 Open Access
Open Access
ARTICLE
From Waste to Biopolymer: Synthesis of P(3HB-co-4HB) from Renewable Fish Oil
1 Institute of Biophysics SB RAS, Federal Research Center “Krasnoyarsk Science Center SB RAS”, 50/50 Akademgorodok, Krasnoyarsk, 660036, Russia
2 Basic Department of Biotechnology, School of Fundamental Biology and Biotechnology, Siberian Federal University, 79 Svobodnyi Av., Krasnoyarsk, 660041, Russia
3 Kaliningrad State Technical University, Sovetsky Avenue, 1, Kaliningrad, 236022, Russia
4 Institute of Chemistry and Chemical Technology, Federal Research Center “Krasnoyarsk Science Center SB RAS”, 50/24 Akademgorodok, Krasnoyarsk, 660036, Russia
* Corresponding Author: Natalia Zhila. Email:
Journal of Renewable Materials 2025, 13(3), 413-432. https://doi.org/10.32604/jrm.2024.058775
Received 20 September 2024; Accepted 10 December 2024; Issue published 20 March 2025
Abstract
The article presents the results of a study on the possibility of synthesizing biodegradable poly(3-hydroxybutyrate-co-4-hydroxybutyrate) [P(3HB-co-4HB)] from renewable waste fish oil (WFO) by the Cupriavidus necator B-10646 bacterium. For the first time, waste oil generated during the processing of Sprattus balticus in the production of sprats was used as the main carbon substrate for the synthesis of P(3HB-co-4HB), and ε-caprolactone was used as a precursor instead of the more expensive γ-butyrolactone. Samples of P(3HB-co-4HB) with a 4HB monomer content from 7.4 to 11.6 mol.% were synthesized, and values of the bacterial biomass yield and the total yield of the copolymer were comparable with the control (where butyric acid was used as carbon source). The following properties of the samples were studied: molecular weight, temperature characteristics, thermal behavior, isothermal crystallization of melts, and the formation of spherulites. The renewable fatty substrate of complex composition was used to synthesize samples of technologically advanced low-crystallinity P(3HB-co-4HB) with significant proportions of 4HB, without impairing the physicochemical properties of the polymer. The biotechnological process involving the use of renewable WFO and ε-caprolactone can be employed to reduce the costs of producing a promising “green” bioplastic and make it more affordable.Graphic Abstract
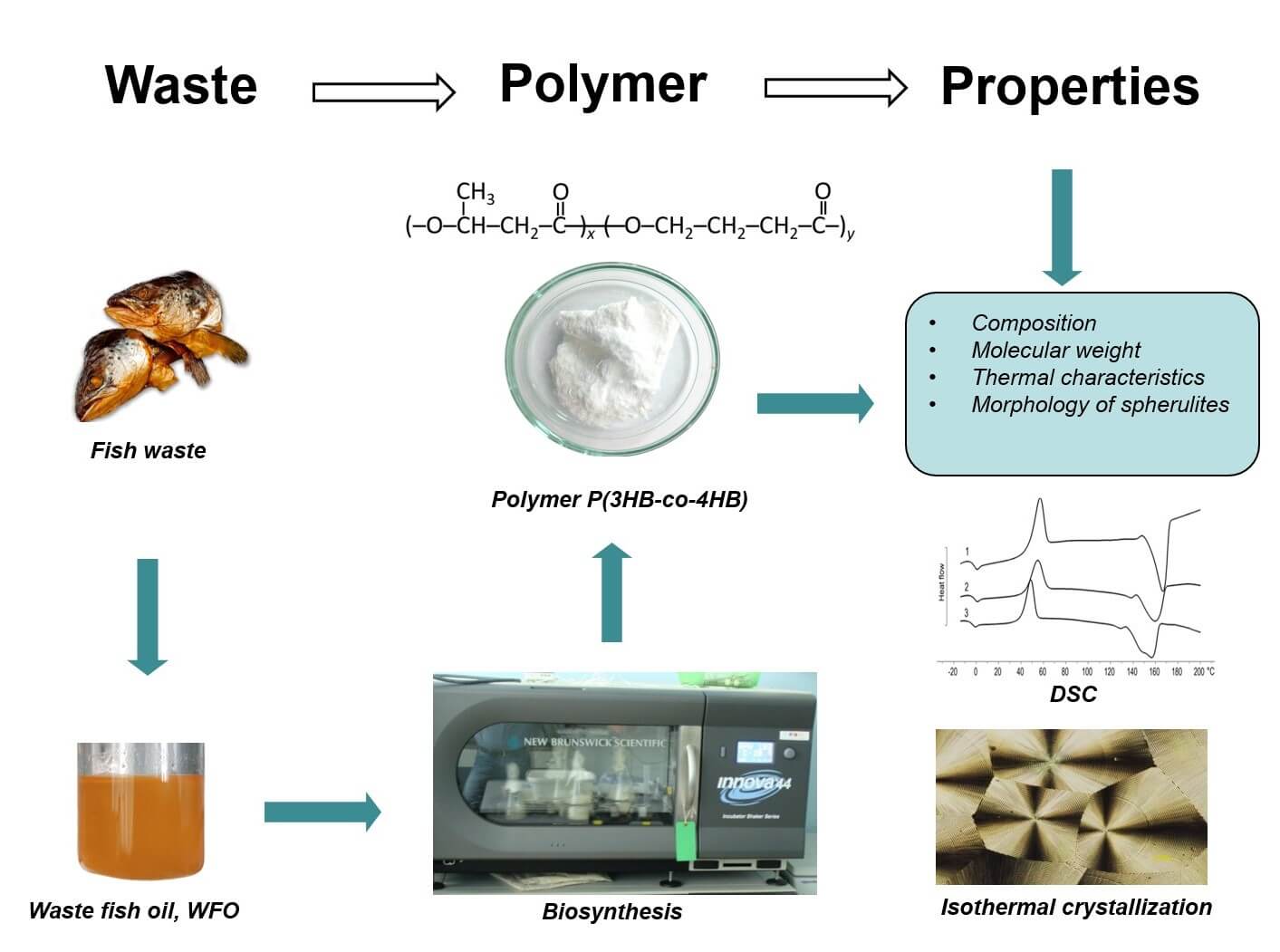
Keywords
Bacterial polymers (polyhydroxyalkanoates, PHAs) play an essential role in the gradual replacement of petroleum-derived synthetic plastics with degradable polymeric materials. PHAs are biodegradable polymers with considerable potential in diverse applications: from agriculture, public utilities, textile and packaging industries to biomedicine and pharmacology [1–5].
Poly(3-hydroxybutyrate) [P(3HB)] homopolymer is the most studied representative of the PHA family. However, this polymer is characterized by the high degree of crystallinity and the small difference between its melting point and thermal degradation temperature. Therefore, P(3HB)-based products lack mechanical strength and are brittle due to formation of large spherulites and secondary crystallization processes [6–10]. A way to remedy the undesirable properties of P(3HB) is to synthesize PHA copolymers containing, in addition to 3HB, monomers of a different structure (4-hydroxybutyrate, 3-hydroxyvalerate, and 3-hydroxyhexanoate, etc.) [11–13].
PHA copolymers consisting of 3- and 4-hydroxybutyrate monomers [P(3HB-co-4HB)] have good consumer properties and stand out from other PHA copolymers. Depending on the 4HB content, the melting point of these copolymers varies considerably (from 40°C–50°C to 150°C–160°C). The degree of crystallinity of such PHAs can be below 20%, and, therefore, products made of P(3HB-co-4HB) copolymers exhibit high elasticity: their elongation at break exceeds the values of this parameter of other PHA types by several orders of magnitude [14–17]. P(3HB-co-4HB) is completely biocompatible because butyric acid is a natural metabolite of living organisms of various levels of organization. These copolymers are subject to destruction in vivo not only by PHA depolymerases, but also by lipases, which accelerate this process [18]. PHAs containing 4HB are considered among the most promising biomaterials intended for medical applications [15].
The 4HB monomer units in the PHAs are described in the study by Professor Y. Doi [19]. The authors found the presence of 4HB monomer units in samples synthesized by Cupriavidus necator (formerly known as Ralstonia eutropha) during growth on 4-hydroxybutyric acid or 4-chlorobutyric acid as a carbon source. After it was shown that Comamonas acidovorans is capable of synthesizing P(3HB-co-4HB) copolymers on sugars using γ-butyrolactone or 1,4-butanediol as a precursor of 4HB, extensive in-depth research of these copolymers was undertaken [20]. The main goal of the research was to find conditions for increasing the 4HB content to 100 mol.%, that is, producing a homopolymer of 4-hydroxybutyrate. Synthesis of the P(4HB) homopolymer was first performed in the culture of a recombinant strain of E. coli [21]. To date, a large body of information has been accumulated on the synthesis of P(3HB-co-4HB) and P(4HB) by recombinant and wild-type strains [14,17].
Resistant wild-type strains of various taxa (Cupriavidus necator, C. malaysiensis, B. sacchari, D. acidovorans, A. lata, H. eudoflava, B. cereus, Aneurinibacillus sp.) are capable of synthesizing P(3HB-co-4HB) copolymers with various 4HB percentages, from just a few mol.% to 96 mol.%. However, an increase in the 4HB content, as a rule, follows an increase in the amount of the added precursors. This reduces the overall production of bacterial biomass and synthesis of copolymers because of the toxicity of the precursors. C. necator is considered as the most promising bacterium for the synthesis of PHAs, including various copolymers [15,19].
Most studies report the synthesis of P(3HB-co-4HB) copolymers from sugars supplemented with γ-butyrolactone as the 4HB precursor. These substrates make up a significant portion of the production costs of these copolymers, increasing their market value and limiting their applications. Economic analysis showed that in order to reduce the cost and increase the accessibility of P(3HB-co-4HB), glucose and γ-butyrolactone should be replaced by cheaper substrates and strains capable of metabolizing such substrates should be found [22].
For P(3HB-co-4HB) synthesis, glucose was replaced by the less expensive sucrose and acetate [23,24], gluconate [25], methane [26]. Renewable wastes of various origins have been also studied as potential substrates. These are glycerol (biodiesel production waste) [27], hydrolysates of renewable agricultural waste [28–30], xylose [31], hydrolysates of wheat straw containing a mixture of pentoses and hexoses [32]. Moreover, P(3HB-co-4HB) was synthesized from fat-containing substrates, including fatty acids [33], soybean oil [34], and crude palm oil [35].
Today, waste fat is being studied as a renewable carbon substrate for biotechnology. The amount of waste fat generated globally every year is about 29 million tons [36]. Waste fat includes fatty acids, low-grade vegetable oils and animal fats, and, more recently, fish processing waste. The prospects for microbial valorization of fat-based substrates for PHA production indicate considerable potential of this renewable source [34,37–39].
Waste fish oil (WFO) is a poorly studied carbon source for PHA synthesis. Renewable fish processing waste (or fish waste) is a by-product generated during the production of food from fish. According to the Technical Regulations of the Eurasian Economic Union TR EAEU 040/2016, “fish waste is the residue generated during the production of fish food products that is unsuitable for the production of fish food products”. This regulation came into force on 01.09.2017 and is a legislative requirement for all EAEU member countries. Fish waste, as a rule, has increased fat content, which varies considerably and depends not only on the fish species and age and time of catch, but also on the habitat and food supply, as well as on the source (heads, entrails, fins, etc.) [40].
There are rather few available studies addressing WFO for PHA synthesis. It was found that PHA synthesis is, in principle, possible from several fish processing wastes, including hydrolyzed pollock fat [41], fatty acids extracted from fish processing waste [42], waste fat from tuna canning production [43,44], wastewater from fish canning production [45], and fatty waste from Basa fish (Pangasius bocourti) (Vietnam) [46,47]. PHA synthesis from waste fish oil generated during the processing of sprat, mackerel, and pike-perch was recently described by our team [48].
The purpose of this work was to study the possibility of synthesizing P(3HB-co-4HB) copolymers using renewable WFO as a growth substrate and ε-caprolactone as a precursor of 4HB and examine their properties.
P(3HB-co-4HB) samples were produced at the Laboratory of Chemoautotrophic Biosynthesis at the Institute of Biophysics of the SB RAS (Siberian Branch of the Russian Academy of Sciences). The wild-type strain C. necator B-10646 capable of synthesizing copolymers of various monomer compositions was used as a producer using the author’s cultivation technology [49].
2.2.1 Cell Cultivation Technique
The bacterial cells were cultivated in aerobic batch mode under sterile conditions at a temperature of 30°C in 1.0 L flasks in the PHA synthesis mode with a reduced nitrogen concentration in the medium (Incubator Shaker Innova, New Brunswick Scientific, Edison, NJ, USA). Schlegel’s salt mineral medium was used [50]. It was based on phosphate buffer (Na2HPO4, 9.1 g/L; KH2PO4, 1.5 g/L) containing a source of magnesium (MgSO4, 0.2 g/L), iron (Fe3C6H5O7, 0.025 g/L), nitrogen source (NH4Cl, 0.7 g/L) and a set of microelements (3 mL solution/L of medium; solution composition (g/L): H3BO3 (0.228), CoCl2 × 6H2O (0.03), CuSO4 × 5H2O (0.008), MnCl2 × 4H2O (0.008), ZnSO4 × 7H2O (0.176), NaMoO4 × 2H2O (0.05), NiCl (0.008). WFO was used as the carbon substrate and its concentration in the nutrient medium was 15 g/L, as described in detail earlier [48]. ɛ-caprolactone (C6H10O2) (Geyer GmbH & Co., Renningen, Germany) was used as a 4HB precursor. To avoid its inhibitory effect, the precursor was added to the nutrient medium fractionally (a single addition varied from 0.5 to 2.0 g/L); the total concentration of the precursor was from 1.0 to 4.0 g/L. Butyric acid at a concentration of 2.0–4.0 g/L as the main carbon source was used as a control (C4H8O2) (Acros Organics, Geel, Belgium). In all experiments, the duration of the cultivation process was 48 h.
2.2.2 Cell Cultivation Technique
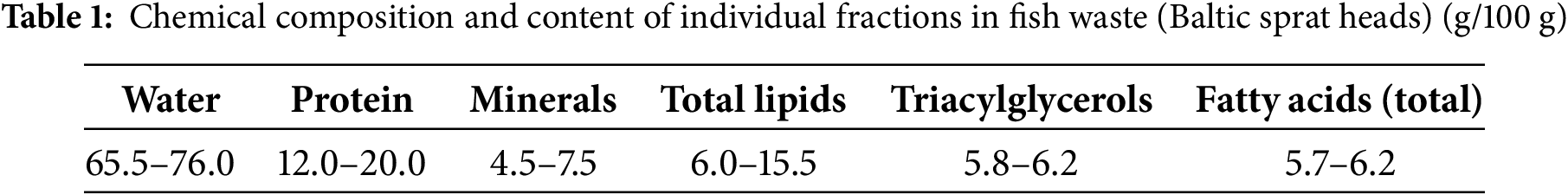
Waste fish oil from canning industry enterprises (the State Concern “Za Rodinu” and JSC “RosCon” Kaliningrad region, Russia) was used as a renewable carbon source. The fat was obtained by a thermal method from Baltic sprat (Sprattus balticus). The general chemical composition and content of individual fractions (and their possible fluctuations) of the used fish waste (Baltic sprat heads) according to data [51,52] are presented in Table 1.
The technological process for extracting fat from fish waste is schematically shown in Fig. 1. Fat was extracted from pre-crushed fish waste (smoked sprat heads), after which it was mixed with water in a 1:1 ratio, heated and kept at a temperature of 90°C with stirring for 15–20 min. The cooled mixture was centrifuged at 3000 g; the fat fraction of the supernatant was separated from the non-fat fraction by decantation [53]. The resulting fat fraction was analyzed and used in the nutrient medium. The total content of lipids, protein, carbohydrates in sprat oil was determined by conventional methods [54].
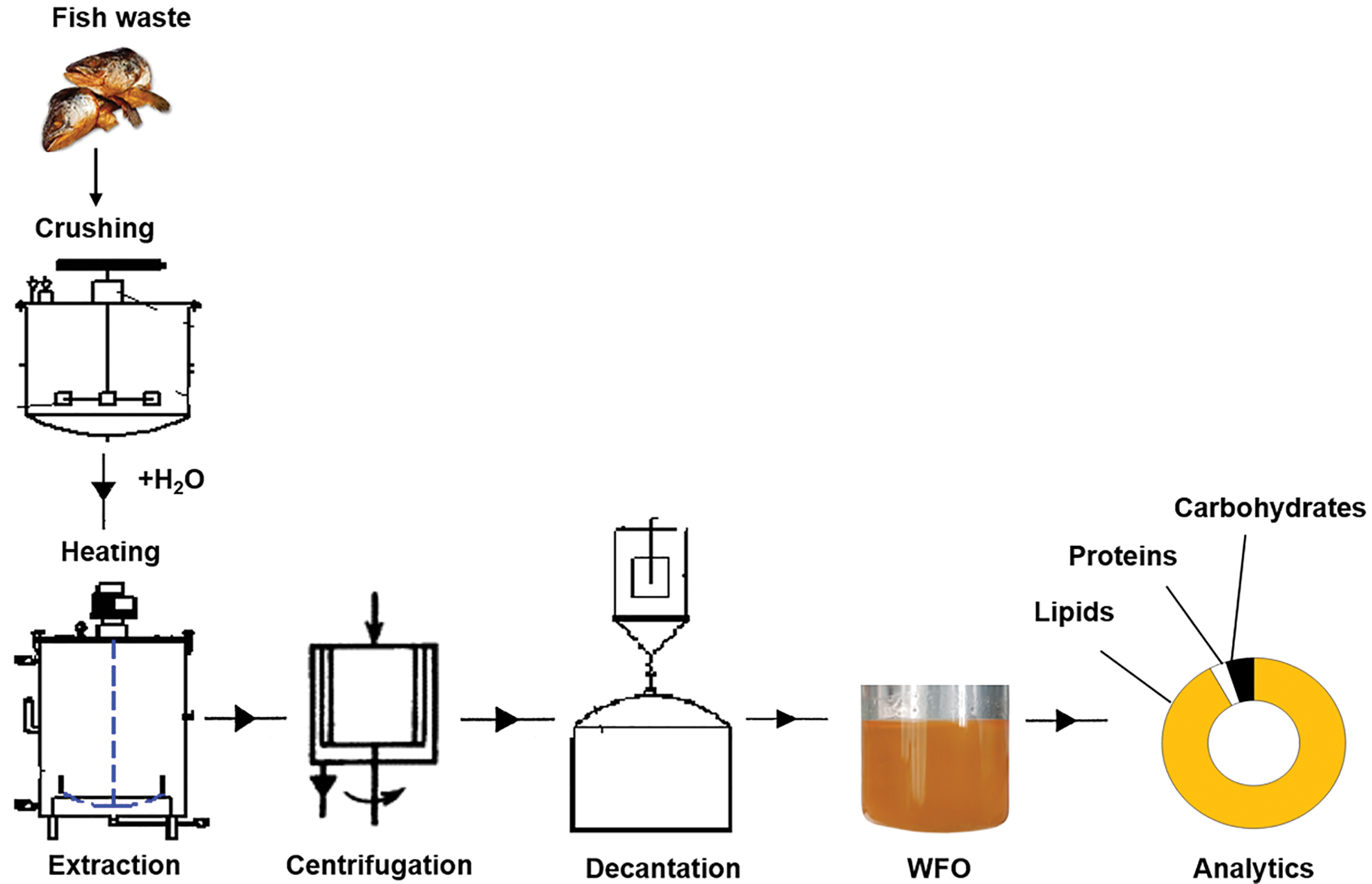
Figure 1: Scheme of stages of thermal method of fat extraction from fish processing waste (smoked sprat heads)
2.2.3 The Parameters of the Bacterial Growth Process
The total copolymer content in cell biomass and samples isolated from cell biomass and purified from cellular metabolites were determined by gas chromatography of the preliminarily derived methyl ester monomers (7890A/5975C Agilent Technologies, Santa Clara, CA, USA). The chromatography conditions were as follows: carrier gas flow (helium) 1 mL/min; starting temperature 120°C, increase to 230°C (5°C/min), isothermal mode for 5 min, increase to 310°C (10°C/min), isothermal mode for 3 min. To correctly determine the ratio of monomers and the content of 4HB in the copolymer, NMR spectroscopy was used. 1H NMR spectra of PHA were recorded at room temperature in CDCl3 on a Bruker Avance III 600 spectrometer (Germany) operating at 600.13 MHz.
Extraction and purification of polymer samples and methods for analyzing physicochemical properties have been described previously [49]. To study the molecular properties the size-exclusion chromatography was applied (Agilent Technologies 1260 Infinity, Waldbronn, Germany; Agilent PLgel Mixed-C column). Molecular-weight characteristics (the weight-average molecular weight (Mw), number-average molecular weight (Mn) and polydispersity (Ð)) were determined. The melting point was determined from the exothermic peaks in the thermograms (STARe software) using a DSC-1 differential scanning calorimeter (Mettler Toledo, Schwerzenback, Switzerland); the thermal degradation temperature was determined by the TGA2 (Mettler Toledo, Schwerzenback, Switzerland). The theoretical degree of crystallinity was calculated using the formula
2.2.4 An Optical Study of Spherulite Formation
P(3HB-co-4HB) samples were subjected to isothermal crystallization at different temperatures, and their morphology and radial growth rate were studied (polarizing optical microscope (Nikon, Eclipse E600 POL, Tokyo, Japan) equipped with a heating stage (Linkam LTS420, Redhill, UK)). The samples were first melted at 195°C for 3 min to destroy the thermal prehistory and then cooled to the desired (studied) crystallization temperature. The growth rate of the spherulites (G) was calculated by measuring the radius (R) as a function of crystallization time (t) and expressed in m/min. The observation was continued until the field of view was completely covered by spherulites.
All experiments were performed in five replicates (n = 5). Standard deviations and arithmetic means were calculated using the generally accepted methods embedded in the Microsoft Excel software package (Ver. 15.0.4420.1017). The Mann–Whitney test was used to compare groups (significance level p ≤ 0.05).
Growth media containing fat-containing substrates are heterophase systems of complex composition, in which fat forms an “oil-in-water” emulsion. Such emulsions are thermodynamically unstable complex systems, which can be separated into water and oil over time [56]. Emulsification of fat is necessary to ensure its availability to microbial cells: metabolism of this substrate depends on the degree of emulsification in the culture medium and the activity of lipolytic enzymes of the microorganisms. It is known that lipases secreted by microbial cells into the medium catalyze hydrolysis of triacylglycerols with the formation of free fatty acids and glycerol, resulting in an emulsion formation in the culture medium at the interface between the fat and water phases; and the original complex substrate becomes available to the cells, enters them and is metabolized [57]. Cupriavidus strains, similar to Bacillus sp. and Halomonas sp., have lipase activity and are capable of hydrolyzing fats of various origins with the formation of free fatty acids accessible to cells, in contrast, for example, to P. putida, for which the fat must first be hydrolyzed [39,58]. Free fatty acids released from fat are the actual growth substrate for microorganisms.
The WFO samples used as the growth carbon substrate contained 97.2 ± 2.9 of fat (total lipids), 0.40 ± 0.02 of carbohydrates, and 2.13 ± 0.15 of “crude” protein (total nitrogen × 6.25) (% of absolutely dry material, ADM). The fat contained 20 fatty acids with different C-chain lengths, from 14 to 24 carbon atoms. The dominant FAs were palmitic (28.0%), oleic (25.3%), docosahexaenoic (16.7%), and eicosapentaenoic (8.7%) FAs. The content of stearic, linoleic, linolenic, and myristic FAs was determined at the level of 2.5%–4.5%. The content of long-chain monounsaturated fatty acids (C20 and C24) does not exceed 1.1%–1.5%. Minor fatty acids (content less than 1%) are represented by saturated C15, C20 and C22, as well as monounsaturated C16:1 and C17:1 FA. The total amounts of saturated and unsaturated fatty acids are 38.5% and 61.5%, respectively.
3.1 Synthesis of P(3HB-co-4HB) Using Waste Fish Oil
The results of C. necator B-10646 cultivation and P(3HB-co-4HB) synthesis using WFO, a fat-containing substrate of complex composition, compared with the control (sole substrate–butyric acid) are presented in Fig. 2 and Table 2. The more affordable ɛ-caprolactone was used as a precursor of 4-hydroxybutyrate [59,60]. The precursor was introduced into the growing bacterial culture at 12 h from the start of the process, fractionally by means of two additions (at 12 and 24 h of growth) in a concentration that did not significantly inhibit bacterial growth (one dose contained 0.5–2.0 g/L of ε-caprolactone). The duration of the process was 48 h, which is significantly shorter than in the case of P(3HB) homopolymer synthesis (60–70 h). This is due to the fact that the highest rate of PHA synthesis in a batch culture of PHA-producer strains, including C. necator B-10646, and the activity of the PHA-synthase enzyme is characteristic of the exponential growth phase (the first 15–20 h from the beginning of the cultivation). It was shown that the inclusion of 4HB monomers into the polymer chain is usually observed 12–24 h after the precursor was added to the culture [61]. Further, as the culture ages and the activity of intracellular PHA depolymerases increases, endogenous breakdown of PHA molecules occurs. Thus, the content of polymer and monomers other than 3HB decreases against the background of an increase in the content of 3HB monomers. Therefore, in the case of the synthesis of copolymer PHAs, it is necessary to sacrifice the total PHA and the cell biomass yield, reducing the duration of the bacterial growth process.
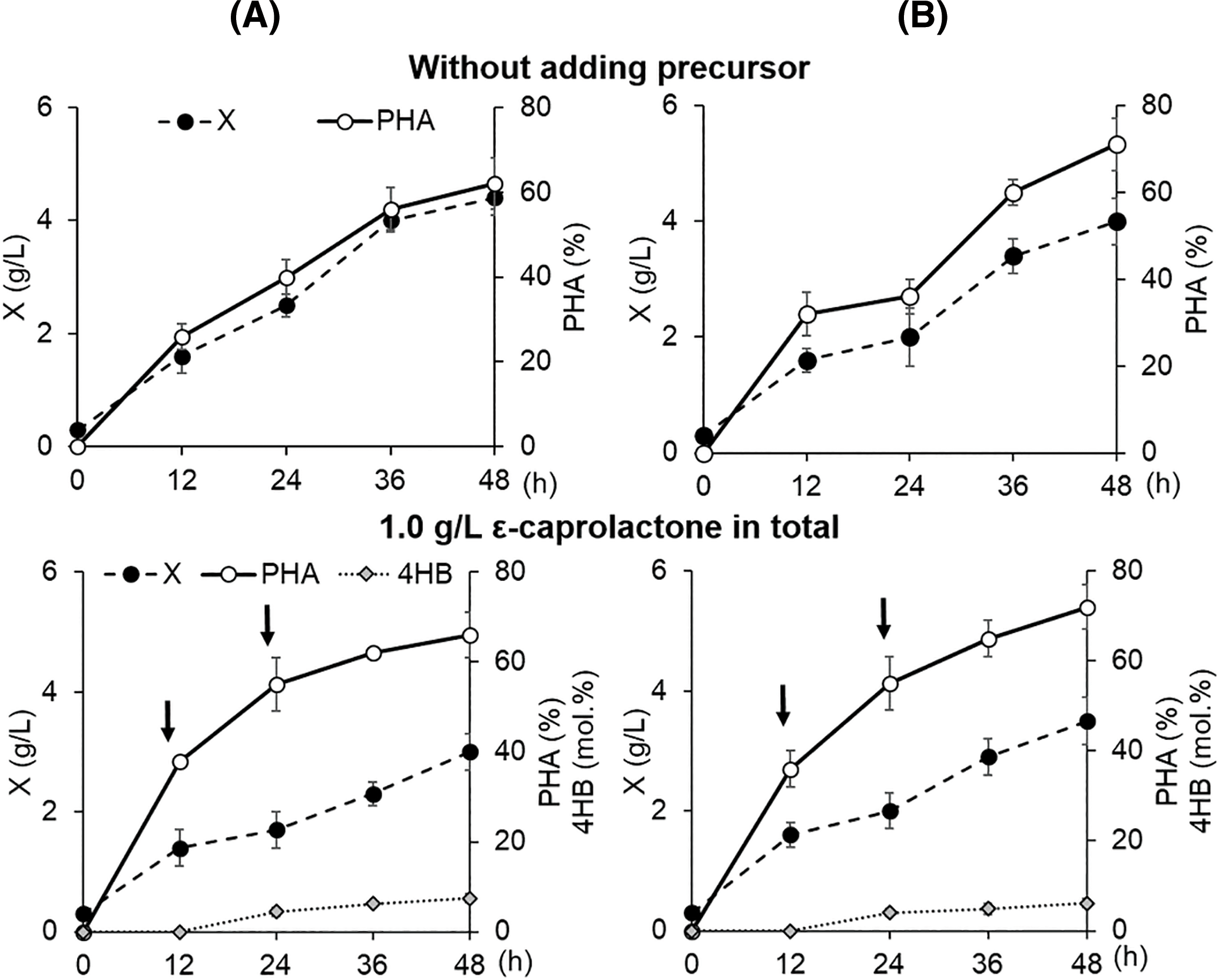
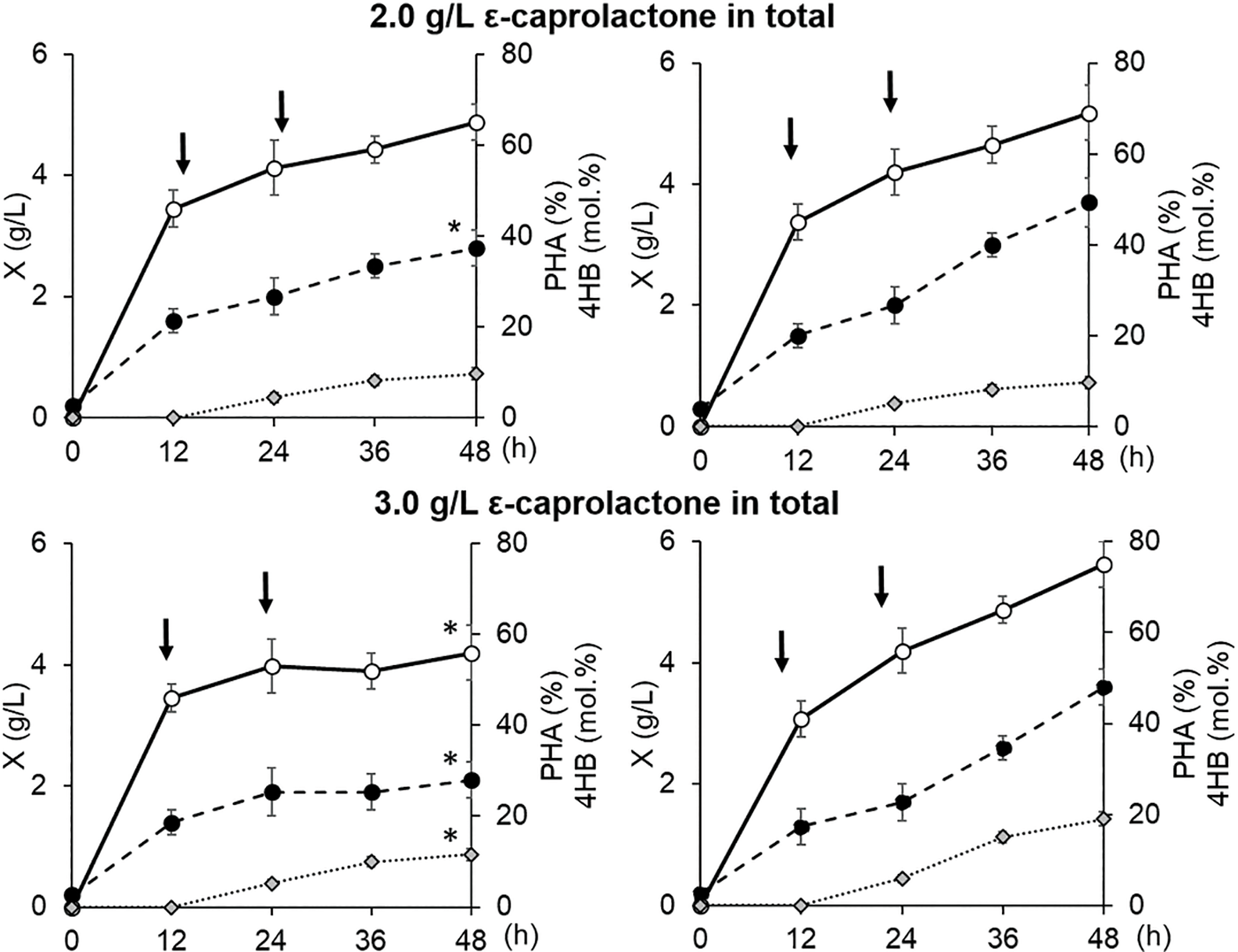
Figure 2: Parameters of the batch culture of Cupriavidus necator B-10646 grown on WFO as the main C-substrate (A) with the addition of ɛ-caprolactone relative to the control (butyric acid) (B): total cell biomass yield, X (g/L), intracellular content of P(3HB-co-4HB), % of CDW; content of 4HB monomers in the copolymer (mol.%). The symbol ≪*» indicates a statistically significant difference from the control when comparing groups using the Mann–Whitney test at a level of p < 0.05, n = 5. Arrows point at the addition of ɛ-caprolactone
The bacterial culture parameters in the control and in the experiment without the use of the precursor were comparable. The precursor dosing mode affected the bacterial biomass yield, P(3HB-co-4HB) yields, and the 3HB/4HB monomer ratio. At the same time, with the same precursor dosing mode in the bacterial culture, the incorporation of 4HB into the polymer chain of 3-hydroxybutyrate occurred differently. At a ε-caprolactone concentration of 1.0 g/L fed to the culture, the cell biomass yield, intracellular content of the copolymer, and 4HB monomers (6.1–7.4 mol.%) did not differ statistically. In the case of a higher precursor concentration (2.0 g/L) in the control (butyric acid) and in the treatment (WFO), the copolymer and 4HB monomer contents were comparable (increasing to 9.6–9.7 mol.%). However, the inhibitory effect of the precursor was evident. That negatively affected the biomass yield in the treatment, in contrast to the control. A further increase in the precursor concentration (up to 3.0 g/L) enhanced the inhibitory effect in the treatment. The value of X (g/L) and the yield of the copolymer decreased. The content of 4HB in the control (19.0 mol.%) statistically significantly exceeded this parameter in the treatment (11.6 mol.%). A further increase in the precursor concentration (up to 4.0 g/L) dramatically inhibited the growth of cells in the treatment. By contrast, in the control, the 4HB content and the total yield of the copolymer, as well as the yield of bacterial biomass, did not change. The use of several more variants of the dosing regimens of ε-caprolactone in the culture of C. necator B-10646 did not allow us to obtain copolymer samples in which the 4HB content would be higher than 11.6 mol.%.
Previously, in the culture of C. necator B-10646, using glucose as the main carbon substrate and γ-butyrolactone as a precursor (the number of additions varied from one to 5-6), samples of P(3HB-co-4HB) with a significantly higher content of 4HB, from 10 to 56 mol.% and 75 mol.%, as well as 3- and 4-component copolymers, respectively, P(3HB-co-4HB-co-3HV) and P(3HB-co-4HB-co-3HV-co-3HHx), in which the 4HB content varied from 9 to 55 mol.% (valeric acid or valeric + hexanoic acids were additionally used as precursors) were obtained [49]. In this paper, the authors did not discuss or take into account the production parameters of the strain in terms of the total bacterial biomass yield, since they mainly considered the composition of the copolymers and their 4HB content. At the same time, it was shown that the bacterial biomass yield with a large amount of precursor additions and high 4HB content was extremely low, about 0.30–0.50 g/L (unpublished data of the authors of the present article).
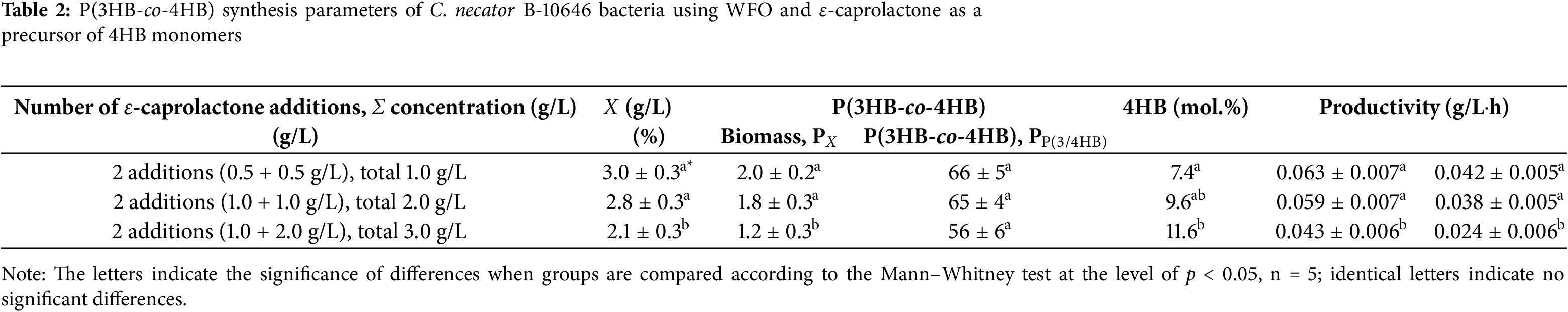
The production parameters of C. necator B-10646 culture grown on WFO with the addition of ε-caprolactone in different concentrations are given in Table 2.
Thus, the production parameters of the C. necator B-10646 bacterial culture in terms of bacterial biomass yield (X (g/L)), total copolymer yield (P(3HB-co-4HB) (g/L), as well as the process productivity in terms of biomass and copolymer (PX and PP(3/4HB)) during growth on WFO decreased with an increase in the precursor concentration to 3.0 g/L (Table 2). At the same time, in general, the possibility of synthesizing P(3HB-co-4HB) copolymers with the inclusion of 4HB monomers from 7.4 to 11.6 mol.% with satisfactory parameters in terms of the total bacterial biomass yield (X (g/L)) was shown, using WFO derived from accessible and renewable fish processing waste.
The review of the literature showed that the ability of wild-type and recombinant Cupriavidus strains to synthesize P(3HB-co-4HB) with a 4HB monomer content from units to 90–96 mol.% when they were grown on sugars and γ-butyrolactone as a precursor has been studied and discussed in many publications, including reviews [14,15,17]. However, data on the synthesis of P(3HB-co-4HB) on fat-containing substrates, mainly on vegetable oils and low-grade animal fats and their waste, are very limited. At the same time, the synthesis of P(3HB-co-4HB) copolymers on fatty waste from fish processing is reported in only one work. The bacterium Salinivibrio sp. M318 (VTCC910086) grown on fish processing waste and glycerol with the addition of 1,4-butanediol, γ-butyrolactone, or sodium 4-hydroxybutyrate synthesized copolymers P(3HB-co-4HB) with yields of bacterial biomass and copolymer, respectively, up to 9.8–10.2 g/L and 49.2%–54.6%, but the content of 4HB did not exceed 5.2–5.9 mol.% [62].
In the present work, P(3HB-co-4HB) productivity in the experiment with C. necator B-10646 grown on WFO in flasks was close to the values found for wild-type Cupriavidus strains, but was somewhat inferior to recombinant strains [27,63]. This is significantly inferior to the productivity parameters for the synthesis of the P(3HB) homopolymer (3.14 g/L·h) [5]. Thus, in general, the synthesis of PHA copolymers is less productive.
In synthesis of copolymers, the affinity of PHA synthases to certain monomers is of great importance for the production of PHA copolymers and the content of monomers of different structures [64]. The results of the P(3HB-co-4HB) synthesis in the Cupriavidus sp. culture demonstrate that the activity of PHA synthase is the “bottleneck” in increasing the 4HB content of copolymers [65]. However, this aspect of PHA biosynthesis still represents an insufficiently studied niche today. Therefore, the study of PHA synthases in new wild-type and engineered PHA producers, as well as in new strategies for conducting biosynthesis processes on complex C-substrates, is important for planning and organizing efficient processes for the production of technological PHAs [14,15,17]. The detected decrease in the incorporation of the studied precursors into 4HB in the case of P(3HB-co-4HB) synthesis by C. necator B-10646 cells during growth on WFO (a complex fatty substrate) compared to a sole substrate (butyric acid) may also be associated with the mechanism of precursor transport into cells. However, this complex issue requires additional and special studies.
3.2 Properties of P(3HB-co-4HB) Synthesized on Waste Fish Oil
PHA properties (temperature characteristics, molecular weight, degree of crystallinity) vary significantly depending on the composition and ratio of monomers; this makes it possible to obtain products with different physical and mechanical characteristics. The chemical composition of polyhydroxyalkanoates and the resulting properties are determined by the physiological and biochemical characteristics of the PHA producer strains and the substrate specificity of PHA synthases responsible for the assembly of monomers into PHAs, as well as the conditions of carbon nutrition and the biochemical pathways by which the C-substrate is metabolized [66].
A few publications show that the polymer usually synthesized from fat-containing substrates, including WFO, is the poly(3-hydroxybutyrate) homopolymer, although isolated cases of copolymer formation have been reported. For example, Salinivibrio sp. M318 (VTCC910086) grown on WFO and glycerol synthesized only poly(3-hydroxybutyrate), but it synthesized poly(3-hydroxybutyrate-co-3-hydroxyvalerate) [P(3HB-co-3HV)] copolymers when propionate was added and P(3HB-co-4HB) copolymers when 1,4-butanediol, γ-butyrolactone, or sodium 4-hydroxybutyrate was added [62]. Some Pseudomonas strains synthesized polymers containing, in addition to 3HB, medium-chain monomers, with 2–3 mol.% of 3-hydroxyhexanoate, and 3-hydroxyoctanoate and 3-hydroxyhexanoate dominated when they were grown on the fatty waste of the Alaska pollock fish [41]. Synthesis of P(3HB-co-3HV) copolymers was detected in C. necator TISTR 1095 cells grown on the wastewater of a tuna cannery [44]. When WFO was used as a carbon substrate for the cultivation of the C. necator B-10646 strain studied in this work, it mainly synthesized P(3HB), but in some cases it synthesized a three-component polymer with a dominant fraction of 3HB monomers and a minor content of 3-hydroxyvalerate and 3-hydroxyhexanoate monomers [48].
The use of WFO or butyric acid (control) as the main growth substrate and ε-caprolactone as the precursor made it possible to synthesize P(3HB-co-4HB) copolymers with different 4HB contents; their chemical composition and properties are illustrated in Table 3 and Fig. 3–7.
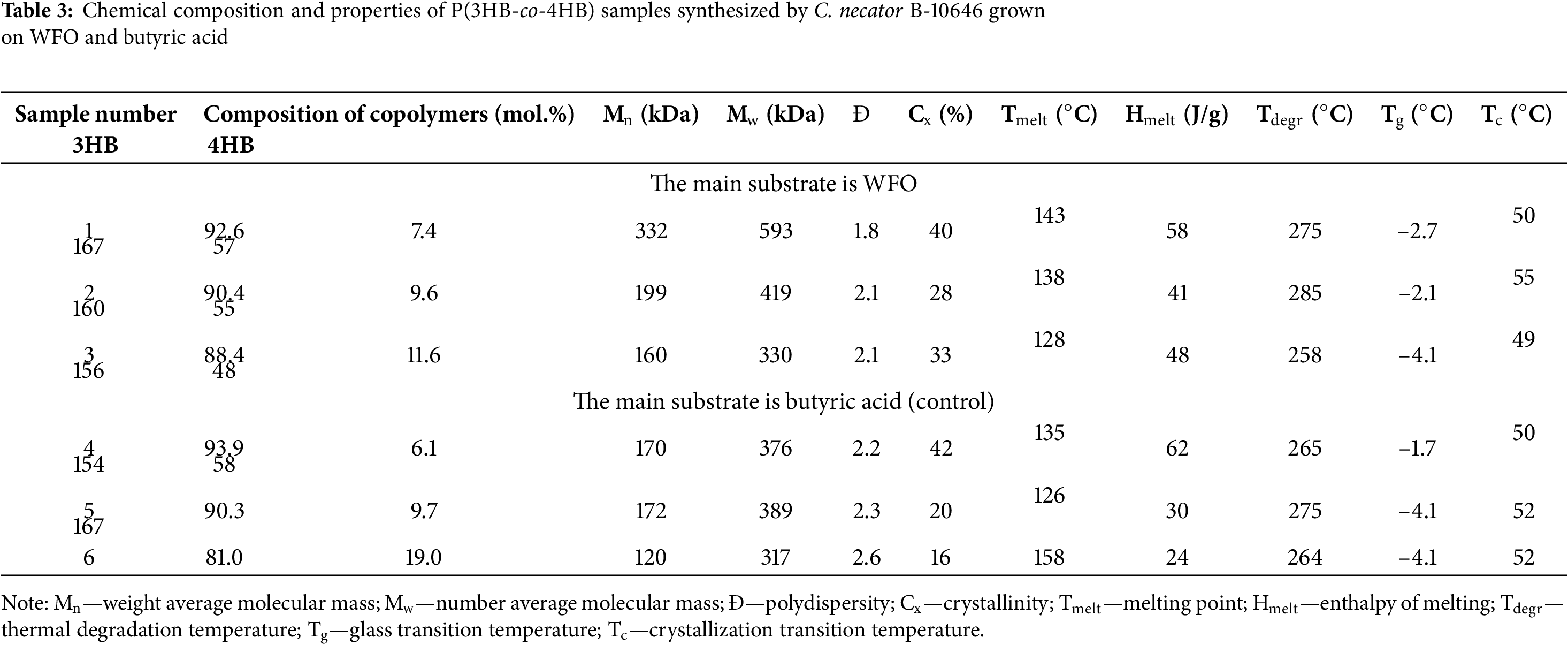
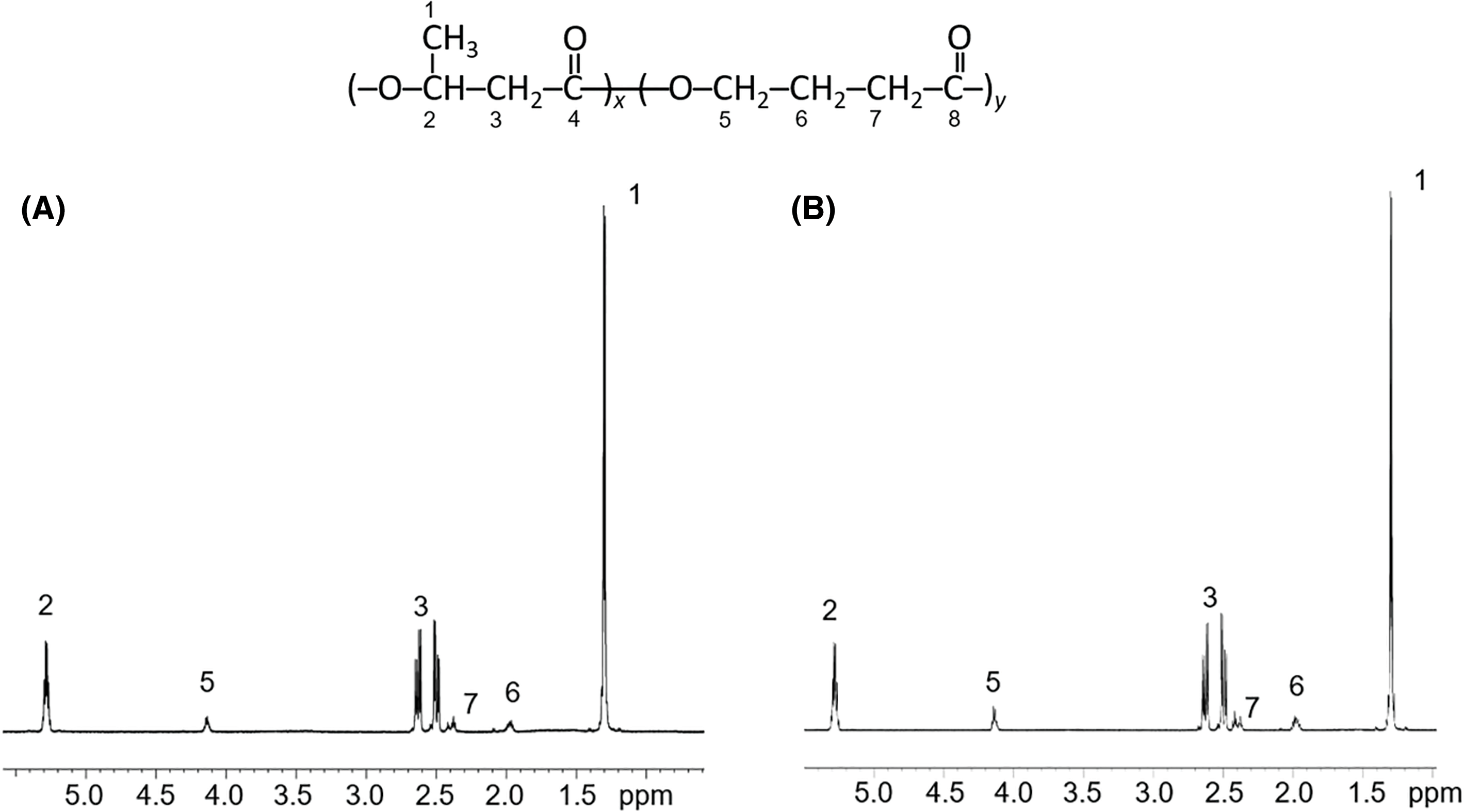
Figure 3: 1H NMR spectra of copolymers synthesized by C. necator B-10646 grown on WFO (A) and butyric acid (B)
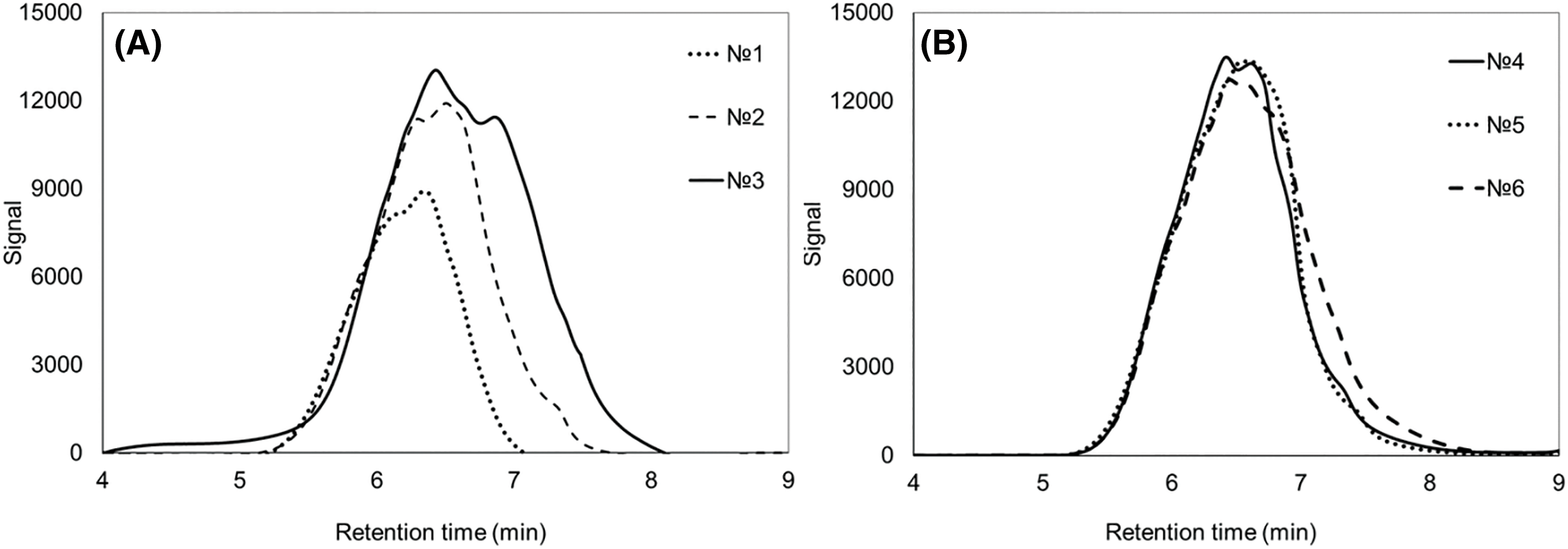
Figure 4: Chromatograms of molecular weight distribution of P(3HB-co-4HB) samples synthesized on WFO (A) and butyric acid (control) (B). Sample numbering corresponds to Table 3
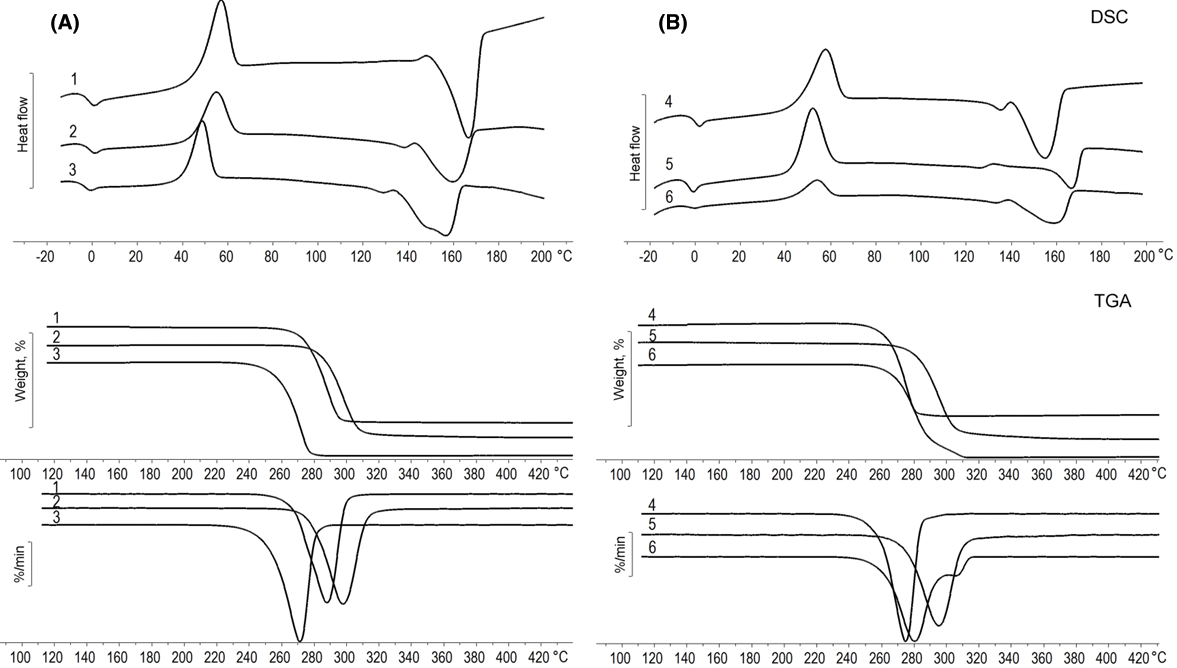
Figure 5: Thermal characteristics (DSC, TGA) of P(3HB-co-4HB) samples synthesized on WFO (A) and butyric acid (control) (B). Sample numbering according to Table 3
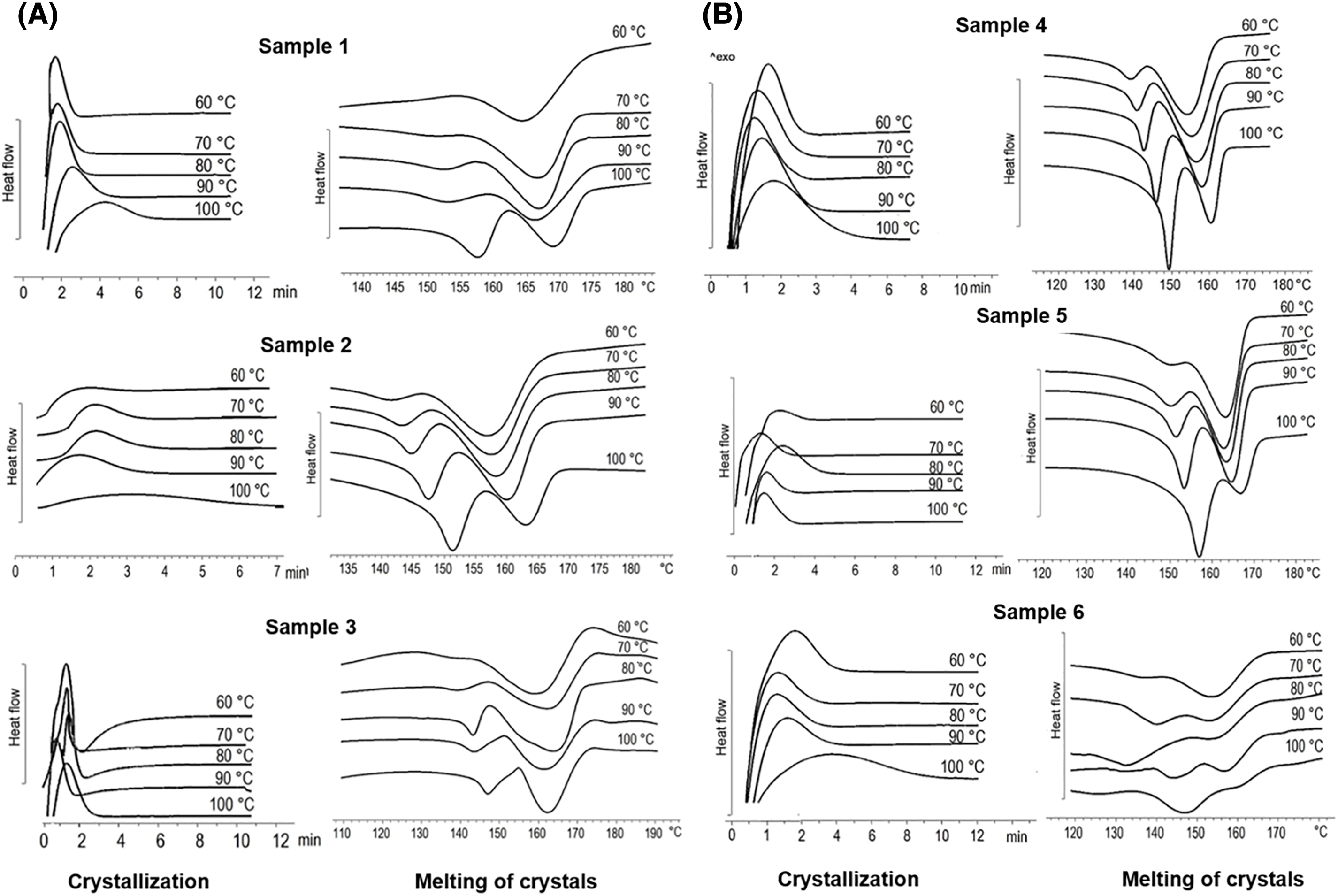
Figure 6: Isothermal crystallization of P(3HB-co-4HB) samples with different 4HB monomer contents synthesized on WFO (A) and butyric acid (control) (B). Sample numbering according to Table 3
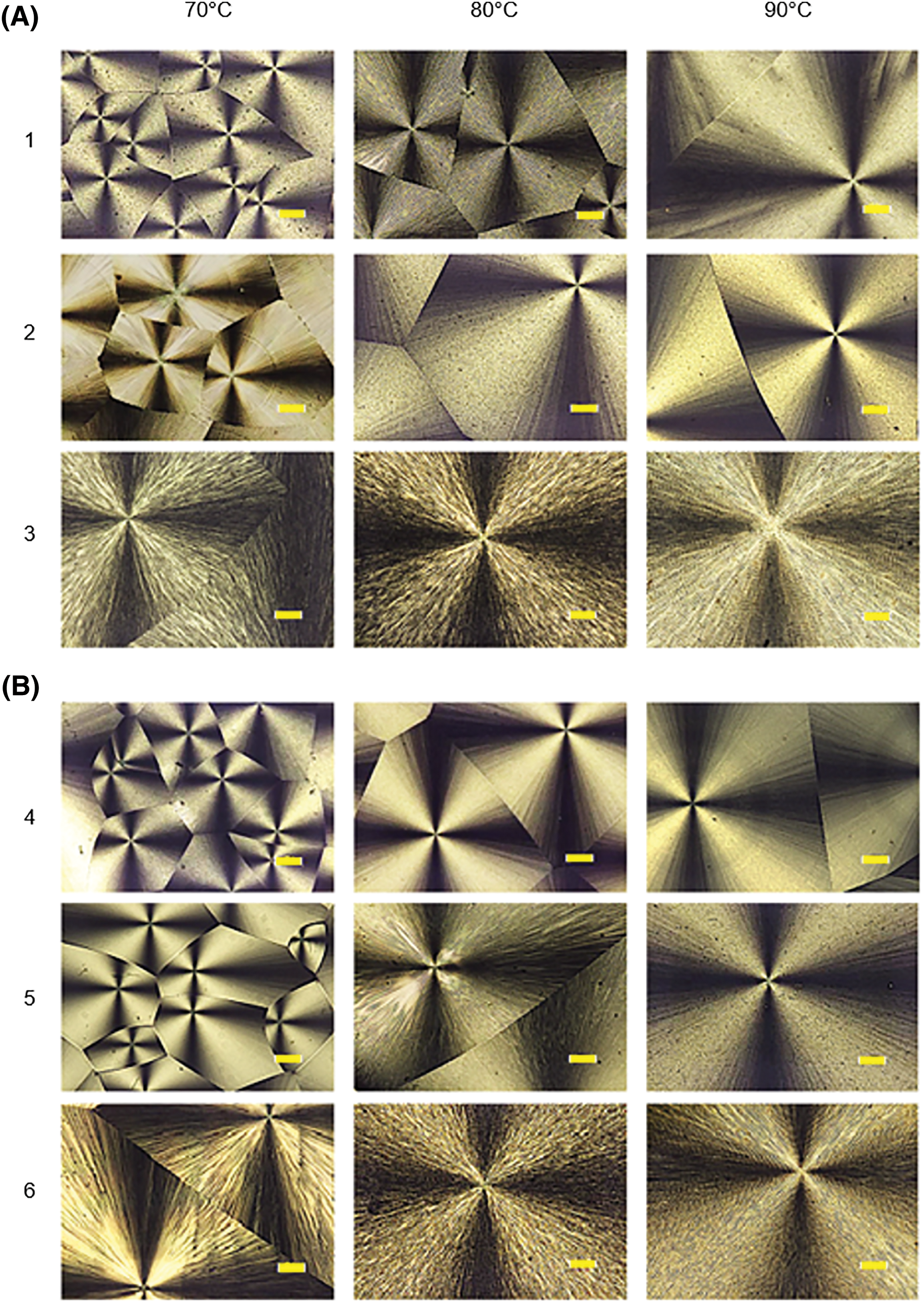
Figure 7: Morphology of spherulites of P(3HB-co-4HB) samples with different 4HB monomer contents, synthesized on WFO (A) and on butyric acid (control) (B), formed during isothermal crystallization of copolymer melts at different temperatures. Bar = 100 μm (sample numbering according to Table 3)
The inclusion of 4HB in the structure of P(3HB-co-4HB) was confirmed by NMR. Fig. 3 shows, as an example, the 1H NMR spectra of P(3HB-co-4HB) samples with similar contents of 4HB (9.6 and 9.7 mol.%), synthesized on WFO and on butyric acid. Both spectra are equivalent and do not depend on the type of substrate used.
For a comparative study of the physicochemical properties of P(3HB-co-4HB) copolymers synthesized on WFO or butyric acid, samples with similar 4HB contents were selected (Table 3).
Analysis of the molecular weight characteristics of the copolymers produced in this study (Table 3, Fig. 4) showed that in the treatment and in the control, as the 4HB content increased, there was a decrease in the molecular weight values. This is typical for the PHA copolymers in contrast to P(3HB). When WFO was used, a decrease in Mn from 332 to 160 kDa (Mw from 593 to 330 kDa) was noted with an insignificant increase in polydispersity (Ð). A similar pattern is typical for the control. At the same time, in general, the molecular weight characteristics of the P(3HB-co-4HB) samples synthesized on WFO are 1.5–2.0 times higher than those of P(3HB-co-4HB) synthesized on butyric acid with a similar content of 4HB.
PHA molecular weight is a highly variable parameter, significantly depending on the cultivation mode and duration, the polymer extraction procedure and other factors; therefore, the data on the molecular weight may differ significantly [67]. The published data on the molecular weight characteristics of PHA containing 4HB synthesized from fat-containing substrates as the main carbon source vary widely [61,68].
On the thermograms double wide melting peaks were recorded for all samples (Fig. 5) and melting points lower compared to P(3HB). In the experimental group, an increase in the 4HB content leads to a decrease in the melting point from 167°C to 156°C and a decrease in the enthalpy of melting (Table 3). A decrease in the enthalpy of melting indicates a decrease in the degree of crystallinity and amorphization of the samples with an increase in the 4HB monomer content. In the control group, the sample with a 4HB content of 9.7 mol.% had the highest melting point, 167°C. A decrease in the glass transition temperature is also noted in both groups with an increase in the 4HB content. The lowest glass transition temperature of –4.1°C was recorded for the sample with a 4HB content of 11.6 mol.% in the treatment group and for the samples containing 9.7 and 19 mol.% (control group). All samples demonstrated good thermal stability; thermal degradation of the samples began when they were heated above 250°C, and the maximum rate of weight loss was reached in the temperature range of 274°C–302°C. In both groups, the best thermal stability was noted for samples with 4HB monomer contents of 9.6 and 9.7 mol.%; the thermal degradation temperature was 285°C and 275°C with a maximum weight loss at 302°C and 298°C, respectively.
Jo et al. points to a decrease in the degree of crystallinity with an increase in the 4HB content, as well as the appearance of cold crystallization peaks, which the authors associate with an increase in chain randomness [69]. Double melting peaks and lower melting point (158°C) than for P(3HB) (170°C) and crystallinity degree of 40% were observed for the copolymer with 4HB content of 19 mol.%, which was synthesized by Comamonas acidovorans on mixed carbon sources (n-butyric acid and 1,4-butanediol) [70].
Crystallization kinetics plays a central role in determining the mechanical properties of polymer products. This indicates the importance of studying this process in PHA. Fig. 6A,B illustrates the crystallization and melting of the obtained P(3HB-co-4HB) crystals with different 4HB contents, under isothermal conditions at different temperatures (60°C, 70°C, 80°C, 90°C, 100°C). For all samples, the crystallization enthalpy rises with increasing crystallization temperature, which may be due to the emergence of nucleation difficulties at higher temperatures. In the melting thermograms of crystals obtained by crystallization at 60°C, the melting peaks have a small shoulder on the side of low temperatures. With increasing crystallization temperature, this shoulder develops into a separate peak, the area of which increases. Thus, all thermograms of crystallization at high temperatures have two peaks: one in the region of low temperatures (138°C–157°C) and the other in the region of higher temperatures (154°C–169°C). The position of the first peak is not constant, and with an increase in the crystallization temperature it shifts towards higher temperatures; the location of the second melting peak on the thermogram does not differ significantly. In many studies, double melting peaks for copolymers of this type are associated with the formation of two phases: a phase enriched in molecules containing 4HB and forming defective crystals and a phase enriched in 3HB, in which more perfect crystals with a higher melting point are formed [71].
This behavior during isothermal crystallization is typical for all PHA copolymers. Increasing the content of monomers other than 3HB disrupts the regularity of the P(3HB) chain, which hinders crystallization, but can also promote crystallization due to the higher flexibility of some monomers compared to 3HB at the corresponding isothermal temperatures. The presence of other monomers in the P(3HB) chain reduces the rate of crystallization; however, an increase in the flexibility of the copolymer chain above a certain content of other monomers can lead to an increase in the rate of crystallization and the formation of defective crystals [67,71–73].
Spherulites are the most common crystalline form of PHAs, formed upon rapid cooling of melts or upon precipitation from a concentrated polymer solution [74]. Typically, PHAs are characterized by the formation of extremely large spherulites under certain crystallization conditions [75]. In semicrystalline PHAs, crystallization occurs near the equilibrium state, at which crystalline lamellae develop into spherulites [76]. It was found that PHAs can form non-striped and striped spherulites by isothermal crystallization from a thin melt at both low and higher crystallization temperatures [77]. The present study shows that the change in the ratio of crystallization and diffusion rates is the reason for the change in spherulite morphology. The shape and size of spherulites in PHA are influenced by many factors, with the key factor being the temperature at which crystallization occurs. The periodicity and regularity of the spherulite band structure can change depending on the crystallization conditions, as well as the molecular weight of the polymer [78].
The morphology of spherulites of P(3HB-co-4HB) samples with different 4HB contents synthesized by C. necator B-10646 cells grown on WFO and butyric acid (control) is shown in Fig. 7.
A typical pattern–“Maltese cross” and concentric bands—was recorded for all the studied P(3HB-co-4HB) samples during crystallization, regardless of the type of the main carbon substrate used and the monomer ratio in the entire studied crystallization temperature range of the copolymer melts (70°C, 80°C and 90°C). The dependences of the change in the spherulite sizes of the studied P(3HB-co-4HB) samples on the crystallization temperature at all values of the content of the molar fraction of 4HB, as well as in the treatment on WFO and in the control (butyric acid), were comparable. Larger spherulites were formed at higher crystallization temperatures and a higher 4HB content of the P(3HB-co-4HB) samples. Thus, at a temperature of 70°C, the size of spherulites varied from 200–300 µm to 1–2 mm, depending on the 4HB content. At a temperature of 90°C, the range of changes in the size of spherulites was significantly wider, ranging between 1.5 and 3.0 mm or more. This is presumably associated with the observed lower nucleation density at a higher crystallization temperature and is consistent with the results obtained by Hobbs et al. [78].
The size of the spherulites increased linearly throughout the process and regardless of the crystallization temperature until the moment of collision. This indicates a constant spherulite growth rate for P(3HB-co-4HB) throughout the crystallization process. The nature of the temperature dependence of the growth rate of spherulites of the P(3HB-co-4HB) samples was practically independent of the 4HB content in the copolymer. However, minor differences were revealed in the maximum value of the spherulite formation rate at similar 4HB contents for samples synthesized on WFO and on butyric acid (control). The maximum values of the rate were recorded at a temperature close to 85°C. For example, for samples with similar 4HB monomer contents (9.6 and 9.7 mol.%), the values were 1.32 and 0.95 μm/min for the samples produced on butyric acid and WFO, respectively. Thus, the type of the main carbon substrate did not have a significant effect on this parameter.
Regardless of the type of the main substrate, the maximum value of the spherulite growth rate was proportional to the 4HB content. A similar nature of spherulite formation was found for both the highly crystalline P(3HB) homopolymer and the PHA copolymers containing 3-hydroxyvalerate (P(3HB-co-3HV)) [73] and 3-hydroxyhexanoate-P(3HB-co-3HHx) [72]. In that case, the maximum spherulite growth rate for P(3HB-co-4HB) significantly differed from this parameter (more than 3 μm/min) for P(3HB). A similar dependence was also described by You [79].
This fact allows us to state that for the P(3HB-co-4HB) copolymers, the effect of 4HB on the crystallization behavior is complex and is determined by the competition between two factors. On the one hand, the crystalline structure of the P(3HB) fraction is disrupted by the inclusion of 4HB in its chain. This leads to a decrease in the crystallization ability of P(3HB). On the other hand, the introduction of 4HB into the 3HB monomer chain can improve the mobility of macromolecular chains. This was also shown by Wen et al. [80]. However, this effect, favorable for crystallization, apparently does not depend on the type of C-substrate used.
In general, the obtained results are consistent with the few studies performed by other authors on the crystallization and formation of spherulites during isothermal crystallization of melts of P(3HB-co-4HB) samples synthesized by other strains on glucose as the main substrate [80].
The present study investigated synthesis of the P(3HB-co-4HB) copolymer, which is one of the most promising bioplastics among the degradable microbial PHAs, using a renewable growth substrate waste fish oil. The aim of the study and the results obtained correspond to the current trend of the wider use of biopolymers that degrade in the environment to form safe substances, and contribute to the modern concept of “circular economy”. P(3HB-co-4HB) copolymers with 4HB ranging between 7.4 and 11.6 mol.% were synthesized for the first time by Cupriavidus necator B-10646 using renewable waste fish oil (WFO) generated in Baltic sprat processing as a growth substrate and ɛ-caprolactone as a 4HB precursor. The physicochemical properties of the copolymers synthesized in the current study were comparable with those of the P(3HB-co-4HB) copolymers produced from a sole substrate (butyric acid), including a reduced degree of crystallinity (up to 16%). The use of renewable WFO and ɛ-caprolactone reduces the costs of producing these promising bioplastics and increases their accessibility without deteriorating their physicochemical properties.
Acknowledgement: The authors would like to express their special thanks to the Krasnoyarsk Regional Center for Collective Use, “Krasnoyarsk Science Center SB RAS” Federal Research Center SB RAS for providing equipment.
Funding Statement: This research was funded by the Russian Science Foundation, grant number 23-64-10007.
Author Contributions: Conceptualization, results analysis: Tatiana Volova; organization of biosynthesis processes: Natalia Zhila; synthesis on WFO: Kristina Sapozhnikova; synthesis on butyric acid: Olga Menshikova; DTA-DSC investigation: Evgeniy Kiselev; study of spherulites: Alexey Sukovatyi; WFO production: Vladimir Volkov; NMR investigation: Ivan Peterson; films production: Natalia Ipatova; discussion of results, writing: Ekaterina Shishatskaya. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: All data are available in the paper.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Saratale RG, Cho SK, Saratale GD, Kadam AA, Ghodake GS, Kumar M, et al. A comprehensive overview and recent advances on polyhydroxyalkanoates (PHA) production using various organic waste streams. Biores Technol. 2021;325(8):124685. doi:10.1016/j.biortech.2021.124685. [Google Scholar] [PubMed] [CrossRef]
2. Meereboer KW, Misra M, Mohanty AK. Review of recent advances in the biodegradability of polyhydroxyalkanoate (PHA) bioplastics and their composites. Green Chem. 2020;22(17):5519–58. doi:10.1039/D0GC01647K. [Google Scholar] [CrossRef]
3. Park H, He H, Yan X, Liu X, Scrutton NS, Chen G-Q. PHA is not just a bioplastic!. Biotechnol Adv. 2024;71(5):108320. doi:10.1016/j.biotechadv.2024.108320. [Google Scholar] [PubMed] [CrossRef]
4. Mitra R, Xu T, Chen G, Xiang H, Han J. An updated overview on the regulatory circuits of polyhydroxyalkanoates synthesis. Microb Biotechnol. 2022;15(5):1446–70. doi:10.1111/1751-7915.13915. [Google Scholar] [PubMed] [CrossRef]
5. Tan D, Wang Y, Tong Y, Chen G-Q. Grand challenges for industrializing polyhydroxyalkanoates (PHAs). Trends Biotechnol. 2021;39(9):953–63. doi:10.1016/j.tibtech.2020.11.010. [Google Scholar] [PubMed] [CrossRef]
6. Koller M, Mukherjee A. A new wave of industrialization of PHA biopolyesters. Bioengineering. 2022;9(2):74. doi:10.3390/bioengineering9020074. [Google Scholar] [PubMed] [CrossRef]
7. Majerczak K, Wadkin‐Snaith D, Magueijo V, Mulheran P, Liggat J, Johnston K. Polyhydroxybutyrate: a review of experimental and simulation studies of the effect of fillers on crystallinity and mechanical properties. Polym Int. 2022;71(12):1398–408. doi:10.1002/pi.6402. [Google Scholar] [CrossRef]
8. Zhang Z, Quinn EC, Olmedo-Martinez JL, Caputo MR, Franklin KA, Muller AJ, et al. Toughening brittle bio-P3HB with synthetic P3HB of engineered stereomicrostructures. Angew Chem Int Edit. 2023;62(49):157. doi:10.1002/anie.202311264. [Google Scholar] [PubMed] [CrossRef]
9. McAdam B, Fournet MB, McDonald P, Mojicevic M. Production of polyhydroxybutyrate (PHB) and factors impacting its chemical and mechanical characteristics. Polymers. 2020;12(12):2908. doi:10.3390/polym12122908. [Google Scholar] [PubMed] [CrossRef]
10. Eesaee M, Ghassemi P, Nguyen DD, Thomas S, Elkoun S, Nguyen-Tri P. Morphology and crystallization behaviour of polyhydroxyalkanoates-based blends and composites: a review. Biochem Eng J. 2022;187(4):108588. doi:10.1016/j.bej.2022.108588. [Google Scholar] [CrossRef]
11. Costa P, Basaglia M, Casella S, Favaro L. Copolymers as a turning point for large scale polyhydroxyalkanoates applications. Int J Biol Macromol. 2024;275:133575. doi:10.1016/j.ijbiomac.2024.133575. [Google Scholar] [PubMed] [CrossRef]
12. García A, Aguirre C, Pérez A, Bahamonde SS, Urtuvia V, Díaz-Barrera A, et al. Recent trends in the production and recovery of bioplastics using polyhydroxyalkanoates copolymers. Microorganisms. 2024;12(11):2135. doi:10.3390/microorganisms12112135. [Google Scholar] [PubMed] [CrossRef]
13. Vicente D, Proença DN, Morais PV. The role of bacterial polyhydroalkanoate (PHA) in a sustainable future: a review on the biological diversity. Int J Environ Res Public Health. 2023;20(4):2959. doi:10.3390/ijerph20042959. [Google Scholar] [PubMed] [CrossRef]
14. Utsunomia C, Ren Q, Zinn M. Poly(4-hydroxybutyratecurrent state and perspectives. Front Bioeng Biotechnol. 2020;8:3624. doi:10.3389/fbioe.2020.00257. [Google Scholar] [PubMed] [CrossRef]
15. Huong K-H, Sevakumaran V, Amirul AA. P(3HB-co-4HB) as high value polyhydroxyalkanoate: its development over recent decades and current advances. Crit Rev Biotechnol. 2021;41(4):474–90. doi:10.1080/07388551.2020.1869685. [Google Scholar] [PubMed] [CrossRef]
16. Kawamura Y, Gan H, Kabe T, Maehara A, Kimura S, Hikima T, et al. Mechanism of elastic properties of biodegradable Poly[(R)-3-hydroxybutyrate-co-4-hydroxybutyrate] films revealed by synchrotron radiation. ACS Omega. 2021;6(11):7387–93. doi:10.1021/acsomega.0c05662. [Google Scholar] [PubMed] [CrossRef]
17. de Macedo MA, Oliveira-Filho ER, Taciro MK, Piccoli RAM, Gomez JGC, Silva LF. Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) [P(3HB-co-4HB)] biotechnological production: challenges and opportunities. Biomass Convers Biorefin. 2022;14(21):26631–50. doi:10.1007/s13399-022-03500-2. [Google Scholar] [CrossRef]
18. Doi Y, Kanesawa Y, Kunioka M, Saito T. Biodegradation of microbial copolyesters: poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and poly(3-hydroxybutyrate-co-4-hydroxybutyrate). Macromolecules. 1990;23(1):26–31. doi:10.1021/ma00203a006. [Google Scholar] [CrossRef]
19. Doi Y, Kunioka M, Nakamura Y, Soga K. Nuclear magnetic resonance studies on unusual bacterial copolyesters of 3-hydroxybutyrate and 4-hydroxybutyrate. Macromolecules. 1988;21(9):2722–7. doi:10.1021/ma00187a012. [Google Scholar] [CrossRef]
20. Lee W-H, Azizan MNM, Sudesh K. Effects of culture conditions on the composition of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) synthesized by Comamonas acidovorans. Polym Degrad Stab. 2004;84(1):129–34. doi:10.1016/j.polymdegradstab.2003.10.003. [Google Scholar] [CrossRef]
21. Hein S, Söhling B, Gottschalk G, Steinbüchel A. Methods for the biosynthesis of polyesters. Alexandria, VA, USA: International Publication; 1998. [Google Scholar]
22. Choi J, Lee SY. Process analysis and economic evaluation for Poly(3-hydroxybutyrate) production by fermentation. Bioprocess Engineer. 1997;17(6):335. doi:10.1007/s004490050394. [Google Scholar] [CrossRef]
23. Miranda De Sousa Dias M, Koller M, Puppi D, Morelli A, Chiellini F, Braunegg G. Fed-batch synthesis of poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-4-hydroxybutyrate) from sucrose and 4-hydroxybutyrate precursors by Burkholderia sacchari strain DSM 17165. Bioengineering. 2017;4(2):36. doi:10.3390/bioengineering4020036. [Google Scholar] [PubMed] [CrossRef]
24. Renner G, Pongratz K, Braunegg G. Production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) by Comamonas testosteronii A3. Food Technol Biotechnol. 1996;34(2–3):91–5. [Google Scholar]
25. Valappil SP, Peiris D, Langley GJ, Herniman JM, Boccaccini AR, Bucke C, et al. Polyhydroxyalkanoate (PHA) biosynthesis from structurally unrelated carbon sources by a newly characterized Bacillus spp. J Biotechnol. 2007;127(3):475–87. doi:10.1016/j.jbiotec.2006.07.015. [Google Scholar] [PubMed] [CrossRef]
26. Nguyen TT, Lee EY. Methane-based biosynthesis of 4-hydroxybutyrate and P(3-hydroxybutyrate-co-4-hydroxybutyrate) using engineered Methylosinus trichosporium OB3b. Bioresour Technol. 2021;335:125263. doi:10.1016/j.biortech.2021.125263. [Google Scholar] [PubMed] [CrossRef]
27. Cavalheiro JMBT, Raposo RS, de Almeida MCMD, Teresa Cesário M, Sevrin C, Grandfils C, et al. Effect of cultivation parameters on the production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) and poly(3-hydroxybutyrate-4-hydroxybutyrate-3-hydroxyvalerate) by Cupriavidus necator using waste glycerol. Bioresour Technol. 2012;111:391–7. doi:10.1016/j.biortech.2012.01.176. [Google Scholar] [PubMed] [CrossRef]
28. Oliveira-Filho ER, Silva JGP, de Macedo MA, Taciro MK, Gomez JGC, Silva LF. Investigating nutrient limitation role on improvement of growth and poly(3-hydroxybutyrate) accumulation by Burkholderia sacchari LMG 19450 from xylose as the sole carbon source. Front Bioeng Biotechnol. 2020;7:633. doi:10.3389/fbioe.2019.00416. [Google Scholar] [PubMed] [CrossRef]
29. da Silva LF, Oliveira-Filho ER, Moniz Piccoli RA, Taciro MK, Cabrera Gomez JG. PHA biosynthesis starting from sucrose and materials from the sugar industry. In: The handbook of polyhydroxyalkanoates. CRC Press; 2020. p. 417–54. doi:10.1201/9780429296635-17. [Google Scholar] [CrossRef]
30. Dietrich K, Oliveira-Filho ER, Dumont M-J, Gomez JGC, Taciro MK, da Silva LF, et al. Increasing PHB production with an industrially scalable hardwood hydrolysate as a carbon source. Ind Crops Prod. 2020;154:112703. doi:10.1016/j.indcrop.2020.112703. [Google Scholar] [CrossRef]
31. Guamán LP, Barba-Ostria C, Zhang F, Oliveira-Filho ER, Gomez JGC, Silva LF. Engineering xylose metabolism for production of polyhydroxybutyrate in the non-model bacterium Burkholderia sacchari. Microb Cell Fact. 2018;17(1):74. doi:10.1186/s12934-018-0924-9. [Google Scholar] [PubMed] [CrossRef]
32. Cesário MT, Raposo RS, de Almeida MCMD, van Keulen F, Ferreira BS, Telo JP, et al. Production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) by Burkholderia sacchari using wheat straw hydrolysates and gamma-butyrolactone. Int J Biol Macromol. 2014;71:59–67. doi:10.1016/j.ijbiomac.2014.04.054. [Google Scholar] [PubMed] [CrossRef]
33. Huong K-H, Mohd Yahya AR, Amirul AA. Pronounced synergistic influence of mixed substrate cultivation on single step copolymer P(3HB-co-4HB) biosynthesis with a wide range of 4HB monomer composition. J Chem Tech Biotechnol. 2014;89(7):1023–9. doi:10.1002/jctb.4195. [Google Scholar] [CrossRef]
34. Park DH, Kim BS. Production of poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-4-hydroxybutyrate) by Ralstonia eutropha from soybean oil. N Biotechnol. 2011;28(6):719–24. doi:10.1016/j.nbt.2011.01.007. [Google Scholar] [PubMed] [CrossRef]
35. Kek Y-K, Chang C-W, Amirul A-A, Sudesh K. Heterologous expression of Cupriavidus sp. USMAA2-4 PHA synthase gene in PHB−4 mutant for the production of poly(3-hydroxybutyrate) and its copolymers. World J Microbiol Biotechnol. 2010;26(9):1595–603. doi:10.1007/s11274-010-0335-5. [Google Scholar] [CrossRef]
36. Maddikeri GL, Pandit AB, Gogate PR. Intensification approaches for biodiesel synthesis from waste cooking oil: a review. Ind Eng Chem Res. 2012;51(45):14610–28. doi:10.1021/ie301675j. [Google Scholar] [CrossRef]
37. Chien Bong CP, Alam MNHZ, Samsudin SA, Jamaluddin J, Adrus N, Mohd Yusof AH, et al. A review on the potential of polyhydroxyalkanoates production from oil-based substrates. J Environ Manage. 2021;298:113461. doi:10.1016/j.jenvman.2021.113461. [Google Scholar] [PubMed] [CrossRef]
38. Wang J, Liu S, Huang J, Qu Z. A review on polyhydroxyalkanoate production from agricultural waste Biomass: development, advances, circular approach, and challenges. Bioresour Technol. 2021;342(206):126008. doi:10.1016/j.biortech.2021.126008. [Google Scholar] [PubMed] [CrossRef]
39. Lim SW, Kansedo J, Tan IS, Tan YH, Nandong J, Lam MK, et al. Microbial valorization of oil-based substrates for polyhydroxyalkanoates (PHA) production—current strategies, status, and perspectives. Process Biochem. 2023;130:715–33. doi:10.1016/j.procbio.2023.05.013. [Google Scholar] [CrossRef]
40. Pinela J, de la Fuente B, Rodrigues M, Pires TCSP, Mandim F, Almeida A, et al. Upcycling fish by-products into bioactive fish oil: the suitability of microwave-assisted extraction. Biomolecules. 2022;13(1):1. doi:10.3390/biom13010001. [Google Scholar] [PubMed] [CrossRef]
41. Ashby RD, Solaiman DKY. Poly(hydroxyalkanoate) biosynthesis from crude alaskan pollock (Theragra chalcogramma) oil. J Polym Environ. 2008;16(4):221–9. doi:10.1007/s10924-008-0108-5. [Google Scholar] [CrossRef]
42. Mohapatra S, Sarkar B, Samantaray DP, Daware A, Maity S, Pattnaik S, et al. Bioconversion of fish solid waste into PHB using Bacillus subtilis based submerged fermentation process. Environ Technol. 2017;38(24):3201–8. doi:10.1080/09593330.2017.1291759. [Google Scholar] [PubMed] [CrossRef]
43. Argiz L, Gonzalez-Cabaleiro R, Correa-Galeote D, Val del Rio A, Mosquera-Corral A. Open-culture biotechnological process for triacylglycerides and polyhydroxyalkanoates recovery from industrial waste fish oil under saline conditions. Sep Purif Technol. 2021;270:118805. doi:10.1016/j.seppur.2021.118805. [Google Scholar] [CrossRef]
44. Sangkharak K, Paichid N, Yunu T, Klomklao S, Prasertsan P. Utilisation of tuna condensate waste from the canning industry as a novel substrate for polyhydroxyalkanoate production. Biomass Convers Biorefin. 2021;11(5):2053–64. doi:10.1007/s13399-019-00581-4. [Google Scholar] [CrossRef]
45. Correa-Galeote D, Argiz L, Val del Rio A, Mosquera-Corral A, Juarez-Jimenez B, Gonzalez-Lopez J, et al. Dynamics of PHA-accumulating bacterial communities fed with lipid-rich liquid effluents from fish-canning industries. Polymers. 2022;14(7):1396. doi:10.3390/polym14071396. [Google Scholar] [PubMed] [CrossRef]
46. Thuoc DV, Anh VTM. Bioconversion of crude fish oil into Poly-3-hydroxybutyrate by Ralstonia sp M91. Appl Biochem Microbiol. 2021;57(2):219–25. doi:10.1134/S0003683821020162. [Google Scholar] [CrossRef]
47. Loan TT, Trang DTQ, Huy PQ, Ninh PX, Van Thuoc D. A fermentation process for the production of poly(3-hydroxybutyrate) using waste cooking oil or waste fish oil as inexpensive carbon substrate. Biotechnol Rep. 2022;33:e00700. doi:10.1016/j.btre.2022.e00700. [Google Scholar] [PubMed] [CrossRef]
48. Zhila NO, Kiselev EG, Volkov VV, Mezenova OY, Sapozhnikova KY, Shishatskaya EI, et al. Properties of degradable polyhydroxyalkanoates synthesized from new waste fish oils (WFOs). Int J Mol Sci. 2023;24(19):14919. doi:10.3390/ijms241914919. [Google Scholar] [PubMed] [CrossRef]
49. Zhila N, Shishatskaya E. Properties of PHA bi-, ter-, and quarter-polymers containing 4-hydroxybutyrate monomer units. Int J Biol Macromol. 2018;111:1019–26. doi:10.1016/j.ijbiomac.2018.01.130. [Google Scholar] [PubMed] [CrossRef]
50. Schlegel HG, Kaltwasser H, Gottschalk G. Ein Submersverfahren zur Kultur wasserstoffoxydierender Bakterien: wachstumsphysiologische Untersuchungen. Arch Mikrobiol. 1961;38(3):209–22. doi:10.1007/BF00422356. [Google Scholar] [CrossRef]
51. Golubev VN, Kutina OI. Handbook of a technologist for processing fish and seafood. Saint Petersburg: GIORD; 2003 (In Russian). [Google Scholar]
52. Baidalinova LS. Biochemistry of aquatic organisms: a tutorial. Moscow: Morbook; 2017 (In Russian). [Google Scholar]
53. Mezenova OY, Agafonova SV, Romanenok NU, Kalinina NS, Volkov VV. Justification of rational modes of thermal extraction of lipids from fat-containing fish waste. Fish Farm. 2023;4:99–106 (In Russian). [Google Scholar]
54. Ermakov AI, Arasimovich VV, Smirnova-Ikonnikova MI, Yarosh NP, Lukovnikova GA. Metody biokhimicheskogo issledovaniya rastenii (Methods of biochemical plant research). Leningrad, Russia: Kolos; 1972 (In Russian). [Google Scholar]
55. Barham PJ, Keller A, Otun EL, Holmes PA. Crystallization and morphology of a bacterial thermoplastic: poly-3-hydroxybutyrate. J Mater Sci. 1984;19(9):2781–94. doi:10.1007/BF01026954. [Google Scholar] [CrossRef]
56. McClements DJ. Food Emulsions: principles, practices and techniques. 2nd Ed. Boca Raton, FL, USA: CRC Press; 2005. [Google Scholar]
57. Cho IJ, Choi KR, Lee SY. Microbial production of fatty acids and derivative chemicals. Curr Opin Biotechnol. 2020;65:129–41. doi:10.1016/j.copbio.2020.02.006. [Google Scholar] [PubMed] [CrossRef]
58. Blunt W, Dartiailh C, Sparling R, Gapes D, Levin DB, Cicek N. Carbon flux to growth or polyhydroxyalkanoate synthesis under microaerophilic conditions is affected by fatty acid chain-length in Pseudomonas putida LS46. Appl Microbiol Biotechnol. 2018;102(15):6437–49. doi:10.1007/s00253-018-9055-9. [Google Scholar] [PubMed] [CrossRef]
59. Merck. ε-caprolactone. Available from: https://www.sigmaaldrich.com/RU/en/product/mm/802801. [Accessed 2024]. [Google Scholar]
60. Merck. γ-butyrolactone. Available from: https://www.sigmaaldrich.com/RU/en/product/mm/801661. [Accessed 2024]. [Google Scholar]
61. Rahayu A, Zaleha Z, Yahya ARM, Majid MIA, Amirul AA. Production of copolymer poly(3-hydroxybutyrate-co-4-hydroxybutyrate) through a one-step cultivation process. World J Microbiol Biotechnol. 2008;24(11):2403–9. doi:10.1007/s11274-008-9764-9. [Google Scholar] [CrossRef]
62. Thuoc DV, My DN, Loan TT, Sudesh K. Utilization of waste fish oil and glycerol as carbon sources for polyhydroxyalkanoate production by Salinivibrio sp. M318. Int J Biol Macromol. 2019;141:885–92. doi:10.1016/j.ijbiomac.2019.09.063. [Google Scholar] [PubMed] [CrossRef]
63. Norhafini H, Huong KH, Amirul AA. High PHA density fed-batch cultivation strategies for 4HB-rich P(3HB-co-4HB) copolymer production by transformant Cupriavidus malaysiensis USMAA1020. Int J Biol Macromol. 2019;125(1):1024–32. doi:10.1016/j.ijbiomac.2018.12.121. [Google Scholar] [PubMed] [CrossRef]
64. Steinbuchel A, Hustede E, Liebergesell M, Pieper U, Timm A, Valentin H. Molecular basis for biosynthesis and accumulation of polyhydroxyalkanoic acids in bacteria. FEMS Microbiol Lett. 1992;103(2–4):217–30. doi:10.1111/j.1574-6968.1992.tb05841.x. [Google Scholar] [PubMed] [CrossRef]
65. Syafiq IM, Huong K-H, Shantini K, Vigneswari S, Aziz NA, Amirul A-AA, et al. Synthesis of high 4-hydroxybutyrate copolymer by Cupriavidus sp. transformants using one-stage cultivation and mixed precursor substrates strategy. Enzyme Microb Technol. 2017;98:1–8. doi:10.1016/j.enzmictec.2016.11.011. [Google Scholar] [PubMed] [CrossRef]
66. Rehm BHA, Steinbüchel A. Biochemical and genetic analysis of PHA synthases and other proteins required for PHA synthesis. Int J Biol Macromol. 1999;25(1–3):3–19. doi:10.1016/S0141-8130(99)00010-0. [Google Scholar] [PubMed] [CrossRef]
67. Laycock B, Halley P, Pratt S, Werker A, Lant P. The chemomechanical properties of microbial polyhydroxyalkanoates. Prog Polym Sci. 2013;38(3–4):536–83. doi:10.1016/j.progpolymsci.2012.06.003. [Google Scholar] [CrossRef]
68. Ramachandran H, Amirul AA. Yellow-pigmented Cupriavidus sp., a novel bacterium capable of utilizing glycerine pitch for the sustainable production of P(3HB-co-4HB). J Chem Tech Biotechnol. 2013;88(6):1030–8. doi:10.1002/jctb.3928. [Google Scholar] [CrossRef]
69. Jo M, Jang Y, Lee E, Shin S, Kang HJ. The modification of Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) by melt blending. Polymers. 2022;14(9):1725. [Google Scholar] [PubMed]
70. Mitomo H, Hsieh WC, Nishiwaki K, Kasuya K. Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) produced by Comamonas acidovorans. Polymer. 2001;42(8):3455–61. [Google Scholar]
71. Zhang T, Jang Y, Jung M, Lee E, Kang HJ. The effect melt processing temperature on the isothermal crystallization of poly(3-hydroxybutyrate-co-4-hydroxybutyrate). Macromol Res. 2024;32(1):1–2. [Google Scholar]
72. Volova TG, Uspenskaya MV, Kiselev EG, Sukovatyi AG, Zhila NO, Vasiliev AD, et al. Effect of monomers of 3-hydroxyhexanoate on properties of copolymers poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Polymers. 2023;15(13):2890. doi:10.3390/polym15132890. [Google Scholar] [PubMed] [CrossRef]
73. Volova TG, Zhila NO, Kiselev EG, Sukovatyi AG, Lukyanenko AV, Shishatskaya EI. Biodegradable polyhydroxyalkanoates with a different set of valerate monomers: chemical structure and physicochemical properties. Int J Mol Sci. 2023;24(18):14082. doi:10.3390/ijms241814082. [Google Scholar] [PubMed] [CrossRef]
74. Wang Q, Xu Y, Xu P, Yang W, Chen M, Dong W, et al. Crystallization of microbial polyhydroxyalkanoates: a review. Int J Biol Macromol. 2022;209:330–43. doi:10.1016/j.ijbiomac.2022.04.018. [Google Scholar] [PubMed] [CrossRef]
75. Gazzano M, Focarete ML, Riekel C, Ripamonti A, Scandola M. Structural investigation of poly(3-hydroxybutyrate) spherulites by microfocus X-ray diffraction. Macromol Chem Phys. 2001;202(8):1405–9. doi:10.1002/1521-3935(20010501)202:8<1405::AID-MACP1405>3.0.CO;2-5. [Google Scholar] [CrossRef]
76. Wunderlich B. Macromolecular physics, crystal melting. New York: Academic Press; 1980. [Google Scholar]
77. Ding G, Liu J. Morphological varieties and kinetic behaviors of poly(3-hydroxybutyrate) (PHB) spherulites crystallized isothermally from thin melt film. Colloid Polym Sci. 2013;291(6):1547–54. doi:10.1007/s00396-012-2882-9. [Google Scholar] [CrossRef]
78. Hobbs JK, Binger DR, Keller A, Barham PJ. Spiralling optical morphologies in spherulites of poly(hydroxybutyrate). J Polym Sci B Polym Phys. 2000;38(12):1575–83. doi:10.1002/(SICI)1099-0488(20000615)38:12<1575::AID-POLB10>3.0.CO;2-Z. [Google Scholar] [CrossRef]
79. You J-W. Influence of hydroxyvalerate content on the crystallization kinetics of poly(hydroxybutyrate-co-hydroxyvalerate). J Polymer Research. 2003;10(1):47–54. doi:10.1023/A:1023958014221. [Google Scholar] [CrossRef]
80. Wen X, Lu X, Peng Q, Zhu F, Zheng N. Crystallization behaviors and morphology of biodegradable poly(3-hydroxybutyrate-co-4-hydroxybutyrate). J Therm Anal Calorim. 2012;109(2):959–66. doi:10.1007/s10973-011-1768-2. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools