

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2022.019410
REVIEW
Phytogenic Synthesis of Metal/Metal Oxide Nanoparticles for Degradation of Dyes
1Department of Biotechnology, School of Engineering & Technology, Sharda University, Greater Noida, 201310, India
2Department of Applied Chemistry, School of Applied Natural Science, Adama Science and Technology University, Adama, 1888, Ethiopia
3Sulaimani Polytechnic University, Slemani, 46001, Iraq
4Department of Horticulture, University of Raparin, Ranya, 46012, Iraq
5Department of Pharmacy, International Islamic University Chittagong, Chittagong, 4318, Bangladesh
6Department of Botany, Mahatma Gandhi Central University, Motihari, 845401, India
*Corresponding Authors: Arpita Roy. Email: arbt2014@gmail.com; Ram Prasad. Email: rpjnu2001@gmail.com
Received: 23 September 2021; Accepted: 11 November 2021
Abstract: Now-a-days nanotechnology is one of the booming fields for the researchers. With the increase in industrialization mainly textile, paper, medicine, plastic industry, there is an increase in concentration of organic dyes as pollutant. Release of harmful dyes in water bodies has become a serious issue, as most of the dyes are carcinogenic and mutagenic in nature and causes various diseases. Therefore, there is a requirement to find out new approaches for efficient treatment of effluent containing dyes. Nanoparticles are one of the potential solutions to this problem. They can be synthesized from different methods, however synthesis of nanoparticles from different plant parts (leaf, root or stem extract) is economical as well as ecofriendly. Phytogenic nanoparticles have various environmental applications and one of them is remediation of dyes. The aim of this review is to provide an overview of last five years studies about catalytic and photocatalytic degradation of various harmful dyes by plant synthesized nanoparticles, mechanism of degradation and advantages and disadvantages of phytogenic synthesis.
Keywords: Nanoparticles; green synthesis; plants; dyes; remediation, metal oxide
Organic, inorganic, and biological contaminants infiltrate into water bodies due to the increase of urbanization and industrialization, which is hazardous to humans and the environment. Environmental pollution is a major concern for public health because it becomes the root cause of various diseases throughout the world [1,2]. In the current situation, clean water is one of the major challenges society faces around the world. The increasing human population is one of the major factors for water pollution. Several physical, chemical, and biological methods are available for wastewater treatment. Current wastewater treatment methods have limitations such as inefficient energy, generation of residuals, etc. [3,4]. Therefore, an alternative way to overcome these problems is required.
One of the main elements of wastewater pollution is the presence of organic dyes. Synthetic dyes are released from various industrial applications such as printing, textile, leather etc., and are considered as detrimental pollutants that contain complex structures and cannot be easily degraded [5]. It is estimated that 10–15% of the dyes are gone in the effluent during the process of dying in the textile industry. Textile industry wastewater is one of the main sources of aromatic amines released into the environment that cause various adverse effects [6]. The presence of coloring-containing dyes is due to the azo bond (–N = N–) and related chromophores, therefore dumping of dyes into surface water not only influences the aesthetic but also produces biotoxicity [7]. The existence of harmful synthetic dyes in the soil as well as water is hazardous for humans and adversely affects our natural ecosystem. These dyes are accumulating with water and soil, and then enter into the food chain; thereby it turns into a serious threat to food security. Dyes have become a major concern compared to other environmental pollutants because these dyes cannot be shattered by natural degradation [8]. The majority of dyes and their breakdown products are carcinogenic, and some reactive dyes induce allergies such as skin irritation, eye infection, and respiratory disorders, etc. [9]. Some also have mutagenic effects, especially azo dyes. There are various methods used for the treatment of textile dye effluents such as chemical methods, physical methods, biological methods by using enzymes, microorganisms [10] (Fig. 1). Traditional remediation methods of cleanup are ineffective in dealing with this toxicity. Hence, there is a demand for an advanced method of degradation of dyes.
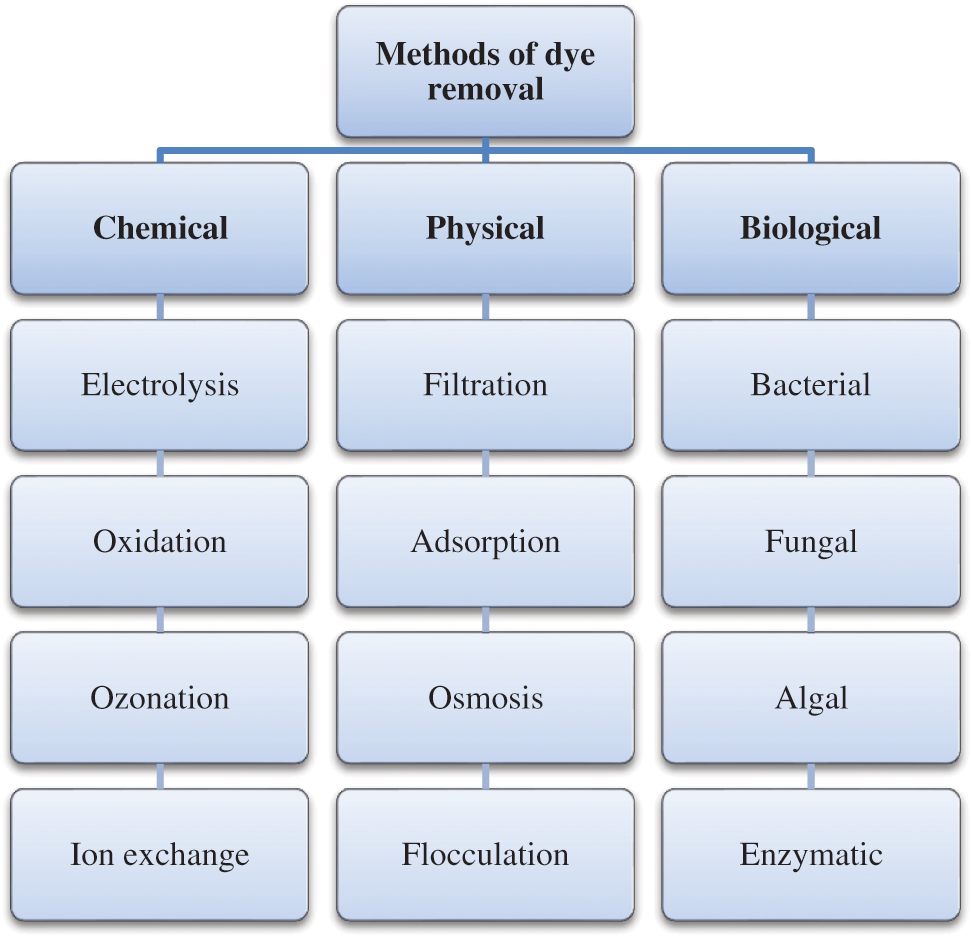
Figure 1: Traditional methods of dye removal
Nanotechnology provides an efficient approach to eliminating various contaminants from wastewater. Integration of nanoscale materials into functional assemblies and in addition to multifunctional devices can be accomplished through a ‘bottom-up’ approach. Nanotechnology has been used in the past few years in numerous fields like cosmetics, medicines, paints, textiles, crafting iridescent glasswork, creation of weapons, etc. [11]. The removal of contaminants from the environment is supposed as rectification. Bioremediation is the term used when biological agents are involved in the rectification process. Specifically, once living plants are concerned with the degradation of pollutants which is referred to as phytoremediation. Nanophytoremediation is a newer technique for degrading toxic pollutants and that blends applied science with living plants [12].
Research on nanomaterial synthesis is of great interest due to its exceptional properties such as magnetic, optoelectronic, and mechanical that are different from bulk materials [13]. These unique emerging features have a potential role in electronics, medicines, and other fields. Due to the small size of nanoparticles, they exhibit enhanced characteristics and are used for different applications [14,15]. Nanoparticles are generally grouped into two types, i.e., organic nanoparticles which include carbon nanoparticles such as fullerenes, and inorganic nanoparticles which include magnetic, metal, and semiconductor nanoparticles [16]. Metal nanoparticles, such as gold and silver, have recently sparked renewed attention due to their superior material characteristics and functional diversity [17,18]. Various reports have suggested the synthesis of different nanoparticles such as copper, gold, silver, zinc, iron, etc., using plant extracts [19,20]. Different researchers have reported utilization of green synthesized nanoparticles for the efficient removal of different dyes such as phenol red, methyl orange, methyl red, eosin yellow, etc. [21–23]. This review summarizes the different plant-derived nanoparticles that were used for the degradation of various dyes.
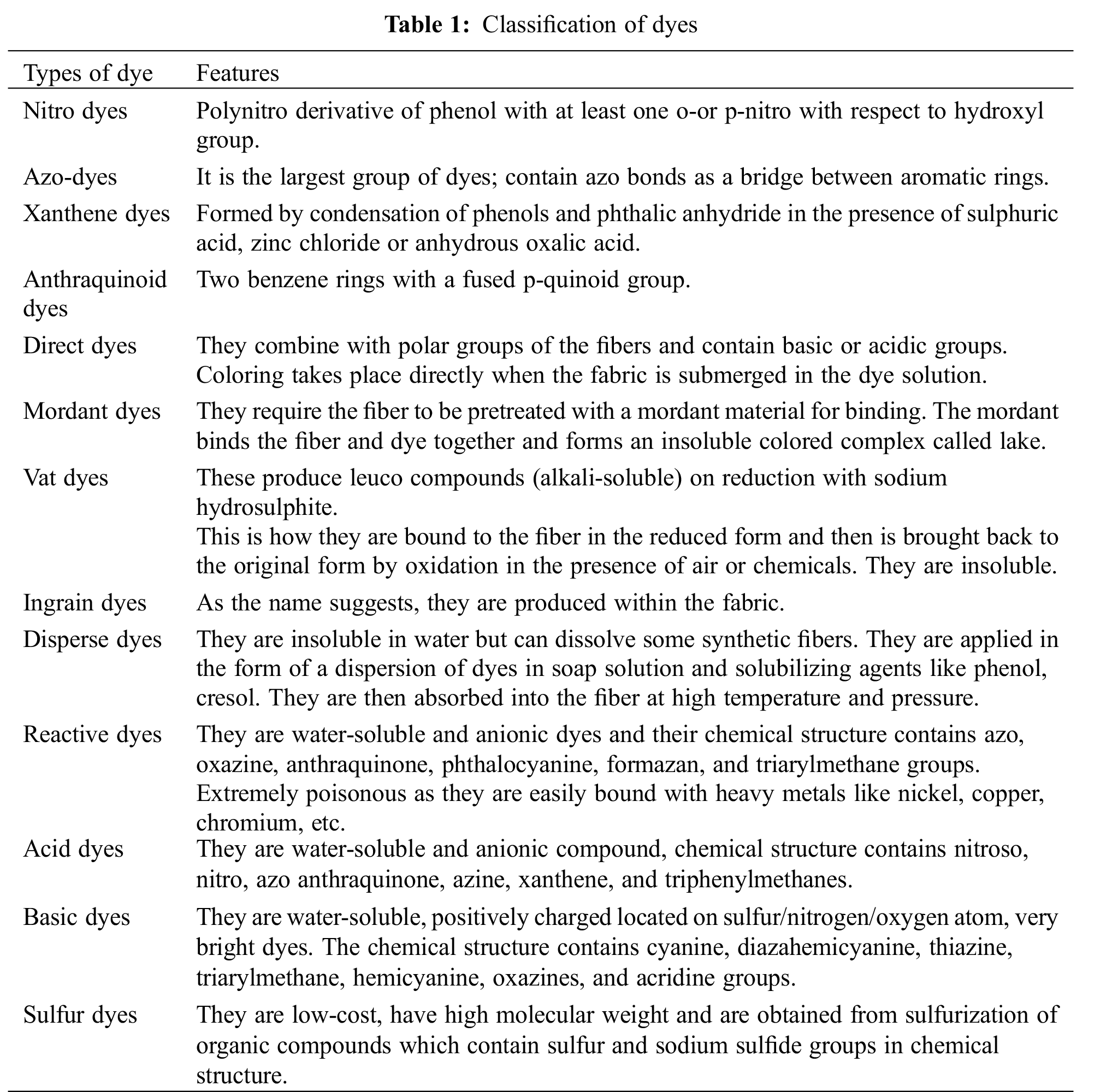
Classification of dyes can be done based on a number of parameters like chemical structure, applications, ionic charge, and color (Table 1).
3 Problems Associated with Dye Contamination
Dyes are substances that are applied to a substrate to give color. Some of the common dyes that act as a pollutant are methyl orange, eosin Y, methylene blue, etc. [24] (Fig. 2). Dyes can be classified based on their color, ingredients, and applications, with dye applications being the most popular approach in nanomaterials [25].
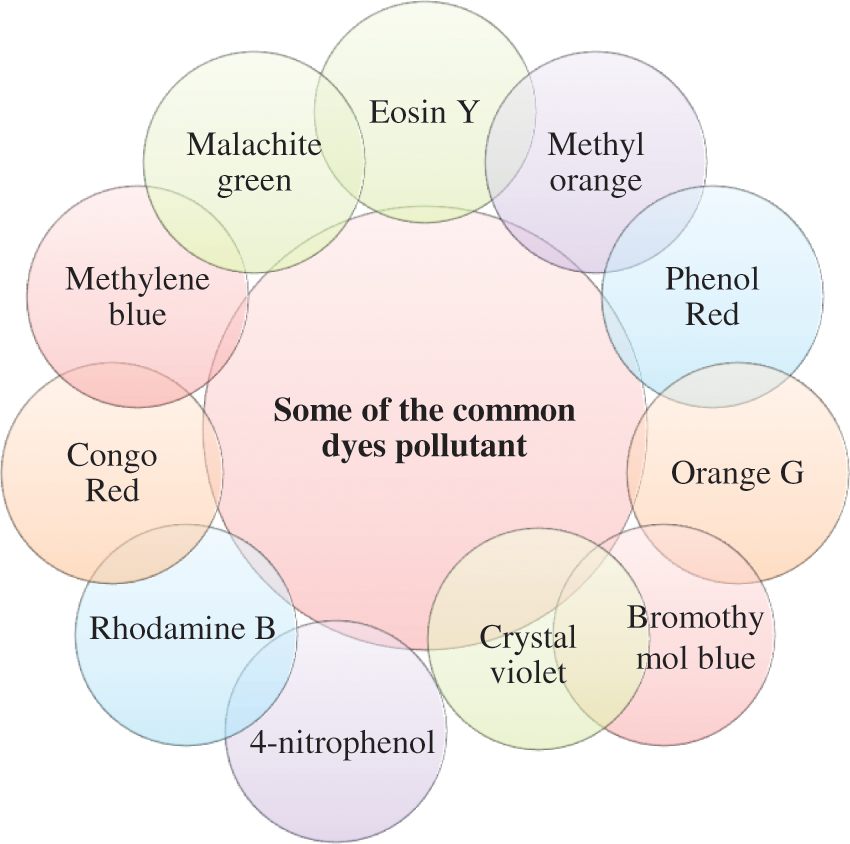
Figure 2: Common dye pollutants
Dyes are utilized in a wide range of industries, including textile, printing, cosmetic, plastic, drug, and food processing. Due to their high solubility, they are found in industrial wastewater. Even tiny quantities of dye as low as 1 ppm are highly noticeable in the case of some dyes and are considered undesirable components for the environment [26]. The dye contamination problem is significant (2%) of total dye that is directly discharged from the aqueous effluent [27,28]. Typically, dyes affect the immune system but can also cause several other problems (Fig. 3). Industrial effluents are rigorously regulated for organic compounds and to keep their limit, removal of dye from wastewater is essential. The occurrence of dyes in the natural resources of water can result in a reduction of sunlight penetration and depletion of dissolved oxygen. Azo dyes are water-soluble and toxic; they pose a potential threat to animals, aquatic organisms, and human beings [29]. In the process of degradation of some azo dyes, they produce carcinogenic aromatic amines. Dyes can also penetrate groundwater and cause contamination of soil. Inhalation of dye particles can cause various respiratory problems which is the most common hazard linked with reactive dyes [30]. Itching, watering of the eyes, sneezing, coughing, and breathlessness are some of the signs associated with this kind of problem (Fig. 3).
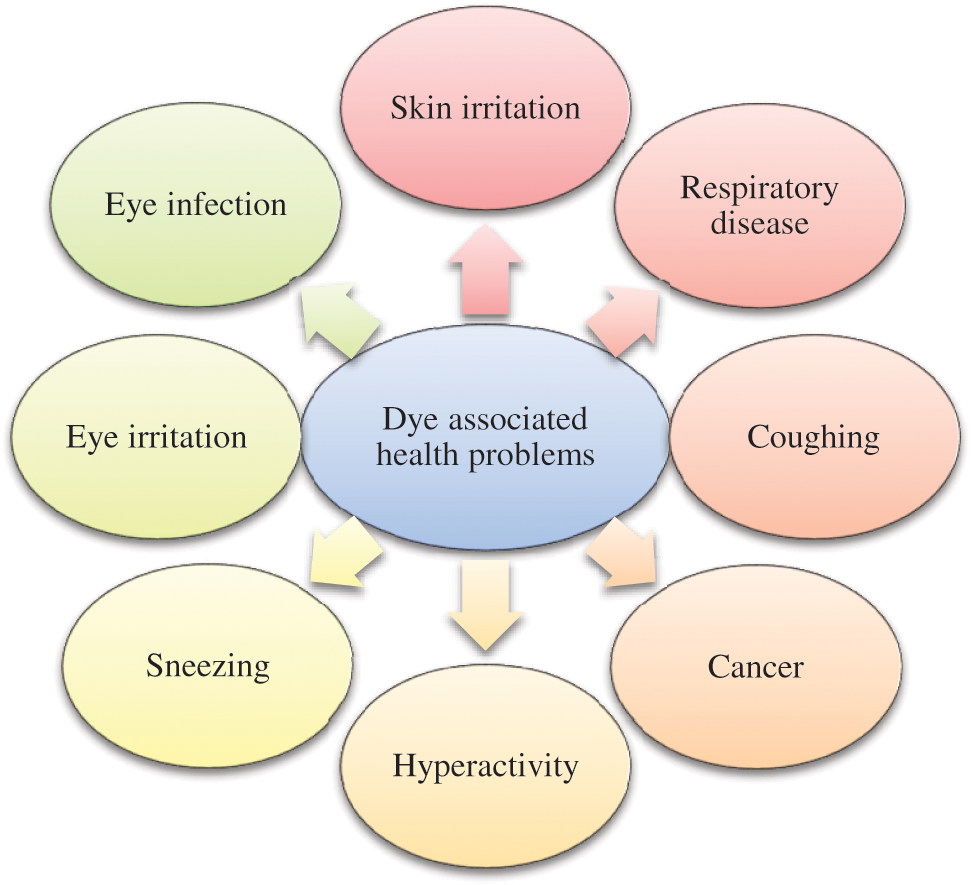
Figure 3: Health problems associated with dyes
4 Green Synthesis of Nanoparticles
For the green production of nanoparticles, the bottom-up technique is applied, and the reaction that occurs is oxidation/reduction [31]. The need for a greener approach arose due to the cost and environmental problems associated with physical and chemical processes [32]. Chemical synthesis occurs in the presence of many harmful chemicals absorbed on the surface and has adverse effects when utilized for medical applications. Because the chemical approach uses that are hazardous to the environment, researchers have switched to the green synthesis of nanoparticles to avoid the toxic impacts of chemicals [33]. To find out a cheaper way for the synthesis of nanoparticles, researchers utilize various biological agents such as enzymes from microbes, fungal extracts, and extracts of plants. Due to their reducing properties, they are generally accountable for metal compounds into their respective nanoparticles. Researchers prefer plants for the synthesis of nanoparticles over bacteria or other biological substances that of its several advantages over other biological entities [34]. Plants are safe to handle, easy to maintain, easily available, and contain a variety of biomolecules such as tannins, alkaloids, terpenoids, etc. Nanoparticle synthesis using plants involves the bioreduction of metal ions. Plant extract and metal precursors are mixed at a suitable reaction condition whereas plant extract acts as a stabilizing and reducing agent during the synthesis process [20,22] (Fig. 4).

Figure 4: Green synthesis of nanoparticles using plants
5 Different Types of Metallic/Metal Oxide Nanoparticles
Generally, metallic nanoparticles are solid colloidal metal particles that have a size from 10–100 nm. Metallic nanoparticles with different shapes and sizes based on exceptional and useful physical and chemical properties have been commonly considered in various applications [19,20]. Metallic/metal-oxide nanoparticles which are commonly used in the environmental remediation process include silver, gold, iron/iron oxide, copper/copper oxide, zinc oxide, etc.
Gold nanoparticles: Gold is a noble metal; it is used for the manufacturing of nanosized materials and possesses several properties such as low toxicity to biological systems, conformational flexibility, etc. [35]. Synthesis of gold nanoparticles can be done by reducing gold in the aqueous phase, which tends to have a quasi-sphere morphology because this shape represents the smallest surface area compared to other morphologies [36]. The suspension of gold nanoparticles shows a ruby-red color due to light scattering by the nanoparticles, but an increase in size or a change in environmental parameters leads to the modification of the optical properties of the colloids [37].
Silver nanoparticles: Silver nanoparticles (AgNPs) are used for various purposes such as medical use, healthcare sector, food industry, etc. It possesses some unique properties such as electrical, optical, thermal, and biological [38]. AgNPs can be synthesized by chemical as well as biological methods. But biological methods are simple, fast, and nontoxic and can produce a well-defined size and morphology under optimized conditions [39].
Iron and iron oxide nanoparticles: Iron nanoparticles show better reactivity with oxygen in comparison with bulk iron particles [40]. Iron nanoparticles are present in ultrahigh purity compared to iron. Iron nanoparticles possess high thermal conductivity, high surface area, and also very high magnetic properties [41]. The reactivity of iron nanoparticles is because of their surface area. Iron nanoparticles can be more useful in a non-oxidizing environment because a large amount of energy is stored in nanoparticles as surface energy [42]. Iron oxide nanoparticles are one of the most important oxides, they have been widely applied in catalysis, biomedicine, magnetic materials, water treatment, and other fields [43].
Copper and copper oxide nanoparticles: Copper nanoparticles have diverse applications in fields such as agriculture, agriculture, industrial engineering, and technological fields [22]. The synthesis of copper nanoparticles has attracted particular interest, compared to other nanoparticles, because of their certain properties, that is, they are less expensive than gold and silver nanoparticles [44]. The copper nanoparticle can be synthesized by numerous methods such as vapor deposition, thermal deposition, radiolysis reduction, chemical reduction of metal salts of copper, electrochemical reduction also room temperature synthesis from hydrazine hydrate and starch [45]. From recent research, it was found that green synthesis of copper nanoparticles can be achieved by using plant extract as well as microorganisms. Due to the effective environmental applications of copper nanoparticles, it has been used in various processes [46]. Copper oxide is a p-type semiconductor with diversified applications [47]. Surface conductivity makes CuO an ideal material for semiconductor resistive gas sensor applications. Copper oxide nanoparticles show a potential role in the degradation of dyes and other environmental pollutants [47].
Zinc oxide nanoparticles: Zinc oxide is known as multifunctional material due to its unique chemical and physical properties [48]. Zinc oxide has applications in solar cells, ceramics, gas sensors, catalysts, and cosmetics because it is a semiconductor in nature [49]. The morphology of zinc oxide nanoparticles depends upon the process by which it has been synthesized. They can be nanoplates, nanorods, nanospheres, hexagonal, tripods, tetrapods, etc. [50]. Environmental applications of zinc oxide nanoparticles include dye degradation and wastewater remediation, etc. [51,52].
Nickel nanoparticles: Nickel nanoparticles have been widely explored due to their various potential applications and their superior ferromagnetic properties such as high coercive forces, magneto-crystalline anisotropy, and chemical stability [53].
6 Characterization Methods of Synthesized Nanoparticles
Various techniques have been used for the characterization purpose which includes UV-visible spectroscopy, i.e., based on surface plasmon resonance (SPR) principle [19]. Another technique is X-ray diffraction (XRD) to determine the crystalline phase [22]. Fourier transform infrared (FTIR) spectroscopy helps to identify the functional groups present in the sample. Characterization of nanoparticles based on size, morphology, and surface charge was performed using atomic force microscopy (AFM), scanning electron microscopy, and transmission electron microscopy [20,23]. Dynamic light scattering is used to measure nanoparticles size.
7 Dye Degradation Using Plant-Derived Nanoparticles
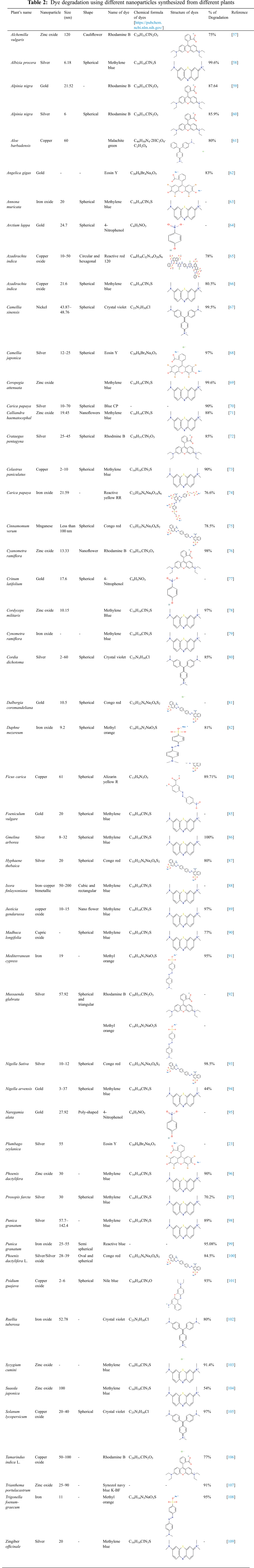
Photocatalytic degradation of dyes and other pollutants has been extensively researched since the mid-twentieth century. Dyes are commonly used in many products such as furniture, clothes, plastics, etc. During the dyeing process, approximately 12% of the dyes are eliminated as waste and 20% of that waste penetrates the environment and can severely affect populations causing serious health hazards [54]. These reactive dyes possess respiratory problems because of the inhalation of dye particles [55]. Phytoremediation using nanomaterials is one of the modern methods for dye degradation. Nanoscale particles are gaining attention for remediation of environmental pollution. Nanoparticles are encouraged as an efficient nutrient source for biomass production from plants because of intensified metabolic activities, and utilization of native nutrients by promoting microbial activities [56]. The main concern of using nanomaterial is reducing the use of harmful chemicals. Both catalytic and photocatalytic degradation of organic dyes is reported in the literature. Photocatalytic degradation has an advantage over other methods, it is environment-friendly, less expensive because it uses solar light, and does not produce any secondary pollutants. Application of various plant-derived nanoparticles in dye degradation has been mentioned in Table 2.
8 Mechanism of Dye Degradation
As a result of the exceptional characteristics of nanoparticles, they can be utilized for the remediation of harmful dyes. Nanoparticles act as catalysts and increase the degradation mechanism. Nanoparticles are irradiated by a light source such as UV light or visible light [21]. Degradation method is either accomplished by directly treating the nanoparticle surface with high-energy light sources or with the assistance of the photosensitization pathway. In the case of direct photocatalytic degradation, electrons excited from the valence band (filled) to the conduction band through energy given by light and it is called photoexcitation. Then photoexcited electrons are utilized by dissolved oxygen that is available in the reaction mixture so that the formation of free radical oxygen can take place. At the same time, photoexcited holes produced in the valence band can oxidize the adsorbed water molecules to produce OH radicals. These free radicals are very reactive and able to degrade dye molecules that have been adsorbed on the photocatalyst [21].
9 Advantages and Disadvantages of Nanoparticles Use in Dye Degradation
Nanoparticles have various applications, and there is great interest in investigating and developing new methods that can help to remediate environmental problems such as dyes [110]. There are different methods available for nanoparticles synthesis, phytogenic synthesis of nanoparticles has various advantages such as easier management, large production, simple downstream processing, and different applications. However, the synthesis procedure is limited to a few metals, some sulfides, and oxides [110,111]. Therefore, there is a requirement to develop newer methods to synthesize nanoparticles from metal oxides and even their carbides and nitrides [111]. More experimental validations are required to understand the mechanism involved in the synthesis process. Plant-derived approaches pose some challenges when it comes to industrial applications. Nanoparticles’ shape and size depending upon their plant localization and metal ions content. Furthermore, isolation, extraction, and purification approaches are not very effective and create problems, which lead to a lower rate of recovery.
Nowadays environmental pollution is a serious concern which increases day by day. The major concern of the remediation process is that the methods themselves will not affect the environment. The use of harsh chemicals and toxic materials must be avoided and also the cost of the process must be below. For a cost-effective and environment-friendly remediation technology, bioremediation and phytoremediation must be preferred. Using plants and microorganisms for remediation technology is good for our environment. For dye degradation nano-phytoremediation is a new achievement. Nanotechnology is growing very fast, because of the small size of nanoparticles, it possesses unique properties which is better than bulk matter. Nanoparticles synthesized from plants are a good alternative for the remediation process and effective for dye degradation. Biological synthesis of nanoparticles using plant material provides an environmentally friendly, quick, and effective track for nanoparticle synthesis. Synthesized nanoparticles have potential applications in the biomedical field, sensor development, and environmental remediations.
Funding Statement: Dr. Arpita Roy is thankful to Sharda University for providing seed fund (Seed fund-4 2001 (SUSF2001/12)).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Ali, H., Khan, E., Ilahi, I. (2019). Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. Journal of Chemistry, 2019, 6730305. DOI 10.1155/2019/6730305. [Google Scholar] [CrossRef]
2. Chahal, S., Rani, N., Kumar, A., Kumar, P. (2020). Electronic structure and photocatalytic activity of samarium doped cerium oxide nanoparticles for hazardous rose bengal dye degradation. Vacuum, 172, 109075. DOI 10.1016/j.vacuum.2019.109075. [Google Scholar] [CrossRef]
3. Chahal, S., Rani, N., Kumar, A., Kumar, P. (2019). UV-irradiated photocatalytic performance of yttrium doped ceria for hazardous Rose Bengal dye. Applied Surface Science, 493, 87–93. DOI 10.1016/j.apsusc.2019.06.284. [Google Scholar] [CrossRef]
4. Hu, H., Li, X., Wu, S., Yang, C. (2020). Sustainable livestock wastewater treatment via phytoremediation: current status and future perspectives. Bioresource Technology, 315, 123809. DOI 10.1016/j.biortech.2020.123809. [Google Scholar] [CrossRef]
5. Sharma, J., Sharma, S., Soni, V. (2021). Classification and impact of synthetic textile dyes on Aquatic Flora: A review. Regional Studies in Marine Science, 45, 101802. DOI 10.1016/j.rsma.2021.101802. [Google Scholar] [CrossRef]
6. Kishor, R., Purchase, D., Saratale, G. D., Saratale, R. G., Ferreira, L. F. R. et al. (2021). Ecotoxicological and health concerns of persistent coloring pollutants of textile industry wastewater and treatment approaches for environmental safety. Journal of Environmental Chemical Engineering, 9(2), 105012. DOI 10.1016/j.jece.2020.105012. [Google Scholar] [CrossRef]
7. Selvaraj, V., Karthika, T. S., Mansiya, C., Alagar, M. (2020). An over review on recently developed techniques, mechanisms and intermediate involved in the advanced azo dye degradation for industrial applications. Journal of Molecular Structure, 1224, 129195. DOI 10.1016/j.molstruc.2020.129195. [Google Scholar] [CrossRef]
8. Rasheed, T., Shafi, S., Bilal, M., Hussain, T., Sher, F. et al. (2020). Surfactants-based remediation as an effective approach for removal of environmental pollutants—A review. Journal of Molecular Liquids, 318, 113960. DOI 10.1016/j.molliq.2020.113960. [Google Scholar] [CrossRef]
9. Mani, S., Chowdhary, P. (2020). Dyes: Industrial applications and toxicity profile. In: Contaminants and clean technologies, pp. 137–148. Boca Raton: CRC Press. [Google Scholar]
10. Adane, T., Adugna, A. T., Alemayehu, E. (2021). Textile industry effluent treatment techniques. Journal of Chemistry, 2021, 5314404. DOI 10.1155/2021/5314404. [Google Scholar] [CrossRef]
11. Fakruddin, M., Hossain, Z., Afroz, H. (2012). Prospects and applications of nanobiotechnology: A medical perspective. Journal of Nanobiotechnology, 10(1), 1–8. DOI 10.1186/1477-3155-10-31. [Google Scholar] [CrossRef]
12. Verma, A., Roy, A., Bharadvaja, N. (2021). Remediation of heavy metals using nanophytoremediation. In: Advanced oxidation processes for effluent treatment plants, pp. 273–296. Netherlands: Elsevier. DOI 10.1016/B978-0-12-821011-6.00013-X. [Google Scholar] [CrossRef]
13. Baig, N., Kammakakam, I., Falath, W. (2021). Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Materials Advances, 2(6), 1821–1871. DOI 10.1039/D0MA00807A. [Google Scholar] [CrossRef]
14. Yaqoob, A. A., Ahmad, H., Parveen, T., Ahmad, A., Oves, M. et al. (2020). Recent advances in metal decorated nanomaterials and their various biological applications: A review. Frontiers in Chemistry, 8, 341. DOI 10.3389/fchem.2020.00341. [Google Scholar] [CrossRef]
15. Zaman, M., Ahmad, E., Qadeer, A., Rabbani, G., Khan, R. H. (2014). Nanoparticles in relation to peptide and protein aggregation. International Journal of Nanomedicine, 12(9), 899–912. DOI 10.2147/IJN.S54171. [Google Scholar] [CrossRef]
16. Ealia, S. A. M., Saravanakumar, M. P. (2017). A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conference Series: Materials Science and Engineering, 263(3), 032019. https://iopscience.iop.org/article/10.1088/17. [Google Scholar]
17. Fadeel, B., Garcia-Bennett, A. E. (2010). Better safe than sorry: Understanding the toxicological properties of inorganic nanoparticles manufactured for biomedical applications. Advanced Drug Delivery Reviews, 62(3), 362–374. DOI 10.1016/j.addr.2009.11.008. [Google Scholar] [CrossRef]
18. Liang, R., Wei, M., Evans, D. G., Duan, X. (2014). Inorganic nanomaterials for bioimaging, targeted drug delivery and therapeutics. Chemical Communications, 50(91), 14071–14081. DOI 10.1039/C4CC03118K. [Google Scholar] [CrossRef]
19. Roy, A., Bharadvaja, N. (2017). Qualitative analysis of phytocompounds and synthesis of silver nanoparticles from Centella asiatica. Innovative Techniques in Agriculture, 1(2), 88–95. [Google Scholar]
20. Roy, A. (2021). Plant derived silver nanoparticles and their therapeutic applications. Current Pharmaceutical Biotechnology, 22(14), 1834–1847. DOI 10.2174/1389201021666201027155708. [Google Scholar] [CrossRef]
21. Mittal, S., Roy, A. (2021). Fungus and plant-mediated synthesis of metallic nanoparticles and their application in degradation of dyes. In: Photocatalytic degradation of dyes, pp. 287–308. Netherlands: Elsevier. DOI 10.1016/B978-0-12-823876-9.00009-3. [Google Scholar] [CrossRef]
22. Mehata, M. S. (2021). Green synthesis of silver nanoparticles using Kalanchoe pinnata leaves (life plant) and their antibacterial and photocatalytic activities. Chemical Physics Letters, 778, 138760. DOI 10.1016/j.cplett.2021.138760. [Google Scholar] [CrossRef]
23. Roy, A., Bharadvaja, N. (2019). Silver nanoparticle synthesis from Plumbago zeylanica and its dye degradation activity. Bioinspired, Biomimetic and Nanobiomaterials, 8(2), 130–140. DOI 10.1680/jbibn.18.00036. [Google Scholar] [CrossRef]
24. Pradeeba, S. J., Sampath, K., Ramadevi, A. (2019). Photo-catalytic degradations of methylene blue, malachite green and Bismarck brown using poly (azomethine)/TiO2 nanocomposite. Cluster Computing, 22(2), 3893–3909. DOI 10.1007/s10586-018-2505-4. [Google Scholar] [CrossRef]
25. Benkhaya, S., M’rabet, S., El Harfi, A. (2020). A review on classifications, recent synthesis and applications of textile dyes. Inorganic Chemistry Communications, 115, 107891. DOI 10.1016/j.inoche.2020.107891. [Google Scholar] [CrossRef]
26. Rahimian, R., Zarinabadi, S. (2020). A review of studies on the removal of methylene blue dye from industrial wastewater using activated carbon adsorbents made from almond bark. Progress in Chemical and Biochemical Research, 3(3), 251–268. DOI 10.33945/SAMI/PCBR.2020.3.8. [Google Scholar] [CrossRef]
27. Tkaczyk, A., Mitrowska, K., Posyniak, A. (2020). Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Science of the Total Environment, 717, 137222. DOI 10.1016/j.scitotenv.2020.137222. [Google Scholar] [CrossRef]
28. Bhatia, D., Sharma, N. R., Singh, J., Kanwar, R. S. (2017). Biological methods for textile dye removal from wastewater: A review. Critical Reviews in Environmental Science and Technology, 47(19), 1836–1876. DOI 10.1080/10643389.2017.1393263. [Google Scholar] [CrossRef]
29. Lellis, B., Fávaro-Polonio, C. Z., Pamphile, J. A., Polonio, J. C. (2019). Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnology Research and Innovation, 3(2), 275–290. DOI 10.1016/j.biori.2019.09.001. [Google Scholar] [CrossRef]
30. Maity, S., Singha, K., Pandit, P. (2021). Chemical risk assessment in textile and fashion. In: Chemical management in textiles and fashion, pp. 53–78. Cambridge: Woodhead Publishing. [Google Scholar]
31. Shah, M., Fawcett, D., Sharma, S., Tripathy, S. K., Poinern, G. E. J. (2015). Green synthesis of metallic nanoparticles via biological entities. Materials, 8(11), 7278–7308. DOI 10.3390/ma8115377. [Google Scholar] [CrossRef]
32. de Marco, B. A., Rechelo, B. S., TÃtoli, E. G., Kogawa, A. C., Salgado, H. R. N. (2019). Evolution of green chemistry and its multidimensional impacts: A review. Saudi Pharmaceutical Journal, 27(1), 1–8. DOI 10.1016/j.jsps.2018.07.011. [Google Scholar] [CrossRef]
33. Saif, S., Tahir, A., Chen, Y. (2016). Green synthesis of iron nanoparticles and their environmental applications and implications. Nanomaterials, 6(11), 209. DOI 10.3390/nano6110209. [Google Scholar] [CrossRef]
34. Damle, S., Sharma, K., Bingi, G., Shah, H. (2016). A comparative study of green synthesis of silver and copper nanoparticles using Smithia sensitiva (DabzellCassia tora (L.) and Colocasia esculenta (L.). Indian Journal of Pure & Applied Biosciences, 4, 275–281. DOI 10.18782/2320-7051.2332. [Google Scholar] [CrossRef]
35. Pan, M., Yang, J., Liu, K., Yin, Z., Ma, T. et al. (2020). Noble metal nanostructured materials for chemical and biosensing systems. Nanomaterials, 10(2), 209. DOI 10.3390/nano10020209. [Google Scholar] [CrossRef]
36. Freitas de Freitas, L., Varca, G. H. C., dos Santos Batista, J. G., Benévolo Lugão, A. (2018). An overview of the synthesis of gold nanoparticles using radiation technologies. Nanomaterials, 8(11), 939. DOI 10.3390/nano8110939. [Google Scholar] [CrossRef]
37. Soni, V., Raizada, P., Singh, P., Cuong, H. N., Rangabhashiyam, S. et al. (2021). Sustainable and green trends in using plant extracts for the synthesis of biogenic metal nanoparticles toward environmental and pharmaceutical advances: A review. Environmental Research, 202, 111622. DOI 10.1016/j.envres.2021.111622. [Google Scholar] [CrossRef]
38. Barkat, M. A., Beg, S., Naim, M., Pottoo, F. H., Singh, S. P. et al. (2018). Current progress in synthesis, characterization and applications of silver nanoparticles: Precepts and prospects. Recent Patents on Anti-Infective Drug Discovery, 13(1), 53–69. DOI 10.2174/1574891X12666171006102833. [Google Scholar] [CrossRef]
39. Abdellatif, A. A., Alturki, H. N., Tawfeek, H. M. (2021). Different cellulosic polymers for synthesizing silver nanoparticles with antioxidant and antibacterial activities. Scientific Reports, 11(1), 1–18. DOI 10.1038/s41598-020-79834-6. [Google Scholar] [CrossRef]
40. Huber, D. L. (2005). Synthesis, properties, and applications of iron nanoparticles. Small, 1(5), 482–501. DOI 10.1002/smll.200500006. [Google Scholar] [CrossRef]
41. Feng, L., Cao, M., Ma, X., Zhu, Y., Hu, C. (2012). Superparamagnetic high-surface-area Fe3O4 nanoparticles as adsorbents for arsenic removal. Journal of Hazardous Materials, 217, 439–446. DOI 10.1016/j.jhazmat.2012.03.073. [Google Scholar] [CrossRef]
42. Wu, J., Yan, M., Lv, S., Yin, W., Bu, H. et al. (2021). Preparation of highly dispersive and antioxidative nano zero-valent iron for the removal of hexavalent chromium. Chemosphere, 262, 127733. DOI 10.1016/j.chemosphere.2020.127733. [Google Scholar] [CrossRef]
43. Kataria, N., Garg, V. K. (2019). Application of EDTA modified Fe3O4/sawdust carbon nanocomposites to ameliorate methylene blue and brilliant green dye laden water. Environmental Research, 172, 43–54. DOI 10.1016/j.envres.2019.02.002. [Google Scholar] [CrossRef]
44. Ingle, A. P., Duran, N., Rai, M. (2014). Bioactivity, mechanism of action, and cytotoxicity of copper-based nanoparticles: A review. Applied Microbiology and Biotechnology, 98(3), 1001–1009. DOI 10.1007/s00253-013-5422-8. [Google Scholar] [CrossRef]
45. Gawande, M. B., Goswami, A., Felpin, F. X., Asefa, T., Huang, X. et al. (2016). Cu and Cu-based nanoparticles: Synthesis and applications in catalysis. Chemical Reviews, 116(6), 3722–3811. DOI 10.1021/acs.chemrev.5b00482. [Google Scholar] [CrossRef]
46. Saratale, R. G., Saratale, G. D., Shin, H. S., Jacob, J. M., Pugazhendhi, A. et al. (2018). New insights on the green synthesis of metallic nanoparticles using plant and waste biomaterials: Current knowledge, their agricultural and environmental applications. Environmental Science and Pollution Research, 25(11), 10164–10183. DOI 10.1007/s11356-017-9912-6. [Google Scholar] [CrossRef]
47. Horti, N. C., Kamatagi, M. D., Patil, N. R., Sannaikar, M. S., Inamdar, S. R. (2020). Synthesis and optical properties of copper oxide nanoparticles: Effect of solvents. Journal of Nanophotonics, 14(4), 046010. DOI 10.1117/1.JNP.14.046010. [Google Scholar] [CrossRef]
48. Kołodziejczak-Radzimska, A., Jesionowski, T. (2014). Zinc oxide—from synthesis to application: A review. Materials, 7(4), 2833–2881. DOI 10.3390/ma7042833. [Google Scholar] [CrossRef]
49. Sabir, S., Arshad, M., Chaudhari, S. K. (2014). Zinc oxide nanoparticles for revolutionizing agriculture: Synthesis and applications. The Scientific World Journal, 2014, 925494. DOI 10.1155/2014/925494. [Google Scholar] [CrossRef]
50. Siddiqi, K. S., ur Rahman, A., Husen, A. (2018). Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Research Letters, 13(1), 1–13. DOI 10.1186/s11671-018-2532-3. [Google Scholar] [CrossRef]
51. Chauhan, A. K., Kataria, N., Garg, V. K. (2020). Green fabrication of ZnO nanoparticles using Eucalyptus spp. leaves extract and their application in wastewater remediation. Chemosphere, 247, 125803. DOI 10.1016/j.chemosphere.2019.125803. [Google Scholar] [CrossRef]
52. Kataria, N., Garg, V. K., Jain, M., Kadirvelu, K. J. A. P. T. (2016). Preparation, characterization and potential use of flower shaped Zinc oxide nanoparticles (ZON) for the adsorption of Victoria Blue B dye from aqueous solution. Advanced Powder Technology, 27(4), 1180–1188. DOI 10.1016/j.apt.2016.04.001. [Google Scholar] [CrossRef]
53. Jaji, N. D., Lee, H. L., Hussin, M. H., Akil, H. M., Zakaria, M. R. et al. (2020). Advanced nickel nanoparticles technology: From synthesis to applications. Nanotechnology Reviews, 9(1), 1456–1480. DOI 10.1515/ntrev-2020-0109. [Google Scholar] [CrossRef]
54. Ali, H. (2010). Biodegradation of synthetic dyes—A review. Water, Air, Soil Pollution, 213(1), 251–273. DOI 10.1007/s11270-010-0382-4. [Google Scholar] [CrossRef]
55. Vakili, M., Rafatullah, M., Salamatinia, B., Abdullah, A. Z., Ibrahim, M. H. et al. (2014). Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: A review. Carbohydrate Polymers, 113, 115–130. DOI 10.1016/j.carbpol.2014.07.007. [Google Scholar] [CrossRef]
56. Marchiol, L., Iafisco, M., Fellet, G., Adamiano, A. (2020). Nanotechnology support the next agricultural revolution: Perspectives to enhancement of nutrient use efficiency. Advances in Agronomy, 161, 27–116. DOI 10.1016/bs.agron.2019.12.001. [Google Scholar] [CrossRef]
57. Rajendrachari, S., Taslimi, P., Karaoglanli, A. C., Uzun, O., Alp, E. et al. (2021). Photocatalytic degradation of Rhodamine B (RhB) dye in waste water and enzymatic inhibition study using cauliflower shaped ZnO nanoparticles synthesized by a novel one-pot green synthesis method. Arabian Journal of Chemistry, 14(6), 103180. DOI 10.1016/j.arabjc.2021.103180. [Google Scholar] [CrossRef]
58. Rafique, M., Sadaf, I., Tahir, M. B., Rafique, M. S., Nabi, G. et al. (2019). Novel and facile synthesis of silver nanoparticles using Albizia procera leaf extract for dye degradation and antibacterial applications. Materials Science and Engineering: C, 99, 1313–1324. DOI 10.1016/j.msec.2019.02.059. [Google Scholar] [CrossRef]
59. Baruah, D., Goswami, M., Yadav, R. N. S., Yadav, A., Das, A. M. (2018). Biogenic synthesis of gold nanoparticles and their application in photocatalytic degradation of toxic dyes. Journal of Photochemistry and Photobiology B: Biology, 186, 51–58. DOI 10.1016/j.jphotobiol.2018.07.002. [Google Scholar] [CrossRef]
60. Baruah, D., Yadav, R. N. S., Yadav, A., Das, A. M. (2019). Alpinia nigra fruits mediated synthesis of silver nanoparticles and their antimicrobial and photocatalytic activities. Journal of Photochemistry and Photobiology B: Biology, 201, 111649. DOI 10.1016/j.jphotobiol.2019.111649. [Google Scholar] [CrossRef]
61. Madiha, B., Zahid, Q., Nida, M., Abdul, S. S. (2018). Studie on malachite green dye degradation by biogenic metal nano Cuo and Cuo/Zno Nano composites. Archives of Nanomedicine: Open Access Journal, 1(4), 119. DOI 10.32474/ANOAJ.2018.01.000119. [Google Scholar] [CrossRef]
62. Chokkalingam, M., Rupa, E. J., Huo, Y., Mathiyalagan, R., Anandapadmanaban, G. et al. (2019). Photocatalytic degradation of industrial dyes using Ag and Au nanoparticles synthesized from Angelica gigas ribbed stem extracts. Optik, 185, 1213–1219. DOI 10.1016/j.ijleo.2019.04.065. [Google Scholar] [CrossRef]
63. Jose, B., Thomas, F. (2020). Photocatalytic degradation of methylene blue using iron oxide nanoparticles synthesized using Annona muricata leaf extract. International Journal of Pharmacy and Pharmaceutical Sciences, 12(10), 46–51, DOI 10.22159/ijpps.2020v12i10.38821. [Google Scholar] [CrossRef]
64. Nguyen, T. T. N., Vo, T. T., Nguyen, B. N. H., Nguyen, D. T., Dang, V. S. et al. (2018). Silver and gold nanoparticles biosynthesized by aqueous extract of burdock root, Arctium lappa as antimicrobial agent and catalyst for degradation of pollutants. Environmental Science and Pollution Research, 25(34), 34247–34261. DOI 10.1007/s11356-018-3322-2. [Google Scholar] [CrossRef]
65. Thirumurugan, A., Harshini, E., Marakathanandhini, B. D., Kannan, S. R., Muthukumaran, P. (2017). Catalytic degradation of reactive red 120 by copper oxide nanoparticles synthesized by Azadirachta indica. In: Bioremediation and sustainable technologies for cleaner environment, pp. 95–102. Springer, Cham. DOI 10.1007/978-3-319-48439-6_9. [Google Scholar] [CrossRef]
66. Rafique, M., Shaikh, A. J., Rasheed, R., Tahir, M. B., Gillani, S. S. A. et al. (2018). Aquatic biodegradation of methylene blue by copper oxide nanoparticles synthesized from Azadirachta indica leaves extract. Journal of Inorganic and Organometallic Polymers and Materials, 28(6), 2455–2462. DOI 10.1007/s10904-018-0921-9. [Google Scholar] [CrossRef]
67. Bibi, I., Kamal, S., Ahmed, A., Iqbal, M., Nouren, S. et al. (2017). Nickel nanoparticle synthesis using Camellia sinensis as reducing and capping agent: Growth mechanism and photo-catalytic activity evaluation. International Journal of Biological Macromolecules, 103, 783–790. DOI 10.1016/j.ijbiomac.2017.05.023. [Google Scholar] [CrossRef]
68. Karthik, R., Govindasamy, M., Chen, S. M., Cheng, Y. H., Muthukrishnan, P. et al. (2017). Biosynthesis of silver nanoparticles by using Camellia japonica leaf extract for the electrocatalytic reduction of nitrobenzene and photocatalytic degradation of Eosin-Y. Journal of Photochemistry and Photobiology B: Biology, 170, 164–172. DOI 10.1016/j.jphotobiol.2017.03.018. [Google Scholar] [CrossRef]
69. Nayak, S. S., Mirgane, N. A., Shivankar, V. S., Pathade, K. B., Wadhawa, G. C. (2021). Degradation of the industrial dye using the nanoparticles synthesized from flowers of plant Ceropegia attenuata. Materials Today: Proceedings, 37, 2427–2431. DOI 10.1016/j.matpr.2020.08.274. [Google Scholar] [CrossRef]
70. Jain, A., Ahmad, F., Gola, D., Malik, A., Chauhan, N. et al. (2020). Multi dye degradation and antibacterial potential of Papaya leaf derived silver nanoparticles. Environmental Nanotechnology, Monitoring Management, 14, 100337. DOI 10.1016/j.enmm.2020.100337. [Google Scholar] [CrossRef]
71. Vinayagam, R., Selvaraj, R., Arivalagan, P., Varadavenkatesan, T. (2020). Synthesis, characterization and photocatalytic dye degradation capability of Calliandra haematocephala-mediated zinc oxide nanoflowers. Journal of Photochemistry and Photobiology B: Biology, 203, 111760. DOI 10.1016/j.jphotobiol.2019.111760. [Google Scholar] [CrossRef]
72. Ebrahimzadeh, M. A., Naghizadeh, A., Amiri, O., Shirzadi-Ahodashti, M., Mortazavi-Derazkola, S. (2020). Green and facile synthesis of Ag nanoparticles using Crataegus pentagyna fruit extract (CP-AgNPs) for organic pollution dyes degradation and antibacterial application. Bioorganic Chemistry, 94, 103425. DOI 10.1016/j.bioorg.2019.103425. [Google Scholar] [CrossRef]
73. Mali, S. C., Dhaka, A., Githala, C. K., Trivedi, R. (2020). Green synthesis of copper nanoparticles using Celastrus paniculatus Willd. leaf extract and their photocatalytic and antifungal properties. Biotechnology Reports, 27, e00518. DOI 10.1016/j.btre.2020.e00518. [Google Scholar] [CrossRef]
74. Bhuiyan, M. S. H., Miah, M. Y., Paul, S. C., Aka, T. D., Saha, O. et al. (2020). Green synthesis of iron oxide nanoparticle using Carica papaya leaf extract: Application for photocatalytic degradation of remazol yellow RR dye and antibacterial activity. Heliyon, 6(8), e04603. DOI 10.1016/j.heliyon.2020.e04603. [Google Scholar] [CrossRef]
75. Kamran, U., Bhatti, H. N., Iqbal, M., Jamil, S., Zahid, M. (2019). Biogenic synthesis, characterization and investigation of photocatalytic and antimicrobial activity of manganese nanoparticles synthesized from Cinnamomum verum bark extract. Journal of Molecular Structure, 1179, 532–539. DOI 10.1016/j.molstruc.2018.11.006. [Google Scholar] [CrossRef]
76. Varadavenkatesan, T., Lyubchik, E., Pai, S., Pugazhendhi, A., Vinayagam, R. et al. (2019). Photocatalytic degradation of Rhodamine B by zinc oxide nanoparticles synthesized using the leaf extract of Cyanometra ramiflora. Journal of Photochemistry and Photobiology B: Biology, 199, 111621. DOI 10.1016/j.jphotobiol.2019.111621. [Google Scholar] [CrossRef]
77. Vo, T. T., Nguyen, T. T. N., Huynh, T. T. T., Vo, T. T. T., Nguyen, T. T. N. et al. (2019). Biosynthesis of silver and gold nanoparticles using aqueous extract from Crinum latifolium leaf and their applications forward antibacterial effect and wastewater treatment. Journal of Nanomaterials, 2019, 1–14. DOI 10.1155/2019/8385935. [Google Scholar] [CrossRef]
78. Li, J. F., Rupa, E. J., Hurh, J., Huo, Y., Chen, L. et al. (2019). Cordyceps militaris fungus mediated zinc oxide nanoparticles for the photocatalytic degradation of Methylene blue dye. Optik, 183, 691–697. DOI 10.1016/j.ijleo.2019.02.081. [Google Scholar] [CrossRef]
79. Bishnoi, S., Kumar, A., Selvaraj, R. (2018). Facile synthesis of magnetic iron oxide nanoparticles using inedible Cynometra ramiflora fruit extract waste and their photocatalytic degradation of methylene blue dye. Materials Research Bulletin, 97, 121–127. DOI 10.1016/j.materresbull.2017.08.040. [Google Scholar] [CrossRef]
80. Bharathi, D., Vasantharaj, S., Bhuvaneshwari, V. (2018). Green synthesis of silver nanoparticles using Cordia dichotoma fruit extract and its enhanced antibacterial, anti-biofilm and photo catalytic activity. Materials Research Express, 5(5), 055404. DOI 10.1088/2053-1591/aac2ef. [Google Scholar] [CrossRef]
81. Umamaheswari, C., Lakshmanan, A., Nagarajan, N. S. (2018). Green synthesis, characterization and catalytic degradation studies of gold nanoparticles against Congo red and methyl orange. Journal of Photochemistry and Photobiology B: Biology, 178, 33–39. DOI 10.1016/j.jphotobiol.2017.10.017. [Google Scholar] [CrossRef]
82. Beheshtkhoo, N., Kouhbanani, M. A. J., Savardashtaki, A., Amani, A. M., Taghizadeh, S. (2018). Green synthesis of iron oxide nanoparticles by aqueous leaf extract of Daphne mezereum as a novel dye removing material. Applied Physics A, 124(5), 1–7. DOI 10.1007/s00339-018-1782-3. [Google Scholar] [CrossRef]
83. Narasaiah, P., Mandal, B. K., Sarada, N. C. (2017). Biosynthesis of copper oxide nanoparticles from drypetes sepiaria leaf extract and their catalytic activity to dye degradation. IOP Conference Series: Materials Science and Engineering, 263(2), 022012. DOI 10.1088/1757-899X/263/2/022012. [Google Scholar] [CrossRef]
84. Usman, M., Ahmed, A., Yu, B., Peng, Q., Shen, Y. et al. (2019). Photocatalytic potential of bio-engineered copper nanoparticles synthesized from Ficus carica extract for the degradation of toxic organic dye from waste water: Growth mechanism and study of parameter affecting the degradation performance. Materials Research Bulletin, 120, 110583. DOI 10.1016/j.materresbull.2019.110583. [Google Scholar] [CrossRef]
85. Choudhary, M. K., Kataria, J., Sharma, S. (2017). A biomimetic synthesis of stable gold nanoparticles derived from aqueous extract of Foeniculum vulgare seeds and evaluation of their catalytic activity. Applied Nanoscience, 7(7), 439–447. DOI 10.1007/s13204-017-0589-4. [Google Scholar] [CrossRef]
86. Saha, J., Begum, A., Mukherjee, A., Kumar, S. (2017). A novel green synthesis of silver nanoparticles and their catalytic action in reduction of Methylene Blue dye. Sustainable Environment Research, 27(5), 245–250. DOI 10.1016/j.serj.2017.04.003. [Google Scholar] [CrossRef]
87. Bello, B. A., Khan, S. A., Khan, J. A., Syed, F. Q., Mirza, M. B. et al. (2017). Anticancer, antibacterial and pollutant degradation potential of silver nanoparticles from Hyphaene thebaica. Biochemical and Biophysical Research Communications, 490(3), 889–894. DOI 10.1016/j.bbrc.2017.06.136. [Google Scholar] [CrossRef]
88. Younas, U., Hassan, S. T., Ali, F., Hassan, F., Saeed, Z. et al. (2021). Radical scavenging and catalytic activity of Fe-Cu bimetallic nanoparticles synthesized from Ixora finlaysoniana extract. Coatings, 11(7), 813. DOI 10.3390/coatings11070813. [Google Scholar] [CrossRef]
89. Vasantharaj, S., Shivakumar, P., Sathiyavimal, S., Senthilkumar, P., Vijayaram, S. et al. (2021). Antibacterial Activity and photocatalytic dye degradation of copper oxide nanoparticles (CuONPs) using Justicia gendarussa. Applied Nanoscience, pp. 1–8. DOI 10.1007/s13204-021-01939-9. [Google Scholar] [CrossRef]
90. Das, P., Ghosh, S., Ghosh, R., Dam, S., Baskey, M. (2018). Madhuca longifolia plant mediated green synthesis of cupric oxide nanoparticles: A promising environmentally sustainable material for waste water treatment and efficient antibacterial agent. Journal of Photochemistry and Photobiology B: Biology, 189, 66–73. DOI 10.1016/j.jphotobiol.2018.09.023. [Google Scholar] [CrossRef]
91. Ebrahiminezhad, A., Taghizadeh, S., Ghasemi, Y., Berenjian, A. (2018). Green synthesized nanoclusters of ultra-small zero valent iron nanoparticles as a novel dye removing material. Science of the Total Environment, 621, 1527–1532. DOI 10.1016/j.scitotenv.2017.10.076. [Google Scholar] [CrossRef]
92. Francis, S., Joseph, S., Koshy, E. P., Mathew, B. (2017). Green synthesis and characterization of gold and silver nanoparticles using Mussaenda glabrata leaf extract and their environmental applications to dye degradation. Environmental Science and Pollution Research, 24(21), 17347–17357. DOI 10.1007/s11356-017-9329-2. [Google Scholar] [CrossRef]
93. Chand, K., Jiao, C., Lakhan, M. N., Shah, A. H., Kumar, V. et al. (2021). Green synthesis, characterization and photocatalytic activity of silver nanoparticles synthesized with Nigella sativa seed extract. Chemical Physics Letters, 763, 138218. DOI 10.1016/j.cplett.2020.138218. [Google Scholar] [CrossRef]
94. Chahardoli, A., Karimi, N., Sadeghi, F., Fattahi, A. (2018). Green approach for synthesis of gold nanoparticles from Nigella arvensis leaf extract and evaluation of their antibacterial, antioxidant, cytotoxicity and catalytic activities. Artificial Cells, Nanomedicine, and Biotechnology, 46(3), 579–588. DOI 10.1080/21691401.2017.1332634. [Google Scholar] [CrossRef]
95. Francis, S., Joseph, S., Koshy, E. P., Mathew, B. (2017). Synthesis and characterization of multifunctional gold and silver nanoparticles using leaf extract of Naregamia alata and their applications in the catalysis and control of mastitis. New Journal of Chemistry, 41(23), 14288–14298. DOI 10.1039/C7NJ02453C. [Google Scholar] [CrossRef]
96. Rambabu, K., Bharath, G., Banat, F., Show, P. L. (2021). Green synthesis of zinc oxide nanoparticles using Phoenix dactylifera waste as bioreductant for effective dye degradation and antibacterial performance in wastewater treatment. Journal of Hazardous Materials, 402, 123560. DOI 10.1016/j.jhazmat.2020.123560. [Google Scholar] [CrossRef]
97. Miri, A., Vahed, H. O. S., Sarani, M. (2018). Biosynthesis of silver nanoparticles and their role in photocatalytic degradation of methylene blue dye. Research on Chemical Intermediates, 44(11), 6907–6915. DOI 10.1007/s11164-018-3529-3. [Google Scholar] [CrossRef]
98. Joshi, S. J., Geetha, S. J., Al-Mamari, S., Al-Azkawi, A. (2018). Green synthesis of silver nanoparticles using pomegranate peel extracts and its application in photocatalytic degradation of methylene blue. Jundishapur Journal of Natural Pharmaceutical Products, 13(3), 1–5. DOI 10.5812/jjnpp.67846. [Google Scholar] [CrossRef]
99. Bibi, I., Nazar, N., Ata, S., Sultan, M., Ali, A. et al. (2019). Green synthesis of iron oxide nanoparticles using pomegranate seeds extract and photocatalytic activity evaluation for the degradation of textile dye. Journal of Materials Research and Technology, 8(6), 6115–6124. DOI 10.1016/j.jmrt.2019.10.006. [Google Scholar] [CrossRef]
100. Laouini, S. E., Bouafia, A., Soldatov, A. V., Algarni, H., Tedjani, M. L. et al. (2021). Green synthesized of Ag/Ag2O nanoparticles using aqueous leaves extracts of Phoenix dactylifera L. and their Azo dye photodegradation. Membranes, 11(7), 468. DOI 10.3390/membranes11070468. [Google Scholar] [CrossRef]
101. Singh, J., Kumar, V., Kim, K. H., Rawat, M. (2019). Biogenic synthesis of copper oxide nanoparticles using plant extract and its prodigious potential for photocatalytic degradation of dyes. Environmental Research, 177, 108569. DOI 10.1016/j.envres.2019.108569. [Google Scholar] [CrossRef]
102. Vasantharaj, S., Sathiyavimal, S., Senthilkumar, P., LewisOscar, F., Pugazhendhi, A. (2019). Biosynthesis of iron oxide nanoparticles using leaf extract of Ruellia tuberosa: Antimicrobial properties and their applications in photocatalytic degradation. Journal of Photochemistry and Photobiology B: Biology, 192, 74–82. DOI 10.1016/j.jphotobiol.2018.12.025. [Google Scholar] [CrossRef]
103. Sadiq, H., Sher, F., Sehar, S., Lima, E. C., Zhang, S. et al. (2021). Green synthesis of ZnO nanoparticles from Syzygium cumini leaves extract with robust photocatalysis applications. Journal of Molecular Liquids, 335, 116567. DOI 10.1016/j.molliq.2021.116567. [Google Scholar] [CrossRef]
104. Shim, Y. J., Soshnikova, V., Anandapadmanaban, G., Mathiyalagan, R., Perez, Z. E. J. et al. (2019). Zinc oxide nanoparticles synthesized by Suaeda japonica Makino and their photocatalytic degradation of methylene blue. Optik, 182, 1015–1020. DOI 10.1016/j.ijleo.2018.11.144. [Google Scholar] [CrossRef]
105. Vaidehi, D., Bhuvaneshwari, V., Bharathi, D., Sheetal, B. P. (2018). Antibacterial and photocatalytic activity of copper oxide nanoparticles synthesized using Solanum lycopersicum leaf extract. Materials Research Express, 5(8), 085403. [Google Scholar]
106. Zaman, M. B., Poolla, R., Singh, P., Gudipati, T. (2020). Biogenic synthesis of CuO nanoparticles using Tamarindus indica L. and a study of their photocatalytic and antibacterial activity. Environmental Nanotechnology, Monitoring Management, 14, 100346. DOI 10.1016/j.enmm.2020.100346. [Google Scholar] [CrossRef]
107. Khan, Z. U. H., Sadiq, H. M., Shah, N. S., Khan, A. U., Muhammad, N. et al. (2019). Greener synthesis of zinc oxide nanoparticles using Trianthema portulacastrum extract and evaluation of its photocatalytic and biological applications. Journal of Photochemistry and Photobiology B: Biology, 192, 147–157. DOI 10.1016/j.jphotobiol.2019.01.013. [Google Scholar] [CrossRef]
108. Radini, I. A., Hasan, N., Malik, M. A., Khan, Z. (2018). Biosynthesis of iron nanoparticles using Trigonella foenum-graecum seed extract for photocatalytic methyl orange dye degradation and antibacterial applications. Journal of Photochemistry and Photobiology B: Biology, 183, 154–163. DOI 10.1016/j.jphotobiol.2018.04.014. [Google Scholar] [CrossRef]
109. Barman, K., Chowdhury, D., Baruah, P. K. (2020). Bio-synthesized silver nanoparticles using Zingiber officinale rhizome extract as efficient catalyst for the degradation of environmental pollutants. Inorganic and Nano-Metal Chemistry, 50(2), 57–65. DOI 10.1080/24701556.2019.1661468. [Google Scholar] [CrossRef]
110. Devendran, P., Selvakumar, D., Ramadoss, G., Sivaramakrishnan, R., Alagesan, T. et al. (2022). A novel visible light active rare earth doped CdS nanoparticles decorated reduced graphene oxide sheets for the degradation of cationic dye from wastewater. Chemosphere, 287, 132091. DOI 10.1016/j.jallcom.2017.11.139. [Google Scholar] [CrossRef]
111. Thiruppathi, M., Kumar, P. S., Devendran, P., Ramalingan, C., Swaminathan, M. et al. (2018). Ce@ TiO2 nanocomposites: An efficient, stable and affordable photocatalyst for the photodegradation of diclofenac sodium. Journal of Alloys and Compounds, 735, 728–734. DOI 10.1016/j.chemosphere.2021.132091. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |