 Open Access
Open Access
ARTICLE
Optimization of Preparation of Fe3O4-L by Chemical Co-Precipitation and Its Adsorption of Heavy Metal Ions
College of Civil Engineering, Liaoning Technical University, Fuxin, 123000, China
* Corresponding Author: Xueying Sun. Email:
(This article belongs to the Special Issue: Porous Materials for Sustainable Development)
Journal of Renewable Materials 2023, 11(5), 2209-2232. https://doi.org/10.32604/jrm.2023.025241
Received 30 June 2022; Accepted 16 August 2022; Issue published 13 February 2023
Abstract
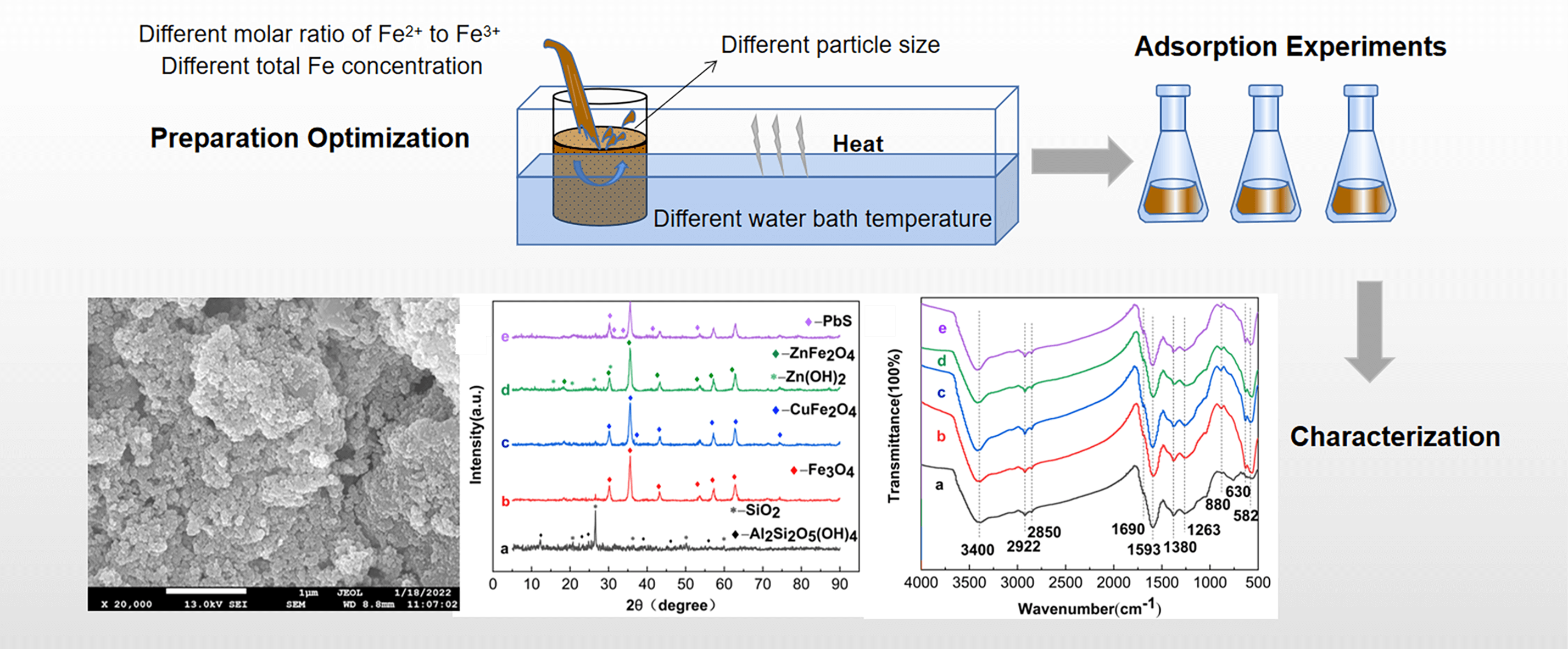
To address the serious pollution of heavy metals in AMD, the difficulty and the high cost of treatment, Fe3O4-L was prepared by the chemical co-precipitation method. Based on the single-factor and RSM, the effects of particle size, total Fe concentration, the molar ratio of Fe2+ to Fe3+ and water bath temperature on the removal of AMD by Fe3O4-L prepared by chemical co-precipitation method were analyzed. Static adsorption experiments were conducted on Cu2+, Zn2+ and Pb2+ using Fe3O4-L prepared under optimal conditions as adsorbents. The adsorption properties and mechanisms were analyzed by combining SEM-EDS, XRD and FTIR for characterization. The study showed that the effects of particle size, total Fe concentration and the molar ratio of Fe2+ to Fe3+ are larger. Obtained by response surface optimization analysis, the optimum conditions for the preparation of Fe3O4-L were a particle size of 250 mesh, a total Fe concentration of 0.5 mol/L, and a molar ratio of Fe2+ to Fe3+ of 1:2. Under these conditions, the removal rates of Cu2+, Zn2+, and Pb2+ were 94.52%, 88.49%, and 96.69% respectively. The adsorption of Cu2+, Zn2+ and Pb2+ by Fe3O4-L prepared under optimal conditions reached equilibrium at 180 min, with removal rates of 99.99%, 85.27%, and 97.48%, respectively. The adsorption reaction of Fe3O4-L for Cu2+ and Zn2+ is endothermic, while that for Pb2+ is exothermic. Fe3O4-L can still maintain a high adsorption capacity after five cycles of adsorption-desorption experiments. Cu2+, Zn2+ and Pb2+ mainly exist as CuFe2O4, Zn(OH)2, ZnFe2O4 and PbS after being adsorbed by Fe3O4-L, which is the result of the combination of physical diffusion, ion exchange and surface complexation reaction.Graphic Abstract
Keywords
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools