 Open Access
Open Access
ARTICLE
Folic Acid-Functionalized Nanocrystalline Cellulose as a Renewable and Biocompatible Nanomaterial for Cancer-Targeting Nanoparticles
1 Nanotechnology & Catalysis Research Center (NANOCAT), Universiti Malaya, Kuala Lumpur, 50603, Malaysia
2 Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Universiti Malaya, Kuala Lumpur, 50603, Malaysia
3 Department of Chemistry, Faculty of Science and Technology, Universitas Airlangga, Campus C, Mulyorejo, Surabaya, 60115, Indonesia
* Corresponding Authors: Mochamad Zakki Fahmi. Email: ; Hwei Voon Lee. Email:
Journal of Renewable Materials 2024, 12(1), 29-43. https://doi.org/10.32604/jrm.2023.043449
Received 03 July 2023; Accepted 30 August 2023; Issue published 23 January 2024
Abstract
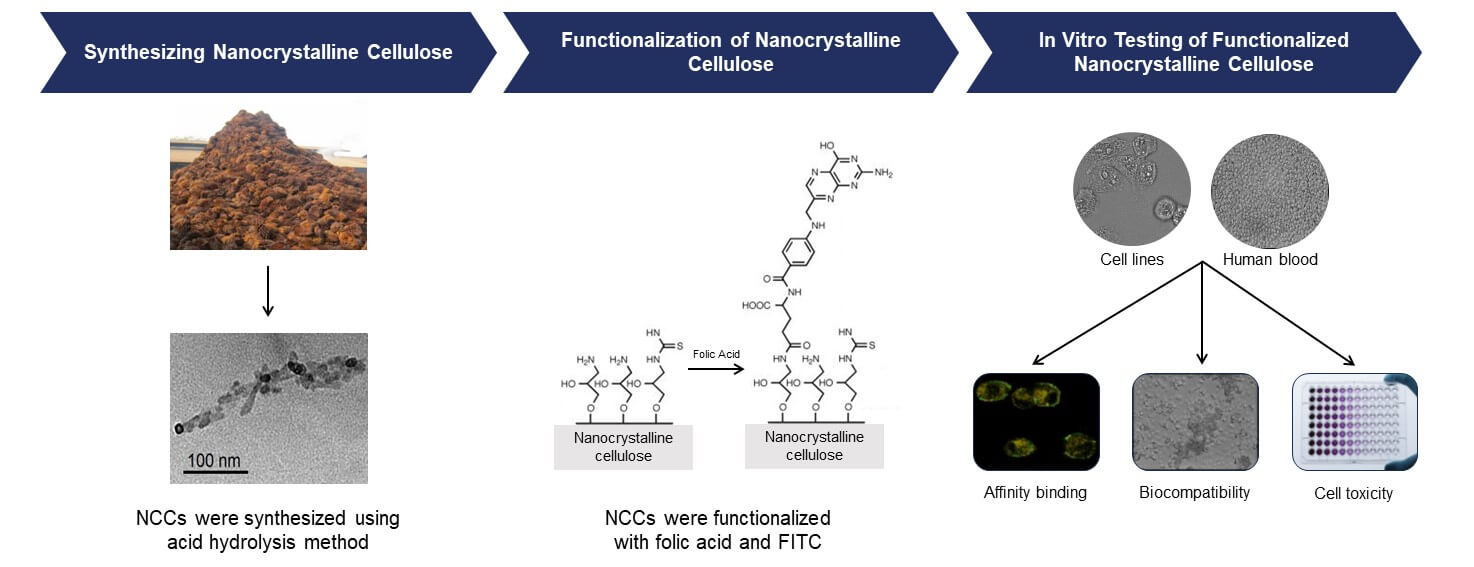
The study focuses on the development of biocompatible and stable FA-functionalized nanocrystalline cellulose (NCC) as a potential drug delivery system for targeting folate receptor-positive cancer cells. The FA-functionalized NCCs were synthesized through a series of chemical reactions, resulting in nanoparticles with favorable properties for biomedical applications. The microstructural analysis revealed that the functionalized NCCs maintained their rod-shaped morphology and displayed hydrodynamic diameters suitable for evading the mononuclear phagocytic system while being large enough to target tumor tissues. Importantly, these nanoparticles possessed a negative surface charge, enhancing their stability and repelling potential aggregation. The binding specificity of FA-functionalized NCCs to folate receptor-positive cancer cells was demonstrated through various assays. The free folic acid inhibition assay showed approximately 30% decrease in the binding of functionalized NCCs in the presence of just 5 mM free FA, confirming their selectivity for folate receptor-positive cells. Confocal microscopy further validated this specificity, as only cancer cells displayed significant binding of functionalized NCCs. Crucially, biocompatibility tests revealed that both NCCs and FA-functionalized NCCs had minimal effects on red blood cells, and they did not induce erythrocyte aggregation. Furthermore, cell viability assays demonstrated functionalized NCCs have selective cytotoxicity against colorectal cancer cells HT-29 and SW-620 (68%–88% cell viability) while sparing noncancerous colon cells CCD-18Co (81%–97% cell viability). In summary, FA-functionalized NCCs exhibit promising characteristics for targeted drug delivery in cancer therapy. Their biocompatibility, stability, and selective cytotoxicity make them an attractive option for delivering therapeutic agents to folate receptor-positive cancer cells, potentially improving the effectiveness of cancer treatments while minimizing harm to healthy tissues.Graphic Abstract
Keywords
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools