 Open Access
Open Access
ARTICLE
Preparation of Peanut Shell Cellulose Double-Network Hydrogel and Its Adsorption Capacity for Methylene Blue
1 Department of College of Environment and Bioengineering, Henan University of Engineering, Zhengzhou, 451191, China
2 School of Mathematics and Statistics, Carleton University, Ontario, K1S 5B6, Canada
* Corresponding Author: Yalin Li. Email:
Journal of Renewable Materials 2023, 11(7), 3001-3023. https://doi.org/10.32604/jrm.2023.026604
Received 15 September 2022; Accepted 23 November 2022; Issue published 05 June 2023
Abstract
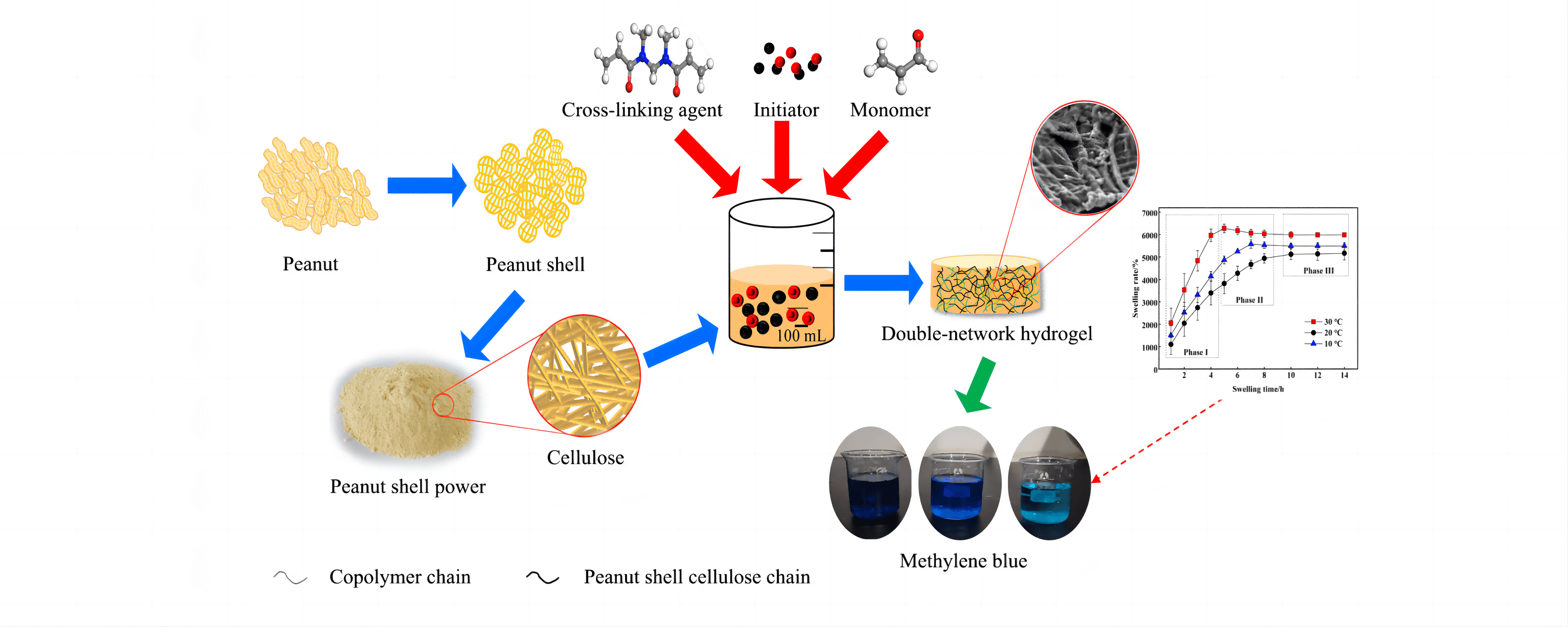
To achieve optimal recovery and value-added utilisation of cellulose in peanut shells, the cellulose in peanut shells was first extracted using the sodium hydroxide-sodium chlorite method. Then, cellulose hydrogel was prepared by graft copolymerisation using N, N’-methylenebisacrylamide as the cross-linking agent, sodium persulfate as the initiator, and acrylic acid as the monomer. Orthogonal optimisation experiments were designed to obtain optimal process parameters for hydrogel preparation with the cellulose dosage of 0.40 g, initiator dosage of 0.20 g, polymerisation temperature of 70°C, cross-linking agent of 0.25 g, and monomer dosage of 3.0 mL. The effect of initiator dosage on hydrogel synthesis was the most significant, followed by monomer dosage and reaction temperature. Characterisation using X-ray diffraction analysis and scanning electron microscopy revealed that the hydrogel was amorphous and exhibited a distinct three-dimensional double network structure. Hydrogel swelling kinetic analysis showed that the hydrogel swelling process was divided into three stages, and fitted the Schott secondary swelling kinetic model. The prepared hydrogel had a good adsorption effect on methylene blue; the adsorption of methylene blue by the hydrogel was 1.259 mg/g at 25°C when the initial concentration of methylene blue was 5 mg/L. The adsorption kinetics of the hydrogel fit the pseudo-first-order kinetic model, pseudo-second-order kinetic model, Eovich model and particle diffusion model. The best fitting effect was obtained with the pseudo-second-order kinetic model. The adsorption isotherm analysis of methylene blue on hydrogel showed that the adsorption process was consistent with Langmuir and Freundlich models. The correlation coefficient of the Freundlich isotherm model was higher, indicating that the adsorption of methylene blue on hydrogel was mainly chemisorption.Graphic Abstract
Keywords
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools