

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2022.016364
ARTICLE
One Step Regioselective Acylation of Polyphenolic Wood Extractive and Its Application for Wood Treatment
Université de Lorraine-Institut National de Recherche Pour l’Agriculture, L’Alimentation et L’Environnement (INRAE), Laboratoire d’Etudes et de Recherche sur le Matériau Bois (LERMAB), Nancy, France
*Corresponding Author: Christine Gerardin-Charbonnier. Email: Christine.Gerardin@univ-lorraine.fr
Received: 01 March 2021; Accepted: 21 July 2021
Abstract: This study evaluated the methods of grafting commercial catechin with fatty acids, namely capric acid (C10), lauric acid (C12), and myristic acid (C14) through esterification. Specimens of beech wood (Fagus sylvatica L.) were impregnated with catechin and modified catechin-fatty acids, separately, at a 5% concentration diluted in ethanol using vacuum pressure treatment and subjected to leaching. The weight percentage gain before leaching (WPG), after leaching (WPGAL), and weight loss due to leaching (PL) were investigated. Both leached and unleached samples were tested against white-rot fungi (Trametes versicolor) in Petri-dishes for twelve weeks. Results show that samples treated with modified catechin-fatty acids provide improved resistance towards leaching. Catechin-C14 was found to be more promising, possibly due to its chain length. The decay weight loss for samples treated with modified catechin-fatty acids does not differ significantly between the samples that leached and not. Despite the antifungal properties of catechin, the treatment with catechin alone was insufficient to protect wood samples from fungi. Further, it is recommended to increase the concentration level of modified catechin to obtain a significant effect on the decay resistance.
Keywords: Antifungal; catechin; chemical modification; fatty acids; wood protection
Nowadays, there has been a lot of research interest in developing a new sustainable wood protection system that reduces biocide utilization. It comes as a result of environmental issues and regulations pushing the development of new technologies to create new sustainable wood preservatives [1–5]. One of those manners was identifying plant extractives compound from wood and its bark, which contains many types of extractable polyphenols or tannins. Several studies have suggested that these compounds could be utilized as an alternative for wood protection agents against several types of wood-decaying fungi. Examples of tested compounds were flavonoids from the wood of Salix caprea L. [6]; condensed tannins from bark complexed with copper [7]; Mimosa sp. and Quebracho sp. extracts [8]; or through synergistic combinations such as tannin borate combinations [9]; and by addition of either a chelator, such as ethylenediaminetetraacetic acid (EDTA), or an antioxidant, such as Irganox 1076 [10–13].
Catechin is a hydrophylic flavanol possessing antioxidant properties [14]. It possess powerful radical scavenging activity due to the presence of a catechol moiety on ring B and hydroxyl groups on ring C [15]. Due to its antimicrobial and antifungal properties, catechin can potentially be applied as wood protection agent [16–18]. It is reported that the antioxidant and free radical scavenging activities of flavonoids, especially catechin, degrade wood polymers by inhibiting free radicals formed by wood rooting fungi, and participate in the natural durability of wood against fungi [19–21].
Even if it is difficult to find a natural antifungal active against a wide range of wood colonizing fungi, previous study carried out by Malterud et al. [6] reported that flavonoids are effective against wood rotting fungi, but not against all microbial agents. Moreover, as stated above, flavonoids are soluble in water and therefore easily leachable from wood, it seems therefore interesting to develop flavonoids derivatives with potential increased biological activity and lower water solubility. In addition to the complexing and antioxidant properties of flavonoids, esterification of these latter ones by fatty acid may confer them hydrophobic properties, which may modify their biological properties (Fig. 1).
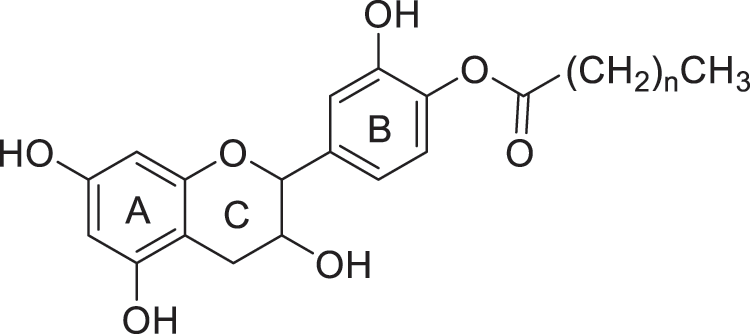
Figure 1: Esterification of catechin by fatty acids
Clausen et al. [22] stated that the fatty acid chain could give hydrophobic properties, which is important to increase the resistance against leaching. Studies conducted by Coleman et al. [23] and Pohl et al. [24] reported that the low molecular weight aliphatic fatty acids such as pentanoic (C5) to decanoic acids (C10), are effective against a wide range of fungal pathogens allowing their use as environmentally friendly antifungal agents [22].
Beech wood is one of the most important commercial tree species in Europe that has been universally used for furniture, plywood, particleboards or bentwood industry [25] presenting low natural durability and suitable for impregnation treatment due to its high treatability [26]. The aims of this study are to modify the catechin’s hydroxyl groups with fatty acid and to evaluate the effect of modified catechins as wood protection agent. Different parameters as weight percent gain after impregnation, resistance to leaching, and decay resistance to the white-rot fungus T. versicolor were investigated to evaluate the modified catechin’s efficacy as a wood protection agent.
(+)-Catechin hydrate, capric acid, myristic acid, lauric acid, and all reactants were obtained from Sigma–Aldrich Chimie, France.
2.2 Procedure for the Reaction of Esterification of Catechin
To a stirred solution of catechin (2 g, 6.89 mmol) in 25 mL acetonitrile, lauric acid 1 equivalent is added directly, and after 10 min DCCI 1 equivalent is added drop by drop to the reaction mixture at 0°C, which is stirred for 3 h at 0°C and 24 h at room temperature under N2 condition. Precipitated urea is then filtered off and the filtrate evaporated down in vacuo. The residue is taken up in ethyl acetate and is washed with saturated NH4Cl solution, saturated NaCl solution, saturated NaHCO3 solution, NaCl saturated solution, and then dried over MgSO4. The solvent is removed by evaporation. Crystalline products can be obtained in pure form by silica gel chromatography (cyclohexane: ethyl acetate/8:2). Esterification using other fatty alkyl chain length were carried out in a similar manner.
2.3 Spectroscopic Characteristics
Products were identified using 1H and 13C Nuclear Magnetic Resonance (1H NMR and 13C NMR) performed on Bruker AC-200 and 400 MHz. FTIR spectra were recorded on a Perkin Elmer Spectrum 2000. All the products and their description are presented in Figs. 2–8.
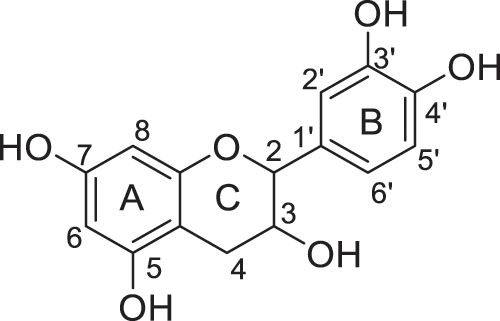
Figure 2: Catechin
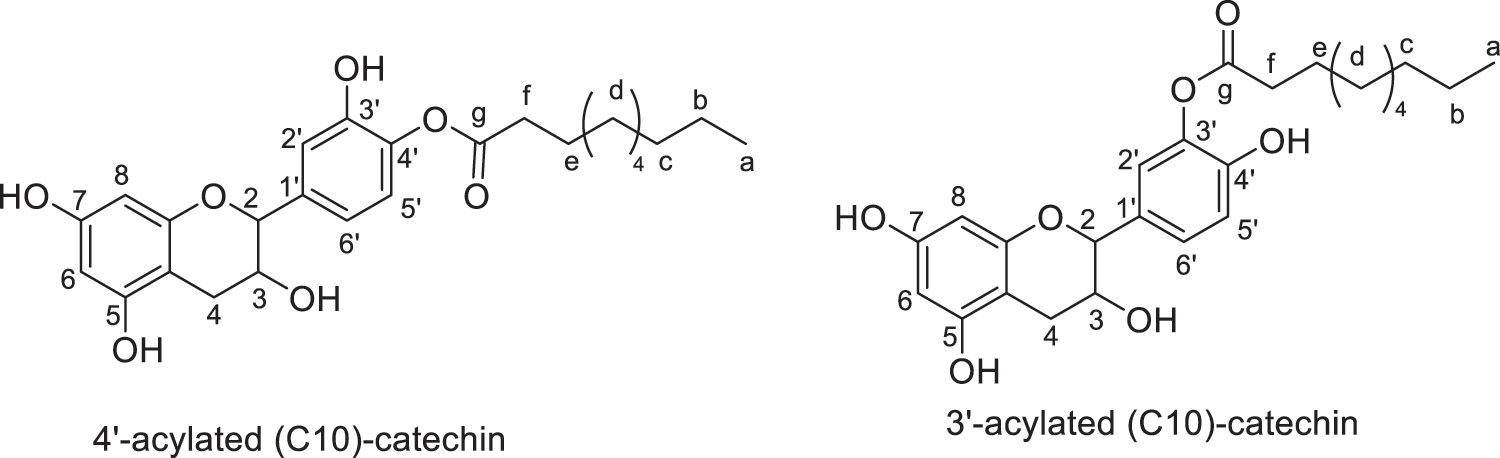
Figure 3: Regio-isomers of acylated catechin with capric acid (monoesters)
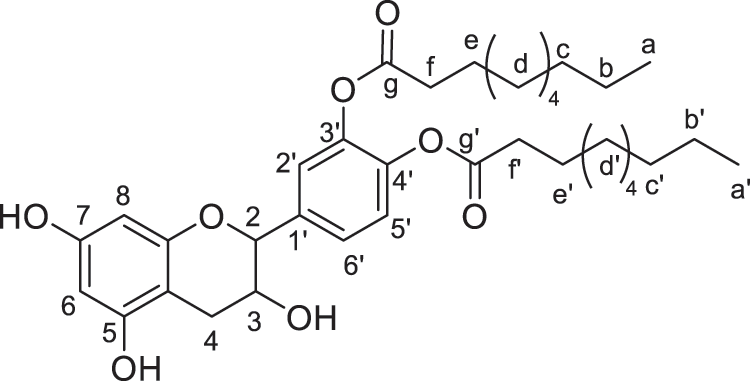
Figure 4: Catechin modified with capric acid (diester)
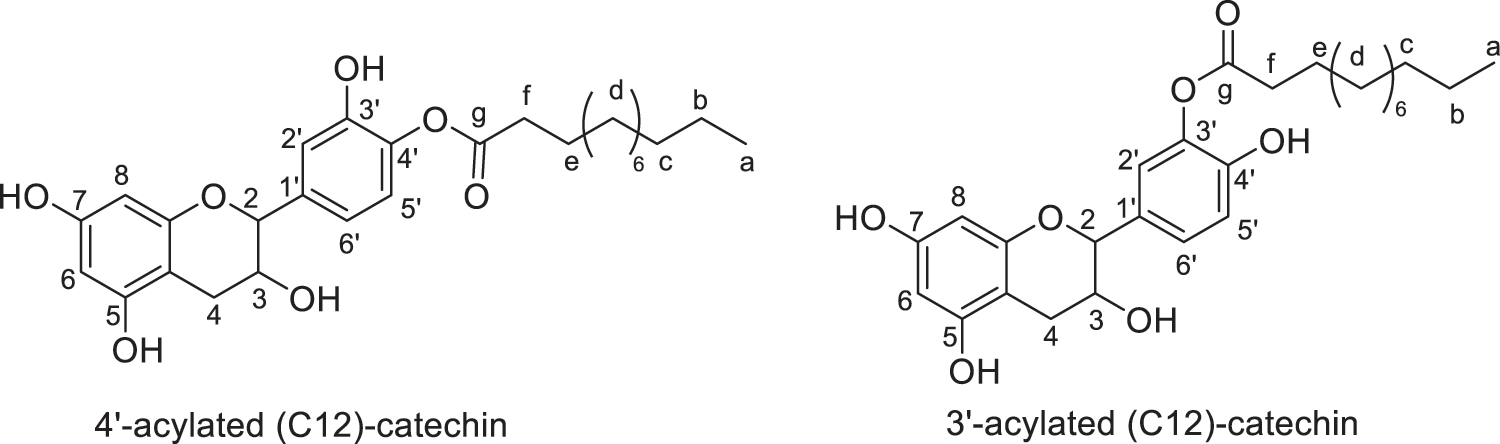
Figure 5: Regio-isomers of acylated catechin modified with lauric acid (monoesters)
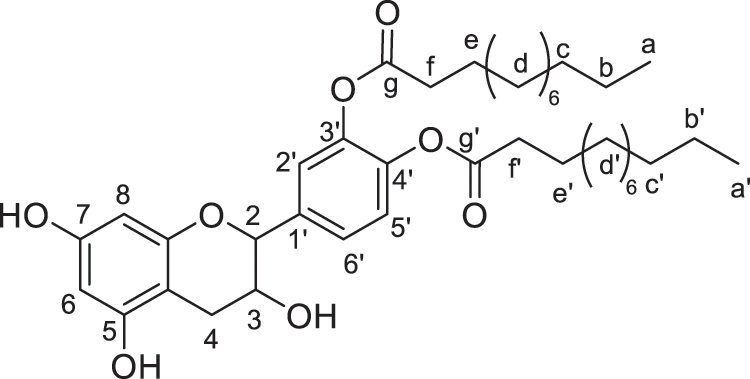
Figure 6: Catechin modified with lauric acid (diester)
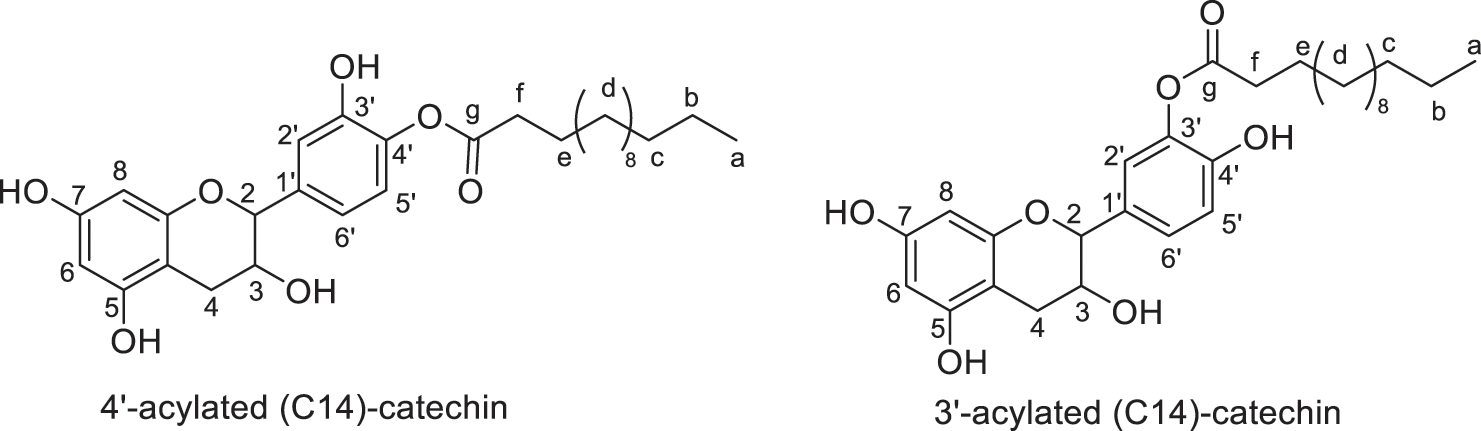
Figure 7: Regio-isomers of acylated catechin modified with myristic acid (monoesters)
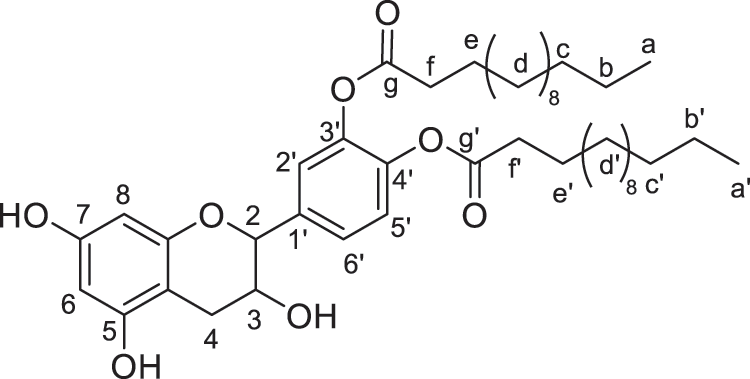
Figure 8: Catechin modified with myristic acid (diester)
1H NMR (DMSO-d6, 400 MHz): δ(ppm): 2, 30 (dd, 1H, J = 8, 1 Hz, J = 16, 1 Hz, 4ax); 2, 67 (dd, 1H, J = 5, 4 Hz, J = 16, 1 Hz, 4eq); 3, 87 (ddd, 1H, J = 5, 4 Hz, J = 8, 1 Hz, J = 7, 5 Hz, 3); 4, 49 (d, 1H, J = 7, 5 Hz, 2); 4.86 (s, 1H, 3-OH); 5, 7 (d, 1H, J = 2, 4 Hz, 6); 5, 9 (d, 1H, J = 2, 4 Hz, 8); 6, 6 (dd, 1H, J = 2.1 Hz, J = 8, 3 Hz, 6’); 6, 69 (d, 1H, J = 8, 3 Hz, 5’); 6, 73 (d, 1H, J = 2, 1 Hz, 2’); 8.84 (s, 1H, 3’-OH’); 8.86 (s, 1H, 4’-OH); 8.95 (s, 1H, 5-OH); 9.18 (s, 1H, 7-OH); 13C NMR (DMSO-d6, 400MHZ): δ(ppm): 80.94 (C2); 66, 26 (C3); 28 (C4); 156.40 (C5); 95.05 (C6); 156.12 (C7); 93.80 (C8); 155.31 (C9); 99.01 (C10); 130.54 (C1’); 114.45 (C2’); 144.79 (C3’); 144.79 (C4’); 115.2 (C5’); 118.6 (C6’).
Monoester:
1H NMR (DMSO-d6, 400 MHz) : δ(ppm) of 4’ regioisomer [3’regioisomer if different]: 0.83 [0.86] (t, 3H, J = 7 Hz, Ha); 1.18–1.32 (m, 12H, Hb, c, d); 1.57–1.65 (m, 2H, He), 2.35 [2.32] (dd, 1H, J = 8, 4 Hz, J = 19 Hz, 4ax); 2.50–2.58 (m, 2H, Hf); 2.68 [2.70] (dd, 1H, J = 5.6 Hz, J = 19 Hz, 4eq); 3.81–3.87 (m, 1H, 3); 4.57 [4.51] (d, 1H, J = 7.6 Hz, 2); 5.03 [4.95] (d, 1H, J = 5.5 Hz, 3-OH); 5.71 [5.68] (d, 1H, J = 2.3 Hz, 6); 5.92 [5.85] (d, 1H, J = 2.3 Hz, 8); 6.77 [6.75] (dd, 1H, J = 2 Hz, J = 8.2 Hz, 6’); 6.93 [6.96] (d, 1H, J = 8.2 Hz, 5’); 7.03[7.06] (d, 1H, J = 2.1 Hz, J = 8.2 Hz, 2’); 8.95 [8.80] (s, 1H, 3’-OH or 4’-OH); 9.22 [9.19] (s, 1H, 5-OH); 9.61 [9.59] (s, 1H, 7-OH); 13C NMR (DMSO-d6, 400 MHZ): δ(ppm): 14.6(Ca); 22.7 (Cb); 25 (Cc); 29.1 (Cd, 4); 31.8 (Ce); 33.8 (Cf); 66.3 (C3); 80.7 (C2); 93.9 (C8); 95.4 (C6); 98.9 (C10); 115.9 (C2’); 125.7 (C5’); 122.2 (C6’); 130.6 (C1’); 138.1 (C3’); 148.7 (C4’); 155.3 (C5); 156.14 (C9); 156.5 (C7); 171.2 (Cg); IR: 1736 cm−1 (O-CO), 2853–2923 cm−1 (aliphatic chain), 3344 cm−1 OH phenolic; mp: 80°C –90°C.
Diester:
1H NMR (DMSO-d6, 400 MHz): δ(ppm): 0.81–0.85 (m, 6H, Ha,a’); 1.18–1.30 (m, 24H, Hb,b,c,c’, d,d’); 1.55–1.62 (m, 4H, He, e’), 2.33 (dd, 1H, J = 8, 4 Hz, J = 19 Hz, 4ax); 2.50 (m, 4H, Hf, f’); 2.68 (dd, 1H, J = 5, 6 Hz, J = 19 Hz, 4eq); 3.81–3.83 (m, 1H, 3); 4.57 [4.49] (d, 1H, J = 7.6 Hz, 2); 5.03 [4.97] (d, 1H, J = 1.5 Hz, 3-OH); 5.71 [5.62] (d, 1H, J = 2, 3 Hz, 6); 5.89 [5.65] (d, 1H, J = 2, 3 Hz, 8); 6.76 (dd, 1H, J = 2 Hz, J = 8, 2 Hz, 6’); 6.93 (d, 1H, J = 8, 2 Hz, 5’); 7.03 (d, 1H, J = 2.1 Hz, J = 8.2 Hz, 2’); 9.22 (s, 1H, 5-OH); 9.61 (s, 1H, 7-OH); 13C NMR (DMSO-d6, 400 MHZ): δ(ppm): 14.4 (Ca,a’); 22.7 (Cb,b’); 25 (Cc,c’); 29.1 (Cd,d’, 4); 32 (Ce,e’); 34 (Cf,f’); 66.9 (C3); 80.8 (C2); 94, 3 (C8); 96, 1 (C6); 99, 6 (C10); 123 (C2’); 123.7 (C5’); 126, 1 (C6’); 130.6 (C1’); 138.9 (C3’); 142 (C4’); 155.5 (C5); 156.6 (C9); 157, 09 (C7); 171.1 (Cg,g’); IR: 1736 cm−1 (O-CO), 2853–2923 cm−1 (aliphatic chain), 3344 cm−1 OH phenolic; mp: 80–90°C.
Monoester:
1H NMR (DMSO-d6, 400 MHz): δ(ppm) of 4’ regioisomer [3’regioisomer if different]: 0.85 [0.90] (t, 3H, J = 7 Hz, Ha); 1.16–1.30 (m, 16H, Hb,c,d); 1.64–1.57 (m, 2H, He); 2.37 [2.30] (dd, 1H, J = 8.4 Hz, J = 19 Hz, 4ax); 2.50 (m, 2H, Hf); 2.68 [2.65] (dd, 1H, J = 5, 6 Hz, J = 19 Hz, 4eq); 3.81–3.83 (m, 1H, 3); 4.57 [4.45] (d, 1H, J = 7.6 Hz, 2); 5.05 [5.10] (d, 1H, J = 1, 5 Hz, 3-OH); 5.72 [5.68](d, 1H, J = 2, 3 Hz, 6); 5.92 [5.88] (d, 1H, J = 2, 3 Hz, 8); 6.76 [6.85] (dd, 1H, J = 2 Hz, J = 8, 2 Hz, 6’); 6.95 (d, 1H, J = 8, 2 Hz, 5’); 7.10 [7.05] (d, 1H, J = 2.1 Hz, J = 8.2 Hz, 2’); 8.85 [8.81] (s, 1H, 3’-OH or 4’-OH); 9.30 [9.22] (s, 1H, 5-OH); 9.65 [9.66] (s, 1H, 7-OH); 13C NMR (DMSO-d6, 400 MHz): δ(ppm): 14 (Ca); 22 (Cb); 24. (Cf); 28.6 (Ce,4); 31.4 (CC); 33.1 (Cg); 66.3 (C3); 80.6 (C2); 93.8 (C8); 95.4 (C6); 98.9 (C10); 115.9 (C2’); 122.2 (C5’); 125.5 (C6’); 130.7 (C1’); 141(C3’); 148.6 (C4’); 155 (C5); 156.18 (C9); 156.5 (C7) 171.6 (Cd); IR: 1736 cm−1 (O-CO), 2853–2922 cm−1 (aliphatic chain), 3365 cm−1 OH phenolic; mp: 100–110°C.
Diester:
1H NMR (CDCl3, 400 MHz): δ(ppm) : 0.80–0.86 (m, 6H, Ha, a’); 1.2–1.30 (m, 32H, Hb, b’,c,c’, d,d’); 1.62–170 (m, 4H, He,e’), 2.36 (dd, 1H, J = 8.8 Hz, J = 16.21 Hz, 4ax); 2, 38–2.43 (m, 4H, Hf,f’); 2, 78 (dd, 1H, J = 5.7 Hz, J = 16.12 Hz, 4eq); 3.84 (ddd, 1H, J = 5.3 Hz, J = 6.8, 3); 4, 66 (d, 1H, J = 8.2 Hz, 2); 5.14 (d, 1H, J = 1.5 Hz, 3-OH); 5, 94 (d, 1H, J = 2, 11 Hz, 6); 5, 74 (d, 1H, J = 2, 17 Hz, 8); 7.23 (dd, 1H, J = 1.5 Hz, J = 6.54 Hz, 6’); 7.25 (d, 1H, 5’); 7.31(d, 1H, J = 2.2 Hz, 2’); 9 (s, 1H, 5-OH); 9.26 (s, 1H, 7-OH); 13C NMR (CDCl3, 400 MHz): δ(ppm): 13.9 (Ca,a’); 22.1 (Cb,b’); 25 (Cc,c’); 28.6 (Cd,d’); 31.4(Ce,e’); 33.2 (Cf,f’); 66.4 (C3); 80.2 (C2); 93.9 (C8); 95.5 (C6); 98.9 (C10); 122.3 (C2’); 123 (C5’); 125.7 (C6’); 138.4 (C1’); 141.6 (C3’,4’); 154.9 (C5); 156.1(C9); 156.5 (C7); 170.4(Cg,g’).
Monoester:
1H NMR (DMSO-d6, 400 MHz): δ(ppm) of 4’ regioisomer [3’regioisomer if different]: 0.84 [0.82] (t, 3H, J = 7 Hz, Ha); 1.36–1.18 (m, 20H, H b,c,d); 1.60 (t, 2H, J = 7 Hz, He), 2.35 [2.36] (dd, 1H, J = 9 Hz, J = 17 Hz, 4ax); 2.46–2.53 (m, 2H, Hf); 2.68 [2.73] (dd, 1H, J = 5.4 Hz, J = 17 Hz, 4eq); 3.88–3.78 (m, 1H, 3); 4.57 [4.49] (d, 1H, J = 7.4 Hz, 2); 5.03 [4.95] (d, 1H, J = 1.5 Hz, 3-OH); 5.70 [5.60] (d, 1H, 6); 5, 88 [5.75] (d, 1H, 8); 6.76 [6.70] (dd, 1H, J = 2 Hz, J = 8.2 Hz, 6’); 6.89 (d, 1H, J = 8, 2 Hz, 5’); 7.03 [6.95] (d, 1H, 2’); 8.96 [8.90] (s, 1H, 3’-OH or 4’-OH); 9.21 [9.15] (s, 1H, 5-OH); 9.60 [9.62] (s, 1H, 7-OH); 13C NMR (DMSO-d6, 400 MHz): δ(ppm): 14 (Ca); 22 (Cb); 24.5(Cc); 28.8 (Cd, 4); 31.3 (Cd); 33.3 (Cf); 68.3 (C3); 82.3 (C2); 95.5 (C8); 96.5 (C6); 100 (C10); 117.1 (C2’); 119.6 (C5’); 123.5 (C6’); 132.3 (C1’); 141 (C3’); 149.6 (C4’); 156.7 (C5); 157.2 (C9); 157.8 (C7) 172.1(Cg); IR: 1736 cm−1 (O-CO), 2852–2922 cm−1 (aliphatic chain), 3367 cm−1 OH phenolic; mp: 130–140°C.
Diester:
1H NMR (CDCl3, 400 MHz): δ(ppm): 0.81–0.85 (m, 6H, J = 6.3 Hz, Ha); 1.23–1, 29 (m, 40H, Hb,c,d); 1.62 (m, 4H, He,e’), 2, 40 (dd, 1H, J = 8, 9 Hz, J = 16 Hz, 4ax); 2.51–2.56 (m, 4H, Hf,f’); 2, 78 (dd, 1H, J = 5, 4 Hz, J = 16 Hz, 4eq); 3.86 (ddd, 1H, J= 6.24 Hz, J= 14.21 Hz, 3); 4, 65 (d, 1H, J = 8.16 Hz, 2); 5.12 (d, 1H, J= 5 Hz, 3-OH); 5, 94 (d, 1H, J = 2, 15 Hz, 6); 5, 74 (d, 1H, J = 2, 15 Hz, 8); 7.27 (dd, 1H, 6.54 Hz, 6’); 7.25 (d, 1H, J = 6.54 Hz, 5’); 7.31(d, 1H, J = 2.2 Hz, 2’); 8.9 (s, 1H, 5-OH); 9.25 (s, 1H, 7-OH); 13C NMR (CDCl3, 400 MHz): δ(ppm): 13.9 (Ca,a’); 22.3 (Cb,b’); 24.6 (Cc,c’); 28.9 (Cd,d’, 4); 31.4(Ce,e’); 33.7 (Cf,f’); 66.4 (C3); 80.1 (C2); 93.8 (C8); 95.5 (C6); 99.1 (C10); 122.3 (C2’); 123.2 (C5’); 125.5 (C6’); 138.6 (C1’); 141.3 (C3’); 141.5 (C4’); 154.9 (C5); 156.1(C9); 156.5 (C7); 170.4 (Cg,g’).
Conditioned sapwood portion of beech wood (Fagus sylvatica L.) samples were cut into dimensions of 2.5 cm × 1.5 cm × 0.5 cm (L × R × T). Prior to impregnation, wood samples were dried at 103 ± 2°C for 48 h to obtain their anhydrous weight (m0). Catechin and modified catechin-fatty acids were used for wood impregnation after dilution at 5% concentration in ethanol (m/v), resulting in four treatments.
Oven-dried wood specimens were placed in a 250 mL beaker inside a desiccator equipped with a two-way tap and subjected to a 90–110 mbar vacuum for one hour. Afterward, the samples were taken out from the beaker and oven-dried at 103 ± 2°C for 24 h until reaching the constant weight (m1). Sixteen replicates were used for each impregnation. The weight percentage gain (WPG) was calculated by the difference between the oven-dried weight of the samples before and after impregnation (Eq. (1))
where m0 is the initial anhydrous weight before impregnation (g) and m1 is the anhydrous weight after impregnation (g).
Leaching procedure was carried out according to the standard NF X41–568 [27]. Eight wood specimens from each treatment were immersed in 90 mL of distilled water and subjected to six successive leaching periods of increasing duration under continuous shaking at 20 ± 2°C. In the first period, the specimens were leached for 1, 2, and 4 h with replacement of water between each leaching cycle. Afterward, the specimens were removed and kept at air for 16 h. A second period of leaching was then continued for 8, 16, and 48 h. After completion of all the leaching cycles, the samples were oven-dried at 103 ± 2°C for 24 h, and their weight measured (m2). Weight percent gain after leaching (WPGAL) and percentage of weight loss due to leaching (PL) were then determined (Eqs. (2) and (3))
where m0 is the initial anhydrous weight before impregnation, m1 is the anhydrous weight after impregnation, and m2 is the anhydrous weight after leaching.
Treated and untreated wood samples were exposed to the white-rot fungus Trametes versicolor, strain CTB 863 A according to a procedure inspired from the EN 113 standard [28]. Sterile culture medium (20 mL) was prepared from malt (25 g) and agar (40 g) in distilled water (1 L). The solution was poured into 9 cm Petri-dish. After the solidification of medium, a small piece of mycelium was inoculated in the center of each Petri dish. Petri dishes were incubated for approximately 15 days at 22 ± 2°C and 70 ± 5% relative humidity to allow colonization of the surface by the mycelium. After being sterilized at 110°C for 20 min, all wood samples, including untreated wood as control and treated wood samples before and after leaching, were put in Petri-dishes and incubated for 12 weeks (3 wood samples for each Petri dish). Each experiment was triplicate. After this period, mycelia were cleaned from the wood specimen, and then oven-dried at 103 ± 2°C for 24 h and weighed. Weight loss (WL) due to fungal attack was determined following Eq. (4):
where ma is the anhydrous weight before being exposed to fungi (g) and mb is the anhydrous weight after being exposed to fungi (g).
T-test analysis was used to compare the data from each specimen and treatment. The data were analyzed to see their groupings and determine whether or not two sets of data from each specimen are significantly different. The analysis was conducted using Microsoft Excel, R [29], and RStudio.
3.1 Reaction of Catechin with Fatty Acids
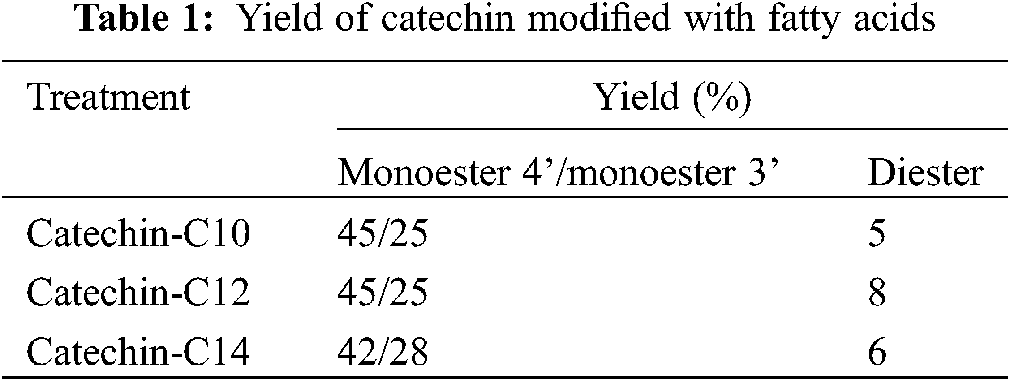
Fig. 9 illustrated the regioselective acylation of catechin. Yields of reactions of catechin with fatty acids are presented in Table 1. Monoesters are obtained in good yields, ranging from 65% to 70%. The highest yield was obtained with C12 fatty acid, leading to 70% of monoester and 8% of diester. The formation of diester could be considered as a secondary product. In these conditions of reaction, the two regioisomers are obtained in the proportions 70/30 with the 4’ regioisomer as the major product regardless of the length of the chain. The determination of this regioselectivity was made by examining the literature and comparing in particular the 1H and 13C NMR spectra [30–34].
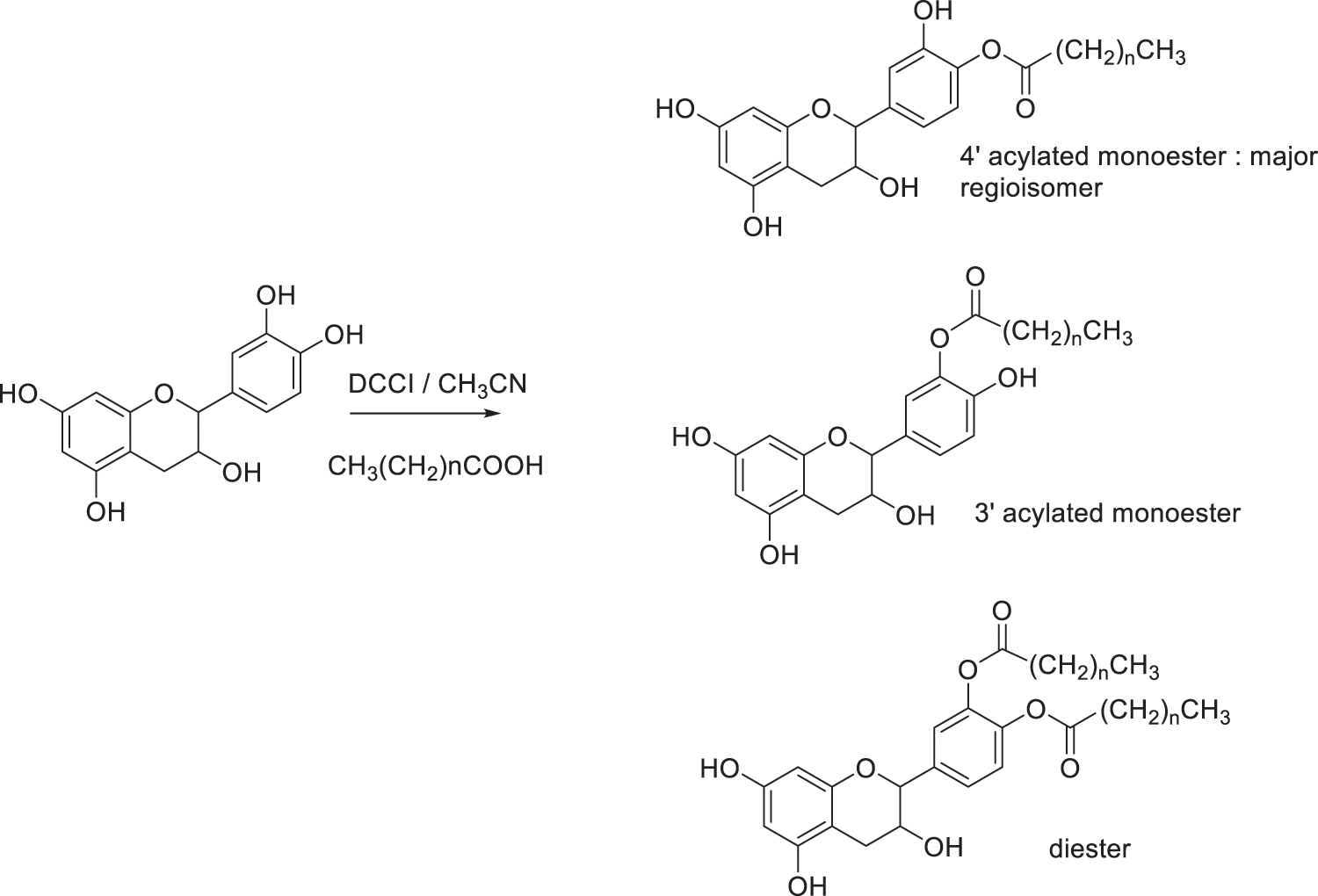
Figure 9: Regioselective acylation of catechin
Regioselectivity of the reaction can be explained based on the acidity of the different phenolic groups. First of all, phenolic groups of catechol moiety (ring B) are more acidic due to the proximity of the two electronegative oxygen atoms compared to the two phenolic groups of ring A. Then, phenol in position 4’ is believed to present a higher acidity compared to phenol in position 3’ due to its distance from the electron donor inductive alkyl group in position 1’. Consequently, reaction take place mainly at the 4’ position due to the easier deprotonation of hydroxyl group by DCCI acting as weak base. Once formed, the monoester may be stabilized by intramolecular hydrogen bonding with hydroxyl group in 3’ position limiting its reactivity. Formation of the diester occurred as a by-product under the reactional conditions used in this study. Such reactivity has already been described in the literature [30–34].
Modification of catechin with fatty acids results in products with an amphiphilic behavior possessing both hydrophilic and hydrophobic properties. Hydrophobic part reduces the release of the compound impregnated in wood, while at the same time, the hydrophilic part allows formation of a stable suspension in water [35].
3.2 Characteristics of Treated Wood and Its Leachability
The results on WPG, WPGAL, and PL are illustrated in Fig. 10, with their statistical grouping presented in Table 2. Treatment with catechin alone produces the lowest WPG (3.02%) and differs significantly from those modified with fatty acids. The WPG increased with the treatment of catechin and fatty acids (3.92%−4.18%); however, there is no significant difference between them (see Table 2).
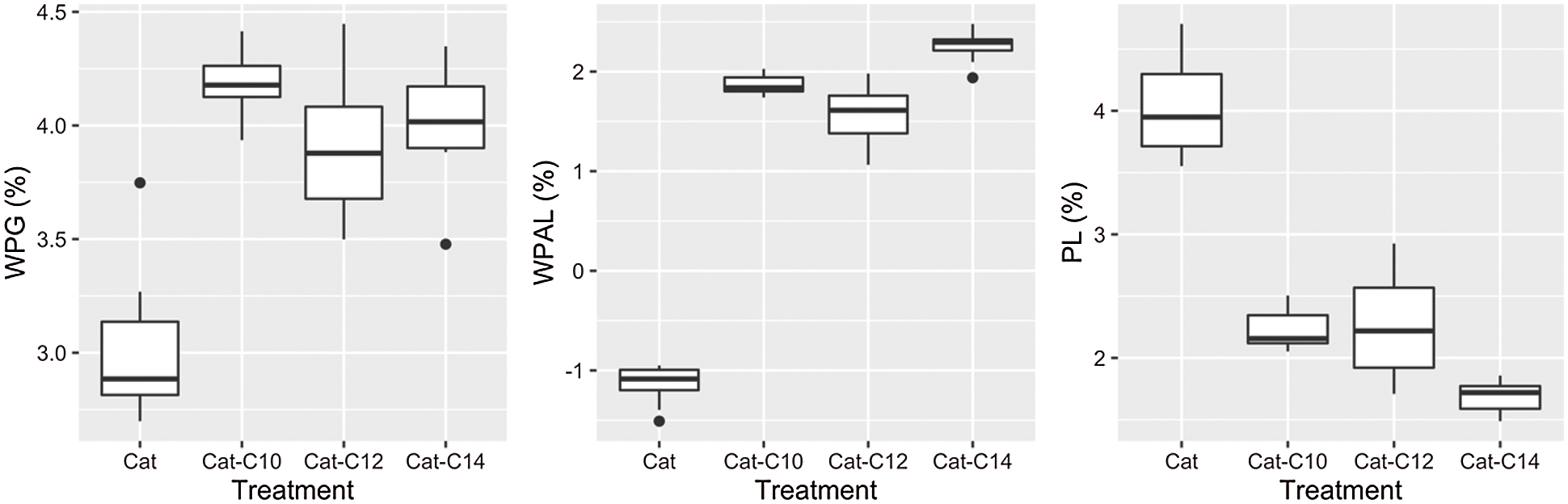
Figure 10: Boxplot results of WPG, WPGAL and PL
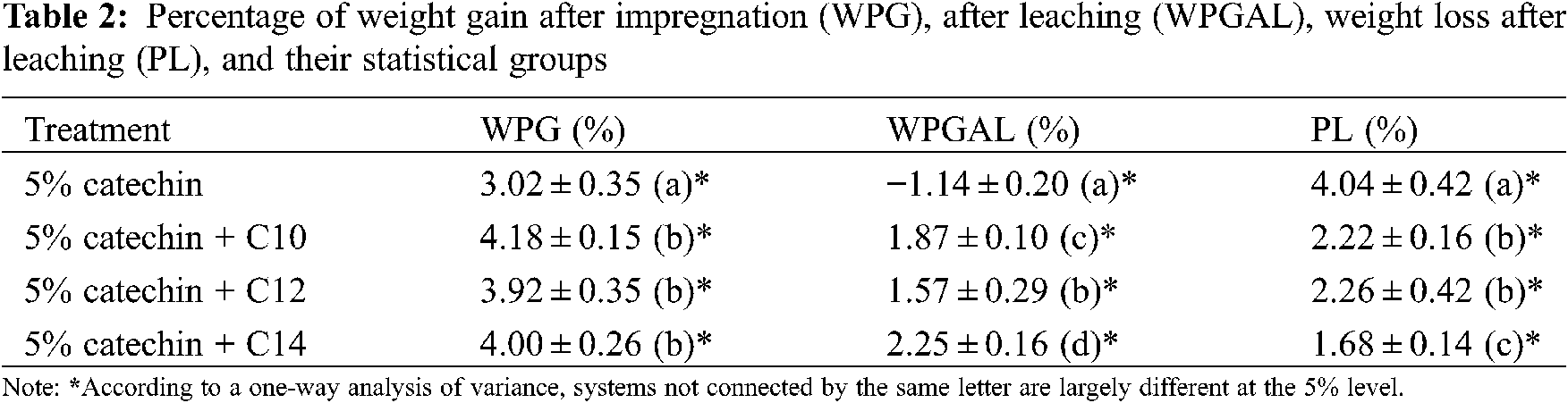
Statistically, the leaching process has a significant effect on all treatments related to the WPGAL results. Samples treated with catechin showed the highest leachability compared to the modified ones. A negative value on WPGAL is possibly caused by some water-soluble extractives that were leached during the test. Results indicated that the leachability decrease evidently when treating catechin with myristic acid, possessing the highest WPGAL and lowest PL. It can be understood because this type of fatty acid has the longest chain, which represents the hydrophobic properties (more resistant to water). This also confirmed that the increase of hydrophobicity with longer chain length might reduce their solubility in water [22–24]. However, no statistical difference was observed for the samples treated with catechin-C10 or catechin-C12 regarding their weight losses (PL). The addition of fatty chains to the catechin is believed to increase the resistance to leaching due to additional hydrophobic properties.
The results of weight loss are evident for the untreated beech wood (51.49%), showing the vigorous fungal activity of T. versicolor under test conditions (Fig. 11). Table 3 presents the weight loss of samples after being exposed to T. versicolor.

Figure 11: Aspect of wood samples after exposure to Trametes versicolor
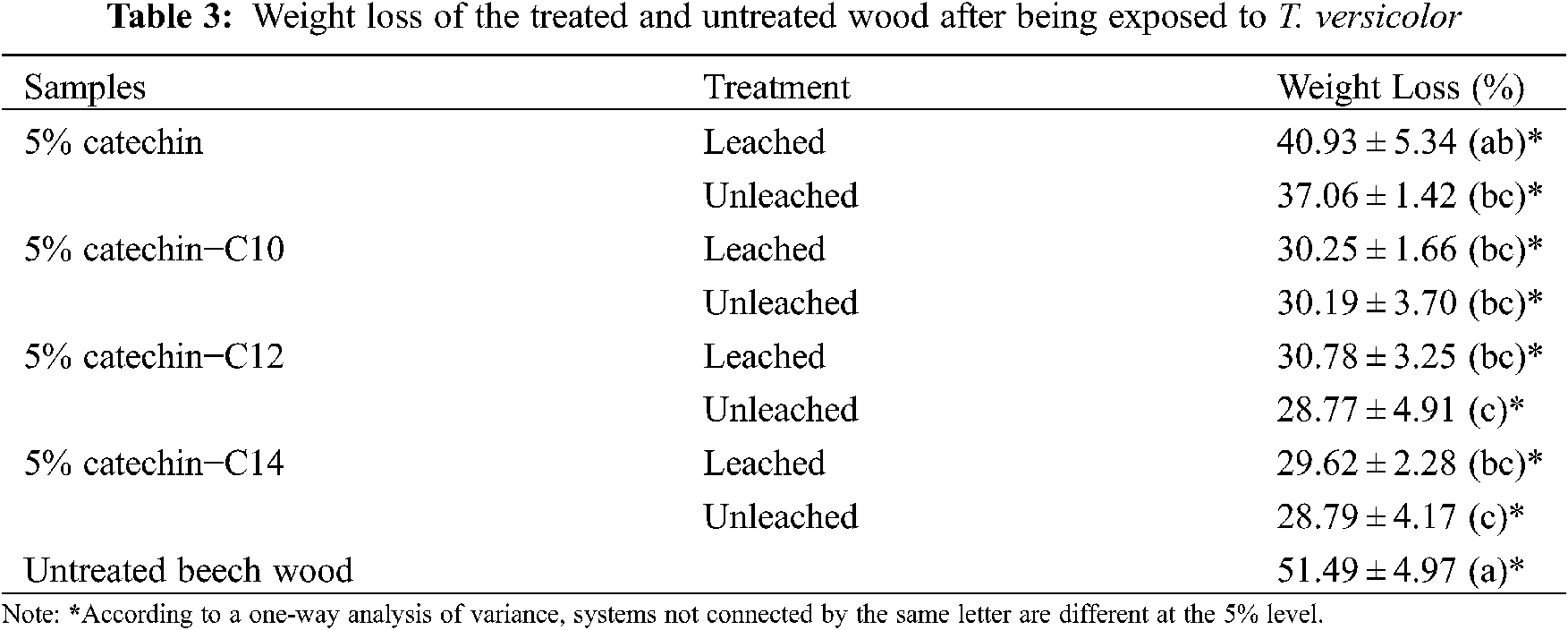
Samples treated with catechin alone presented lower weight losses, 40.93% and 37.06% for the leached and unleached samples respectively, compared to untreated beech wood, which presented weight loss of 51.49%. In a previous experiments conducted by Laks et al. [7], samples treated by catechin at 4% concentration presented weight loss up to 42% and did not differ significantly from their controls. Another study conducted by Tascioglu et al. [8] showed the same trend, while use of plant extracts used at 3% and 6% such as mimosa and quebracho lead to high percentage of weight losses up to 37%–51%. It is obvious that catechin alone at 5% concentration was inadequate to protect wood efficiently.
While the modification of catechin and fatty acids seem to improve their leachability, it can be noted that the decay weight loss does not differ significantly between the samples that leached and not. The specimens treated with catechin and C10 fatty acid experience the highest weight loss (30.25% and 30.19%), while specimens treated with catechin and C14 fatty acid have the lowest weight loss (29.62% and 28.79%). However, there is no significant difference between them.
Literature studies reported that the hydrophobic groups of saturated fatty acids possess an important role in bioactivity, with an increase of the chain length led to increased antifungal efficiency [36,37]. In our case, the addition of fatty chains to catechin tends to reduce leachability of modified catechin as the chain length increases, while the chain length seems to have less effect on decay durability contrary to literature results. Nevertheless, further studies need to be conducted to obtain the threshold level of catechin against wood-decaying fungi.
The potential of catechin for wood protection can be optimized by selectively change its properties through chemical modification, notably to reduce its leachability. The combination of antifungal and hydrophobic properties may improve efficiency of catechin derivatives. Results suggested that catechin modified with myristic acid (C14) provides better resistance towards leaching. Moreover, no significant differences were observed in the decay weight loss for the samples treated with modified catechin with fatty acids before or after leaching. It is believed that the addition of fatty alkyl chain increased resistance to leaching. However, it is recommended to increase the concentration of modified catechin to obtain significant effect on decay resistance. It would also be interesting to consider the combination of these compounds with already used wood preservatives in order to increase the effectiveness of the treatment solution through a potential synergistic effect due to the antioxidant effect of catechin derivatives.
Acknowledgement: The authors acknowledge support of “LERMaB” by the “Impact Biomolecules” Project of the “Lorraine Université d’Excellence” (Investissements d’avenir–ANR 15-004) and of the French Ministery of Agriculture and the Lorraine-FEDER for the support of “EXTRAFOREST” Project.
Funding Statement: Authors gratefully acknowledge the “Ministère des Affaires étrangères et du développement international (MAEDI)” and the “Ministère de l’Education nationale, de l’Enseignement supérieur et de la recherche (MENESR)” for the financial support through Bio-Asie Program (2015–2016). LERMaB is supported by a grant overseen by the French National Research Agency (ANR) as part of the “Investissements d’Avenir” Program (ANR-11-LABX-0002-01. Lab of Excellence ARBRE).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Schultz, T. P., Nicholas, D. D., Preston, A. F. (2007). A brief review of the past, present and future of wood preservation. Pest Management Science, 63(8), 784–788. DOI 10.1002/ps.1386. [Google Scholar] [CrossRef]
2. Obounou-Akong, F., Pasc, A., Emo, M., Gérardin-Charbonnier, C. (2013). A supramolecular hydrogel based on an original pseudopeptidic catanionic surfactant. New Journal of Chemistry, 37(3), 559–562. DOI 10.1039/C2NJ40960G. [Google Scholar] [CrossRef]
3. Obounou-Akong, F., Gérardin, P., Thévenon, M. F., Gérardin-Charbonnier, C. (2015). Hydrogel-based boron salt formulations for wood preservation. Wood Science and Technology, 49(3), 443–456. DOI 10.1007/s00226-015-0701-4. [Google Scholar] [CrossRef]
4. Pizzi, A. (2016). Wood products and green chemistry. Annals of Forest Science, 73(1), 185–203. DOI 10.1007/s13595-014-0448-3. [Google Scholar] [CrossRef]
5. Grosse, C., Noël, M., Thévenon, M. F., Rautkari, L., Gérardin, P. (2018). Influence of water and humidity on wood modification with lactic acid. Journal of Renewable Materials, 6(3), 259–269. DOI 10.7569/JRM.2017.634176. [Google Scholar] [CrossRef]
6. Malterud, K. E., Bremnes, T. E., Faegri, A., Moe, T., Dugstad, E. K. S. et al. (1985). Flavonoids from the wood of salix caprea as inhibitors of wood-destroying fungi. Journal of Natural Products, 48(4), 559–563. DOI 10.1021/np50040a007. [Google Scholar] [CrossRef]
7. Laks, P. E., McKaig, P. A., Hemingway, R. W. (1988). Flavonoid biocides: Wood preservatives based on condensed tannins. Holzforschung, 42(5), 299–306. DOI 10.1515/hfsg.1988.42.5.299. [Google Scholar] [CrossRef]
8. Tascioglu, C., Yalcin, M., Sen, S., Akcay, C. (2013). Antifungal properties of some plant extracts used as wood preservatives. International Biodeterioration & Biodegradation, 85, 23–28. DOI 10.1016/j.ibiod.2013.06.004. [Google Scholar] [CrossRef]
9. Thevenon, M. F., Tondi, G., Pizzi, A. (2009). High performance tannin resin-boron wood preservatives for outdoor end-uses. European Journal of Wood and Wood Products, 67(1), 89–93. DOI 10.1007/s00107-008-0290-0. [Google Scholar] [CrossRef]
10. Mabicka, A., Dumarçay, S., Rouhier, N., Linder, M., Jacquot, J. P. et al. (2005). Synergistic wood preservatives involving EDTA, Irganox 1076 and 2-hydroxypyridine-N-oxide. International Biodeterioration & Biodegradation, 55(3), 203–211. DOI 10.1016/j.ibiod.2005.01.002. [Google Scholar] [CrossRef]
11. Diouf, P. N., Delbarre, N., Perrin, D., Gérardin, P., Rapin, C. et al. (2002). Influence of tropolone on Poria placenta wood degradation. Applied and Environmental Microbiology, 68(9), 4377–4382. DOI 10.1128/AEM.68.9.4377-4382.2002. [Google Scholar] [CrossRef]
12. Baya, M., Soulounganga, P., Gelhaye, E., Gérardin, P. (2001). Fungicidal activity of beta-thujaplicin analogues. Pest Management Science, 57(9), 833–838. DOI 10.1002/ps.379. [Google Scholar] [CrossRef]
13. Mounanga, K., Gerardin, T. K., Poaty, P., Perrin, B., Gérardin, D. C. (2008). Synthesis and properties of antioxidant amphiphilic ascorbate salts. Colloids and Surfaces A–Physicochemical and Engineering Aspects, 318(1–3), 134–140. DOI 10.1016/j.colsurfa.2007.12.048. [Google Scholar] [CrossRef]
14. Aditya, N. P., Aditya, S., Yang, H., Kim, H. W., Park, S. O. et al. (2015). Co-delivery of hydrophobic curcumin and hydrophilic catechin by a water-in-oil-in-water double emulsion. Food Chemistry, 173, 7–13. DOI 10.1016/j.foodchem.2014.09.131. [Google Scholar] [CrossRef]
15. Gadkari, P. V., Balaraman, M. (2015). Catechins: Sources, extraction and encapsulation: A review. Food and Bioproducts Processing, 93, 122–138. DOI 10.1016/j.fbp.2013.12.004. [Google Scholar] [CrossRef]
16. Harun, J., Labosky, P. (1985). Antitermitic and antifungal properties of selected bark extractives. Wood and Fiber Science, 17(3), 327–335. [Google Scholar]
17. Kawamura, F., Ramle, S. F. M., Sulaiman, O., Hashim, R., Ohara, S. (2011). Antioxidant and antifungal activities of extracts from 15 selected hardwood species of Malaysian timber. European Journal of Wood and Wood Products, 69(2), 207–212. DOI 10.1007/s00107-010-0413-2. [Google Scholar] [CrossRef]
18. Vek, V., Oven, P., Poljanšek, I. (2013). Quantitative HPLC analysis of catechin in wound-associated wood and knots of beech. Drvna Industrija, 64(3), 231–238. DOI 10.5552/drind.2013.1307. [Google Scholar] [CrossRef]
19. Saha Tchinda, J. B., Abia, D., Dumarcay, S., Ndikontar, N. K., Gérardin, P. et al. (2013). Antioxidant activities, total phenolic contents and chemical compositions of extracts from four Cameroonian woods: Padouk (Pterocarpus soyauxii Taubbtali (Erythrophleum suaveolensmoabi (Baillonella toxispermaand movingui (Distemonanthus benthamianus). Industrial Crops and Products, 41, 71–77. DOI 10.1016/j.indcrop.2012.04.012. [Google Scholar] [CrossRef]
20. Wijayanto, A., Dumarcay, S., Gérardin-Charbonnier, C., Kartika Sari, R., Syafi, W. et al. (2015). Phenolic and lipophilic extractives in Pinus merkusii Jungh et de Vries knots and stemwood. Industrial Crops and Products, 69, 466–471. DOI 10.1016/j.indcrop.2015.02.061. [Google Scholar] [CrossRef]
21. Say Anouhe, J. B., Bobelé Niamké, F., Milcard, F., Virieux, D., Pirat, J. L. et al. (2018). The role of extractives in the natural durability of the heartwood of Dicorynia guianensis Amsh: New insights in antioxydant and antifungal properties. Annals of Forest Science, 75(1). DOI 10.1007/s13595-018-0691-0. [Google Scholar] [CrossRef]
22. Clausen, C. A., Coleman, R. D., Yang, V. W. (2010). Fatty acid–based formulations for wood protection against mold and sapstain. Forest Products Journal, 60(3), 301–304. DOI 10.13073/0015-7473-60.3.301. [Google Scholar] [CrossRef]
23. Coleman, R., Yang, V., Woodward, B., Lebow, P., Clausen, C. (2016). Efficacy of fatty acid chemistry: candidate mold and decay fungicides. One Hundred Sixth Annual Meeting of the American Wood Protection Association, vol. 106, pp. 287–297. Savannah, Georgia, American Wood Protection Association, Birmingham, Ala. [Google Scholar]
24. Pohl, C. H., Kock, J. L., Thibane, V. S. (2011). Antifungal free fatty acids: A review. Science Against Microbial Pathogens: Current Research and Technological Advances, 1, 61–71. [Google Scholar]
25. Gryc, V., Vavrčík, H., Gomola, Š. (2008). Selected properties of european beech (Fagus sylvatica L.). Journal of Forest Science, 54(9), 418–425. DOI 10.17221/JFS. [Google Scholar] [CrossRef]
26. EN 350 (2016). Durability of wood and wood-based products. In: Testing and classification of the durability to biological agents of wood and wood-based materials. CEN, European Standardisation Institute, Brussels, Belgium. [Google Scholar]
27. NF X 41-568 (2014). Wood preservatives. Laboratory method for obtaining samples for analysis to measure losses by leaching water or synthetic sea water. AFNOR. [Google Scholar]
28. Bravery, A. F. (1979). A miniaturised wood-block test for the rapid evaluation of wood preservative fungicides. In: Screening techniques for potential wood preservative chemicals (Principles of a Special Seminar), pp. 57–65. Stockholm, Sweden: Swedish Wood Preservation Institute. [Google Scholar]
29. R Core Team (2019). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. http://www.r-project.org/index.html. [Google Scholar]
30. Guangzhi, J., Hisashi, Y. (2005). Synthesis of lipophilic poly-lauroyl-(+)-Catechins and radical-scavenging activity. Bioscience, Biotechnology and Biochemistry, 69, 440V447. DOI 10.1271/bbb.69.440. [Google Scholar] [CrossRef]
31. Shan, H., Songbai, L. (2016). Targeted acylation for all the hydroxyls of (+)-catechin and evaluation of their individual contribution to radical scavenging activity. Food Chemistry, 197, 415–421. DOI 10.1016/j.foodchem.2015.10.134. [Google Scholar] [CrossRef]
32. Hong, S., Wang, S., Cai, H., Liu, S. (2019). Regiospecific methylation of all the hydroxyls in (+)-catechin by a stepwise differentiation strategy. Journal of the Science of Food and Agriculture, 99, 3785–3791. DOI 10.1002/jsfa.9594. [Google Scholar] [CrossRef]
33. Cren-Olive, C., Wieruszeski, J. M., Maes, E., Rolando, C. (2002). Catechin and epicatechin deprotonation followed by 13C NMR. Tetrahedron Letters, 43, 4545–4549. DOI 10.1016/S0040-4039(02)00745-1. [Google Scholar] [CrossRef]
34. Salma, U., Chen, N., Richter, D. L., Filson, P. B., Dawson-Andoh, B. et al. (2010). Amphiphilic core/shell nanoparticles to reduce biocide leaching from treated wood, 1–leaching and biological efficacy. Macromolecular Materials and Engineering, 295(5), 442–450. DOI 10.1002/mame.200900250. [Google Scholar] [CrossRef]
35. Ding, X., Richter, D. L., Matuana, L. M., Heiden, P. A. (2011). Efficient one-pot synthesis and loading of self-assembled amphiphilic chitosan nanoparticles for low-leaching wood preservation. Carbohydrate Polymers, 86(1), 58–64. DOI 10.1016/j.carbpol.2011.04.002. [Google Scholar] [CrossRef]
36. Sado-Kamdem, S. L., Vannini, L., Guerzoni, M. E. (2009). Effect of α-linolenic, capric and lauric acid on the fatty acid biosynthesis in Staphylococcus aureus. International Journal of Food Microbiology, 129(3), 288–294. DOI 10.1016/j.ijfoodmicro.2008.12.010. [Google Scholar] [CrossRef]
37. Wang, L. L., Johnson, E. A. (1992). Inhibition of listeria monocytogenes by fatty acids and monoglycerides. Applied and Environmental Microbiology, 58(2), 624–629. DOI 10.1128/aem.58.2.624-629.1992. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |