagnani M, Soon WL, Mezzenga R. Turning food protein waste into sustainable technologies. Chem Rev. 2022;123(5):2112–54. doi:10.1021/acs.chemrev.2c00236. [Google Scholar] [PubMed] [CrossRef]
11. Ribeiro-Oliveira R, Martins ZE, Sousa JB, Ferreira IM, Diniz C. The health-promoting potential of peptides from brewing by-products: an up-to-date review. Tren Food Sci Technol. 2021;118:143–53. doi:10.1016/j.tifs.2021.09.019. [Google Scholar] [CrossRef]
12. Mullins AP, Arjmandi BH. Health benefits of plant-based nutrition: focus on beans in cardiometabolic diseases. Nutrients. 2021;13(2):519. doi:10.3390/nu13020519. [Google Scholar] [PubMed] [CrossRef]
13. Jakubczyk A, Karaś M, Rybczyńska-Tkaczyk K, Zielińska E, Zieliński D. Current trends of bioactive peptides—new sources and therapeutic effect. Foods. 2020;9(7):846. doi:10.3390/foods9070846. [Google Scholar] [PubMed] [CrossRef]
14. Pérez-Gregorio R, Soares S, Mateus N, de Freitas V. Bioactive peptides and dietary polyphenols: two sides of the same coin. Molecules. 2020;25(15):3443. doi:10.3390/molecules25153443. [Google Scholar] [PubMed] [CrossRef]
15. Hadidi M, Aghababaei F, Gonzalez-Serrano DJ, Goksen G, Trif M, McClements DJ, et al. Plant-based proteins from agro-industrial waste and by-products: towards a more circular economy. Int J Biol Macromol. 2024;261:129576. doi:10.1016/j.ijbiomac.2024.129576. [Google Scholar] [PubMed] [CrossRef]
16. Peighambardoust SH, Karami Z, Pateiro M, Lorenzo JM. A review on health-promoting, biological, and functional aspects of bioactive peptides in food applications. Biomolecules. 2021;11(5):631. doi:10.3390/biom11050631. [Google Scholar] [PubMed] [CrossRef]
17. Amigo L, Hernández-Ledesma B. Current evidence on the bioavailability of food bioactive peptides. Molecules. 2020;25(19):4479. doi:10.3390/molecules25194479. [Google Scholar] [PubMed] [CrossRef]
18. Cruz-Casas DE, Aguilar CN, Ascacio-Valdés JA, Rodríguez-Herrera R, Chávez-González ML, Flores-Gallegos AC. Enzymatic hydrolysis and microbial fermentation: the most favorable biotechnological methods for the release of bioactive peptides. Food Chem: Molecul Sci. 2021;3:100047. [Google Scholar]
19. Parlasca MC, Qaim M. Meat consumption and sustainability. Annu Rev Resour Econ. 2022;14(1):17–41. doi:10.1146/resource.2022.14.issue-1. [Google Scholar] [CrossRef]
20. Yang L, Guo Z, Wei J, Han L, Yu Q-L, Chen H, et al. Extraction of low molecular weight peptides from bovine bone using ultrasound-assisted double enzyme hydrolysis: impact on the antioxidant activities of the extracted peptides. LWT. 2021;146:111470. doi:10.1016/j.lwt.2021.111470. [Google Scholar] [CrossRef]
21. López-Pedrouso M, Zaky AA, Lorenzo JM, Camiña M, Franco D. A review on bioactive peptides derived from meat and by-products: extraction methods, biological activities, applications and limitations. Meat Sci. 2023;204:109278. doi:10.1016/j.meatsci.2023.109278. [Google Scholar] [PubMed] [CrossRef]
22. Romero-Garay MG, Montalvo-González E, Hernández-González C, Soto-Domínguez A, Becerra-Verdín EM, García-Magaña MDL. Bioactivity of peptides obtained from poultry by-products: a review. Food Chem: X. 2022;13:100181. [Google Scholar] [PubMed]
23. Zhu L, Xiong H, Huang X, Guyonnet V, Ma M, Chen X, et al. Identification and molecular mechanisms of novel antioxidant peptides from two sources of eggshell membrane hydrolysates showing cytoprotection against oxidative stress: a combined in silico and in vitro study. Food Res Intern. 2022;157:111266. doi:10.1016/j.foodres.2022.111266. [Google Scholar] [PubMed] [CrossRef]
24. Wongngam W, Mitani T, Katayama S, Nakamura S, Yongsawatdigul J. Production and characterization of chicken blood hydrolysate with antihypertensive properties. Poult Sci. 2020;99(10):5163–74. doi:10.1016/j.psj.2020.07.006. [Google Scholar] [PubMed] [CrossRef]
25. Liu H, Guo YJ, Xu X, Li X, Zhang HR, Qi LW, et al. Preparation, physicochemical characterization and bioactivity comparison of different livestock and poultry bone peptides. Scientia Agricul Sinica. 2022;55(13):2629–42. [Google Scholar]
26. Ding D, Du B, Zhang C, Zaman F, Huang Y. Isolation and identification of an antioxidant collagen peptide from skipjack tuna (Katsuwonus pelamis) bone. RSC Adv. 2019;9(46):27032–41. doi:10.1039/C9RA04665H. [Google Scholar] [PubMed] [CrossRef]
27. Cipolari OC, de Oliveira Neto XA, Conceição K. Fish bioactive peptides: a systematic review focused on sting and skin. Aquaculture. 2020;515:734598. doi:10.1016/j.aquaculture.2019.734598. [Google Scholar] [CrossRef]
28. Bi J, Tian C, Jiang J, Zhang G-L, Hao H, Hou H-M. Antibacterial activity and potential application in food packaging of peptides derived from turbot viscera hydrolysate. J Agric Food Chem. 2020;68(37):9968–77. doi:10.1021/acs.jafc.0c03146. [Google Scholar] [PubMed] [CrossRef]
29. Mhina CF, Jung HY, Kim JK. Recovery of antioxidant and antimicrobial peptides through the reutilization of Nile perch wastewater by biodegradation using two Bacillus species. Chemosphere. 2020;253:126728. doi:10.1016/j.chemosphere.2020.126728. [Google Scholar] [PubMed] [CrossRef]
30. Ketnawa S, Martínez-Alvarez O, Gómez-Estaca J, del Carmen Gómez-Guillén M, Benjakul S, Rawdkuen S. Obtaining of functional components from cooked shrimp (Penaeus vannamei) by enzymatic hydrolysis. Food Biosci. 2016;15:55–63. doi:10.1016/j.fbio.2016.05.005. [Google Scholar] [CrossRef]
31. Trung TS, Phuong PTD. Bioactive compounds from by-products of shrimp processing industry in Vietnam. J Food Drug Anal. 2012;20(1):194–7. [Google Scholar]
32. Joshi I, Janagaraj K, Nazeer RA. Isolation and characterization of angiotensin I-converting enzyme (ACE-I) inhibition and antioxidant peptide from by-catch shrimp (Oratosquilla woodmasoni) waste. Biocatal Agric Biotechnol. 2020;29(7):101770. doi:10.1016/j.bcab.2020.101770. [Google Scholar] [CrossRef]
33. Rashidian G, Abedian Kenari A, Nikkhah M. Evaluation of antioxidative and antibacterial activities of fractionated hydrolysate from shrimp Litopenaeus vannamei head wastes against aquatic pathogenic bacteria. Aquac Res. 2021;52(8):3696–704. doi:10.1111/are.15214. [Google Scholar] [CrossRef]
34. Mathew GM, Mathew DC, Sukumaran RK, Sindhu R, Huang C-C, Binod P, et al. Sustainable and eco-friendly strategies for shrimp shell valorization. Environ Pollut. 2020;267:115656. doi:10.1016/j.envpol.2020.115656. [Google Scholar] [PubMed] [CrossRef]
35. Kim SH, Lee JY, Balolong MP, Kim J-E, Paik H-D, Kang D-K. Identification and characterization of a novel antioxidant peptide from bovine skim milk fermented by Lactococcus lactis SL6. Korean J Food Sci Anim Resour. 2017;37(3):402. doi:10.5851/kosfa.2017.37.3.402. [Google Scholar] [PubMed] [CrossRef]
36. Yu X-C, Li Z, Liu X-R, Hu J-N, Liu R, Zhu N, et al. The antioxidant effects of whey protein peptide on learning and memory improvement in aging mice models. Nutrients. 2021;13(6):2100. doi:10.3390/nu13062100. [Google Scholar] [PubMed] [CrossRef]
37. Guha S, Sharma H, Deshwal GK, Rao PS. A comprehensive review on bioactive peptides derived from milk and milk products of minor dairy species. Food Product Process Nutrit. 2021;3:1–21. [Google Scholar]
38. Fontoura R, Daroit DJ, Corrêa APF, Moresco KS, Santi L, Beys-da-Silva WO, et al. Characterization of a novel antioxidant peptide from feather keratin hydrolysates. New Biotechnol. 2019;49:71–6. doi:10.1016/j.nbt.2018.09.003. [Google Scholar] [PubMed] [CrossRef]
39. Carrera-Alvarado G, Toldrá F, Mora L. DPP-IV inhibitory peptides GPF, IGL, and GGGW obtained from chicken blood hydrolysates. Int J Mol Sci. 2022;23(22):14140. doi:10.3390/ijms232214140. [Google Scholar] [PubMed] [CrossRef]
40. Abou-Diab M, Thibodeau J, Fliss I, Dhulster P, Nedjar N, Bazinet L. Impact of conductivity on the performances of electro-acidification and enzymatic hydrolysis phases of bovine hemoglobin by electrodialysis with bipolar membranes for the production of bioactive peptides. Sep Purif Technol. 2021;269:118650. doi:10.1016/j.seppur.2021.118650. [Google Scholar] [CrossRef]
41. Zhu L, Li Z, Ma M, Huang X, Guyonnet V, Xiong H. Exploration of novel DPP-IV inhibitory peptides from discarded eggshell membrane: an integrated in silico and in vitro study. Food Biosci. 2024;59:104036. doi:10.1016/j.fbio.2024.104036. [Google Scholar] [CrossRef]
42. Zhao Q-C, Zhao J-Y, Ahn DU, Jin Y-G, Huang X. Separation and identification of highly efficient antioxidant peptides from eggshell membrane. Antioxidants. 2019;8(10):495. doi:10.3390/antiox8100495. [Google Scholar] [PubMed] [CrossRef]
43. Yuan G, Li W, Pan Y, Wang C, Chen H. Shrimp shell wastes: optimization of peptide hydrolysis and peptide inhibition of α-amylase. Food Biosci. 2018;25:52–60. doi:10.1016/j.fbio.2018.07.008. [Google Scholar] [CrossRef]
44. Dong Y, Yan W, Zhang Y-Q, Dai Z-Y. A novel angiotensin-converting enzyme (ACE) inhibitory peptide from tilapia skin: preparation, identification and its potential antihypertensive mechanism. Food Chem. 2024;430:137074. doi:10.1016/j.foodchem.2023.137074. [Google Scholar] [PubMed] [CrossRef]
45. Rozi P, Mamattohti W, Yang X, Kelimu A, Askar G, Ma S, et al. Optimization of ultrafiltration membrane separation technology and characterization of peptides from bovine bone marrow. Int J Pept Res Ther. 2021;27:703–17. doi:10.1007/s10989-020-10119-2. [Google Scholar] [CrossRef]
46. Hu G, Wang D, Sun L, Su R, Corazzin M, Sun X, et al. Isolation, purification and structure identification of a calcium-binding peptide from sheep bone protein hydrolysate. Foods. 2022;11(17):2655. doi:10.3390/foods11172655. [Google Scholar] [PubMed] [CrossRef]
47. Chopada K, Basaiawmoit B, Sakure AA, Maurya R, Bishnoi M, Kondepudi KK, et al. Purification and characterization of novel antihypertensive and antioxidative peptides from whey protein fermentate: in vitro, in silico, and molecular interactions studies. J Am Nutr Assoc. 2023;42(6):598–617. [Google Scholar] [PubMed]
48. Xia Y, Yu J, Xu W, Shuang Q. Purification and characterization of angiotensin-I-converting enzyme inhibitory peptides isolated from whey proteins of milk fermented with Lactobacillus plantarum QS670. J Dairy Sci. 2020;103(6):4919–28. doi:10.3168/jds.2019-17594. [Google Scholar] [PubMed] [CrossRef]
49. Piovesana S, Capriotti AL, Cavaliere C, La Barbera G, Montone CM, Chiozzi RZ, et al. Recent trends and analytical challenges in plant bioactive peptide separation, identification and validation. Anal Bioanal Chem. 2018;410(15):3425–44. doi:10.1007/s00216-018-0852-x. [Google Scholar] [PubMed] [CrossRef]
50. Junttila MH. Extraction of brewers’ spent grain in near subcritical conditions: a method to obtain high protein contents extracts. J Agricul Food Res. 2022;10:100378. doi:10.1016/j.jafr.2022.100378. [Google Scholar] [CrossRef]
51. Abeynayake R, Zhang S, Yang W, Chen L. Development of antioxidant peptides from brewers’ spent grain proteins. LWT. 2022;158:113162. doi:10.1016/j.lwt.2022.113162. [Google Scholar] [CrossRef]
52. Kotecka-Majchrzak K, Sumara A, Fornal E, Montowska M. Proteomic analysis of oilseed cake: a comparative study of species-specific proteins and peptides extracted from ten seed species. J Sci Food Agric. 2021;101(1):297–306. doi:10.1002/jsfa.v101.1. [Google Scholar] [CrossRef]
53. Zhang M, Wang O, Cai S, Zhao L, Zhao L. Composition, functional properties, health benefits and applications of oilseed proteins: a systematic review. Food Res Intern. 2023;171:113061. doi:10.1016/j.foodres.2023.113061. [Google Scholar] [PubMed] [CrossRef]
54. Wang Y, Xu K, Lu F, Wang Y, Ouyang N, Ma H. Increasing peptide yield of soybean meal solid-state fermentation of ultrasound-treated Bacillus amyloliquefaciens. Innovat Food Sci Emerg Technol. 2021;72:102704. doi:10.1016/j.ifset.2021.102704. [Google Scholar] [CrossRef]
55. Kumari R, Sharma N, Sharma S, Samurailatpam S, Padhi S, Singh SP, et al. Production and characterization of bioactive peptides in fermented soybean meal produced using proteolytic Bacillus species isolated from kinema. Food Chem. 2023;421:136130. doi:10.1016/j.foodchem.2023.136130. [Google Scholar] [PubMed] [CrossRef]
56. Lu F, Alenyorege EA, Ouyang N, Zhou A, Ma H. Simulated natural and high temperature solid-state fermentation of soybean meal: a comparative study regarding microorganisms, functional properties and structural characteristics. LWT. 2022;159:113125. doi:10.1016/j.lwt.2022.113125. [Google Scholar] [CrossRef]
57. Xu F, Zhang J, Wang Z, Yao Y, Atungulu GG, Ju X, et al. Absorption and metabolism of peptide WDHHAPQLR derived from rapeseed protein and inhibition of HUVEC apoptosis under oxidative stress. J Agric Food Chem. 2018;66(20):5178–89. doi:10.1021/acs.jafc.8b01620. [Google Scholar] [PubMed] [CrossRef]
58. Sadh PK, Duhan S, Duhan JS. Agro-industrial wastes and their utilization using solid state fermentation: a review. Bioresour Bioprocess. 2018;5(1):1–15. doi:10.1186/s40643-017-0187-z. [Google Scholar] [CrossRef]
59. Moayedi A, Hashemi M, Safari M. Valorization of tomato waste proteins through production of antioxidant and antibacterial hydrolysates by proteolytic Bacillus subtilis: optimization of fermentation conditions. J Food Sci Technol. 2016;53(1):391–400. doi:10.1007/s13197-015-1965-2. [Google Scholar] [PubMed] [CrossRef]
60. Moayedi A, Mora L, Aristoy MC, Safari M, Hashemi M, Toldrá F. Peptidomic analysis of antioxidant and ACE-inhibitory peptides obtained from tomato waste proteins fermented using Bacillus subtilis. Food Chem. 2018;250:180–7. doi:10.1016/j.foodchem.2018.01.033. [Google Scholar] [PubMed] [CrossRef]
61. Wen C, Zhang J, Feng Y, Duan Y, Ma H, Zhang H. Purification and identification of novel antioxidant peptides from watermelon seed protein hydrolysates and their cytoprotective effects on H2O2-induced oxidative stress. Food Chem. 2020;327:127059. doi:10.1016/j.foodchem.2020.127059. [Google Scholar] [PubMed] [CrossRef]
62. Mazloomi SN, Mora L, Aristoy M-C, Mahoonak AS, Ghorbani M, Houshmand G, et al. Impact of simulated gastrointestinal digestion on the biological activity of an alcalase hydrolysate of orange seed (Siavaraze, Citrus sinensis) by-products. Foods. 2020;9(9):1217. doi:10.3390/foods9091217. [Google Scholar] [PubMed] [CrossRef]
63. Wang Z, Cui Y, Liu P, Zhao Y, Wang L, Liu Y, et al. Small peptides isolated from enzymatic hydrolyzate of fermented soybean meal promote endothelium-independent vasorelaxation and ACE inhibition. J Agric Food Chem. 2017;65(50):10844–50. doi:10.1021/acs.jafc.7b05026. [Google Scholar] [PubMed] [CrossRef]
64. Montone CM, Capriotti AL, Cavaliere C, La Barbera G, Piovesana S, Chiozzi RZ, et al. Characterization of antioxidant and angiotensin-converting enzyme inhibitory peptides derived from cauliflower by-products by multidimensional liquid chromatography and bioinformatics. J Funct Foods. 2018;44(3):40–7. doi:10.1016/j.jff.2018.02.022. [Google Scholar] [CrossRef]
65. Esteve C, Marina M, García M. Novel strategy for the revalorization of olive (Olea europaea) residues based on the extraction of bioactive peptides. Food Chem. 2015;167:272–80. doi:10.1016/j.foodchem.2014.06.090. [Google Scholar] [PubMed] [CrossRef]
66. Cermeño M, Connolly A, O’Keeffe MB, Flynn C, Alashi AM, Aluko RE, et al. Identification of bioactive peptides from brewers’ spent grain and contribution of Leu/Ile to bioactive potency. J Funct Foods. 2019;60(4):103455. doi:10.1016/j.jff.2019.103455. [Google Scholar] [CrossRef]
67. White BL, Sanders TH, Davis JP. Potential ACE-inhibitory activity and nanoLC-MS/MS sequencing of peptides derived from aflatoxin contaminated peanut meal. LWT-Food Sci Technol. 2014;56(2):537–42. doi:10.1016/j.lwt.2013.11.039. [Google Scholar] [CrossRef]
68. Wang X, Chen H, Fu X, Li S, Wei J. A novel antioxidant and ACE inhibitory peptide from rice bran protein: biochemical characterization and molecular docking study. LWT. 2017;75:93–9. doi:10.1016/j.lwt.2016.08.047. [Google Scholar] [CrossRef]
69. Rozi P, Maimaiti P, Abuduwaili A, Wali A, Yili A, Akber Aisa H. Isolation and evaluation of bioactive protein and peptide from domestic animals’ bone marrow. Molecules. 2018;23(7):1673. doi:10.3390/molecules23071673. [Google Scholar] [PubMed] [CrossRef]
70. Sonklin C, Laohakunjit N, Kerdchoechuen O. Assessment of antioxidant properties of membrane ultrafiltration peptides from mungbean meal protein hydrolysates. PeerJ. 2018;6:e5337. doi:10.7717/peerj.5337. [Google Scholar] [PubMed] [CrossRef]
71. Sonklin C, Alashi MA, Laohakunjit N, Kerdchoechuen O, Aluko RE. Identification of antihypertensive peptides from mung bean protein hydrolysate and their effects in spontaneously hypertensive rats. J Funct Foods. 2020;64:103635. doi:10.1016/j.jff.2019.103635. [Google Scholar] [CrossRef]
72. Sheih I-C, Fang TJ, Wu T-K, Lin P-H. Anticancer and antioxidant activities of the peptide fraction from algae protein waste. J Agric Food Chem. 2010;58(2):1202–7. doi:10.1021/jf903089m. [Google Scholar] [PubMed] [CrossRef]
73. Mandal D, Shirazi AN, Parang K. Self-assembly of peptides to nanostructures. Organ Biomolec Chem. 2014;12(22):3544–61. doi:10.1039/C4OB00447G. [Google Scholar] [PubMed] [CrossRef]
74. Bussmann BM, Reiche S, Marí-Buyé N, Castells-Sala C, Meisel HJ, Semino CE. Chondrogenic potential of human dermal fibroblasts in a contractile, soft, self-assembling, peptide hydrogel. J Tissue Eng Regen Med. 2016;10(2):E54–E62. doi:10.1002/term.1766. [Google Scholar] [PubMed] [CrossRef]
75. Wan S, Borland S, Richardson SM, Merry CL, Saiani A, Gough JE. Self-assembling peptide hydrogel for intervertebral disc tissue engineering. Acta Biomater. 2016;46(18):29–40. doi:10.1016/j.actbio.2016.09.033. [Google Scholar] [PubMed] [CrossRef]
76. Pezeshk S, Ojagh SM, Rezaei M, Shabanpour B. Fractionation of protein hydrolysates of fish waste using membrane ultrafiltration: investigation of antibacterial and antioxidant activities. Probio Antimicrob Proteins. 2019;11(3):1015–22. doi:10.1007/s12602-018-9483-y. [Google Scholar] [PubMed] [CrossRef]
77. Hsu K-C, Li-Chan EC, Jao C-L. Antiproliferative activity of peptides prepared from enzymatic hydrolysates of tuna dark muscle on human breast cancer cell line MCF-7. Food Chem. 2011;126(2):617–22. doi:10.1016/j.foodchem.2010.11.066. [Google Scholar] [CrossRef]
78. Kaewsahnguan T, Noitang S, Sangtanoo P, Srimongkol P, Saisavoey T, Reamtong O, et al. A novel angiotensin I-converting enzyme inhibitory peptide derived from the trypsin hydrolysates of salmon bone proteins. PLoS One. 2021;16(9):e0256595. doi:10.1371/journal.pone.0256595. [Google Scholar] [PubMed] [CrossRef]
79. Ishak NH, Shaik MI, Yellapu NK, Howell NK, Sarbon NM. Purification, characterization and molecular docking study of angiotensin-I converting enzyme (ACE) inhibitory peptide from shortfin scad (Decapterus macrosoma) protein hydrolysate. J Food Sci Technol. 2021;58(12):4567–77. doi:10.1007/s13197-020-04944-y. [Google Scholar] [PubMed] [CrossRef]
80. Li KH, Zhang ZF, Li DP, Zhang WS, Yu XQ, Liu W, et al. Biomimetic ultralight, highly porous, shape-adjustable, and biocompatible 3D graphene minerals via incorporation of self-assembled peptide nanosheets. Adv Funct Mater. 2018;28(29):1801056. doi:10.1002/adfm.201801056. [Google Scholar] [CrossRef]
81. Werle M, Bernkop-Schnürch A. Strategies to improve plasma half life time of peptide and protein drugs. Amino Acids. 2006;30(4):351–67. doi:10.1007/s00726-005-0289-3. [Google Scholar] [PubMed] [CrossRef]
82. Mäde V, Els-Heindl S, Beck-Sickinger AG. Automated solid-phase peptide synthesis to obtain therapeutic peptides. Beilstein J Org Chem. 2014;10(1):1197–212. doi:10.3762/bjoc.10.118. [Google Scholar] [PubMed] [CrossRef]
83. Tsai W-B, Chen Y-R, Li W-T, Lai J-Y, Liu H-L. RGD-conjugated UV-crosslinked chitosan scaffolds inoculated with mesenchymal stem cells for bone tissue engineering. Carbohydr Polym. 2012;89(2):379–87. doi:10.1016/j.carbpol.2012.03.017. [Google Scholar] [PubMed] [CrossRef]
84. Sridhar K, Inbaraj BS, Chen B-H. Recent developments on production, purification and biological activity of marine peptides. Food Res Intern. 2021;147:110468. doi:10.1016/j.foodres.2021.110468. [Google Scholar] [PubMed] [CrossRef]
85. Xie H, Wang Y, Zhang J, Chen J, Wu D, Wang L. Study of the fermentation conditions and the antiproliferative activity of rapeseed peptides by bacterial and enzymatic cooperation. Int J Food Sci Technol. 2015;50(3):619–25. doi:10.1111/ijfs.12682. [Google Scholar] [CrossRef]
86. Ferrazzano L, Catani M, Cavazzini A, Martelli G, Corbisiero D, Cantelmi P, et al. Sustainability in peptide chemistry: current synthesis and purification technologies and future challenges. Green Chem. 2022;24(3):975–1020. doi:10.1039/D1GC04387K. [Google Scholar] [CrossRef]
87. Cermeño M, Kleekayai T, Amigo-Benavent M, Harnedy-Rothwell P, FitzGerald RJ. Current knowledge on the extraction, purification, identification, and validation of bioactive peptides from seaweed. Electrophoresis. 2020;41(20):1694–717. doi:10.1002/elps.v41.20. [Google Scholar] [CrossRef]
88. Chang C-H, Chang H-Y, Rappsilber J, Ishihama Y. Isolation of acetylated and unmodified protein N-terminal peptides by strong cation exchange chromatographic separation of TrypN-digested peptides. Molecul Cell Proteom. 2021;20:100003. doi:10.1074/mcp.TIR120.002148. [Google Scholar] [PubMed] [CrossRef]
89. Lafarga T, Sánchez-Zurano A, Villaró S, Morillas-España A, Acién G. Industrial production of spirulina as a protein source for bioactive peptide generation. Tren Food Sci Technol. 2021;116:176–85. doi:10.1016/j.tifs.2021.07.018. [Google Scholar] [CrossRef]
90. Wan Q-H. Mixed-mode. Chromatography: Springer; 2021. [Google Scholar]
91. Bäurer S. Characterization and application of mixed mode stationary phases in pharmaceutical and biochemical analysis using one-and two-dimensional liquid chromatography (Dissertation). Universität Tübingen: Tübingen, Germany; 2020. [Google Scholar]
92. de Castro RJS, Sato HH. Biologically active peptides: processes for their generation, purification and identification and applications as natural additives in the food and pharmaceutical industries. Food Res Intern. 2015;74:185–98. doi:10.1016/j.foodres.2015.05.013. [Google Scholar] [PubMed] [CrossRef]
93. Yan Y, Li Y, Zhang Z, Wang X, Niu Y, Zhang S, et al. Advances of peptides for antibacterial applications. Coll Surf B: Biointerf. 2021;202:111682. doi:10.1016/j.colsurfb.2021.111682. [Google Scholar] [PubMed] [CrossRef]
94. Rabail R, Khan MR, Mehwish HM, Rajoka MSR, Lorenzo JM, Kieliszek M, et al. An overview of chia seed (Salvia hispanica L.) bioactive peptides’ derivation and utilization as an emerging nutraceutical food. Front Biosci. 2021;26(9):643–54. doi:10.52586/4973. [Google Scholar] [PubMed] [CrossRef]
95. Li S, Wang Y, Xue Z, Jia Y, Li R, He C, et al. The structure-mechanism relationship and mode of actions of antimicrobial peptides: a review. Tren Food Sci Technol. 2021;109:103–15. doi:10.1016/j.tifs.2021.01.005. [Google Scholar] [CrossRef]
96. Trabi M, Schirra HJ, Craik DJ. Three-dimensional structure of RTD-1, a cyclic antimicrobial defensin from Rhesus macaque leukocytes. Biochemistry. 2001;40(14):4211–21. doi:10.1021/bi002028t. [Google Scholar] [PubMed] [CrossRef]
97. Liscano Y, Salamanca CH, Vargas L, Cantor S, Laverde-Rojas V, Oñate-Garzón J. Increases in hydrophilicity and charge on the polar face of alyteserin 1c helix change its selectivity towards gram-positive bacteria. Antibiotics. 2019;8(4):238. doi:10.3390/antibiotics8040238. [Google Scholar] [PubMed] [CrossRef]
98. Li J, Hu S, Jian W, Xie C, Yang X. Plant antimicrobial peptides: structures, functions, and applications. Bot Stud. 2021;62(1):5. doi:10.1186/s40529-021-00312-x. [Google Scholar] [PubMed] [CrossRef]
99. Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3(3):238–50. doi:10.1038/nrmicro1098. [Google Scholar] [PubMed] [CrossRef]
100. Zhang L-J, Gallo RL. Antimicrobial peptides. Curr Biol. 2016;26(1):R14–9. doi:10.1016/j.cub.2015.11.017. [Google Scholar] [PubMed] [CrossRef]
101. Epand RM, Epand RF. Bacterial membrane lipids in the action of antimicrobial agents. J Pept Sci. 2011;17(5):298–305. doi:10.1002/psc.1319. [Google Scholar] [PubMed] [CrossRef]
102. Qin X, Xu X, Guo Y, Shen Q, Liu J, Yang C, et al. A sustainable and efficient recycling strategy of feather waste into keratin peptides with antimicrobial activity. Waste Manag. 2022;144(22):421–30. doi:10.1016/j.wasman.2022.04.017. [Google Scholar] [PubMed] [CrossRef]
103. Osman A, Goda HA, Abdel-Hamid M, Badran SM, Otte J. Antibacterial peptides generated by Alcalase hydrolysis of goat whey. 2016;65:480–6. doi:10.1016/j.lwt.2015.08.043. [Google Scholar] [CrossRef]
104. Liu D, Liu M, Meng D, Mu Y, Wang T, Lv Z. Harsh sensitivity and mechanism exploration of an antibacterial peptide extracted from walnut oil residue derived from agro-industrial waste. J Agric Food Chem. 2022;70(24):7460–70. doi:10.1021/acs.jafc.2c02699. [Google Scholar] [PubMed] [CrossRef]
105. Pavlicevic M, Marmiroli N, Maestri E. Immunomodulatory peptides—a promising source for novel functional food production and drug discovery. Peptides. 2022;148(4):170696. doi:10.1016/j.peptides.2021.170696. [Google Scholar] [PubMed] [CrossRef]
106. Wang W, Wang J, Dong S-F, Liu C-H, Italiani P, Sun S-H, et al. Immunomodulatory activity of andrographolide on macrophage activation and specific antibody response. Acta Pharmacol Sin. 2010;31(2):191–201. doi:10.1038/aps.2009.205. [Google Scholar] [PubMed] [CrossRef]
107. Mao R, Wu L, Zhu N, Liu X, Hao Y, Liu R, et al. Immunomodulatory effects of walnut (Juglans regia L.) oligopeptides on innate and adaptive immune responses in mice. J Funct Foods. 2020;73(5):104068. doi:10.1016/j.jff.2020.104068. [Google Scholar] [CrossRef]
108. Maestri E, Marmiroli M, Marmiroli N. Bioactive peptides in plant-derived foodstuffs. J Proteomics. 2016;147(6):140–55. doi:10.1016/j.jprot.2016.03.048. [Google Scholar] [PubMed] [CrossRef]
109. de Oliveira Viana IM, Roussel S, Defrêne J, Lima EM, Barabé F, Bertrand N. Innate and adaptive immune responses toward nanomedicines. Acta Pharmaceutica Sinica B. 2021;11(4):852–70. doi:10.1016/j.apsb.2021.02.022. [Google Scholar] [PubMed] [CrossRef]
110. Bielecka M, Cichosz G, Czeczot H. Antioxidant, antimicrobial and anticarcinogenic activities of bovine milk proteins and their hydrolysates—a review. International Dairy Journal. 2022;127:105208. doi:10.1016/j.idairyj.2021.105208. [Google Scholar] [CrossRef]
111. Saint-Sauveur D, Gauthier SF, Boutin Y, Montoni A. Immunomodulating properties of a whey protein isolate, its enzymatic digest and peptide fractions. Int Dairy J. 2008;18(3):260–70. doi:10.1016/j.idairyj.2007.07.008. [Google Scholar] [CrossRef]
112. Yoshikawa M, Sugimoto K. A specific binding site on soybean membranes for a phytoalexin elicitor released from fungal cell walls by rβ-1, 3-endoglucanase. Plant Cell Physiol. 1993;34(8):1229–37. [Google Scholar]
113. Jiun-rong C, Suetsuna K, Yamauchi F. Isolation and characterization of immunostimulative peptides from soybean. J Nutr Biochem. 1995;6(6):310–3. doi:10.1016/0955-2863(95)00022-R. [Google Scholar] [CrossRef]
114. Takahashi M, Moriguchi S, Yoshikawa M, Sasaki R. Isolation and characterization of oryzatensin: a novel bioactive peptide with ileum-contracting and immunomodulating activities derived from rice albumin. Biochem Mol Biol Int. 1994;33(6):1151–8. [Google Scholar] [PubMed]
115. Hou H, Fan Y, Li B, Xue C, Yu G, Zhang Z, et al. Purification and identification of immunomodulating peptides from enzymatic hydrolysates of Alaska pollock frame. Food Chem. 2012;134(2):821–8. doi:10.1016/j.foodchem.2012.02.186. [Google Scholar] [PubMed] [CrossRef]
116. He XQ, Cao WH, Pan GK, Yang L, Zhang CH. Enzymatic hydrolysis optimization of Paphia undulata and lymphocyte proliferation activity of the isolated peptide fractions. J Sci Food Agric. 2015;95(7):1544–53. doi:10.1002/jsfa.2015.95.issue-7. [Google Scholar] [CrossRef]
117. Nelson R, Katayama S, Mine Y, Duarte J, Matar C. Immunomodulating effects of egg yolk low lipid peptic digests in a murine model. Food Agric Immunol. 2007;18(1):1–15. doi:10.1080/09540100601178623. [Google Scholar] [CrossRef]
118. Lozano-Ojalvo D, Molina E, López-Fandiño R. Regulation of exacerbated immune responses in human peripheral blood cells by hydrolysed egg white proteins. PLoS One. 2016;11(3):e0151813. doi:10.1371/journal.pone.0151813. [Google Scholar] [PubMed] [CrossRef]
119. Chiangjong W, Chutipongtanate S, Hongeng S. Anticancer peptide: physicochemical property, functional aspect and trend in clinical application. Int J Oncol. 2020;57(3):678–96. doi:10.3892/ijo. [Google Scholar] [CrossRef]
120. Liscano Y, Oñate-Garzón J, Delgado JP. Peptides with dual antimicrobial-anticancer activity: strategies to overcome peptide limitations and rational design of anticancer peptides. Molecules. 2020;25(18):4245. doi:10.3390/molecules25184245. [Google Scholar] [PubMed] [CrossRef]
121. Song R, Wei RB, Luo HY, Yang ZS. Isolation and identification of an antiproliferative peptide derived from heated products of peptic hydrolysates of half-fin anchovy (Setipinna taty). J Funct Foods. 2014;10:104–11. doi:10.1016/j.jff.2014.06.010. [Google Scholar] [CrossRef]
122. Kim SE, Kim HH, Kim JY, Im Kang Y, Woo HJ, Lee HJ. Anticancer activity of hydrophobic peptides from soy proteins. Biofactors. 2000;12(1–4):151–5. [Google Scholar] [PubMed]
123. Wang W, Rupasinghe SG, Schuler MA, Gonzalez de Mejia E. Identification and characterization of topoisomerase II inhibitory peptides from soy protein hydrolysates. J Agric Food Chem. 2008;56(15):6267–77. doi:10.1021/jf8005195. [Google Scholar] [PubMed] [CrossRef]
124. Kannan A, Hettiarachchy NS, Lay JO, Liyanage R. Human cancer cell proliferation inhibition by a pentapeptide isolated and characterized from rice bran. Peptides. 2010;31(9):1629–34. doi:10.1016/j.peptides.2010.05.018. [Google Scholar] [PubMed] [CrossRef]
125. Vital DAL, De Mejía EG, Dia VP, Loarca-Piña G. Peptides in common bean fractions inhibit human colorectal cancer cells. Food Chem. 2014;157:347–55. doi:10.1016/j.foodchem.2014.02.050. [Google Scholar] [PubMed] [CrossRef]
126. Wang L, Zhang J, Yuan Q, Xie H, Shi J, Ju X. Separation and purification of an anti-tumor peptide from rapeseed (Brassica campestris L.) and the effect on cell apoptosis. Food Funct. 2016;7(5):2239–48. doi:10.1039/C6FO00042H. [Google Scholar] [PubMed] [CrossRef]
127. Kim E-K, Kim Y-S, Hwang J-W, Lee JS, Moon S-H, Jeon B-T, et al. Purification and characterization of a novel anticancer peptide derived from Ruditapes philippinarum. Process Biochem. 2013;48(7):1086–90. doi:10.1016/j.procbio.2013.05.004. [Google Scholar] [CrossRef]
128. Hung C-C, Yang Y-H, Kuo P-F, Hsu K-C. Protein hydrolysates from tuna cooking juice inhibit cell growth and induce apoptosis of human breast cancer cell line MCF-7. J Funct Foods. 2014;11:563–70. doi:10.1016/j.jff.2014.08.015. [Google Scholar] [CrossRef]
129. Kannan A, Hettiarachchy NS, Marshall M, Raghavan S, Kristinsson H. Shrimp shell peptide hydrolysates inhibit human cancer cell proliferation. J Sci Food Agric. 2011;91(10):1920–4. doi:10.1002/jsfa.4464. [Google Scholar] [PubMed] [CrossRef]
130. Aondona MM, Ikya JK, Ukeyima MT, Gborigo TWJ, Aluko RE, Girgih AT. In vitro antioxidant and antihypertensive properties of sesame seed enzymatic protein hydrolysate and ultrafiltration peptide fractions. J Food Biochem. 2021;45(1):e13587. [Google Scholar] [PubMed]
131. Chaipoot S, Punfa W, Ounjaijean S, Phongphisutthinant R, Kulprachakarn K, Parklak W, et al. Antioxidant, anti-diabetic, anti-obesity, and antihypertensive properties of protein hydrolysate and peptide fractions from black sesame cake. Molecules. 2022;28(1):211. doi:10.3390/molecules28010211. [Google Scholar] [PubMed] [CrossRef]
132. Majumder K, Chakrabarti S, Morton JS, Panahi S, Kaufman S, Davidge ST, et al. Egg-derived ACE-inhibitory peptides IQW and LKP reduce blood pressure in spontaneously hypertensive rats. J Funct Foods. 2015;13:50–60. doi:10.1016/j.jff.2014.12.028. [Google Scholar] [CrossRef]
133. Xiang L, Qiu Z, Zhao R, Zheng Z, Qiao X. Advancement and prospects of production, transport, functional activity and structure-activity relationship of food-derived angiotensin converting enzyme (ACE) inhibitory peptides. Crit Rev Food Sci Nutr. 2023;63(10):1437–63. doi:10.1080/10408398.2021.1964433. [Google Scholar] [PubMed] [CrossRef]
134. Morais HA, Silvestre MP, Amorin LL, Silva VD, Silva MR, Simoes e Silva AC, et al. Use of different proteases to obtain whey protein concentrate hydrolysates with inhibitory activity toward angiotensin-converting enzyme. J Food Biochem. 2014;38(1):102–9. doi:10.1111/jfbc.12032. [Google Scholar] [CrossRef]
135. Lin Z, Lai J, He P, Pan L, Zhang Y, Zhang M, et al. Screening, ACE-inhibitory mechanism and structure-activity relationship of a novel ACE-inhibitory peptide from Lepidium meyenii (Maca) protein hydrolysate. Food Biosci. 2023;52:102374. doi:10.1016/j.fbio.2023.102374. [Google Scholar] [CrossRef]
136. Memarpoor-Yazdi M, Asoodeh A, Chamani J. A novel antioxidant and antimicrobial peptide from hen egg white lysozyme hydrolysates. J Funct Foods. 2012;4(1):278–86. doi:10.1016/j.jff.2011.12.004. [Google Scholar] [CrossRef]
137. Wu J, Liao W, Udenigwe CC. Revisiting the mechanisms of ACE inhibitory peptides from food proteins. Tren Food Sci Technol. 2017;69:214–9. doi:10.1016/j.tifs.2017.07.011. [Google Scholar] [CrossRef]
138. García MC, Endermann J, Gonzalez-Garcia E, Marina ML. HPLC-Q-TOF-MS identification of antioxidant and antihypertensive peptides recovered from cherry (Prunus cerasus L.) subproducts. J Agric Food Chem. 2015;63(5):1514–20. doi:10.1021/jf505037p. [Google Scholar] [PubMed] [CrossRef]
139. González-García E, Marina ML, García MC. Plum (Prunus Domestica L.) by-product as a new and cheap source of bioactive peptides: extraction method and peptides characterization. J Funct Foods. 2014;11:428–37. doi:10.1016/j.jff.2014.10.020. [Google Scholar] [CrossRef]
140. Mäkinen S, Johannson T, Gerd EV, Pihlava JM, Pihlanto A. Angiotensin I-converting enzyme inhibitory and antioxidant properties of rapeseed hydrolysates. J Funct Foods. 2012;4(3):575–83. doi:10.1016/j.jff.2012.03.003. [Google Scholar] [CrossRef]
141. Vinod A, Sanjay M, Suchart S, Jyotishkumar P. Renewable and sustainable biobased materials: an assessment on biofibers, biofilms, biopolymers and biocomposites. J Clean Prod. 2020;258:120978. doi:10.1016/j.jclepro.2020.120978. [Google Scholar] [CrossRef]
142. Ding X, Zhao H, Li Y, Lee AL, Li Z, Fu M, et al. Synthetic peptide hydrogels as 3D scaffolds for tissue engineering. Adv Drug Deliv Rev. 2020;160:78–104. doi:10.1016/j.addr.2020.10.005. [Google Scholar] [PubMed] [CrossRef]
143. Yang J, An H-W, Wang H. Self-assembled peptide drug delivery systems. ACS Appl Bio Mat. 2020;4(1):24–46. [Google Scholar]
144. Xu X, Farach-Carson MC, Jia X. Three-dimensional in vitro tumor models for cancer research and drug evaluation. Biotechnol Adv. 2014;32(7):1256–68. doi:10.1016/j.biotechadv.2014.07.009. [Google Scholar] [PubMed] [CrossRef]
145. Peela N, Truong D, Saini H, Chu H, Mashaghi S, Ham SL, et al. Advanced biomaterials and microengineering technologies to recapitulate the stepwise process of cancer metastasis. Biomaterials. 2017;133(1):176–207. doi:10.1016/j.biomaterials.2017.04.017. [Google Scholar] [PubMed] [CrossRef]
146. Sun W, Gregory DA, Zhao X. Designed peptide amphiphiles as scaffolds for tissue engineering. Adv Colloid Interface Sci. 2023;314(3):102866. doi:10.1016/j.cis.2023.102866. [Google Scholar] [PubMed] [CrossRef]
147. Gagner JE, Kim W, Chaikof EL. Designing protein-based biomaterials for medical applications. Acta Biomater. 2014;10(4):1542–57. doi:10.1016/j.actbio.2013.10.001. [Google Scholar] [PubMed] [CrossRef]
148. Sieminski A, Semino C, Gong H, Kamm R. Primary sequence of ionic self-assembling peptide gels affects endothelial cell adhesion and capillary morphogenesis. J Biomed Mat Res Part A. 2008;87(2):494–504. doi:10.1002/jbm.a.31785. [Google Scholar] [PubMed] [CrossRef]
149. Tao H, Wu Y, Li H, Wang C, Zhang Y, Li C, et al. BMP7-based functionalized self-assembling peptides for nucleus pulposus tissue engineering. ACS Appl Mat Interf. 2015;7(31):17076–87. doi:10.1021/acsami.5b03605. [Google Scholar] [PubMed] [CrossRef]
150. Noel S, Fortier C, Murschel F, Belzil A, Gaudet G, Jolicoeur M, et al. Co-immobilization of adhesive peptides and VEGF within a dextran-based coating for vascular applications. Acta Biomater. 2016;37:69–82. doi:10.1016/j.actbio.2016.03.043. [Google Scholar] [PubMed] [CrossRef]
151. Jing J, Fournier A, Szarpak-Jankowska A, Block MR, Auzély-Velty R. Type, density, and presentation of grafted adhesion peptides on polysaccharide-based hydrogels control preosteoblast behavior and differentiation. Biomacromolecules. 2015;16(3):715–22. doi:10.1021/bm501613u. [Google Scholar] [PubMed] [CrossRef]
152. Li D, Feng X, Chen L, Ding J, Chen X. One-step synthesis of targeted acid-labile polysaccharide prodrug for efficiently intracellular drug delivery. ACS Biomat Sci Eng. 2018;4(2):539–46. doi:10.1021/acsbiomaterials.7b00856. [Google Scholar] [PubMed] [CrossRef]
153. Jia Y, Yan X, Li J. Schiff base mediated dipeptide assembly toward nanoarchitectonics. Angew Chem Int Ed. 2022;61(43):e202207752. doi:10.1002/anie.202207752. [Google Scholar] [PubMed] [CrossRef]
154. De Simone C, Picariello G, Mamone G, Stiuso P, Dicitore A, Vanacore D, et al. Characterisation and cytomodulatory properties of peptides from Mozzarella di Bufala Campana cheese whey. J Peptide Sci. 2009;15(3):251–8. doi:10.1002/psc.1093. [Google Scholar] [PubMed] [CrossRef]
155. Marczak ED, Usui H, Fujita H, Yang Y, Yokoo M, Lipkowski AW, et al. New antihypertensive peptides isolated from rapeseed. Peptides. 2003;24(6):791–8. doi:10.1016/S0196-9781(03)00174-8. [Google Scholar] [PubMed] [CrossRef]
156. Ketnawa S, Suwal S, Huang JY, Liceaga AM. Selective separation and characterisation of dual ACE and DPP-IV inhibitory peptides from rainbow trout (Oncorhynchus mykiss) protein hydrolysates. Int J Food Sci Technol. 2019;54(4):1062–73. doi:10.1111/ijfs.13939. [Google Scholar] [CrossRef]
157. Berillo D, Yeskendir A, Zharkinbekov Z, Raziyeva K, Saparov A. Peptide-based drug delivery systems. Medicina. 2021;57(11):1209. doi:10.3390/medicina57111209. [Google Scholar] [PubMed] [CrossRef]
158. Eskandari S, Guerin T, Toth I, Stephenson RJ. Recent advances in self-assembled peptides: implications for targeted drug delivery and vaccine engineering. Adv Drug Deliv Rev. 2017;110:169–87. [Google Scholar] [PubMed]
159. Lee H-J, Huang Y-W, Aronstam RS. Intracellular delivery of nanoparticles mediated by lactoferricin cell-penetrating peptides in an endocytic pathway. J Nanosci Nanotechnol. 2019;19(2):613–21. doi:10.1166/jnn.2019.15751. [Google Scholar] [PubMed] [CrossRef]
160. Zhou A, Chen S, He B, Zhao W, Chen X, Jiang D. Controlled release of TGF-beta 1 from RADA self-assembling peptide hydrogel scaffolds. Drug Des Devel Ther. 2016;10:3043–51. doi:10.2147/DDDT. [Google Scholar] [CrossRef]
161. Dókus LE, Lajkó E, Ranđelović I, Mező D, Schlosser G, Kőhidai L, et al. Phage display-based homing peptide-daunomycin conjugates for selective drug targeting to PANC-1 pancreatic cancer. Pharmaceutics. 2020;12(6):576. doi:10.3390/pharmaceutics12060576. [Google Scholar] [PubMed] [CrossRef]
162. Zhang C, Pan D, Li J, Hu J, Bains A, Guys N, et al. Enzyme-responsive peptide dendrimer-gemcitabine conjugate as a controlled-release drug delivery vehicle with enhanced antitumor efficacy. Acta Biomater. 2017;55:153–62. doi:10.1016/j.actbio.2017.02.047. [Google Scholar] [PubMed] [CrossRef]
163. Jiang Y, Guan C, Liu X, Wang Y, Zuo H, Xia T, et al. Doxorubicin-loaded CuS nanoparticles conjugated with GFLG: a novel drug delivery system for lymphoma treatment. Nano. 2019;14(1):1950013. doi:10.1142/S1793292019500139. [Google Scholar] [CrossRef]
164. Hossein-Nejad-Ariani H, Althagafi E, Kaur K. Small peptide ligands for targeting EGFR in triple negative breast cancer cells. Sci Rep. 2019;9(1):2723. doi:10.1038/s41598-019-38574-y. [Google Scholar] [PubMed] [CrossRef]
165. Zhu L, Ding Z, Li X, Wei H, Chen Y. Research progress of radiolabeled Asn-Gly-Arg (NGR) peptides for imaging and therapy. Mol Imaging. 2020;19(3):1536012120934957. doi:10.1177/1536012120934957. [Google Scholar] [PubMed] [CrossRef]
166. Han H, Hou Y, Chen X, Zhang P, Kang M, Jin Q, et al. Metformin-induced stromal depletion to enhance the penetration of gemcitabine-loaded magnetic nanoparticles for pancreatic cancer targeted therapy. J Am Chem Soc. 2020;142(10):4944–54. doi:10.1021/jacs.0c00650. [Google Scholar] [PubMed] [CrossRef]
167. Huang W, Zhao H, Wan J, Zhou Y, Xu Q, Zhao Y, et al. pH-and photothermal-driven multistage delivery nanoplatform for overcoming cancer drug resistance. Theranostics. 2019;9(13):3825. doi:10.7150/thno.33958. [Google Scholar] [PubMed] [CrossRef]
168. Conte C, Longobardi G, Barbieri A, Palma G, Luciano A, Dal Poggetto G, et al. Non-covalent strategies to functionalize polymeric nanoparticles with NGR peptides for targeting breast cancer. Int J Pharm. 2023;633:122618. doi:10.1016/j.ijpharm.2023.122618. [Google Scholar] [PubMed] [CrossRef]
169. Mahmoudi R, Ashraf Mirahmadi-Babaheidri S, Delaviz H, Fouani MH, Alipour M, Jafari Barmak M, et al. RGD peptide-mediated liposomal curcumin targeted delivery to breast cancer cells. J Biomater Appl. 2021;35(7):743–53. doi:10.1177/0885328220949367. [Google Scholar] [PubMed] [CrossRef]
170. Ding X-W, Robinson M, Li R, Aldhowayan H, Geetha T, Babu JR. Mitochondrial dysfunction and beneficial effects of mitochondria-targeted small peptide SS-31 in Diabetes Mellitus and Alzheimer’s disease. Pharmacol Res. 2021;171:105783. doi:10.1016/j.phrs.2021.105783. [Google Scholar] [PubMed] [CrossRef]
171. Morroni G, Sante LD, Simonetti O, Brescini L, Kamysz W, Kamysz E, et al. Synergistic effect of antimicrobial peptide LL-37 and colistin combination against multidrug-resistant Escherichia coli isolates. Future Microbiol. 2021;16(4):221–7. doi:10.2217/fmb-2020-0204. [Google Scholar] [PubMed] [CrossRef]
172. Raphael B, Khalil T, Workman VL, Smith A, Brown CP, Streuli C, et al. 3D cell bioprinting of self-assembling peptide-based hydrogels. Mater Lett. 2017;190(7):103–6. doi:10.1016/j.matlet.2016.12.127. [Google Scholar] [CrossRef]
173. Firipis K, Nisbet DR, Franks SJ, Kapsa RM, Pirogova E, Williams RJ, et al. Enhancing peptide biomaterials for biofabrication. Polymers. 2021;13(16):2590. doi:10.3390/polym13162590. [Google Scholar] [PubMed] [CrossRef]
174. Dasgupta A, Das D. Designer peptide amphiphiles: self-assembly to applications. Langmuir. 2019;35(33):10704–24. doi:10.1021/acs.langmuir.9b01837. [Google Scholar] [PubMed] [CrossRef]
175. Zhang Z, Ai S, Yang Z, Li X. Peptide-based supramolecular hydrogels for local drug delivery. Adv Drug Deliv Rev. 2021;174:482–503. doi:10.1016/j.addr.2021.05.010. [Google Scholar] [PubMed] [CrossRef]
176. Li A, Guo C, Li X, Li P, Yang X, Guo Y. Gelation mechanism and physical properties of glucono-δ-lactone induced alginate sodium/casein composite gels. Food Hydrocoll. 2021;118(6):106775. doi:10.1016/j.foodhyd.2021.106775. [Google Scholar] [CrossRef]
177. Wang J, Chen X-Y, Zhao Y, Yang Y, Wang W, Wu C, et al. pH-switchable antimicrobial nanofiber networks of hydrogel eradicate biofilm and rescue stalled healing in chronic wounds. ACS Nano. 2019;13(10):11686–97. doi:10.1021/acsnano.9b05608. [Google Scholar] [PubMed] [CrossRef]
178. Sather NA, Sai H, Sasselli IR, Sato K, Ji W, Synatschke CV, et al. 3D printing of supramolecular polymer hydrogels with hierarchical structure. Small. 2021;17(5):2005743. doi:10.1002/smll.202005743. [Google Scholar] [PubMed] [CrossRef]
179. Susapto HH, Alhattab D, Abdelrahman S, Khan Z, Alshehri S, Kahin K, et al. Ultrashort peptide bioinks support automated printing of large-scale constructs assuring long-term survival of printed tissue constructs. Nano Lett. 2021;21(7):2719–29. doi:10.1021/acs.nanolett.0c04426. [Google Scholar] [PubMed] [CrossRef]
180. Cofiño C, Perez-Amodio S, Semino CE, Engel E, Mateos-Timoneda MA. Development of a self-assembled peptide/methylcellulose-based bioink for 3D bioprinting. Macromol Mater Eng. 2019;304(11):1900353. doi:10.1002/mame.201900353. [Google Scholar] [CrossRef]
181. Hedegaard CL, Collin EC, Redondo-Gómez C, Nguyen LT, Ng KW, Castrejón-Pita AA, et al. Hydrodynamically guided hierarchical self-assembly of peptide-protein bioinks. Adv Funct Mater. 2018;28(16):1703716. doi:10.1002/adfm.201703716. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF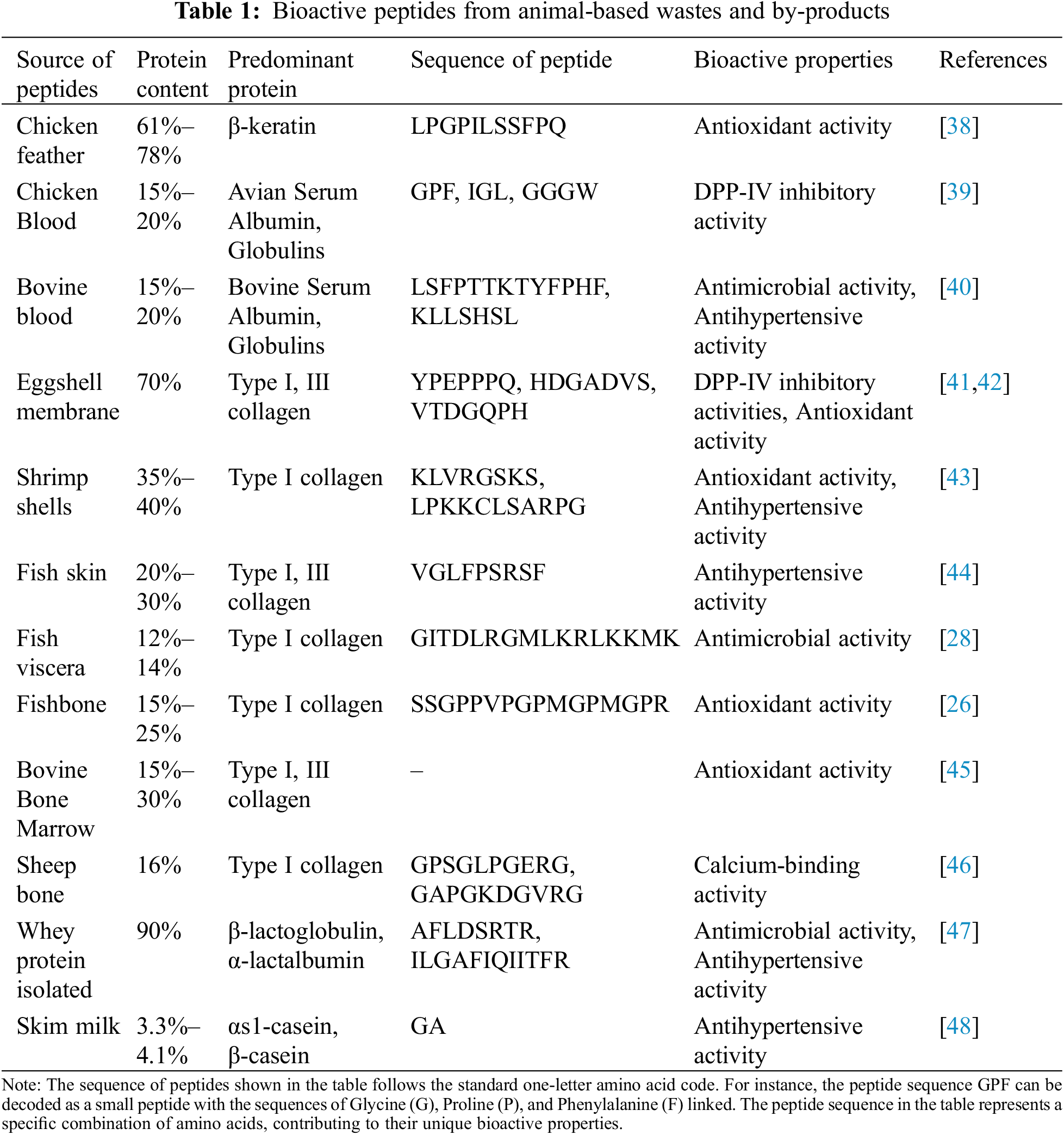


 Downloads
Downloads
 Citation Tools
Citation Tools