 Open Access
Open Access
ARTICLE
A New Exploration of Artificially Induced Spalted Wood of Two Fungi: Hypoxylon and Sistotrema
The Key Laboratory of State Forestry Adminstration for Highly-Efficient Utilization of Forestry Biomass Resources in Southwest
China, Kunming, 650224, China
*
Corresponding Author: Lei Qin. Email: qinlei9999@163.com
Journal of Renewable Materials 2023, 11(11), 3907-3916. https://doi.org/10.32604/jrm.2023.028099
Received 29 November 2022; Accepted 10 February 2023; Issue published 31 October 2023
Abstract
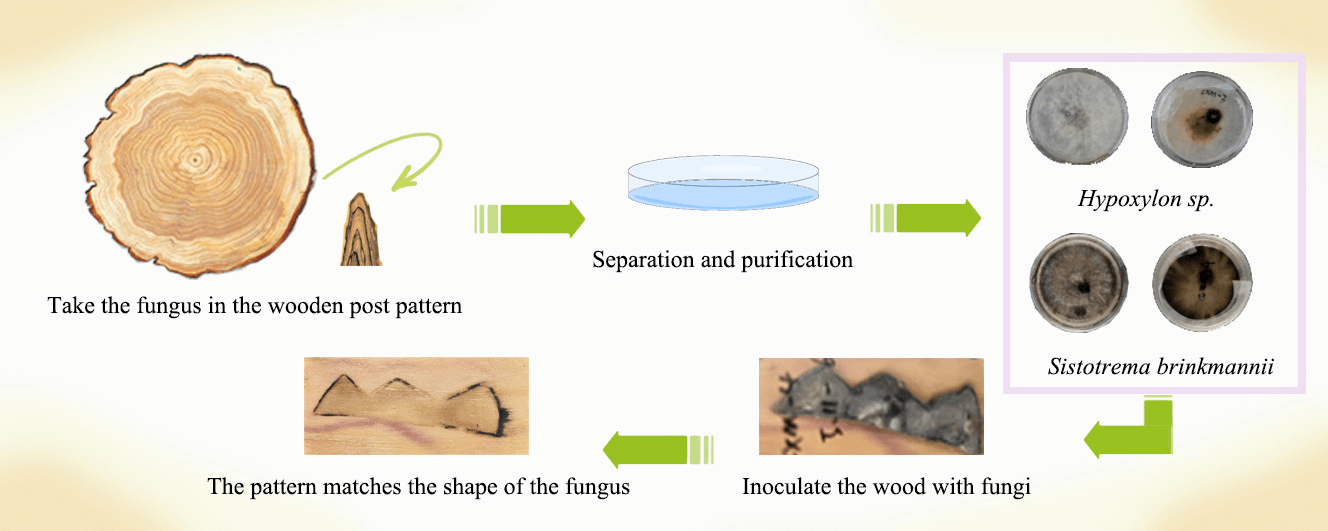
One strain of Hypoxylon sp. CXM-3 and one strain of Sistotrema brinkmannii CXM-4 were inoculated onto sterilized cherry, poplar, birch, and basswood sheets in a certain shape and incubated at constant temperature and humidity for 4, 8, 12, and 16 weeks, respectively, to analyze whether the grain pattern formed by the zone lines was consistent with the predetermined pattern. The results showed that the zone lines of CXM-3 of Hypoxylon were free, delicate, and soft, with brown lines and black staining, mostly accompanied by black and brown dots, facets, and clusters, while the zone lines of CXM-4 of Sistotrema brinkmannii grew along the predetermined grain, with strong lines and a clean surface. After inoculation and incubation at a constant temperature of 25°C ± 2°C and humidity of 60%, Sistotrema brinkmannii CXM-4, on basswood veneer at 4–8 weeks and cherry veneer at 4– 16 weeks, were able to develop zone lines following a predetermined grain. Artificially induced spalted wood can only maintain a large shape, which cannot guarantee that the pattern of large-scale production of spalted wood is exactly the same and cannot be accurate to the minute details. The artificial induction can thus result in the formation of a predetermined grain pattern of the mottled wood, thus enhancing the product value and artistic value of solid wood furniture and crafts.Graphic Abstract
Keywords
Nomenclature
| Term 1 | Interpretation 1 |
| Term 2 | Interpretation 2 |
| e.g. | |
| | Porosity |
| s | Skin factor |
Wood is a natural organic compound with a variety of carbohydrates in its cell wall components and cell cavities that are susceptible to a variety of fungal infestations under certain conditions. The fungal infestation of wood results in patches of varying colors and natural grain patterns, known as spalted wood [1,2]. Depending on the grain presented by the wood, spalted wood can be divided into three categories: intricate zone lines, stains, and decay [3,4].
As research progresses, the common fungi that can independently form zone lines are Basidiomycota and Ascomycota, and zone lines fungi have little damage to the mechanical properties of wood at the beginning of the formation of linear staining [5,6]. Unlike China, where minerals are mostly used as colored pigments for frescoes and ancient buildings, Europe and America use fungal painting techniques for mosaics and furniture veneers, and some famous European churches have frescoes and other artworks that are still brightly colored after more than half a century [7]. The black and dark brown lines formed by the type of zone lines are mainly made up of melanin, which is chemically stable [8–10]. Its ability to enhance the wood’s decorative qualities while avoiding the use of environmentally polluting chemical pigments has made furniture and crafts made from fungus wood popular in Europe and the USA. Robinson at Oregon State University in the USA has done extensive research into the modern use of spalted wood, including the selection of species, strain combinations, staining methods, and sterilization conditions [11–13]. The earliest documented introduction of the name “spalted wood” to China is the translation of spalted grain in the book “Wood Decay and Maintenance” by Professor Menglin Guo of Iowa State University and Professor Jian Qiu of our university [14,15]. In China, Professor Jian Qiu’s team at Southwest Forestry University has been the first to research spalted wood since 2007, extensively investigating and collecting wood-rotting fungal resources, screening zone lines fungi, optimizing the conditions for cultivating spalted wood, granting several invention patents and building a complete technical system for the laboratory preparation of spalted wood. The Sun Jianping of Guangxi University team has conducted pigment extraction, stability analysis, and composition analysis of extracellular pigments produced by a single mosaic fungus to study the application properties of the pigments and provide theoretical support for the application of mosaic fungal pigments [16,17].
The basic form of zone lines on wood is irregular circles, smooth irregular lines like brush strokes constitute a complex and varied pattern, extremely decorative aesthetic, is the type of high decorative value, artificial cultivation and natural formation of zone lines have obvious differences in appearance characteristics, natural common wood to form colorful spots, including red, yellow, blue, green, grey, However, improper combinations can also give an unattractive appearance. Therefore, it is necessary to use the fungal lines as the focus of artificially cultivated spalted wood and artificially screened strains to form beautiful spalted that align with people’s aesthetic needs rather than naturally formed ones [18].
In this study, a Hypoxylon sp. fungi, experiment number CXM-3, and a Sistotrema brinkmannii fungi, experiment number CXM-4, were inoculated onto sterilized cherry (Prunus spp.), poplar (Populus spp.), birch (Betula spp.), and basswood (Tilia spp.) and incubated in the dark for 4, 8, 12, and 16 weeks at constant humidity to observe the grain orientation [19].
Experimental material: The experiments were carried out with strains of Charcoal Cluster Fungus spp. CXM-3 and Sistotrema spp. CXM-4, which were isolated from Vietnamese sedum wood. The woods selected were air-dried cherry, poplar, birch, and basswood.
Other materials: potatoes, glucose, agar, filtered water.
Chemical reagents: 75% alcohol, 95% alcohol, 0.1% liter of mercury.
2.2 Experimental Apparatus and Equipment
Experimental facilities: Vertical pressure steam sterilizer, clean bench, Constant temperature and humidity incubator, Digital display air drying oven, flatted scanner, Sander, electronic balance.
Other instruments: 250 ml conical flask, Petri dish, glass rod, beaker, culture flask, dissecting knife, forceps, culture flask.
In the first group, the strain CXM-3 was inoculated in a predetermined pattern onto four types of wood: cherry, basswood, birch, and poplar and incubated for 4, 8, 12, and 16 weeks to observe the grain orientation. In the second group, strain CXM-4 was inoculated in a predetermined pattern on four kinds of wood, cherry, basswood, birch, and poplar, and incubated for 4, 8, 12, and 16 weeks to observe the grain orientation.
Predetermined pattern: A straight mountain pattern is formed by a straight line with a bottom edge and a straight line with three bands of peaks, or a half-moon bud curved bottom edge and a straight line with three bands of peaks.
Cherry, basswood, birch, and poplar wood were sawn into 5 cm × 10 cm × 0.3 cm sheets and placed in transparent incubators of 8 cm diameter and 18 cm height. The average moisture content of the wood samples was 10.76% (sample replicates 5, standard deviation 0.0016, coefficient of variation 1.52%). In order to adjust the moisture content of the wood to 64%–75% and 90% suitable for the growth of strains CXM-3 and CXM-4 (experiments showed that CXM-3 grew the most spots at a moisture content of 64%–75% without the addition of vermiculite. CXM-4 increased the area of spots with increasing moisture content at a moisture content of 90% or more) Moisture content [9]. In a vertical pressure steam sterilizer (121°C, 0.1 MPa) for 30 min, sterilize and remove.
2.5 Fungal Inoculation and Cultivation Methods
Fungal activation and expansion: The woody substrate material collected from Vietnam was issued with a scalpel, together with the fungal substrates growing on it, cut into small pieces with a side length of 0.3–0.7 cm, sterilized by soaking in 75% alcohol for 10–15 s, then sterilized by soaking in 0.1% mercury for 3–7 min, and washed 3 times with distilled water in a sterilizing pot. Subsequently, placed in an enrichment medium in the dark at 22°C–28°C for 7–15 days to grow zone lines, enrichment medium for PDA medium, to obtain strains Charcoal Clusters CXM-3, CXM-4; then will be to four around the hairy, loose filamentous, white, brown, flat edge, the formation of colonies picked, inoculated into the enrichment medium at The inoculum was obtained by incubation at 22°C–28°C in an HWS constant temperature and humidity incubator (temperature 25°C ± 2°C, humidity 60%, time 7 d), and the proliferation medium was PDA medium.
Fungal preservation: Pick the most vigorous colonies from the proliferation medium and inoculate them into the preservation medium, incubate them for 5–12 days at 22°C–28°C in the dark, and freeze them at 4°C in the refrigerator. The preservation medium is PDA medium.
The wood-based material to be inoculated was sterilized and then fully contacted with the activated, expanded strain, and a specific pattern was outlined with a scalpel. The inoculated sheets were placed in a constant temperature and humidity chamber for 4, 8, 12, and 16 weeks and then removed to observe if a predetermined pattern of mycelial lines was produced [20,21].
Cherry, basswood, birch, and poplar sheets prepared at 4, 8, 12, and 16 weeks were compared and analyzed for the percentage of fungi patterns in relation to the total area using Scion Image software. The surface of the wood samples was scanned, the images were converted to “TIFF” format, the mouse was clicked on the grey scale image and “Analyze” in the software menu bar and “Measure” was selected. “Analyze” in the software’s menu bar to obtain the flecks’ pixels. The total number of pixels obtained and the pixel data of the spots are entered into an Excel sheet. And the percentage of the area of the spots is obtained by dividing the pixels of the spots by the total number of pixels.
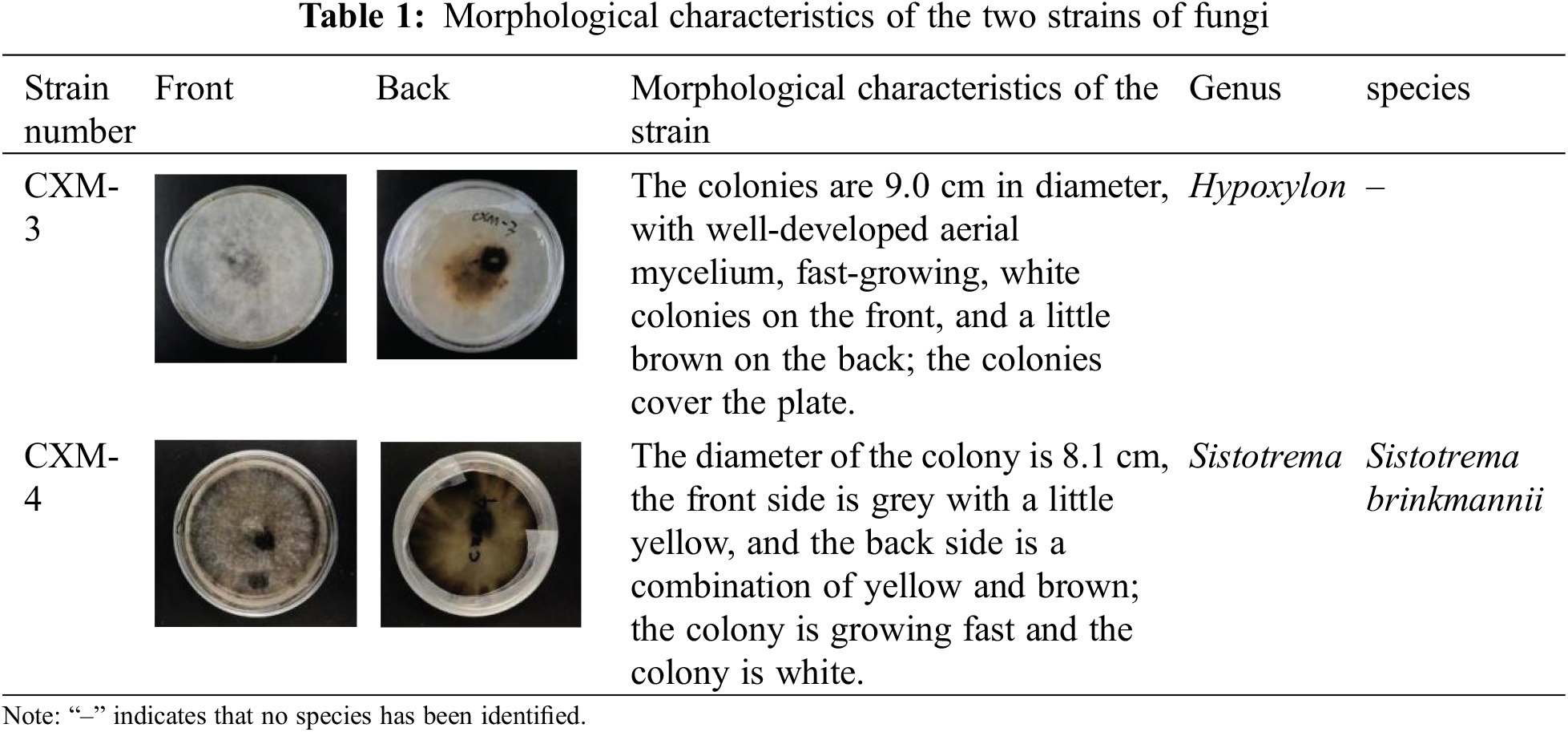
3.1 Morphological Characterisation of Fungi
The morphological characteristics of the two strains of fungi selected for this experiment, after 2 weeks of incubation, are analyzed as shown in Table 1.
3.2 Analysis of Artificially Induced Spatled Wood Fungal Texture
As shown in Fig. 1, after 4 weeks of incubation, CXM-3 produced black and brown staining in dots and facets on all four kinds of wood, with only one closed line on cherry, intermittent non-closed lines on poplar and birch, and penetration of 0.3 cm of veneer. CXM-4 forms a preset pattern of closed zone lines on all four types of wood. The clear orientation, clean layout, and strong decorative effect are accompanied by a purple color on poplar and basswood, a grey dot stain on cherry, and a light grey, brown dot stain on birch.
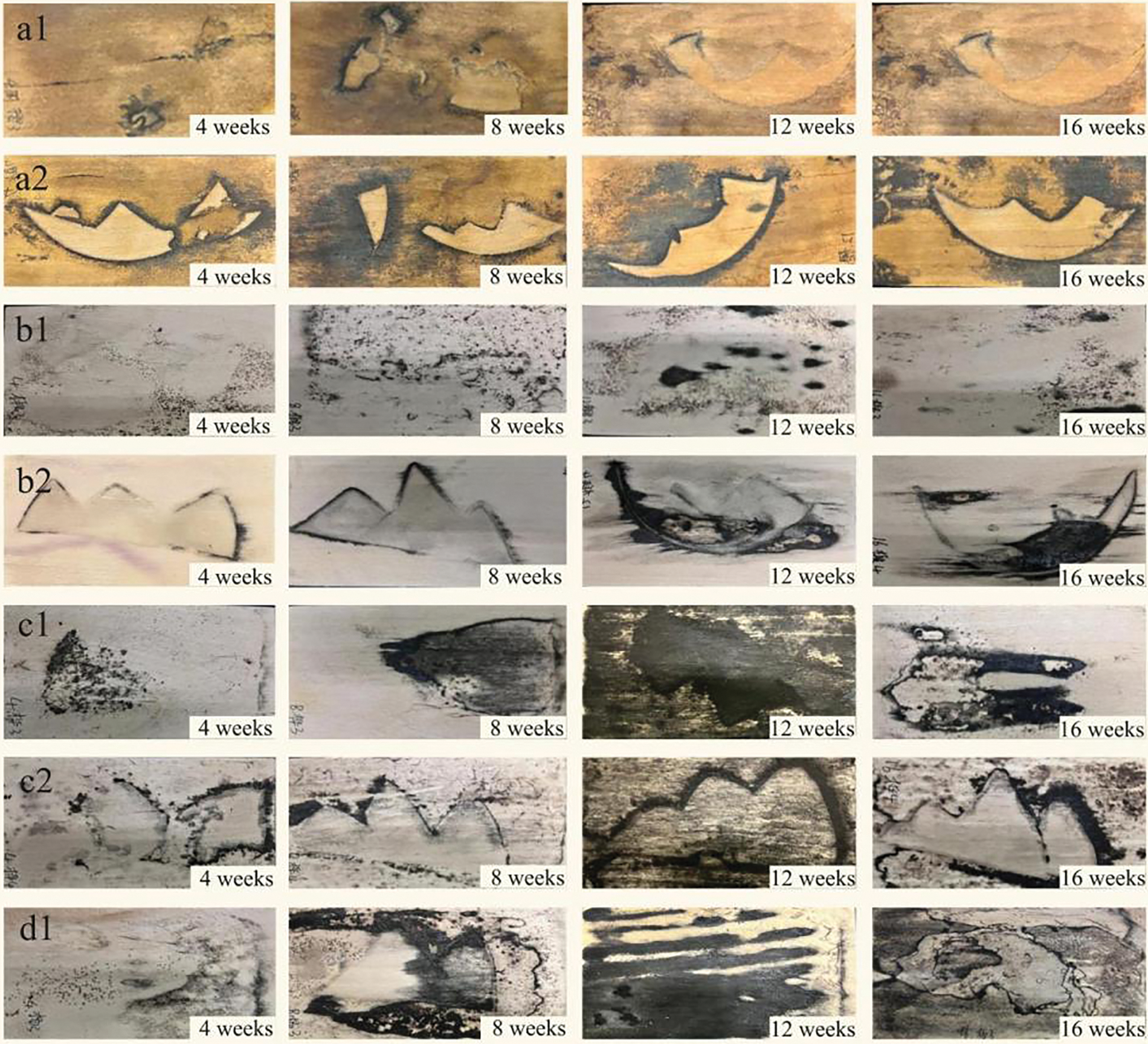
Figure 1: Comparison of the zone lines formed by CXM-3 and CXM-4 (a is cherry wood, b is basswood wood, c is birch wood, d is poplar wood; 1 is spalted prepared by strain CXM-3, 2 is spalted prepared by CXM-4)
After 8 weeks of incubation, CXM-3 formed non-closed mycelium lines on all four kinds of wood that were not restricted to a specific shape and penetrated the laminate, accompanied by extensive black staining on birch and poplar, brown dot staining on basswood, and reddish-brown non-closed mycelium lines on the back of the laminate (as shown in Fig. 4b). CXM-4 forms black clear stain as preset; on basswood and poplar it is still accompanied by light purple staining; on birch, it is accompanied by many dots and fine lines of black staining.
After 12 weeks of incubation, CXM-3 formed a large amount of black staining on all four kinds of wood and penetrated the lamellae, with only a little non-closed-shaped black staining on cherry and a large amount of grey-black spindle staining accompanying it on basswood in addition to brown dotted staining. CXM-4 forms closed black fungal lines with strong lines on cherry, basswood, and birch (As shown in Fig. 2b). The purple staining disappears from the poplar and basswood veneers and is accompanied by black staining along the longitudinal course of the preset grain on the basswood.

Figure 2: (a) Textural characteristics of CXM-3 zone lines, (a)a linear, (a)b fusiform, (a)c,d closed linear; (b) Textural characteristics of CXM-4 zone lines, (b)a, b, c, d PDA preset pattern
After 16 weeks of incubation, the zone lines cultivated by CXM-3 penetrated the lamellae. They formed delicate, soft color shades of rhythmic butterfly wing-like closed patterns on poplar only and more intense black staining on cherry, basswood, and birch. CXM-4 formed predetermined grain patterns on cherry, linden, and birch, while a large amount of intense black staining, linden by the regular longitudinal direction of the accompanying black staining accompanied cherry. A large amount of black spot staining still accompanies Birch.
CXM-4 survived on poplar wood for only four weeks, possibly because the viability of CXM-4 inoculated onto poplar wood had been weakened by multiple generations of expansion, or because the fungus was scalded to death by the hot scalpel after being sterilized by heat from an alcohol lamp during inoculation, due to improper handling.
CXM-3 and CXM-4 were inoculated onto birch wood after 12 weeks of incubation and the wood was covered with black staining on both sides. Probably because the air holes in the caps of the culture bottles were close to the humidifier holes of the constant temperature and humidity chamber, which kept the humidity at 60% continuously, the fungus grew vigorously on the thin plates and the spores spread all over the wood.
CXM-4 was inoculated on basswood and poplar wood for 4 weeks and showed black zone lines along with a purple stain running through the lamellae. It is suspected that the purple staining may be a metabolic product secreted by the fungus during the pre-growth phase.
3.3 Characterisation of the Zone Lines Texture of the Spalted Wood
Strains CXM-3 and CXM-4 both formed zone lines on all four kinds of wood. The zone lines of CXM-3 were randomly distributed in the wood in an episodic pattern, with uneven thickness and no specific direction, and the changes were not repetitive and carried an unexpected effect. The fusiform black staining appeared on the linden wood, and the zone lines on the spalted wood prepared by CXM-4 largely matched the shape cut by the PDA.
3.4 Contrastive Analysis of Zone Lines Area
The percentage of spalted area on the surface of thin sheets formed by CXM-3 and CXM-4 strains on the four kinds of wood is shown in Fig. 3. Due to the thinner zone line of CXM-3, the zone line area formed by CXM-3 is generally less than that of CXM-4. As can be seen from Fig. 4, CXM-3 has the largest proportion of effective zone lines area in the poplar spalted sheet prepared. The effective zone lines area was the smallest in the basswood spatled sheet, and CXM-4 had approximately the same zone lines area percentage in all three kinds of wood sheets prepared [22].
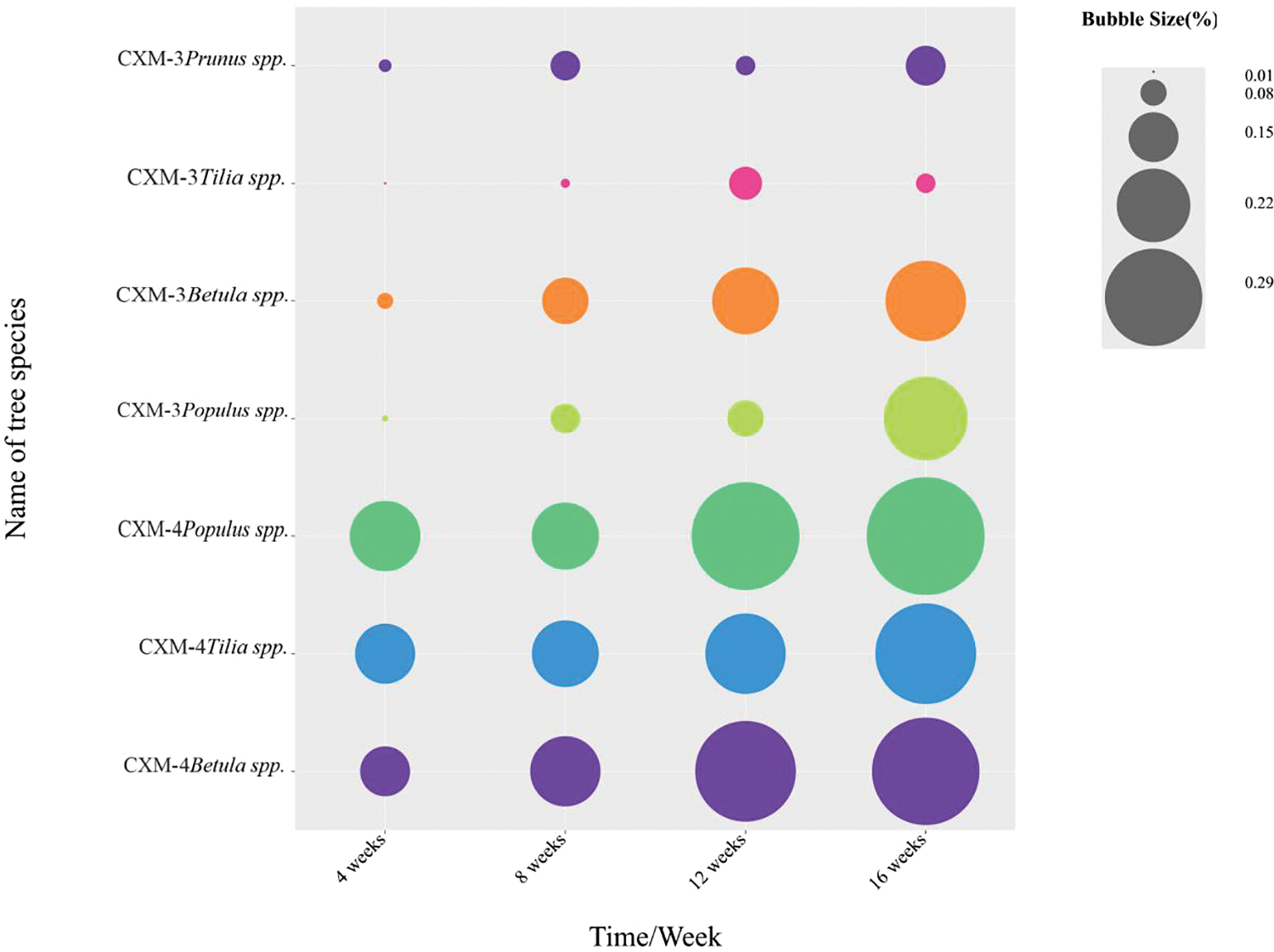
Figure 3: Comparison bubble diagram of the area of spalted wood prepared by the two strains of fungi
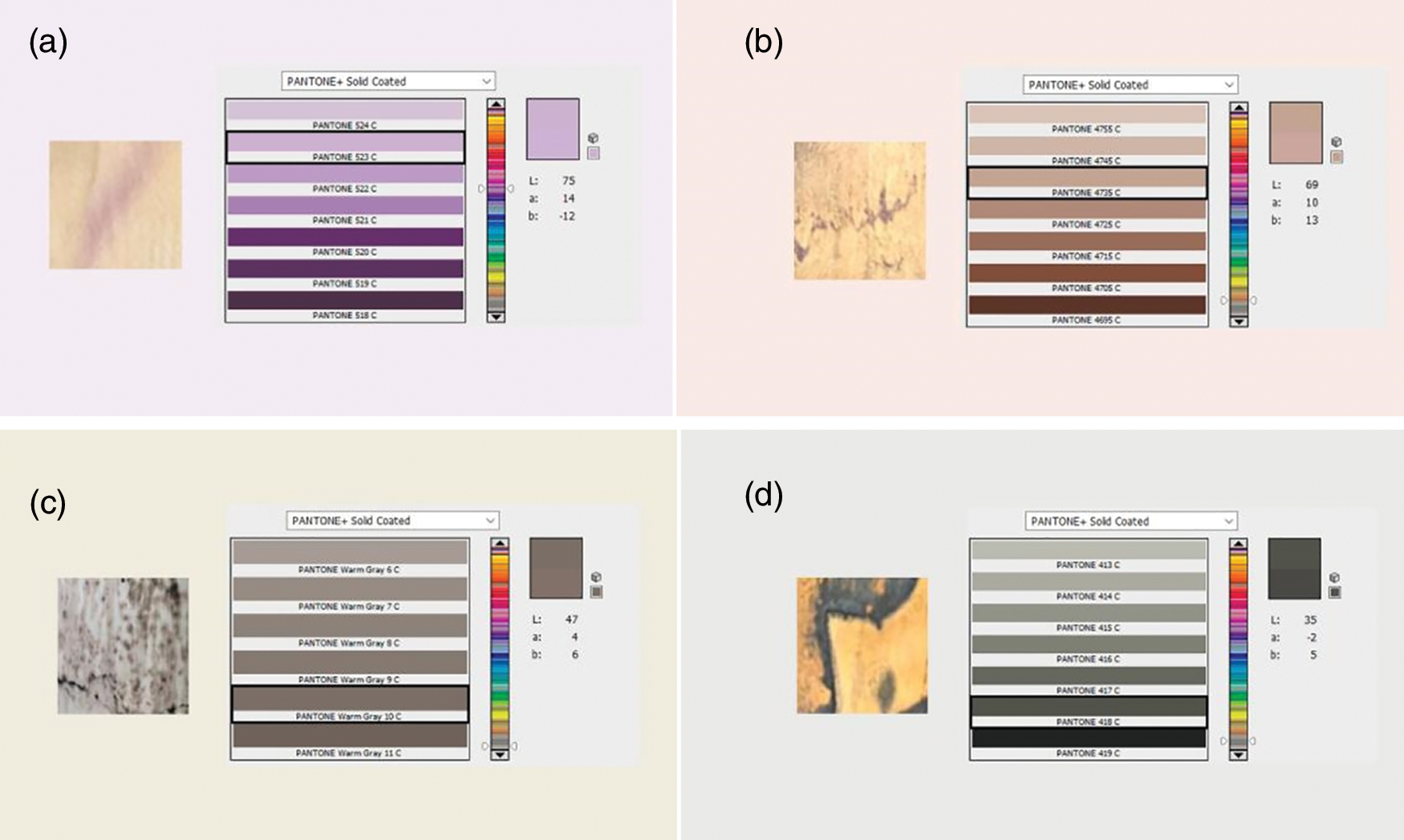
Figure 4: Pantone colours in spalted wood prepared by two strains of fungi
3.5 Characterisation of the Colour of the Spalted Wood Fungal Pattern
Two mechanisms cause the staining inside the wood, one is the secretion of pigment by the fungus outside the cell, the other is accompanied by the growth of the fungus itself inside the wood, and the pigment is attached to the cell wall, the latter is mainly caused by the cyanobacteria of the genus Coracoid, although some other genera can also cause this effect [23,24]. Stained wood can have varying degrees of toughness. Stained fungi increase permeability by removing the perforated membrane, thus allowing the wood to gain moisture more quickly. However, this makes the wood susceptible to rot. Studies on staining fungi in recent years have shown that Scytalidium cuboideum produces red pigment and Scytalidium ganodermophthorum produces yellow pigment, but the specific chemical composition is not clear [25]. Recent studies in the Peruvian Amazon have identified the Green, brown, purple, orange, and red zone lines. We have proposed four Pantone colors from two strains of fungi, Fig. 4a shows the purple color produced by CXM-4 after four weeks of incubation in basswood veneer; Fig. 4b shows the reddish-brown staining produced by CXM-3 grown on the back of an eight-week-cherry-wood-veneer; Fig. 4c shows the brown staining produced by CXM-3 on an eight-week basswood veneer, and Fig. 4d shows the dark grey staining produced by CXM-4 on a twelve-week cherry wood veneer. The brown staining produced by CXM-3 varies with the shade of wood color [26].
Qiu Jian’s team from Southwest Forestry University proposed that the reasons for endophytic fungi to form flower markings on wood include three parts: first, to resist adverse environment. Second, to maintain moisture in their growth environment. And third, to form flower markings on wood to form fruity bodies for reproduction [27]. At present, the two strains of fungi can be safely used in the laboratory. After two hours of sterilization by an ultraviolet lamp on the ultra-clean workbench, the fungi are inactive and no longer grow. The spalted wood prepared by strain CXM-3 does not have the ability to shape. Its lines are black and brown, free, delicate, and soft, mostly accompanied by black and brown dotted and faceted staining. Strain CXM-4 is effective in shaping the texture of the wood substrate material, producing specific shaped black patterns on the surface of the wood substrate material in a very short period of time, and using the solid inoculation method, specific stained patterns were produced on the surface of the substrate in the 4th week of treatment.
When the temperature was 25°C ± 2°C, humidity 60%, and wood moisture 90%, the zone lines could grow along the preset grain on basswood sheet within 8 weeks and spread black staining along the preset grain longitudinally within 8–16 weeks. Within 4 weeks, the zone lines grew along the preset grain on cherry wood and spread black staining irregularly along the preset grain within 4–16 weeks. In terms of shape characteristics, CXM-3 is a random episodic form and CXM-4 is a PDA shape; CXM-3 form is smaller in area than the thin boards prepared by CXM-4; CXM-3 produces brown and black zone lines, and CXM-4 pre-produces purple staining on light colored wood, forming grey and black zone lines. The same fungus can produce different colors on the same kind of wood over time, and the same fungus can produce different colors on different tree species, which may be due to the different endophytic fungi of tree species, and CXM-3 and CXM-4 antagonize them, resulting in different colors.
As a new, natural way of staining, fungal staining technology has a greater aesthetic value and overall utilization of wood. There is no specific directionality to the fungal lines, some follow the direction of the wood fibers, and some intersect with them to form a specific line potential, In the previous study, we observed the polarization and fluorescence characteristics of the wood slicing experiment to analyze the degradation degree of lignin and cellulose. The results showed that there was no significant difference in the brightness between mycelium wood and normal wood, no mycelium was observed, and no obvious degradation of lignin and cellulose was observed [28]. Therefore, the artificially induced formation of mottled wood enhances solid wood furniture and handicrafts’ product value and artistic value. The aim is to enable wood product practitioners, designers, and the general public to understand the aesthetic characteristics of the material and apply them to product development, improve the comprehensive utilization of wood, and broaden the creative development and shaping of wood products [29,30].
Funding Statement: The study was supported by the National Natural Science Foundation of China (No. 32160347) and the Key Laboratory of State Forestry Adminstration for Highly-Efficient Utilization of Forestry Biomass Resources in Southwest China (Southwest Forestry University) (No. 2019-KF14).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Song, T. Z. (2020). Screening and pigmentation of wood mottling fungi (Master’s Thesis). China: Guangxi University. [Google Scholar]
2. Vega, G. S., Robinson, S. C. (2020). Complexity of biodegradation patterns in spalted wood and its influence on the perception of US woodturners. European Journal of Wood and Wood Products, 78(1), 173–178. [Google Scholar]
3. Song, L. Q., Song, T. Z., Zhu, X. W., Cheng, F. C., Sun, J. P. (2022). Research progress on effects and application of wood spalted fungi on wood. Chinese Journal of Applied and Environmental Biology, 28, 805–812. [Google Scholar]
4. He, H. S. (2015). Study on the formation mechanism of flower spotted wood (Master’s Thesis). China: Southwest Forestry University. [Google Scholar]
5. He, H, S., Gan, C., T., Hao, J. Q., Qiu, J. (2019). Screening experiments on the conditions of two strains of mesocosm fungus for cultivating spalted wood of white mullein. Journal of Southwest Forestry University (Natural Science), 39, 184–188. [Google Scholar]
6. Sarath, M., Vega, G., Sara, C., Robinson, S. C. (2017). Microscopic analysis of pigments extracted from spalting fungi. Journal of Fungi, 3(1), 15. [Google Scholar]
7. Ako, T., Gentarow, O. (2022). Color in medieval castle architecture in present-day Poland and Czech Republic. Arts, 11(1), 1–15. [Google Scholar]
8. Gessler, N. N., Egorova, A. S., Belozerskaya, T. A. (2014). Melanin pigments of fungi under extreme environmental conditions (Review). Applied Biochemistry and Microbiology, 50(2), 105–113. [Google Scholar]
9. Zhang, J. W., Meng, M. L., Mitchell Hugh, D., Gaffrey Matthew, J., Orr, G. et al. (2022). Distinctive carbon repression effects in the carbohydrate-selective wood decay fungus Rhodonia placenta. Fungal Genetics and Biology, 5, 34–37. [Google Scholar]
10. Tudor, D., Robinson, S. C., Krigstin, T. L. (2014). Microscopic investigation on fungal pigment formation and its morphology in wood substrates. The Open Mycology Journal, 8(1), 174–186. [Google Scholar]
11. Robinson, S. C., Tudor, D., Hipson, S. (2013). Methods of inoculating Acer spp., Populus tremuloides, and Fagus grandifolia logs for commercial spalting application. Journal of Wood Science, 59, 351–357. [Google Scholar]
12. Robinson, S., Tudor, D., Zhang, W. (2014). Ability of three yellow pigment producing fungi to colour wood under controlled conditions. International Wood Products Journal, 5(2), 103–107. [Google Scholar]
13. Robinson, S. C., Weber, G., Hinsch, E. (2014). Utilizing extracted fungal pigments for wood spalting: A comparis on of induced fungal pigmentation to fungal dyeing. Journal of Coatings, 2014, 759073. [Google Scholar]
14. Robinson, S. C., Tudor, D., Cooper, P. A. (2012). Utilizing pigment-producing fungi to add commercial value to american beech (Fagus grandifolia). Applied Microbiology and Biotechnology, 93, 1041–1048. [Google Scholar] [PubMed]
15. He, R., Qiu, J., He, H. S., Luo, B. (2019). Current status and development trend of wood fungal staining research. Journal of Forestry Engineering, 4(3), 19–24. [Google Scholar]
16. Robinson, S. C. (2022). Cultures of spalting. Encyclopedia, 2(3), 1395–1407. [Google Scholar]
17. Zhong, Z. X., Wu, S. D., Duan, Y. K. (2018). Estimation of timber volume consumption of fungus coated wood. Journal of Southwest Forestry University (Natural Science), 38, 217–220. [Google Scholar]
18. He, H. S., Qiu, J., Gan, C. T. (2013). Current status and outlook of the research on flametree. Journal of Southwest Forestry (Natural Science), 33(6), 94–98. [Google Scholar]
19. He, H. S., Qiu, J., Gan, C. T., Guo, M. L., Pan, Z. H. (2014). A survey of zone lines line forming prone tree species. Forestry Research, 27(6), 776–780. [Google Scholar]
20. University of Würzburg (2020). Impulse for research on fungi. NewsRx Health & Science, 20, 1–3. [Google Scholar]
21. Arya, U., Peterson, W., Sadeghi, F., Meinel, A., Grillenberger, H. (2023). Investigation of oil flow in a ball bearing using bubble image velocimetry and CFD modeling. Tribology International, 3, 177. [Google Scholar]
22. Vega, G. S., Robinson, S. C. (2017). Microscopic analysis of pigments extracted from spalting fungi. Journal of Fungi, 3(1), 15–17. [Google Scholar]
23. Bai, Y. F., Wang, Y., Shi, L., Jiang, J. J. (2022). Pen for the needle, line for the ink. International Journal of Frontiers in Sociology, 4, 3–7. [Google Scholar]
24. Vega, G. S., Robinson, S. C. (2016). Spalting fungi: Genetic identification, material interaction and microscopical characteristics of extracted pigment. Journal of Oregon State University, 16, 20–25. [Google Scholar]
25. Qiu, J., Li, Z., Jia, H. W., He, H. S. (2021). New direction in formation mechanism of spalted wood. Journal of Southwest Forestry University (Natural Science), 41, 1–7. [Google Scholar]
26. Song, T. Z. (2020). Stain capacity of three fungi on two fast-growing wood. Journal of Forestry Research, 6, 32–37. [Google Scholar]
27. Yan, Y., Qin, L., Huang, Q. T., Zhang, L. M., Qiu, J. (2022). Polarization-fluorescence analysis of three species of spalted wood rubberwood. Journal of Northwest Forestry University, 37(6), 161–166. [Google Scholar]
28. Sun, F. L., Zhang, J. P., Lin, H. K. (2003). Research situation and development trend of wood dyeing. The Journal of Northwest Forestry University, 18(3), 96–98. [Google Scholar]
29. Peng, W. X., Cui, X. Y., Wang, J. E., Zhang, Z. L. (2005). Research status and development of wood dyeing technology. Wood Industry, 19(6), 1–3. [Google Scholar]
30. Cui, X. L., He, S. H. (2013). Research status and development trend of wood dyeing. Forestry Machinery & Woodworking Equipment, 41(3), 12–14. [Google Scholar]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools