 Open Access
Open Access
ARTICLE
Preparation of Amide-Containing Insecticidal Derivatives from the Renewable Natural Product β-Pinene
1 East China Woody Fragrance and Flavor Engineering Research Center of National Forestry and Grassland Administration, Camphor Engineering Research Center of National Forestry and Grassland Administration, College of Forestry, Jiangxi Agricultural University, Nanchang, 330045, China
2 College of Plant Protection, Northwest Agriculture and Forestry University, Yangling, 712100, China
3 College of Chemical Engineering, Huaqiao University, Xiamen, 361021, China
4 Institute of Chemical Industry of Forest Products, China Academy of Forestry; National Engineering Laboratory for Biomass Chemical Utilization; State Forestry Administration’s Key and Open Laboratory for Forest Chemical Engineering; Jiangsu Provincial Key Laboratory for Biomass Energy and Material, Nanjing, 210042, China
* Corresponding Authors: Hongyan Si. Email: ; Shengliang Liao. Email:
(This article belongs to the Special Issue: Renewable Material from Agricultural Waste and By-Product and Its Applications)
Journal of Renewable Materials 2023, 11(5), 2367-2379. https://doi.org/10.32604/jrm.2023.026333
Received 30 August 2022; Accepted 18 October 2022; Issue published 13 February 2023
Abstract
The preparation of bioactive derivatives from the renewable natural product pinene is a hot research topic in the deep processing and utilization of pinene. In this study, β-pinene was used to develop novel molecules as a promising new precursor of insecticide. A series of amide-containing derivatives of β-pinene were synthesized and characterized. The insecticidal activities of these derivatives against Mythimna separate and Semiaphis heraclei were tested. The structure characterization results showed that the characterization data of amide-containing derivatives were in full agreement with their proposed structures. The insecticidal activities evaluation results indicated that amide-containing derivatives exhibited weak insecticidal activity against Mythimna separate, but exhibited moderate to good insecticidal activity against Semiaphis heraclei. After testing for 72 h, the corrected mortality against Semiaphis heraclei of compounds 5c, 5e, 5f, 5 h, 5j, and 5 m was 100% at 1000 mg/L. The structure-activity relationship analysis results showed that the introduction of an amide group into the structure of derivatives improved their insecticidal activity against Semiaphis heraclei. Meanwhile, the amide-containing derivatives containing the F and NO2 substituted benzene ring might improve their insecticidal activity against Semiaphis heraclei. This study will be helpful for the high value-added utilization of the natural renewable resource β-pinene and the development of novel insecticides.Keywords
Nomenclature
| Term 1 | Interpretation 1 |
| Term 2 | Interpretation 2 |
| e.g. | |
| | Porosity |
| s | Skin factor |
β-pinene is a renewable resource derived from pine trees. The resin canal of the pine tree secretes resin. After a series of separation processes, the solid part of the resin is known as rosin and the liquid part is known as turpentine. β-pinene is one of the main components of turpentine. It has a special molecular structure in which a double bond, a bridged ring, and two chiral carbon atoms are present (see Fig. 1). The molecular structure of β-pinene endows it with special properties, and among these, the biological activity of β-pinene is valuable, such as its antibacterial [1–3], antitumor [4], antiviral [5], antioxidant [2,6], antibacterial [7], photoprotective [8], and anti-inflammatory [9] activity. Recently, studies on the biological activity of β-pinene have shown that it exhibits certain insecticidal activity [10–13]. However, compared with commercial pesticides, β-pinene has weaker insecticidal activity. Thus, β-pinene cannot be directly used as an insecticide but can be used as a precursor compound to synthesize derivatives with better insecticidal activity through certain structural modifications [14].
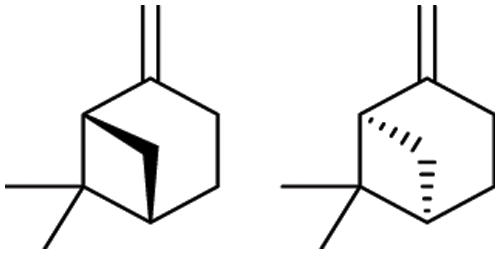
Figure 1: Molecular structure of β-pinene
Amide is an important organic compound and has attracted much attention for its rich biological activity [15–18]. In the field of insecticides, amide compounds exhibit excellent insecticidal activity, and several typical amide insecticides, including Chlorantraniliprole (Fig. 2a), Flubendiamide (Fig. 2b) and Cyantraniliprole (Fig. 2c), are widely used for crop pest control [19–21]. Based on the good insecticidal activity of the amide compound, it can be hypothesized that when the amide structure is introduced into the β-pinene skeleton to prepare β-pinene derivatives containing amide structures, some derivatives with good insecticidal activity are expected to be screened out. Unfortunately, the amide-containing derivatives of β-pinene having insecticidal activity have been rarely reported.

Figure 2: Three typical amide insecticides (a): Chlorantraniliprole; (b): Flubendiamide; (c): Cyantraniliprole
In this study, in order to develop a new insecticidal compound from the natural renewable resource β-pinene, a series of amide-containing derivatives of β-pinene were synthesized, and the insecticidal activity of these synthesized compounds against two agricultural pests was tested. This study demonstrates the positive effect of the high value-added utilization of β-pinene and is also of great significance for the development of new insecticides.
All the reactions were traced by thin layer chromatography (TLC). The compound (–)-β-pinene was purchased from the spice company Jiangxi Jishui Hongda Natural Perfume Co., Limited (Ji’an, China), and the other reagents were of analytical grade; no additional processing was required. The insects Mythimna separate and Semiaphis heraclei were provided by the College of Plant Protection, Northwest Agriculture and Forestry University.
According to the procedures described in our previous reports [22–24], (-)-cis-myrtanol (compound 2), myrtanyl acid (compound 3), and amide-containing derivatives 5a–5o were prepared. Their synthesis routes are shown in Fig. 3.

Figure 3: Synthesis route of the β-pinene-based derivatives
The structures of these amide-containing derivatives were characterized by Fourier transform infrared spectroscopy (FT-IR), nuclear magnetic resonance spectroscopy (1H-NMR and 13C-NMR), and mass spectrometry (MS), and the spectra are included in the Supplementary Materials available online.
2.3 Insecticidal Activity Evaluation
The insecticidal activity of the amide-containing derivatives of β-pinene against Mythimna separate and Semiaphis heraclei was tested by the leaf dipping method and the spraying method, respectively. The details of these methods were as follows.
Leaf dipping method (test insect: Mythimna separate): The test compound was dissolved in DMF to prepare a 2.5% (m/m) mother solution. The prepared mother solution was diluted to a 1000 mg/L test solution using 1‰ Tween-80 aqueous solution. Cabbage leaves were dipped into the test solution for 10 s and air-dried. Five treated leaves were placed in each test vessel (plastic cup) with 10 third instar larvae that were starved for 2 h beforehand. The test vessels were sealed with plastic wrap and vents were poked in it. Then the test vessels were observed for 72 h in an environment with a photoperiod of 16(L)∶8(D), temperature of 25oC, and humidity of 70%. The survival of the test insects was examined at 48 and 72 h. In observing the test results, insects with weak growth, slow movement, and less feeding were judged to be dead. Chlorantraniliprole was used as a positive control, and water was used as a blank control. All the tests were repeated three times. Insect mortality and corrected mortality were calculated according to the following Eqs. (1) and (2).
Spraying method (test insect: Semiaphis heraclei): The test compound was dissolved in DMF to prepare a 2.5% (m/m) mother solution. The prepared mother solution was diluted to a 1000 mg/L test solution using 1‰ Tween-80 aqueous solution. Celery leaves with 30 test insects were placed in a Petri dish. The prepared 1000 mg/L test solution was homogeneously sprayed on the leaves and air-dried. The test Petri dishes were sealed with plastic wrap, and a few holes were poked in it. Then the test Petri dishes were observed for 72 h in an environment with a photoperiod of 16(L)∶8(D), temperature of 25oC, and humidity of 70%. The survival of the test insects was examined at 48 and 72 h. Insects with weak growth, slow movement, and less feeding were judged to be dead. Chlorantraniliprole was used as a positive control, and water was used as a blank control. All the tests were repeated three times. The insect mortality and corrected mortality were calculated according to the following Eqs. (1) and (2).
First, β-pinene was converted to (-)-cis-myrtanol (compound 2) by a hydroboration-oxidation reaction. The FT-IR spectra of compound 2 (see Supplementary Materials Table S1) showed an absorption band at 3313 cm−1 owing to the newly formed OH group. In the 1H-NMR spectra of compound 2, the proton signal of OH was not observed. However, the proton signal of the CH2 group attached to the OH group was observed at δ 3.55 ppm as a doublet of doublets. In the 13C-NMR spectra of compound 2, the carbon signal of the CH2 group attached to the OH group was observed at δ 66.95 ppm. The mass spectra of compound 2 exhibited an expected molecular ion peak at m/z 154.1 [M]+ corresponding to the molecular formula C10H18O.
Then the (-)-cis-myrtanol (compound 2) was oxidized to myrtanyl acid (compound 3) by an oxidation reaction. In the FT-IR spectra of compound 3 (see Supplementary Materials Table S1), absorption bands related to OH and C=O were observed at 3660 and 1675 cm−1, respectively. The carboxyl OH proton was observed at δ 11.91 ppm as a singlet, and the carboxyl carbon signal was observed at δ 183.04 ppm.
Finally, based on the substructure splicing principle, a series of amide derivatives were synthesized by splicing an amide moiety onto the pinane skeleton (see Supplementary Materials Table S1). The FT-IR spectrum for all the β-pinene-based amide derivatives showed the expected frequencies of NH, C=O, benzene ring, and C-N at 3000–3500, 1650–1710, 1400–1600, and 1280–1320 cm−1. In the 1H-NMR spectrum of β-pinene-based amide derivatives, the proton signal of NH was recorded at δ 7.3–11.91 ppm. In the 13C-NMR spectrum of β-pinene-based amide derivatives, the carboxyl carbon signal was observed at δ 173.46–181.14 ppm. The mass spectrum of β-pinene-based amide derivatives exhibited an expected molecular ion peak corresponding to their molecular formula.
In conclusion, these results indicated that the characterization data of amide-containing derivatives of β-pinene were in full agreement with their proposed structures.
3.2 Insecticidal Activity Evaluation
3.2.1 Insecticidal Activity Evaluation of Mythimna Separate
The insecticidal activity of amide-containing derivatives of β-pinene against Mythimna separate was tested by the leaf dipping method. The results are listed in Table 1.
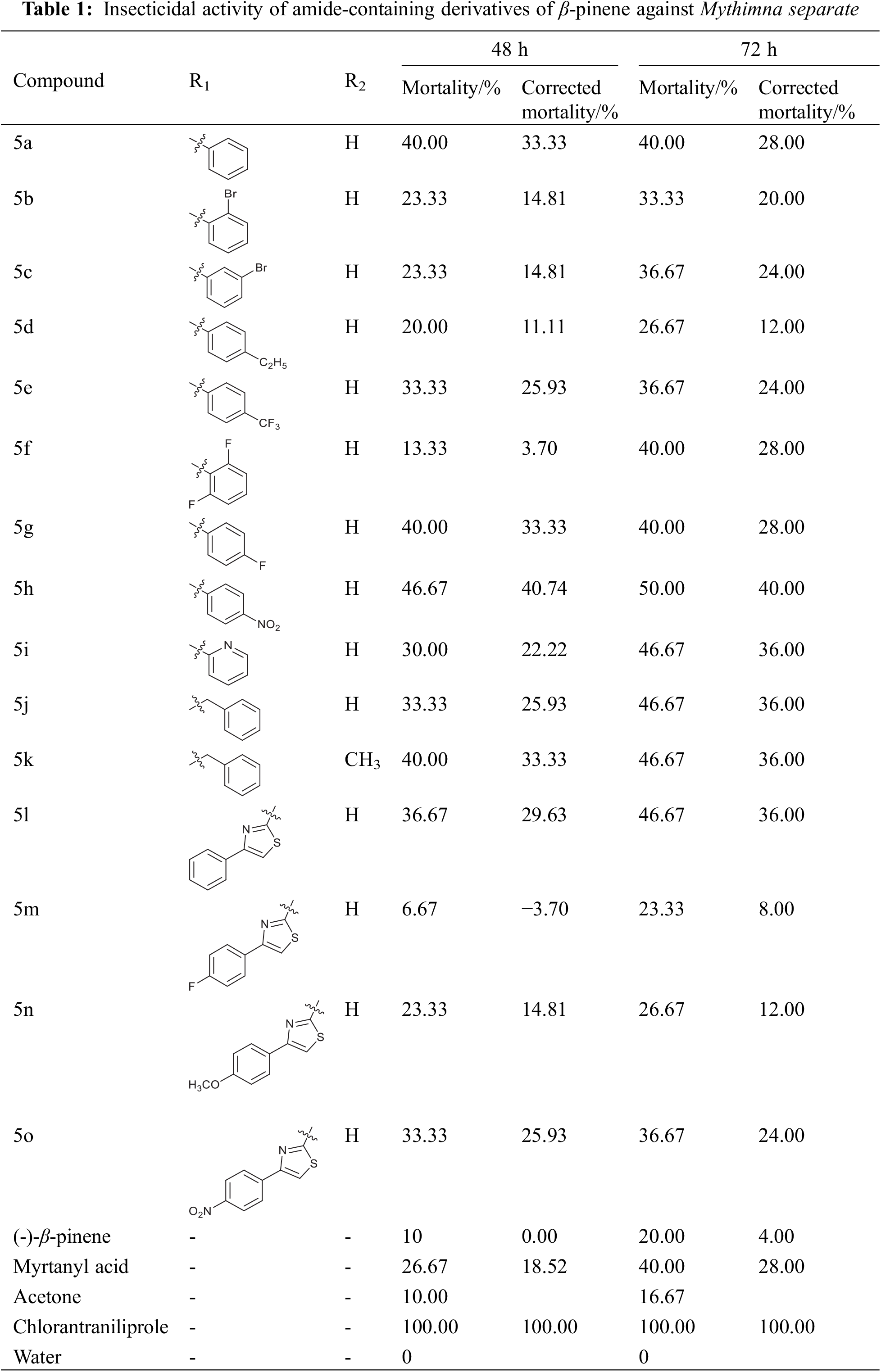
As seen in Table 1, after 72 h of test observation, the initial compound β-pinene and the intermediate compound myrtanyl acid exhibited weak insecticidal activity against Mythimna separate; their corrected mortality rates were 4.00% and 28.00%, respectively. Compared to myrtanyl acid, the amide derivatives of myrtanyl acid did not show significant insecticidal activity improvement against Mythimna separate. Compound 5 h with a nitro group at para-position in the benzene ring showed the best insecticidal activity against Mythimna separate; its corrected mortality was 40.00%.
3.2.2 Insecticidal Activity Evaluation of Semiaphis Heraclei
The insecticidal activity of amide-containing derivatives of β-pinene against Semiaphis heraclei was tested by the spraying method. The results are listed in Table 2.
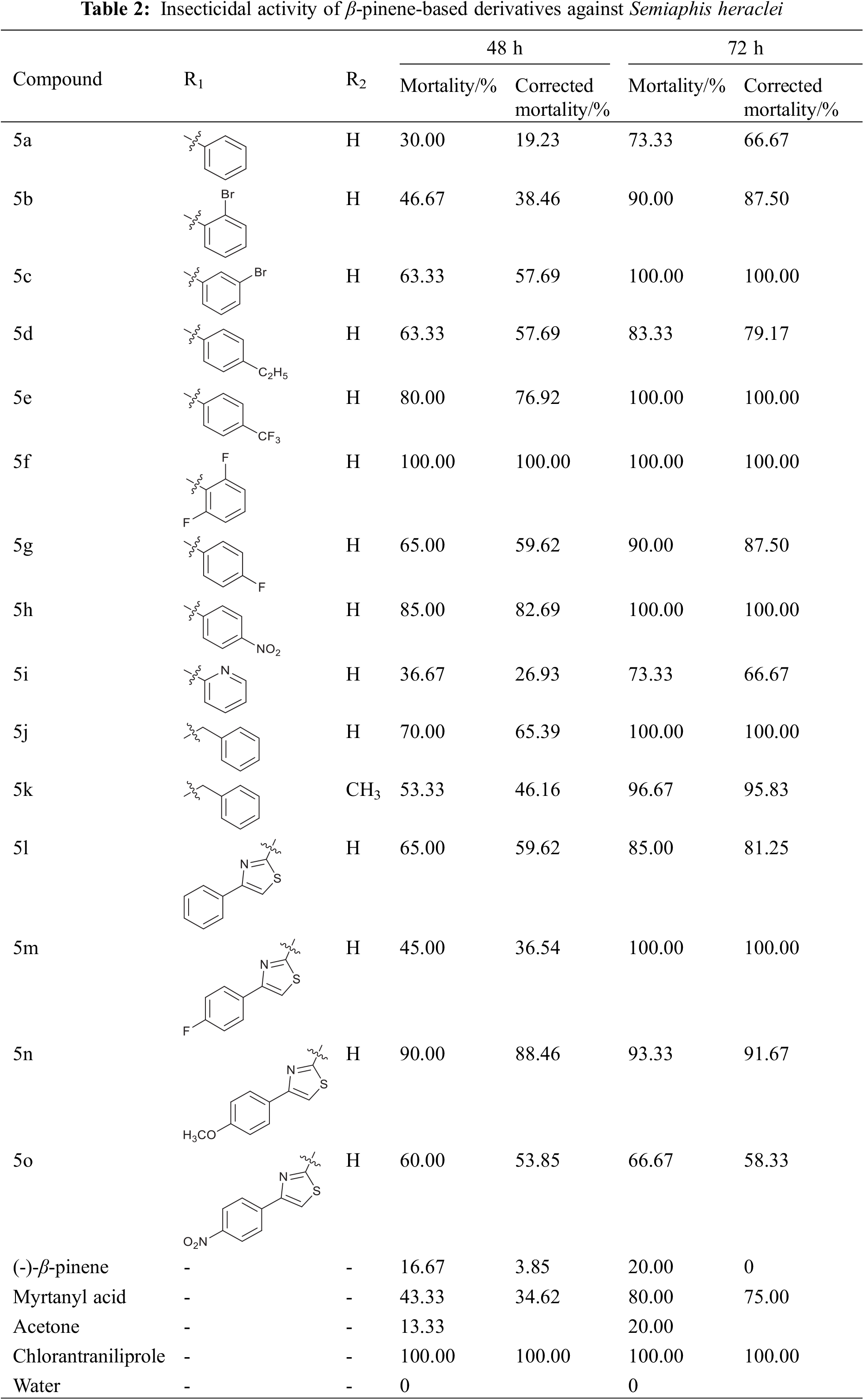
As seen in Table 2, the intermediate compound myrtanyl acid exhibited better insecticidal activity against Semiaphis heraclei than the initial compound β-pinene. At 1000 mg/L, the corrected mortality of myrtanyl acid against Semiaphis heraclei was 75.00% after 72 h. Furthermore, compared to myrtanyl acid, most amide-containing derivatives showed better insecticidal activity against Semiaphis heraclei. After 72 h, the corrected mortality rate of compounds 5c, 5e, 5f, 5 h, 5j, and 5m was 100%, and the corrected mortality rates of compounds 5k and 5n were 95.83% and 91.67%, respectively. By analyzing the data in Table 2, we obtained the following relationship between the structure of the derivatives and the activity against Semiaphis heraclei.
First, most of the amide-containing derivatives showed better insecticidal activity against Semiaphis heraclei than myrtanyl acid, demonstrating that the introduction of the amide group into the structure of derivatives improved their insecticidal activity against Semiaphis heraclei. The studies of Paula et al. [25–27] also found that the insecticidal activities of piperine, matrine, and phenylpyrazole carboxylic acid derivatives were improved by the introduction of amide groups, which suggests that amide groups are the ideal motif for insecticide molecules.
Second, the substituents on the benzene ring of the derivatives had a significant effect on the insecticidal activity of the derivatives. The electron-withdrawing group on the benzene (compound 5 h with a nitro group) ring was more favorable for enhancing the insecticidal activity of the derivative than the electron-donating group on the benzene ring (compound 5d with an ethyl group).
Third, the introduction of a halogen substituent on the benzene ring enhanced the insecticidal activity of the derivatives (compounds 5b, 5c, 5e, 5f, and 5g). For compounds 5l–5o, the introduction of a halogen substituent (fluorine atom) and an electron-donating group (methoxy group) on the benzene ring was advantageous for increasing the insecticidal activity. Lv et al. [28] and Wang et al. [26] also found that the fluorine atom in the amide derivatives has an important influence on the insecticidal activity of amide derivatives, which further verified our experimental results.
Although the mechanism of action of the amide-containing derivatives was not investigated in this study, it was speculated that the insecticidal mechanism of these derivatives may be similar to that of some amide-containing insecticides (e.g., Chlorantraniliprole), and their possible targets were insect ryanodine receptors [29,30].
In our previous study, we found that the pinene skeleton has potential insecticidal activity, and the amide group may improve the insecticidal activity of pinene derivatives. Therefore, a hypothesis was proposed that the fusion of the pinene skeleton and the amide group would produce potent insecticidal derivatives. Based on this hypothesis, a series of amide-containing derivatives of β-pinene were prepared in this study, the insecticidal activities of Mythimna separate and Semiaphis heraclei were evaluated, and several amide derivatives of β-pinene with high insecticidal activity were screened, which validated our hypothesis. The mechanism of action of compounds with good insecticidal activity can be studied in the next step. This study will encourage the high value-added utilization of the natural renewable resource β-pinene and the development of novel insecticides.
Funding Statement: This work is financially supported by the Youth Talent Project of Major Academic and Technical Leaders Training Program of Jiangxi Province (Grant No. 20204BCJL23045), the National Natural Science Foundation of China (Grant No. 31800493), the Special Research Project on Camphor Tree (KRPCT) of Jiangxi Forestry Department (Grant No. 2020CXZX07), and the Innovative Leading Talent Short-Term Project in the Natural Science Area of Jiangxi Province (jxsq2018102072).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Leite, A. M., Lima, E. D. O., Souza, E. L. D., Diniz, M. D. F. F. M., Trajano, V. N. et al. (2007). Inhibitory effect of beta-pinene, alpha-pinene and eugenol on the growth of potential infectious endocarditis causing gram-positive bacteria. Revista Brasileira de Ciências Farmacêuticas, 43(1), 121–126. DOI 10.1590/S1516-93322007000100015. [Google Scholar] [CrossRef]
2. Rodrigues, P. R., Junior, L. M., de Souza, W. F. C., Sato, H. H., Alves, R. M. V. et al. (2021). O-ATRP synthesized poly (β-pinene) blended with chitosan for antimicrobial and antioxidant bio-based films production. International Journal of Biological Macromolecules, 193(A), 425–432. [Google Scholar]
3. Julaeha, E., Herlina, T., Nurzaman, M., Mayanti, T., Kurnia, D. et al. (2021). The antibacterial effect of β-pinene derived from citrus aurantifolia peel against oral streptococcus mutans ATCC 25175. Padjadjaran Journal of Dentistry, 33(1), 88–93. DOI 10.24198/pjd.vol33no1.29200. [Google Scholar] [CrossRef]
4. Zhang, Z., Guo, S., Liu, X., Gao, X. (2015). Synergistic antitumor effect of α-pinene and β-pinene with paclitaxel against non-small-cell lung carcinoma (NSCLC). Drug Research, 65(4), 214–218. DOI 10.1055/s-00023610. [Google Scholar] [CrossRef]
5. Astani, A., Schnitzler, P. (2014). Antiviral activity of monoterpenes beta-pinene and limonene against herpes simplex virus in vitro. Iranian Journal of Microbiology, 6(3), 149. [Google Scholar]
6. Kaur, S., Chowhan, N., Sharma, P., Rathee, S., Singh, H. P. et al. (2022). β-Pinene alleviates arsenic (As)-induced oxidative stress by modulating enzymatic antioxidant activities in roots of oryza sativa. Ecotoxicology and Environmental Safety, 229, 113080. DOI 10.1016/j.ecoenv.2021.113080. [Google Scholar] [CrossRef]
7. Guzmán-Gutiérrez, S. L., Gómez-Cansino, R., García-Zebadúa, J. C., Jiménez-Pérez, N. C., Reyes-Chilpa, R. (2012). Antidepressant activity of litsea glaucescens essential oil: Identification of β-pinene and linalool as active principles. Journal of Ethnopharmacology, 143(2), 673–679. DOI 10.1016/j.jep.2012.07.026. [Google Scholar] [CrossRef]
8. Bitterling, H., Lorenz, P., Vetter, W., Kammerer, D. R., Stintzing, F. C. (2022). Photo-protective effects of selected furocoumarins on β-pinene, R-(+)-limonene and γ-terpinene upon UV-A irradiation. Journal of Photochemistry and Photobiology A: Chemistry, 424, 113623. DOI 10.1016/j.jphotochem.2021.113623. [Google Scholar] [CrossRef]
9. Santos, E. S., Abrantes Coelho, G. L., Saraiva Fontes Loula, Y. K., Saraiva Landim, B. L., Fernandes Lima, C. N. et al. (2022). Hypoglycemic, hypolipidemic, and anti-inflammatory effects of beta-pinene in diabetic rats. Evidence-Based Complementary and Alternative Medicine, 2022, 1–8. DOI 10.1155/2022/8173307. [Google Scholar] [CrossRef]
10. Lucia, A., Audino, P. G., Seccacini, E., Licastro, S., Zerba, E. et al. (2007). Larvicidal effect of eucalyptus grandis essential oil and turpentine and their major components on aedes aegypti larvae. Journal of the American Mosquito Control Association, 23(3), 299–303. DOI 10.2987/8756-971X(2007)23[299:LEOEGE]2.0.CO;2. [Google Scholar] [CrossRef]
11. Pavela, R., Morshedloo, M. R., Lupidi, G., Carolla, G., Barboni, L. et al. (2020). The volatile oils from the oleo-gum-resins of ferula assa-foetida and ferula gummosa: A comprehensive investigation of their insecticidal activity and eco-toxicological effects. Food and Chemical Toxicology, 140, 111312. DOI 10.1016/j.fct.2020.111312. [Google Scholar] [CrossRef]
12. Liu, Z., Li, Q. X., Song, B. (2022). Pesticidal activity and mode of action of monoterpenes. Journal of Agricultural and Food Chemistry, 70(15), 4556–4571. DOI 10.1021/acs.jafc.2c00635. [Google Scholar] [CrossRef]
13. Sarma, R., Adhikari, K., Khanikor, B. (2022). Evaluation of efficacy of pinene compounds as mosquitocidal agent against Aedes aegypti linn. (Diptera: culicidae). International Journal of Tropical Insect Science, 42(3), 2567–2577. DOI 10.1007/s42690-022-00784-9. [Google Scholar] [CrossRef]
14. Gao, Y., Li, J., Shang, S., Wang, D. (2015). Synthesis and insecticidal activity of acylthiourea derivatives from β-pinene. Letters in Drug Design & Discovery, 12(3), 241–249. DOI 10.2174/1570180811666141009234615. [Google Scholar] [CrossRef]
15. Gumbo, M., Beteck, R. M., Mandizvo, T., Seldon, R., Warner, D. F. et al. (2018). Cinnamoyl-oxaborole amides: Synthesis and their in vitro biological activity. Molecules, 23(8), 2038. DOI 10.3390/molecules23082038. [Google Scholar] [CrossRef]
16. Bi, F., Ji, S., Venter, H., Liu, J., Semple, S. J. et al. (2018). Substitution of terminal amide with 1H-1, 2, 3-triazole: Identification of unexpected class of potent antibacterial agents. Bioorganic & Medicinal Chemistry Letters, 28(5), 884–891. DOI 10.1016/j.bmcl.2018.02.001. [Google Scholar] [CrossRef]
17. Aguiar, A. R., Alvarenga, E. S., Silva, E. M., Farias, E. S., Picanço, M. C. (2019). Synthesis, insecticidal activity, and phytotoxicity of novel chiral amides. Pest Management Science, 75(6), 1689–1696. DOI 10.1002/ps.5289. [Google Scholar] [CrossRef]
18. Gill, J. P. K., Sethi, N., Mohan, A. (2018). Synthesis, characterization and herbicidal activity of amide derivatives of glyphosate. Oriental Journal of Chemistry, 34(5), 2378–2383. DOI 10.13005/ojc/340519. [Google Scholar] [CrossRef]
19. Lai, T., Su, J. (2011). Effects of chlorantraniliprole on development and reproduction of beet armyworm, Spodoptera exigua (Hübner). Journal of Pest Science, 84(3), 381–386. DOI 10.1007/s10340-011-0366-1. [Google Scholar] [CrossRef]
20. Tohnishi, M., Nakao, H., Furuya, T., Seo, A., Kodama, H. et al. (2005). Flubendiamide, a novel insecticide highly active against lepidopterous insect pests. Journal of Pesticide Science, 30(4), 354–360. DOI 10.1584/jpestics.30.354. [Google Scholar] [CrossRef]
21. Tiwari, S., Stelinski, L. L. (2013). Effects of cyantraniliprole, a novel anthranilic diamide insecticide, against asian citrus psyllid under laboratory and field conditions. Pest Management Science, 69(9), 1066–1072. DOI 10.1002/ps.3468. [Google Scholar] [CrossRef]
22. Liao, S. L., Shang, S. B., Si, H. Y., Shen, M. G., Rao, X. P. et al. (2015). Synthesis and antibacterial activity of myristyl carboxylate. Chemistry & Industry of Forest Products, 35, 33–38. [Google Scholar]
23. Liao, S., Rao, X., Shen, M., Si, H., Song, J. et al. (2020). New hybrids derived from the natural compound (-)-β-pinene and amides or acylthioureas as antitumor agents. Letters in Drug Design & Discovery, 17(3), 271–284. DOI 10.2174/1570180816666181107094427. [Google Scholar] [CrossRef]
24. Shi, Y. F., Si, H. Y., Wang, P., Chen, S., Shang, S. B. et al. (2019). Derivatization of natural compound β-pinene enhances its in vitro antifungal activity against plant pathogens. Molecules, 24(17), 3144. DOI 10.3390/molecules24173144. [Google Scholar] [CrossRef]
25. Paula, V. F., Barbosa, L. C. A., Demuner, A. J., And, P. V., Picanço, M. C. (2000). Synthesis and insecticidal activity of new amide derivatives of piperine. Pest Management Science, 56(2), 168–174. DOI 10.1002/(ISSN)1526-4998. [Google Scholar] [CrossRef]
26. Wang, B., Wang, H., Liu, H., Xiong, L., Yang, N. et al. (2020). Synthesis and structure-insecticidal activity relationship of novel phenylpyrazole carboxylic acid derivatives containing fluorine moiety. Chinese Chemical Letters, 31(3), 739–745. DOI 10.1016/j.cclet.2019.07.064. [Google Scholar] [CrossRef]
27. Xu, H., Xu, M., Sun, Z., Li, S. (2019). Preparation of matrinic/oxymatrinic amide derivatives as insecticidal/acaricidal agents and study on the mechanisms of action against Tetranychus cinnabarinus. Journal of Agricultural and Food Chemistry, 67(44), 12182–12190. DOI 10.1021/acs.jafc.9b05092. [Google Scholar] [CrossRef]
28. Lv, M., Liu, G., Jia, M., Xu, H. (2018). Synthesis of matrinic amide derivatives containing 1, 3, 4-thiadiazole scaffold as insecticidal/acaricidal agents. Bioorganic Chemistry, 81, 88–92. DOI 10.1016/j.bioorg.2018.07.034. [Google Scholar] [CrossRef]
29. Lahm, G. P., Cordova, D., Barry, J. D. (2009). New and selective ryanodine receptor activators for insect control. Bioorganic & Medicinal Chemistry, 17(12), 4127–4133. DOI 10.1016/j.bmc.2009.01.018. [Google Scholar] [CrossRef]
30. Nauen, R. (2006). Insecticide mode of action: Return of the ryanodine receptor. Pest Management Science: Formerly Pesticide Science, 62(8), 690–692. DOI 10.1002/(ISSN)1526-4998. [Google Scholar] [CrossRef]
Supplementary Materials
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools