 Open Access
Open Access
ARTICLE
Role of Calcination Temperature on Isosorbide Production from Sorbitol Dehydration over the Catalyst Derived from Ce(IV) Sulfate
1
Department of Chemical Engineering, Faculty of Engineering, Khon Kaen University, Khon Kaen University, Khon Kaen, Thailand
2
Research Center for Environmental and Hazardous Substance Management (EHSM), Khon Kaen University, Khon Kaen, Thailand
3
Center of Excellence on Hazardous Substance Management (HSM), Pathumwan, Bangkok, Thailand
4
Center of Excellence on Catalysis and Catalytic Reaction Engineering (CECC) Chemical Engineering, Faculty of Engineering,
Chulalongkorn University, Patumwan, Bangkok, Thailand
5
Energy Management and Conservation Office, Faculty of Engineering, Khon Kaen University, Khon Kaen, Thailand
* Corresponding Author: Sutasinee Neramittagapong. Email:
Journal of Renewable Materials 2023, 11(7), 2985-3000. https://doi.org/10.32604/jrm.2023.026397
Received 20 September 2022; Accepted 19 October 2022; Issue published 05 June 2023
Abstract
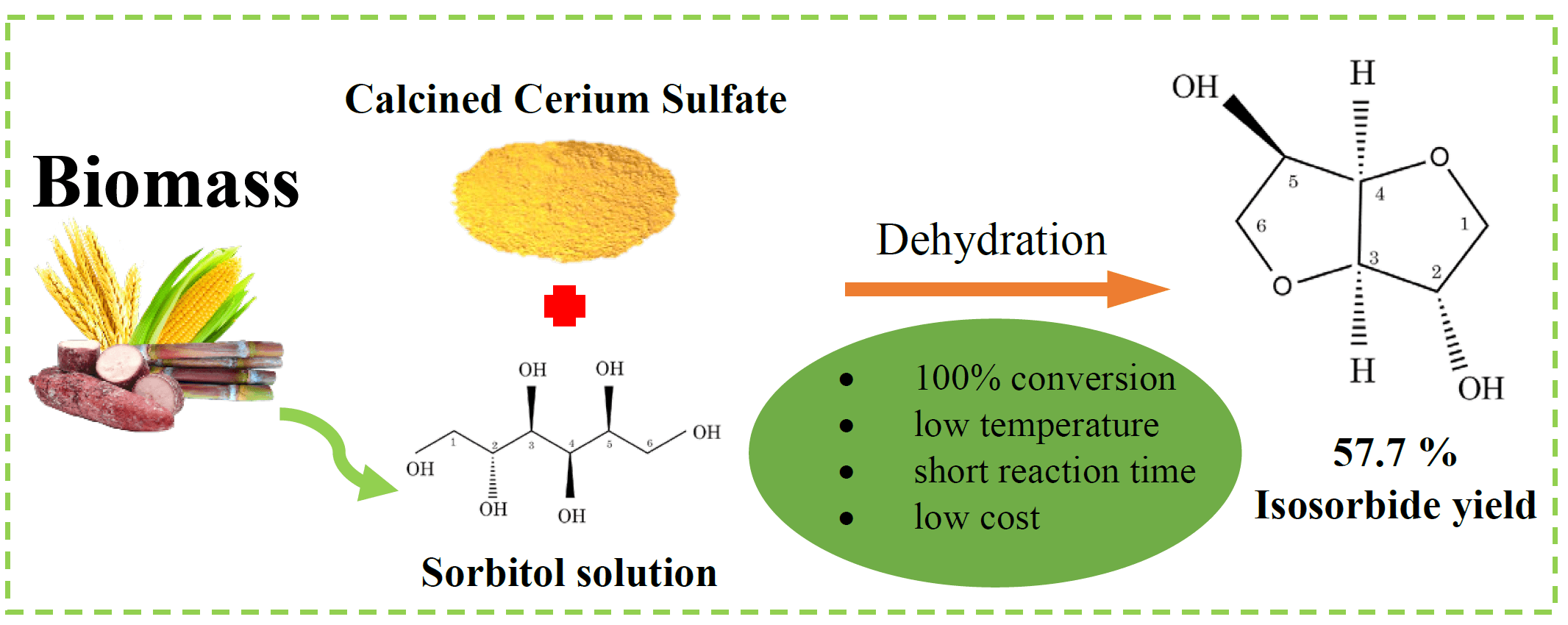
Isosorbide is a multi-purpose chemical that can be produced from renewable resources. Specifically, it has been investigated as a replacement for toxic bisphenol A (BPA) in the production of polycarbonate (PC). In this study, the synthesis of isosorbide by sorbitol dehydration using a cerium-based catalyst derived from calcined cerium (IV) sulfate (300°C, 400°C, 450°C, 500°C, and 650°C) was investigated. The reaction occurred in a high-pressure reactor containing nitrogen gas. Advanced instrumental techniques were applied to analyze the characteristics of the calcined catalyst. The results showed that the calcined catalysts demonstrated different crystalline structures and sulfate species at different temperatures. However, the acidic properties (strength and amount) of the catalyst did not change with the calcination temperature. The cerium (IV) sulfate calcined at 400°C exhibited the best catalytic performance, achieving the highest isosorbide yield (55.7%) and complete conversion of sorbitol at 180°C, 20 bar of N2, and 6 h using CeSO-400. The presence of a sulfate group on the catalyst was the most important factor in determining the catalytic performance of sorbitol dehydration to isosorbide. This work suggests that CeSO-400 catalysts may play an important role in reducing reaction conditions.Graphic Abstract
Keywords
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools