

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2022.019372
ARTICLE
A Vanillin-Derived, DOPO-Contained Bisphenol as a Reactive Flame Retardant for High-Performance Epoxy Thermosets
1School of Materials Science and Engineering, Shaanxi Normal University, Xi’an, 710062, China
2Engineering Research Center of Historical and Cultural Heritage Protection, Ministry of Education, Shaanxi Normal University, Xi’an, 710062, China
3Hangzhou First Applied Material Co., Ltd., Hangzhou, 311300, China
*Corresponding Authors: Juanli Wang. Email: wangjuanli@snnu.edu.cn; Jintao Wan. Email: wanjintao@snnu.edu.cn
#The authors contribute equally
Received: 20 September 2021; Accepted: 22 October 2021
Abstract: Quest for bio-based halogen-free green flame retardant has attracted many concerns in recent years. Herein a reactive functional flame retardant containing phosphorus VDP is synthesized from vanillin, 9,10-dihydro-9-oxa-10-phosphophene-10-oxide (DOPO) and phenol via a facile way. VDP is characterized with 1H NMR, 31P NMR, FTIR and Time of Flight Mass Spectrometry, and used as a new reactive flame retardant for bisphenol epoxy thermosets. Thermogravimetry analysis shows that when the VDP loading is only 0.5P% (based on phosphorus content), the residue increases from 14.2% to 21.1% at 750°C in N2 compare with neat DGEBA. Correspondingly, the limit oxygen index increased to 29.6%, and flame retardancy reaches UL-94 V0 grade. Micro combustion calorimetry (MCC) and cone calorimetry analyses demonstrate that VDP can significantly lower flammability of the epoxy thermoset. With only 0.5P% of VDP, the heat release rate, total heat release rate and smoke production are reduced markedly. At the same time, the mechanical properties of the modified epoxy thermosets are also improved. The impact strength increases by 34% and the flexural strength increased by 23%, with 1.5P% of VDP. In short, VDP not only improves the flame retardancy, but also improves the mechanical properties of the epoxy thermosets.
Keywords: Epoxy resin; bio-based flame retardant; vanillin; properties of thermosets
Issues related to resources and environment push the development of polymerizable monomers, polymeric materials and modifiers derivable from renewable natural resources. The related research attracts more and more interest from academia and industries. Epoxy resins are a kind of important thermosetting polymeric materials, which have good processability, mechanical, thermal and electrical properties. They are widely used in various fields such as industries, civil, military, aerospace and so on. The vast majority of epoxy resins commercially available are bisphenol A epoxy resin (DGEBA), which is produced and applied for multiple purposes in huge volume annually. However, they are poor in flame retardancy and combustible materials, so that their applications in certain fields are limited especially where good flame retardancy is demanding and compulsive. Therefore, researchers conduct numerous studies to modify epoxy resins to expand their applications, for example, by adding inorganic and organic flame retardants. Compared with inorganic flame retardants, organic flame retardants, especially organophosphorus flame retardants have good compatibility with epoxy matrices, without need of special processing techniques, and their efficiency is generally much higher. In recent years, halogen-free flame retardants become the focus of intensive studies, especially the development of flame retardants by using renewable bio-based resources. For example, researchers have developed a variety of natural phenolic flame retardants for epoxy thermosets, for instance, such based on cardanol, eugenol, vanillin, guaiacol, bisphenol acid, syringaldehyde, ellagic acid, and so on, and some of them have shown their good promise for special applications [1–8].
Vanillin is an important natural substituted phenolic compound, which can be extracted from the plant vanilla or obtained from transformation of lignin [9–11]. Today, vanillin becomes an important bio-derivable platform compound. Vanillin molecules have active aldehyde groups and phenolic hydroxyl groups which can undergo many chemical reactions to attach many functional groups to endow the different reactivities. Therefore, vanillin has become an important renewable raw material to synthesize many different kinds of bio-based monomers and polymers with some interesting properties obtained [12–22]. Especially, vanillin-based epoxy thermosets have attracted extensive attention in academic circles [3,18,19,23–46]. Of the particular interest are the flame-retardant epoxy thermosets based on vanillin. Vanillin-based epoxy prepolymers and curing agents containing s-triazine [46], DOPO (9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide) [16,29], diphenyl acyl phosphine [47], diethyl phosphite [39], cyclotriphosphazene [48], aromatic diamine [41], and triazole [49] moieties have been developed with good flame retardancy achieved for the resultant epoxy thermosets.
In this report, a bisphenol compound (VDP) [16] is synthesized from vanillin, DOPO and phenol via a facile one-pot way. The reaction condition is mild, the time reaction is short, unreactive raw materials can be recycled easily, and the yield is high. VDP contains not only DOPO moieties but also the two phenolic hydroxyl group which can react with the epoxy group of epoxy resin like DGEBA to expand the molecular chain and thus improve the compatibility and hopefully final properties of cured thermosets. Note that DOPO is commercialized and wide applied flame retardant for many polymers. A number of recent studies address DOPO-derived molecules used to improve the flame retardancy of epoxy thermosets [21,50–52]. Moreover, the raw materials of VDP are in a relatively cost low, which makes VDP more interesting for practical applications. However, to our knowledge, VDP is still not evaluated as a reactive flame retardant for standard epoxy thermosetting materials. Especially, their effects on the flame retardancy, thermal and mechanical properties of the epoxy thermosets are still little known. To this end, here VDP is used as a reactive flame retardant to modify epoxy thermosets. Our results will demonstrate that VDP can impart the cured epoxy resin with excellent flame retardancy, good thermal resistance, and superior mechanical properties, paving a new way to develop efficient and green flame-retardant epoxy materials.
Vanillin, 9,10-dihydro-9-oxa-10-phosphine-phenanthrene-10-oxide (DOPO), p-toluenesulfonic acid (p-TSA) and diaminodiphenylsulfone (DDS) were purchased from the Energy Chemicals. Phenol and ethanol were obtained from Sinopharm Chemical Reagent Co., Ltd. Epoxy resin (DGEBA, trade name E54) was obtained from a commercial source. All chemicals and solvents were used directly without an additional treatment.
2.2 Synthesis of Addition Product from Vanillin, DOPO and Phenol (VDP)
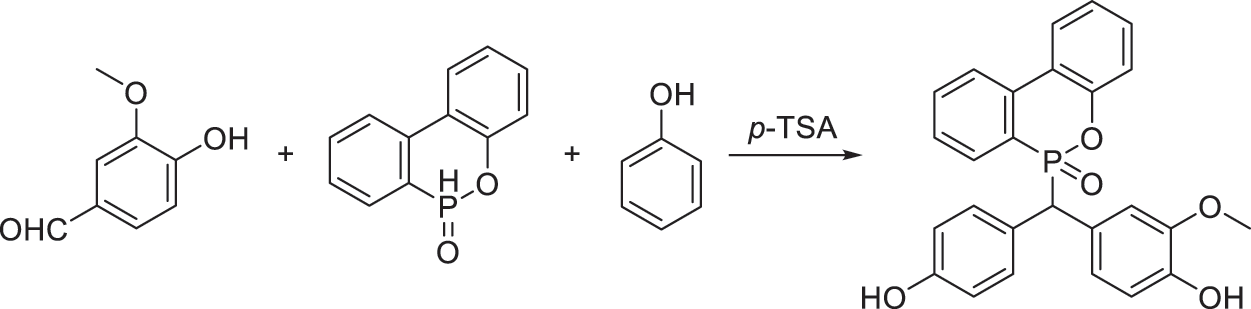
Scheme 1: Synthesis of VDP from vanillin, DOPO and VDP in a “one-pot” way
As illustrated in Scheme 1, VDP is the trimolecular adduct of vanillin, DOPO and phenol, and the synthesis of VDP is in reference to [16] with a certain improvement. Herein, the excessive phenol plays a dual role: reactant and solvent. And most phenol can be recovered after reaction. To illustrate, vanillin (0.25 mol, 38.05 g), DOPO (0.25 mol, 54.05 g), phenol (1.25 mol, 117.65 g) and p-TSA (4 wt% of DOPO) were added into a 500 ml round bottom flask equipped with a condenser, a thermometer and a magnetic stirrer. The reaction was carried out at 110°C for 12 h. Then, unreacted phenol was recycled by distillation under reduced pressure. The obtained crude product was dissolved in ethanol, and then poured it into hot water to remove the remaining phenol. The obtained product was dried in a vacuum oven at 120°C for 3 h to obtain white powder as VDP in a 83% yield.
2.3 Preparation of Shaped Thermosets
The synthesized VDP is used to modify DGEBA with DDS as the curing agent. Preparation of the DGEBA/DDS/VDP thermosets with varied VDP loadings are as follows. A certain equivalent ratio of VDP (based on phosphorus content) was added to the DGEBA and stirred at 195°C until the VDP was completely dissolved to form a transparent solution. After that, the solution was cooled to 170°C, and DDS was added with stirring until the mixture became transparent. After that the reaction mixture was poured into a preheated steel mold coated with a layer of demolding agent. Debubbling was performed under reduced pressure at 170°C for serval minutes. After that, the epoxy formulation was cured at 180°C for 2 h and post-cured at 200°C for 4 h. The mold was cooled to room temperature, then the shaped epoxy specimens were uploaded, polished and subjected to further testing.
Fourier transform infrared spectroscopy (FTIR). FTIR spectra of the samples in the wavelength range of 4000–500 cm−1 were obtained with potassium bromide as the substrate.
Nuclear magnetic resonance (NMR). 1H NMR (400 MHz) spectra of the samples dissolved in deuterated dimethyl sulfoxide (DMSO-d6) with tetramethylsilane as an internal standard were recorded at 60°C on a 400 MHz NMR spectrometer (JEOL, JNMECZ400S/L1).
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS). The molecular weight of VDP was measured with a MALDI-TOF-MS spectrometer (Autoflex Speed, Bruker). The samples were dissolved in acetone, then mixed with saturated 2,5-dihydroxybenzoic acid solution, and dropped on the target to evaporate the solvent.
Thermogravimetric analysis (TG). The epoxy thermosets were measured with a TG analyzer (SDT Q600, TA Instruments) in a dynamic N2/air flow of 100 ml/min. ~5 mg of the sample was heated from 25 to 800°C at 20 °C/min.
Micro combustion calorimetry (MCC). The flammability at a microscale was investigated using a microcalorimeter (Fire Test Technique) based upon ASTM D7309. ~5 mg of the sample was measured with the heating rate of 50 °C/min up to 700°C in an O2-N2 mixture (20:80, by volume). A JF-5 oxygen index tester (Fujian Measurement Instrument Equipment, China), was applied to measure the limit oxygen index (LOI) of the samples in terms of ISO 4589-1996. The sample of dimension was 100 × 10 × 4 mm. UL-94 vertical burning test was carried out on the shaped epoxy thermosets and the size of the specimen was 125 × 12.5 × 3.2 mm, according to the ANSI/UL-94-1985 standard. Combustion and charring behaviors were studied with a cone calorimeter (Fire Testing Technology) according to ISO 5660. The cured epoxy samples (100 × 100 × 4 mm) were tested with the heat flux of 35 KW/m2. Each sample was tested twice to give the average.
The glass transition temperature (Tg). Tg of the cured epoxy samples was determined using a differential scanning calorimeter (DSC Q1000, TA Instruments) under N2 atmosphere, and the samples were heated from room temperature to 280°C, then cooled to room temperature, and finally heated again to 280°C at 20 K/min to determine Tg as the middle point temperature of the specific heat fluctuation during the glass transition.
Flexural properties. Flexural properties of specimens (80 × 10 × 4 mm) were tested on a RGM-2010 universal material testing machine (REGER Instruments Co., Ltd., China) on GB/T 2567-2008. The resting speed was 10 mm/min.
Impact strength. 2 mm depth V-notched specimens (80 × 10 × 4 mm) made and examined using a XJJD-5 simple branched beam impact test machine (Chengde Jinjian Test Instrument, China) for the impact strength test. The standard adopted is GB/T 2567-2008. At least five test specimens from the same batch were tested.
Lap shear strength. DGEBA/DDS/VDP was used to formulate the epoxy adhesives, and tested with the aforementioned testing machine on GB/T 7124-2008 to obtain the lap shear strength. The tensile speed was 5 mm/min. The adhesives were applied to bond two stainless steel pieces with surface are of 12.5 × 25 mm. The samples were cured at 180°C for 2 h and 200°C for 4 h prior to testing.
Fracture mechanics. To characterize the fracture mechanical properties of the materials, here the critical stress factor (KIC) and a critical strain energy release rate (GIC) are examined. KIC and GIC of the specimens were measured in a one-sided incisional bending mode of ASTM D5045. A 4 mm-notch with a sharp pre-crack was made onto rectangular specimens (60 × 10 × 5 mm). The crack dimension was measured accurately using a digital microscope. Notched specimens were bent at 10 mm/min until fracture occurs. KIC and GIC were calculated from Eqs. (1) and (2), respectively. Here, P is the bending force at fracture, W is the specimen width, B is the thickness, a is the crack length, X is a/W, and U is the integrated area under the bending curves.
where
Scanning electron microscopy (SEM). A SEM microscope (SU3500, Hitachi) was used to observe the morphology of the impact section of the epoxy bars and the residual of the epoxy sample after combustion in air. Before the test, thin gold layer was coated on the sample surface, and then observed with the acceleration voltage of 10 KV.
3.1 Spectrum Characterization of VDP
Fig. 1A compares the H-NMR spectra (in DMSO-d6) of the starting materials (vanillin and DOPO) against the synthesized VDP. The NMR resonance of proton of the aldehyde group (-CHO) of vanillin appears at 10.2 ppm, where this resonance disappears in the case of VDP. In addition, the signal due to the methoxy (-OCH3) at ~3.8 ppm in vanillin also appears in VDP, but its position has moved to a higher field, at ~3.6 ppm. Moreover, DOPO shows the signal at 8.8 ppm attributed to P-H bond, whereas VDP does not show such resonance. Meanwhile, an additional signal at 4.4 ppm is observed for VDP, indicating the nucleophilic addition of the aldehyde of vanillin to phosphorus-hydrogen bond. The signals at 6.5–8.2 ppm belong to those of the hydrogen located at the benzene ring. The relative integral areas of 1H NMR spectrum of VDP and the 31P NMR spectrum are shown in the Supporting Materials, which agree with its molecular structure. Fig. 1B shows the FTIR spectra of vanillin, DOPO and VDP, from which the P-H bond absorption peak at 1668 cm−1 and -CHO at 2435 cm−1 disappears in VDP, resulting from nucleophilic addition of the aldehyde to the P-H bond. And the concomitant appearance of P=O bond absorption at 1230 cm−1, P-Ph at 1118 cm−1, P-O-Ph at 917 and 754 cm−1, respectively [53], further confirming the successful integration of DOPO units into the VDP molecules. Fig. 1C presents the MALDI-TOF-MS of VDP, which further confirms that the determined molecular weight (M + 2 = 464 Da, M + Na + = 468 Da) is consistent with the theoretical (M = 444 Da). On the basis of 1H NMR, 31P NMR, IR and MALDI-TOF-MS analyses, the target compound VDP has been successfully synthesized in a good purity.
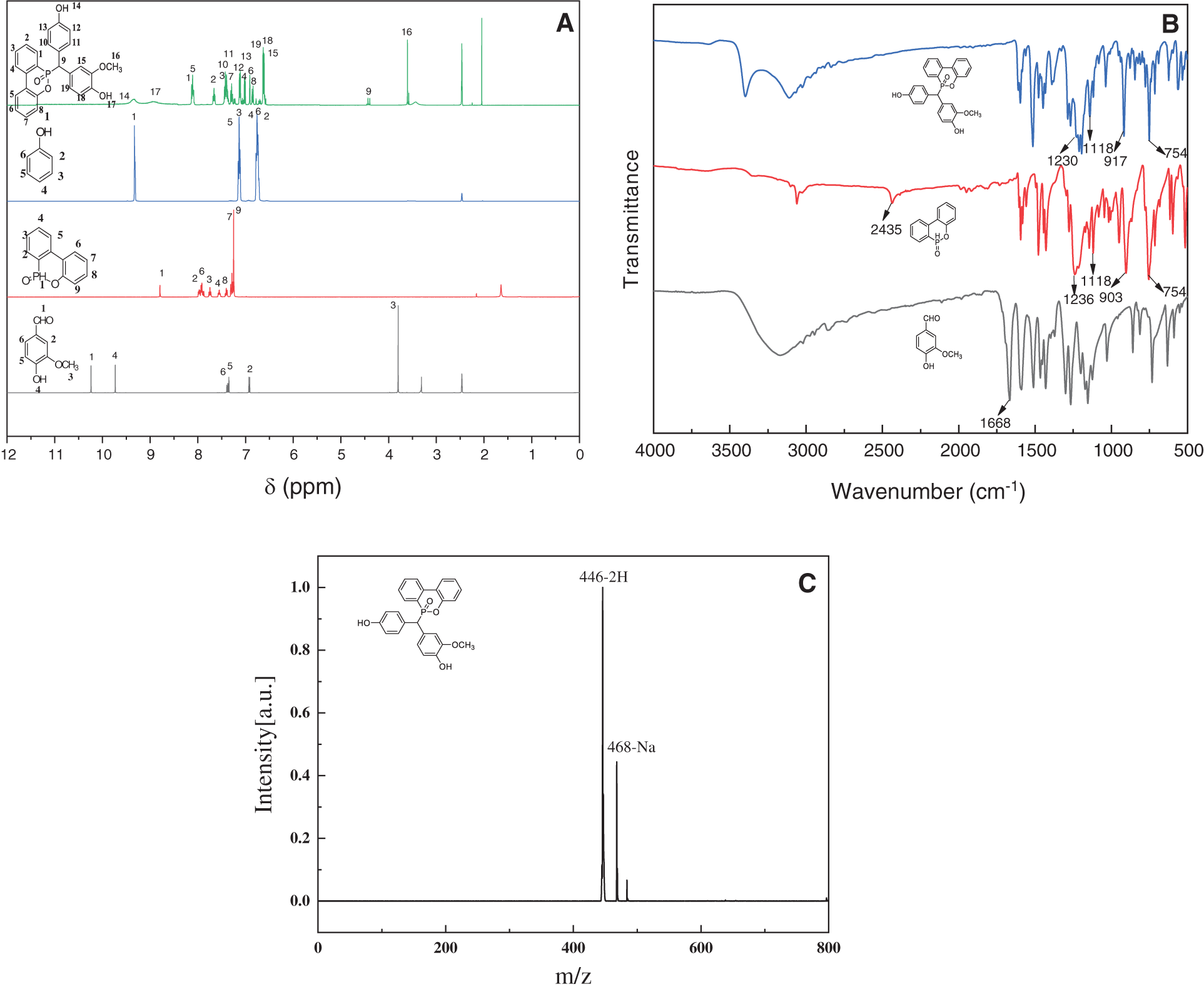
Figure 1: Spectra analysis of VDP. (A) FTIR, (B) 1H NMR (in DMSO-d6) and (C) MALDI-TOF-MS
3.2 Thermal Decomposition Behaviors
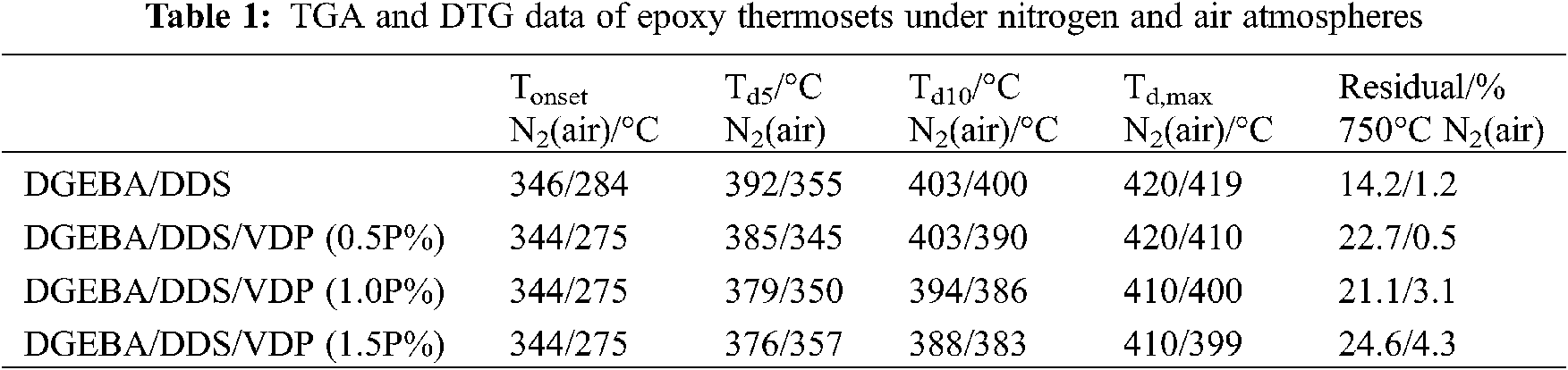
Thermogravimetric analysis (TGA) is used to investigate the thermal decomposition behavior of the epoxy thermosets in nitrogen and air atmospheres to extract such information as thermal stability, pyrolysis and charring process. With the focus on the influence of flame retardants on the thermal degradation process. The TGA and DTG curves of the different epoxy systems in nitrogen and air are presented in Fig. 2, while the temperatures at onset (Tonset), at 5% weight loss (Td5), 10% weight loss (Td10) and for maximum decomposition rate (Td,max) and the residual yield at 750°C are listed in Table 1. Under a nitrogen atmosphere, the thermosets decompose likely in a one-step manner, and the decomposition mainly occurs between 350–500°C. Compared with pristine DGEBA/DDS, the Td5, Td10, and Td,max of DGEBA/DDS/VDP thermosets decrease gradually with increasing VDP loading, because VDP contains P-C, O-P=O and -OCH3 bonds which are less thermally stable, and thus are easier to undergo the thermal cleavage. In addition, the thermal decomposition of the phosphorus moieties will produce phosphoric acid, as polyphosphoric acids, and these generated acid species are able to accelerate the thermal decomposition of the remaining epoxy matrix [54]. However, the residual yield at 750°C increases by 73%, from 14.2% for DGEBA/DDS to 24.6% for DGEBA/DDS/VDP (1.5P%).
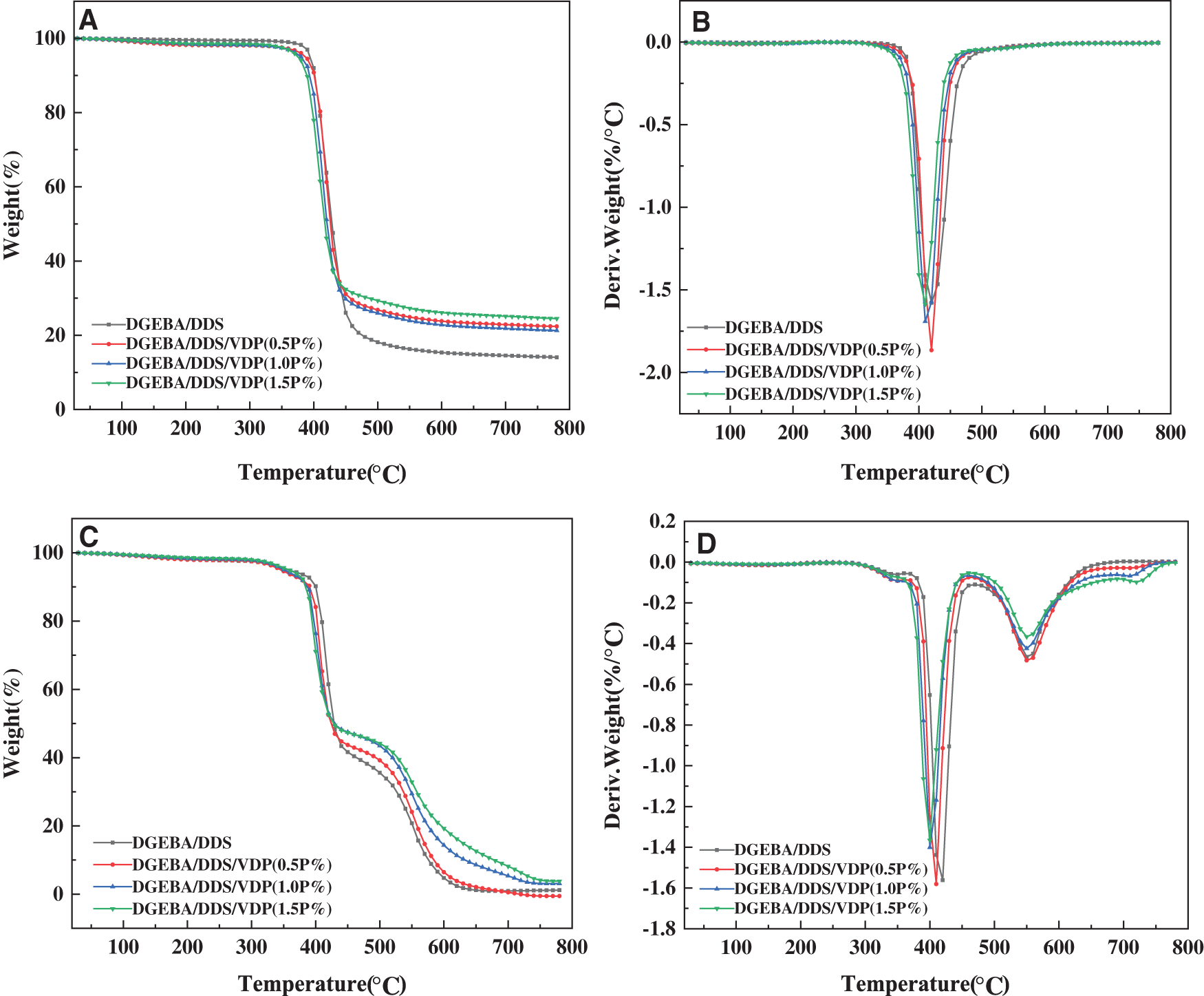
Figure 2: Thermogravimetric and differential thermogravimetric curves of epoxy thermosets in nitrogen (A and B) and air atmospheres (C and D)
On the other hand, there are three stages during the decomposition of the thermosets in an air atmosphere (Figs. 2C and 2D). The first stage will produce phosphoric and polyphosphoric acid species at a relatively low temperature because of the less thermal stability of phosphorus containing groups [52]. The second stage could be attributed to the dehydration, decomposition, and coke of thermosets catalyzed by phosphoric and polyphosphoric acid species. The third stage corresponds to further oxidative decomposition of the previously formed char [2]. The degradation of the neat epoxy is divided into two stages. The first stage occurs between 330 and 460°C, which is likely related to the scission of isopropylidene linkage and the formation of carbon residue in a specific temperature range. The second stage occurs between 460 and 650°C, which is mainly the thermal oxidation decomposition of carbon residue formed in the first stage [21]. Overall, DGEBA/DDS/VDP exhibits the slightly lower thermal stability than DGEBA/DDS dose. Nevertheless, the decreased thermal stability is associated with phosphorus containing units may be necessary in favor of the molecules recombination and carbonization to improve the flame retardancy.
3.3 Glass Transition Temperature of Thermosets
The glass transition temperature (Tg) of cured epoxy thermosets was measured using differential scanning calorimetry (DSC). Fig. 3 shows the DSC thermograms of the cured epoxy products. Tg value of unmodified DGEBA/DDS is 228.9°C. Note that there are some differences in previous literature reporting the Tg values of DGEBA/DDS systems [55], which may be caused by many factors such as epoxy values, curing conditions, epoxy-hardener ratios, different measurement techniques, and testing conditions. For example, the increased heating rate will lead to the shift of the glass transition temperature to a high temperature range. For DGEBA/DDS/VDP thermosets, Tg gradually decreases with the increase of VDP content. For example, Tg of DGEBA/DDS/VDP (1.5P%) is 47.6°C lower than that of DGEBA/DDS. VDP is rich in rigid aromatic cyclic units which should have a positive effect on Tg [56]. The decrease in Tg is attributed to the decreased crosslinking density of epoxy thermosets, resulting from bulky VDP that increases the molecular weight between crosslinks in the cured epoxy network. Nevertheless, the epoxy system still maintains a high glass transition temperature, especially when the VDP loading is low, so that it has good thermal resistance for high-performance applications.
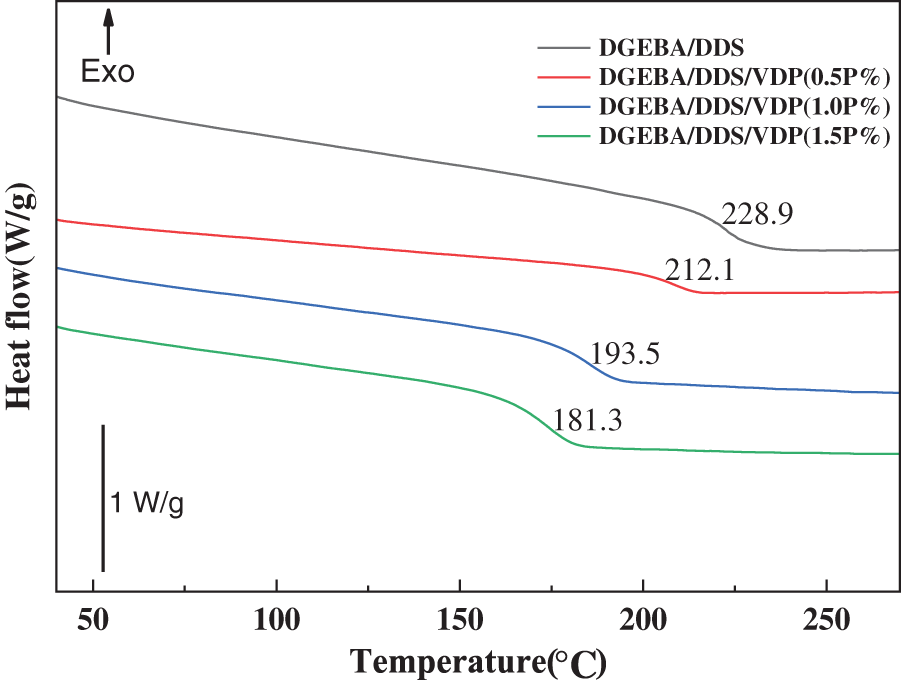
Figure 3: Dynamic DSC thermographs of DGEBA/DDS and DGEBA/DDS/VDP with varied phosphorus contents (heating rate: 20 K/min). Tg is determined as the middle point of the change in heat capacity
3.4 Mechanical Properties of Thermosets
The mechanical properties of DGEBA/DDS and DGEBA/DDS/VDP systems are studied in a compared way, including the lap shear strength, impact strength, flexural properties, fracture toughness and fracture energy. The obtained experimental data are listed in Table 2. Compared with DGEBA/DDS, an increase in VDP loading, the shear strength of the resulting cured epoxy products increases from 19.91 ± 0.46 MPa to 22.01 ± 1.85 MPa, and then decreases to 20.44 ± 2.91 MPa. However, the change is not so much, indicating that VDP little affects the shear strength. On the other hand, the notched impact strength of DGEBA/DDS is 1.60 ± 0.03 kJ/m2, whereas that of DGEBA/DDS/VDP (1.5P%) increases to 2.42 ± 0.23 kJ/m2, an 33.8% enhancement. VDP is attractive to improve the brittleness of the epoxy thermosets. The increased impact strength may be due to the decrease in crosslinking density with the increasing VDP content, leading to the increased plastic deformation ability of matrix and thus improve the resistance to high-speed impact stress.
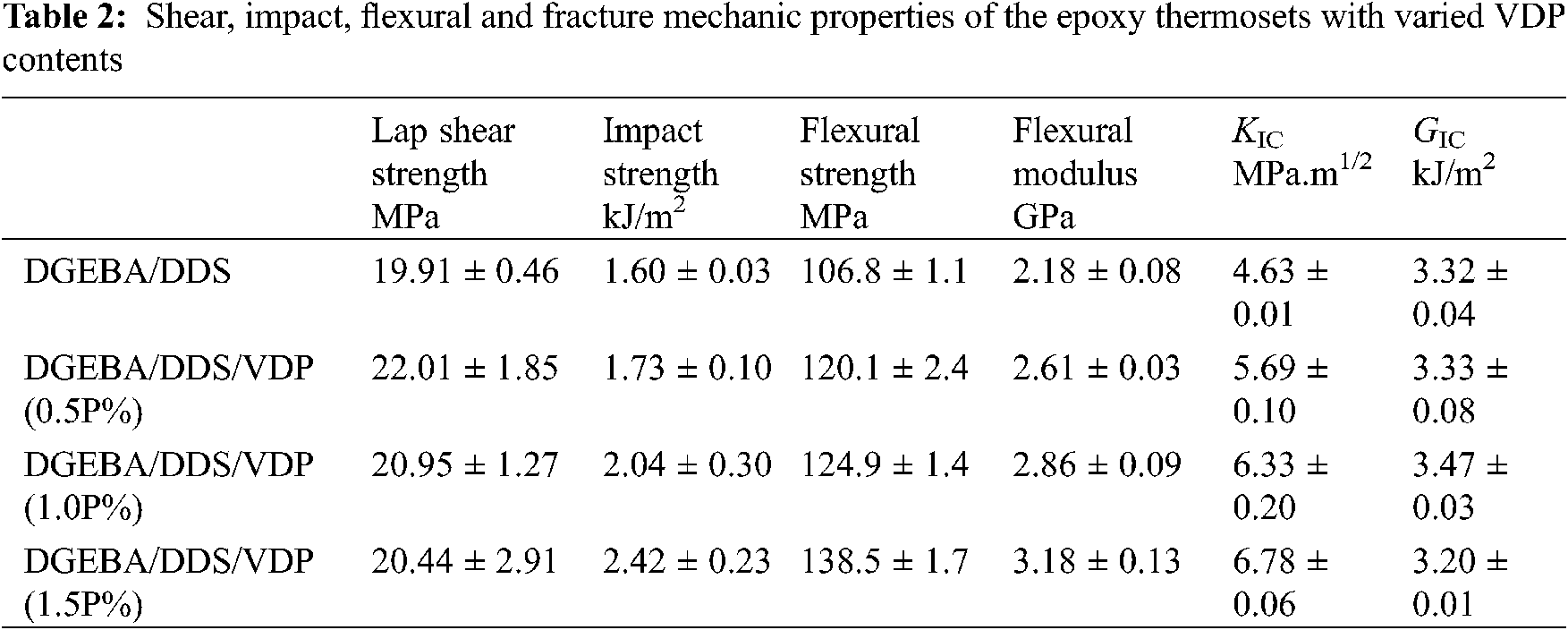
SEM images of impact section of the broken epoxy thermosets are given in Fig. 4. The impact surface of DGEBA/DDS shows scaly cracks, but the crack tip is sharper, and the degree of bending and deformation of the resin matrix are lower, indicating that the impact energy absorbed during the fracture of the materials is relatively low. In contrast, for DGEBA/DDS/VDP (0.5P%), the front of the crack of impact surface shows a certain bending deformation and distortion, indicating that more impact energy has been absorbed before failure. The increased impact strength is related to the reaction between the phenolic hydroxyl groups of VDP and the epoxy group, which leads to the chain extension in the cured epoxy thermoset, thereby improving the ability of the molecular network segment to slide and plastically deform under high-speed impact loads. Therefore, the impact strength is improved with the increased contents of VDP.
Figure 4: SEM images of impact sections of DGEBA/DDS (left) and DGEBA/DDS/VDP (0.5P%) (right)
The flexural strength and modulus of unmodified DGEBA/DDS are 106.8 ± 1.1 MPa and 2.18 ± 0.08 GPa, respectively. As the content of VDP (phosphorus based) in DGEBA/DDS/VDP systems is increased gradually to 1.5P%, and the flexural and modulus increase systematically from 106.8 ± 1.1 to 138.5 ± 1.7 MPa and from 2.18 ± 0.08 to 3.18 ± 0.13 GPa, respectively. Compared with DGEBA/DDS, the flexural strength increased by 23%, and meanwhile the modulus is increased by 41%. The improved flexural properties are also closely related to highly rigid molecular structure of VDP and the strong intramolecular hydrogen bond formed by polar hydroxyl groups. The fracture toughness (KIC) of DGEBA/DDS is 4.63 ± 0.01 MPa·m1/2, while that of DGEBA/DDS/VDP (1.5P%) is increased to 6.78 ± 0.06 MPa·m1/2, a 32% increment. Increased KIC indicates that VDP has the ability to improve the crack endurance of the epoxy thermoset, which important for long-term safety of the high-performance epoxy composites especially for aircraft industry. In terms of fracture energy (GIC), GIC of the epoxy thermoset does not change much with the increased VDP content, which likely indicates that fracture of the materials from a sharp crack to create new surfaces will absorb close energy.
Micro combustion calorimetry (MCC) is used to evaluate the flammability of the epoxy thermosets at a microscale. Table 3 compares the total heat release (Total HR), heat release rate (HHR), heat release capacity and maximum heat release temperature of DGEBA/DDS with varied VDP contents. Fig. 5 shows the relationship between HHR and temperature of the thermosets. The results show that all the systems have the maximum exothermic process in the temperature range of 403–428°C. Compared with Tmax from TG analysis, the same sample shows a slight deviation, which may be caused by the different testing methods of TG and MCC and different heating rate applied. With the increase of the VDP contents, HRR and Total HR decrease gradually. The peak heat release rate (Peak HRR) of DGEBA/DDS/VDP (1.5P%) decreases from 470.8 to 343.7 J/(g·K), a 37% reduction, compared with DGEBA/DDS. Meanwhile, Total HR decreases from 25.3 to 21.9 kJ/g. The results show DGEBA/DDS/VDP has a lower flammability than DGEBA/DDS does. Furthermore, the limit oxygen index (LOI) of DGEBA/DDS and DGEBA/DDS/VDP are compared in Table 3. Modified DGEBA/DDS has a much higher LOI value than that of unmodified DGEBA/DDS, but the LOI of the epoxy system having even higher VDP content (1.5P%) is slightly decreased. Furthermore, when the thermosets contain 0.5, 1.0 and 1.5P%, they can pass the UL-94 vertical burning test with V0 grade achieved. Without any VDP, DGEBA/DDS burns out and fails in the UL 94 test.
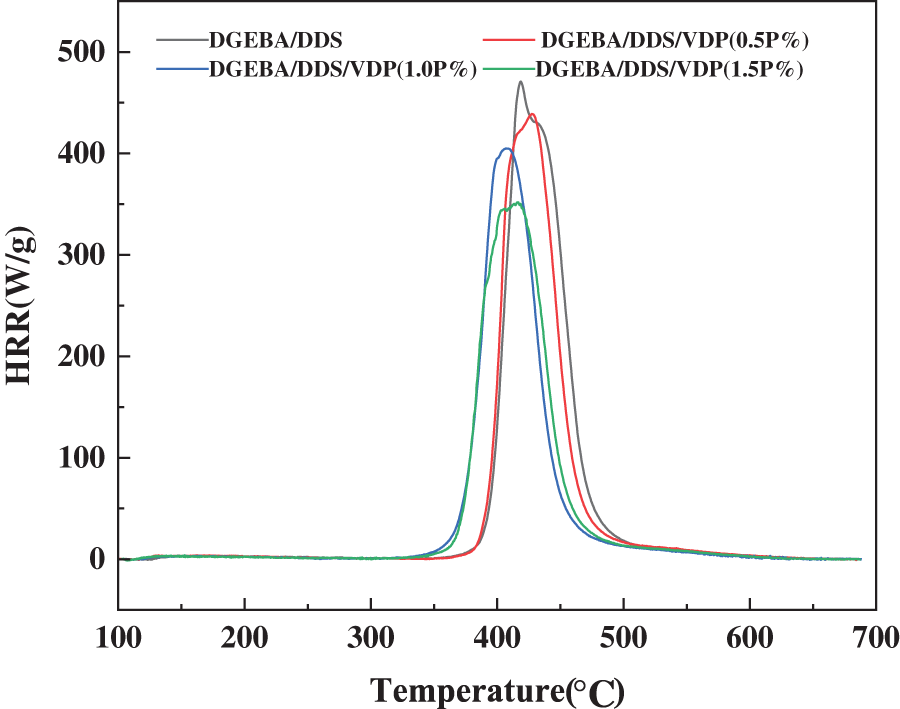
Figure 5: Comparison of the flammability of DGEBA/DDS and DGEBA/DDS/VDP thermosets from MCC tests
Cone calorimeter is recognized as the most powerful instrument to more quantitatively characterize the combustion performance of materials, owing to testing conditions is comparable to the real burning conditions of materials in fire. Fig. 6 shows the heat release rate (HRR), total heat release (THR), residual mass, and total smoke production (TSP) as function of time for the neat and VDP-modified epoxy thermosets; the relevant data are given in Table 4. HRR is an important parameter to characterize fire intensity. The higher HRR and peak HHR, the faster the heat release from combustion and the more intensive the fire. From Fig. 6, the peak HRR of the DGEBA/DDS/VDP (0.5P%) is significantly lower than that of DGEBA/DDS. This indicates that the addition of VDP can greatly reduce fire hazard of the materials. The reason is that the DOPO-derived moieties can not only catalyze charring and but also action as in gaseous phase inhibiter to capture high-energy free radicals and terminate the chain reaction [52,57,58]. After combination, the residue of DGEBA/DDS is 16%, and the residue of DGEBA/DDS/VDP (0.5P%) increases to 33%. The smoke produced in a fire is proportional to the possibility of suffocation and is the main reason leading to casualties in a fire disaster. TSP of DGEBA/DDS is 33 m2/Kg, while the smoke yield is reduced to 28 m2/Kg by adding 0.5P% of VDP only. The smoke suppression effect is attributed to the increased charring ability of the polymer matrix. In Table 4, the ignition time of DGEBA/DDS/VDP (0.5P%) is lower than that of DGEBA/DDS. Because the stability of P-C bond and P=O bond of VDP has relatively lower bond energies, which will lead to the formation of gaseous flammables [52]. Moreover, the OCH3 of VDP is also easer to detach from the epoxy network to form combustible in this stage [59]. In general, the flame-retardant property of the thermosetting resin modified by VDP is improved with the suppressed smoke production.
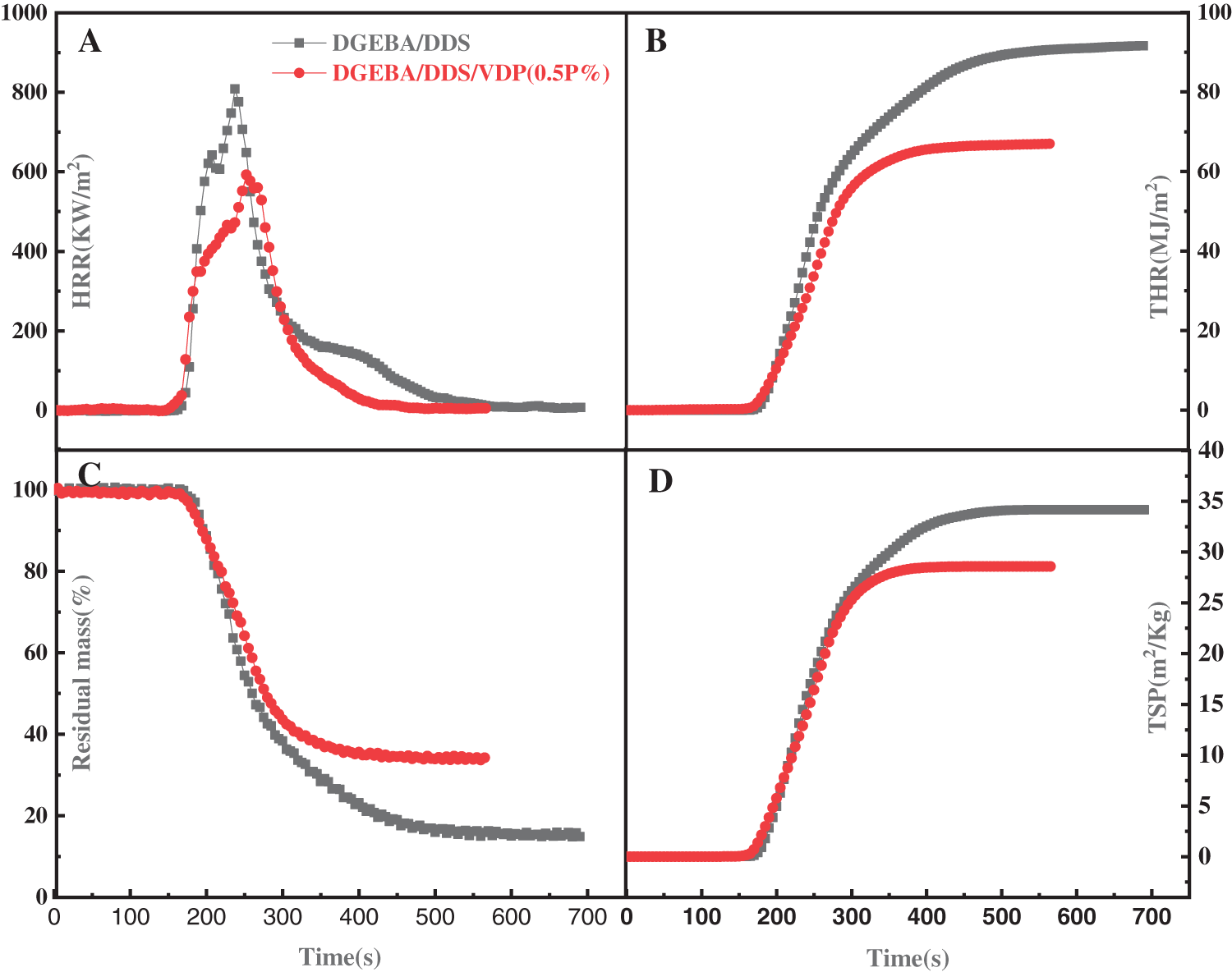
Figure 6: (A) HRR (heat release rate), (B) THR (Total heat release), (C) Residual mass, and (D) Total smoke production (TSP) as a function of time for DGEBA/DDS and DGEBA/DDS/VDP (0.5P%)
Fig. 7 compares the effect of VDP on the morphology of the residual char obtained after combustion of the epoxy thermosets in air. DGEBA/DDS shows a cellular morphology with a large number of open holes, and its char layer surface is relatively smoother, indicating the melting of the thermoset due to significant chain scission during the thermal decomposition of the matrix resin. In this way, the mass transfer rate of combustible gases generated and air is much faster, and the heat generated by combustion is easier to be transmitted to the non-decomposed polymer layer, so as to accelerate the further thermal decomposition and combustion. Therefore, the flame retardancy of the pristine epoxy thermosets is inferior [32]. In contrast, the char residual of DGEBA/DDS/VDP (0.5P%) represents a spongy structures, which can slow down the mass transfer of the combustible gases and air and produce much greater thermal resistance to retard thermal transfer, so the flame retardancy property of the material is improved greatly [60–62]. Furthermore, the surfaces of DGEBA/DDS/VDP (1.0P%) and DGEBA/DDS/VDP (1.5P%)’s residual shows an even denser spongy morphology, indicating that formed char structure has the more notable insulation effect to retard diffusion of air and combustibles.
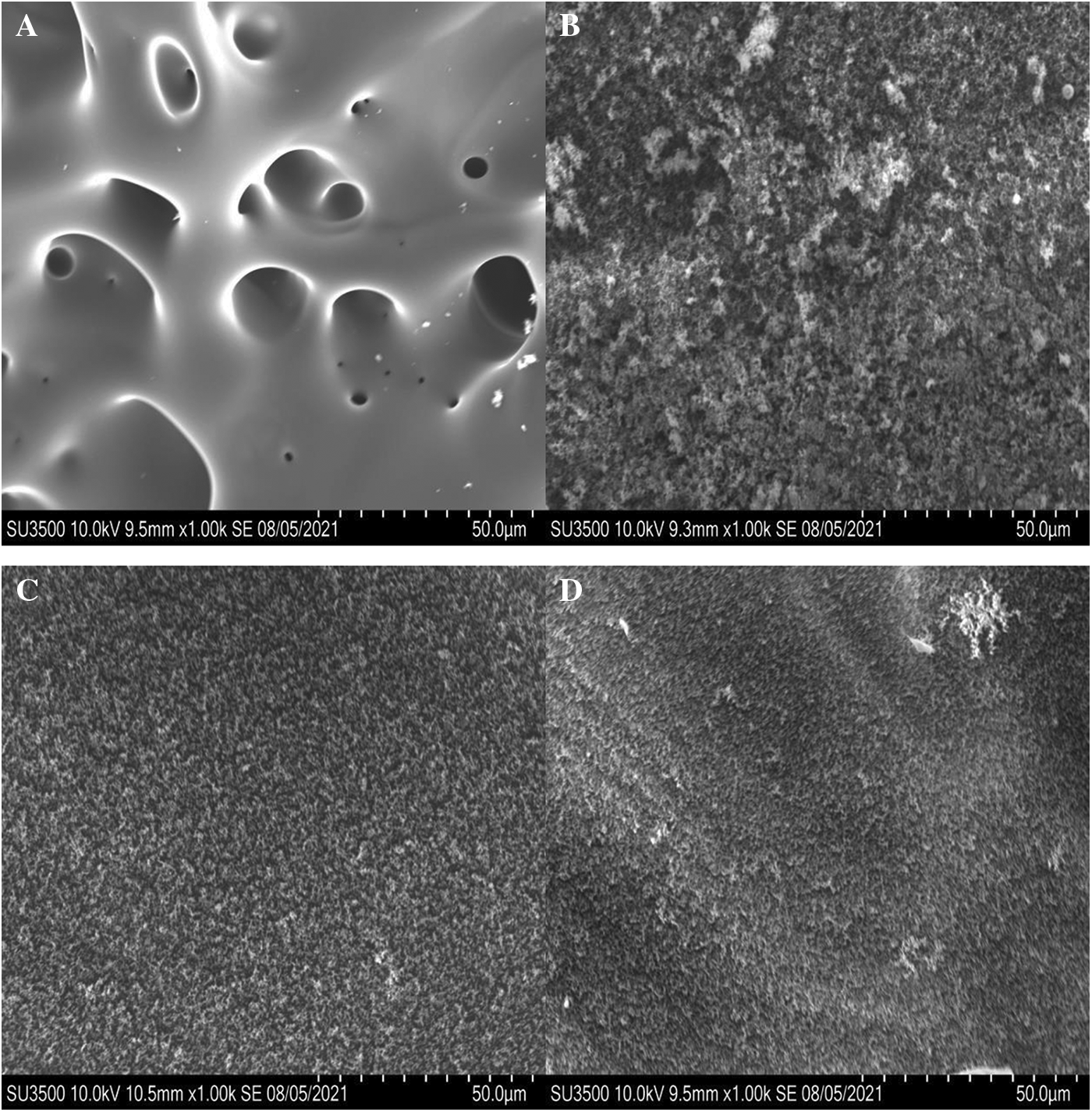
Figure 7: SEM photographs (magnification: 1000 times) of residual of (A) DGEBA/DDS, (B) DGEBA/DDS/VDP (0.5P%), (C) DGEBA/DDS/VDP (1.0P%), and (D) DGEBA/DDS/VDP (1.5P%) after burning in air
A halogen-free, phosphorus-contained flame retardant (VDP) was synthesized from vanillin, DOPO and phenol in a simple and scalable way. VDP was used to modify DGEBA which greatly affected the thermal degradation, mechanical properties and flame retardancy of the resulting epoxy thermosets. Compared with DGEBA/DDS, introduction of VDP slightly decreased the thermal stability of DGEBA/DDS/VDP, but the increased char yield in nitrogen at 750°C from 14.2% for DGEBA/DDS to 21.1% for DGEBA/DDS/VDP (1.5P%). Tg of the thermosets decreased as the VDP content increased, but maintained a high value of >180°C at a 1.5P% VDP loading. The MCC test showed that the heat release rate, total heat release rate and heat capacity of DGEBA/DDS/VDP (1.5P%) were reduced by 27%, 13% and 24%, respectively, compared with DGEBA/DDS. Cone calorimeter test showed that only 0.5P% of VDP led to the significantly reduced that heat release rate (HRR) and total heat release (THR) with the markedly increased fraction of the burnt residual and the reduced total smoke production (TSP). Also, the modified thermosets had a much greater LOI value, increased from 22.6% for DGEBA/DDS to 29.6% for that with 0.5P% VDP loading, with UL94 V0 reached. Moreover, VDP little affected the lap shear strength, but could notably increase the impact strength, flexural properties and fracture toughness of the materials. To conclude, VDP can be readily synthesized in a high yield with a good purity; VDP endows the cured epoxy thermosets not only excellent flame retardancy but superior mechanical properties and good thermal resistance as well. VDP is hopeful as a new flame retardant for high-performance epoxy thermosets.
Funding Statement: This work is supported by the National Natural Science Foundation of China (NSFC) under the agreements of 21875131 and 21773150. The Natural Science Basic Research Plan in Shaanxi Province of China (2020JM-283) and the Fundamental Research Funds for the Central Universities (GK202003044 and GK201902014) are also acknowledged for partial support.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Wang, X., Kalali, E. N., Wan, J. T., Wang, D. Y. (2017). Carbon-family materials for flame retardant polymeric materials. Progress in Polymer Science, 69, 22–46. DOI 10.1016/j.progpolymsci.2017.02.001. [Google Scholar] [CrossRef]
2. Chi, Z., Guo, Z., Xu, Z., Zhang, M., Li, M. et al. (2020). A DOPO-based phosphorus-nitrogen flame retardant bio-based epoxy resin from diphenolic acid: Synthesis, flame-retardant behavior and mechanism. Polymer Degradation and Stability, 176, 109151. DOI 10.1016/j.polymdegradstab.2020.109151. [Google Scholar] [CrossRef]
3. Wan, J., Zhao, J., Zhang, X., Fan, H., Zhang, J. et al. (2020). Epoxy thermosets and materials derived from bio-based monomeric phenols: Transformations and performances. Progress in Polymer Science, 108(2), 101287. DOI 10.1016/j.progpolymsci.2020.101287. [Google Scholar] [CrossRef]
4. Huo, S., Song, P., Yu, B., Ran, S., Chevali, V. S. et al. (2021). Phosphorus-containing flame retardant epoxy thermosets: Recent advances and future perspectives. Progress in Polymer Science, 114, 101366. DOI 10.1016/j.progpolymsci.2021.101366. [Google Scholar] [CrossRef]
5. Wang, X., Niu, H., Guo, W., Song, L., Hu, Y. (2021). Cardanol as a versatile platform for fabrication of bio-based flame-retardant epoxy thermosets as dgeba substitutes. Chemical Engineering Journal, 421(5), 129738. DOI 10.1016/j.cej.2021.129738. [Google Scholar] [CrossRef]
6. Nabipour, H., Wang, X., Song, L., Hu, Y. (2021). A high performance fully bio-based epoxy thermoset from a syringaldehyde-derived epoxy monomer cured by furan-derived amine. Green Chemistry, 23(1), 501–510. DOI 10.1039/D0GC03451G. [Google Scholar] [CrossRef]
7. Nabipour, H., Qiu, S., Wang, X., Song, L., Hu, Y. (2021). Phosphorus-free ellagic acid-derived epoxy thermosets with intrinsic antiflammability and high glass transition temperature. ACS Sustainable Chemistry & Engineering, 9(32), 10799–10808. DOI 10.1021/acssuschemeng.1c02434. [Google Scholar] [CrossRef]
8. Guo, W., Wang, X., Huang, J., Mu, X., Cai, W. et al. (2021). Phosphorylated cardanol-formaldehyde oligomers as flame-retardant and toughening agents for epoxy thermosets. Chemical Engineering Journal, 423, 130192. DOI 10.1016/j.cej.2021.130192. [Google Scholar] [CrossRef]
9. Fache, M., Boutevin, B., Caillol, S. (2016). Vanillin production from lignin and its use as a renewable chemical. ACS Sustainable Chemistry & Engineering, 4(1), 35–46. DOI 10.1021/acssuschemeng.5b01344. [Google Scholar] [CrossRef]
10. Banerjee, G., Chattopadhyay, P. (2019). Vanillin biotechnology: The perspectives and future. Journal of the Science of Food and Agriculture, 99(2), 499–506. DOI 10.1002/jsfa.9303. [Google Scholar] [CrossRef]
11. Zirbes, M., Quadri, L. L., Breiner, M., Stenglein, A., Bomm, A. et al. (2020). High-temperature electrolysis of kraft lignin for selective vanillin formation. ACS Sustainable Chemistry & Engineering, 8(19), 7300–7307. DOI 10.1021/acssuschemeng.0c00162. [Google Scholar] [CrossRef]
12. Fache, M., Boutevin, B., Caillol, S. (2015). Vanillin, a key-intermediate of biobased polymers. European Polymer Journal, 68(6), 488–502. DOI 10.1016/j.eurpolymj.2015.03.050. [Google Scholar] [CrossRef]
13. Fache, M., Darroman, E., Besse, V., Auvergne, R., Caillol, S. et al. (2014). Vanillin, a promising biobased building-block for monomer synthesis. Green Chemistry, 16(4), 1987–1998. DOI 10.1039/C3GC42613K. [Google Scholar] [CrossRef]
14. Tao, Y., Zhou, J., Fang, L., Wang, Y., Chen, X. et al. (2019). Fluoro-containing polysiloxane thermoset with good thermostability and acid resistance based on the renewable multifunctional vanillin. ACS Sustainable Chemistry & Engineering, 7(7), 7304–7311. DOI 10.1021/acssuschemeng.9b00370. [Google Scholar] [CrossRef]
15. Llevot, A., Grau, E., Carlotti, S., Grelier, S., Cramail, H. (2015). Admet polymerization of bio-based biphenyl compounds. Polymer Chemistry, 6(44), 7693–7700. DOI 10.1039/C5PY01232E. [Google Scholar] [CrossRef]
16. Lin, C. H., Chou, Y. C., Shiao, W. F., Wang, M. W. (2016). High temperature, flame-retardant, and transparent epoxy thermosets prepared from an acetovanillone-based hydroxyl poly (ether sulfone) and commercial epoxy resins. Polymer, 97(4), 300–308. DOI 10.1016/j.polymer.2016.05.035. [Google Scholar] [CrossRef]
17. Llevot, A., Grau, E., Carlotti, S., Grelier, S., Cramail, H. (2016). From lignin-derived aromatic compounds to novel biobased polymers. Macromolecular Rapid Communications, 37(1), 9–28. DOI 10.1002/marc.201500474. [Google Scholar] [CrossRef]
18. Geng, H., Wang, Y., Yu, Q., Gu, S., Zhou, Y. et al. (2018). Vanillin-based polyschiff vitrimers: Reprocessability and chemical recyclability. ACS Sustainable Chemistry & Engineering, 6(11), 15463–15470. DOI 10.1021/acssuschemeng.8b03925. [Google Scholar] [CrossRef]
19. Yuan, W., Ma, S., Wang, S., Li, Q., Wang, B. et al. (2018). Research progress on vanillin-based thermosets. Current Green Chemistry, 5(3), 138–149. DOI 10.2174/2213346105666180727104452. [Google Scholar] [CrossRef]
20. Bassett, A. W., Honnig, A. E., Breyta, C. M., Dunn, I. C., La Scala, J. J. et al. (2020). Vanillin-based resin for additive manufacturing. ACS Sustainable Chemistry & Engineering, 8(14), 5626–5635. DOI 10.1021/acssuschemeng.0c00159. [Google Scholar] [CrossRef]
21. Zhang, J., Mi, X., Chen, S., Xu, Z., Zhang, D. et al. (2020). A bio-based hyperbranched flame retardant for epoxy resins. Chemical Engineering Journal, 381(12), 122719. DOI 10.1016/j.cej.2019.122719. [Google Scholar] [CrossRef]
22. Xu, Y., Odelius, K., Hakkarainen, M. (2020). Photocurable, thermally reprocessable, and chemically recyclable vanillin-based imine thermosets. ACS Sustainable Chemistry & Engineering, 8(46), 17272–17279. DOI 10.1021/acssuschemeng.0c06248. [Google Scholar] [CrossRef]
23. Amarasekara, A. S., Garcia-Obergon, R., Thompson, A. K. (2019). Vanillin-based polymers: Iv. Hydrovanilloin epoxy resins. Journal of Applied Polymer Science, 136(4), 47000. DOI 10.1002/app.47000. [Google Scholar] [CrossRef]
24. Fache, M., Auvergne, R., Boutevin, B., Caillol, S. (2015). New vanillin-derived diepoxy monomers for the synthesis of biobased thermosets. European Polymer Journal, 67, 527–538. DOI 10.1016/j.eurpolymj.2014.10.011. [Google Scholar] [CrossRef]
25. Fache, M., Boutevin, B., Caillol, S. (2016). Epoxy thermosets from model mixtures of the lignin-to-vanillin process. Green Chemistry, 18(3), 712–725. DOI 10.1039/C5GC01070E. [Google Scholar] [CrossRef]
26. Fache, M., Viola, A., Auvergne, R., Boutevin, B., Caillol, S. (2015). Biobased epoxy thermosets from vanillin-derived oligomers. European Polymer Journal, 68(5), 526–535. DOI 10.1016/j.eurpolymj.2015.03.048. [Google Scholar] [CrossRef]
27. Fang, Z., Nikafshar, S., Hegg, E. L., Nejad, M. (2020). Biobased divanillin as a precursor for formulating biobased epoxy resin. ACS Sustainable Chemistry & Engineering, 8(24), 9095–9103. DOI 10.1021/acssuschemeng.0c02351. [Google Scholar] [CrossRef]
28. Jiang, H., Sun, L., Zhang, Y., Liu, Q., Ru, C. et al. (2019). Novel biobased epoxy resin thermosets derived from eugenol and vanillin. Polymer Degradation and Stability, 160, 45–52. DOI 10.1016/j.polymdegradstab.2018.12.007. [Google Scholar] [CrossRef]
29. Liu, J., Dai, J., Wang, S., Peng, Y., Cao, L. et al. (2020). Facile synthesis of bio-based reactive flame retardant from vanillin and guaiacol for epoxy resin. Composites Part B: Engineering, 190(22), 107926 /1–107926 /13. DOI 10.1016/j.compositesb.2020.107926. [Google Scholar] [CrossRef]
30. Mai, V. D., Shin, S. R., Lee, D. S., Kang, I. (2019). Thermal healing, reshaping and ecofriendly recycling of epoxy resin crosslinked with schiff base of vanillin and hexane-1,6-diamine. Polymers, 11(2), 293. DOI 10.3390/polym11020293. [Google Scholar] [CrossRef]
31. Memon, H., Liu, H., Rashid, M. A., Chen, L., Jiang, Q. et al. (2020). Vanillin-based epoxy vitrimer with high performance and closed-loop recyclability. Macromolecules, 53(231), 621–630. DOI 10.1021/acs.macromol.9b02006. [Google Scholar] [CrossRef]
32. Nabipour, H., Wang, X., Batool, S., Song, L., Hu, Y. (2021). A phosphaphenanthrene-containing vanillin derivative as co-curing agent for flame-retardant and antibacterial epoxy thermoset. Polymer, 217, 123460. DOI 10.1016/j.polymer.2021.123460. [Google Scholar] [CrossRef]
33. Nal, P., Mestry, S., Mapari, S., Mhaske, S. (2019). Eugenol/vanillin-derived novel triarylmethane-based crosslinking agent for epoxy coating. Iranian Polymer Journal, 28(8), 685–695. DOI 10.1007/s13726-019-00736-0. [Google Scholar] [CrossRef]
34. Savolainen, M., Harvey, B., Chafin, A., Garrison, M., Lamb, J. et al. (2017). High temperature thermosetting polyimide oligomers and epoxy resins derived from biosynthetic vanillin and resveratrol. Abstracts of Papers of the American Chemical Society, 254, 1. [Google Scholar]
35. Savonnet, E., Coz, C. L., Grau, E., Grelier, S., Defoort, B. et al. (2019). Divanillin-based aromatic amines: Synthesis and use as curing agents for fully vanillin-based epoxy thermosets. Frontiers in Chemistry, 7, 606/1–606/6. DOI 10.3389/fchem.2019.00606. [Google Scholar] [CrossRef]
36. Savonnet, E., Grau, E., Grelier, S., Defoort, B., Cramail, H. (2018). Divanillin-based epoxy precursors as DGEBA substitutes for biobased epoxy thermosets. ACS Sustainable Chemistry & Engineering, 6(8), 11008–11017. DOI 10.1021/acssuschemeng.8b02419. [Google Scholar] [CrossRef]
37. Shibata, M., Ohkita, T. (2017). Fully biobased epoxy resin systems composed of a vanillin-derived epoxy resin and renewable phenolic hardeners. European Polymer Journal, 92, 165–173. DOI 10.1016/j.eurpolymj.2017.05.007. [Google Scholar] [CrossRef]
38. Shimasaki, T., Yoshihara, S., Shibata, M. (2012). Preparation and properties of biocomposites composed of sorbitol-based epoxy resin, pyrogallol-vanillin calixarene, and wood flour. Polymer Composites, 33(10), 1840–1847. DOI 10.1002/pc.22327. [Google Scholar] [CrossRef]
39. Wang, S., Ma, S., Xu, C., Liu, Y., Dai, J. et al. (2017). Vanillin-derived high-performance flame retardant epoxy resins: Facile synthesis and properties. Macromolecules, 50(5), 1892–1901. DOI 10.1021/acs.macromol.7b00097. [Google Scholar] [CrossRef]
40. Wang, Z., Gnanasekar, P., Sudhakaran Nair, S., Farnood, R., Yi, S. et al. (2020). Biobased epoxy synthesized from a vanillin derivative and its reinforcement using lignin-containing cellulose nanofibrils. ACS Sustainable Chemistry & Engineering, 8 (3011215–11223. DOI 10.1021/acssuschemeng.0c02559. [Google Scholar] [CrossRef]
41. Xu, X., Wang, S., Ma, S., Yuan, W., Li, Q. et al. (2019). Vanillin-derived phosphorus-containing compounds and ammonium polyphosphate as green fire-resistant systems for epoxy resins with balanced properties. Polymers for Advanced Technologies, 30(2), 264–278. DOI 10.1002/pat.4461. [Google Scholar] [CrossRef]
42. Mora, A. S., Tayouo, R., Boutevin, B., David, G., Caillol, S. (2018). Vanillin-derived amines for bio-based thermosets. Green Chemistry, 20(17), 4075–4084. DOI 10.1039/C8GC02006J. [Google Scholar] [CrossRef]
43. Upton, B. M., Kasko, A. M. (2016). Strategies for the conversion of lignin to high-value polymeric materials: Review and perspective. Chemical Reviews, 116(4), 2275–2306. DOI 10.1021/acs.chemrev.5b00345. [Google Scholar] [CrossRef]
44. Lin, C. M., Chen, C. H., Lin, C. H., Juang, T. Y. (2018). High-performance bio-based benzoxazines derived from phosphinated biphenols and furfurylamine. European Polymer Journal, 108(11), 48–56. DOI 10.1016/j.eurpolymj.2018.08.024. [Google Scholar] [CrossRef]
45. Yu, A. Z., Serum, E. M., Renner, A. C., Sahouani, J. M., Sibi, M. P. et al. (2018). Renewable reactive diluents as practical styrene replacements in biobased vinyl ester thermosets. ACS Sustainable Chemistry & Engineering, 6(10), 12586–12592. DOI 10.1021/acssuschemeng.8b03356. [Google Scholar] [CrossRef]
46. Qi, Y., Weng, Z., Kou, Y., Song, L., Li, J. et al. (2021). Synthesize and introduce bio-based aromatic s-triazine in epoxy resin: Enabling extremely high thermal stability, mechanical properties, and flame retardancy to achieve high-performance sustainable polymers. Chemical Engineering Journal, 406, 126881. DOI 10.1016/j.cej.2020.126881. [Google Scholar] [CrossRef]
47. Gnanasekar, P., Feng, M., Yan, N. (2020). Facile synthesis of a phosphorus-containing sustainable biomolecular platform from vanillin for the production of mechanically strong and highly flame-retardant resins. ACS Sustainable Chemistry & Engineering, 8(47), 17417–17426. DOI 10.1021/acssuschemeng.0c05610. [Google Scholar] [CrossRef]
48. Huang, Y., Ma, T., Wang, Q., Guo, C. (2019). Synthesis of biobased flame-retardant carboxylic acid curing agent and application in wood surface coating. ACS Sustainable Chemistry & Engineering, 7(17), 14727–14738. DOI 10.1021/acssuschemeng.9b02645. [Google Scholar] [CrossRef]
49. Niu, H., Nabipour, H., Wang, X., Song, L., Hu, Y. (2021). Phosphorus-free vanillin-derived intrinsically flame-retardant epoxy thermoset with extremely low heat release rate and smoke emission. ACS Sustainable Chemistry & Engineering, 9(15), 5268–5277. DOI 10.1021/acssuschemeng.0c08302. [Google Scholar] [CrossRef]
50. Huo, S., Yang, S., Wang, J., Cheng, J., Zhang, Q. et al. (2020). A liquid phosphaphenanthrene-derived imidazole for improved flame retardancy and smoke suppression of epoxy resin. ACS Applied Polymer Materials, 2(8), 3566–3575. DOI 10.1021/acsapm.0c00577. [Google Scholar] [CrossRef]
51. Yang, S., Huo, S., Wang, J., Zhang, B., Wang, J. et al. (2021). A highly fire-safe and smoke-suppressive single-component epoxy resin with switchable curing temperature and rapid curing rate. Composites Part B: Engineering, 207(4), 108601. DOI 10.1016/j.compositesb.2020.108601. [Google Scholar] [CrossRef]
52. Wang, X., Hu, Y., Song, L., Xing, W., Lu, H. et al. (2010). Flame retardancy and thermal degradation mechanism of epoxy resin composites based on a DOPO substituted organophosphorus oligomer. Polymer, 51(11), 2435–2445. DOI 10.1016/j.polymer.2010.03.053. [Google Scholar] [CrossRef]
53. Dai, J. Y., Teng, N., Peng, Y. Y., Liu, Y., Cao, L. J. et al. (2018). Biobased benzoxazine derived from daidzein and furfurylamine: Microwave-assisted synthesis and thermal properties investigation. Chemsuschem, 11(18), 3175–3183. DOI 10.1002/cssc.201801404. [Google Scholar] [CrossRef]
54. Kasemsiri, P., Neramittagapong, A., Chindaprasirt, P. (2015). Curing kinetic, thermal and adhesive properties of epoxy resin cured with cashew nut shell liquid. Thermochimica Acta, 600, 20–27. DOI 10.1016/j.tca.2014.11.031. [Google Scholar] [CrossRef]
55. Perez, R. M., Sandler, J. K. W., Altstädt, V., Hoffmann, T., Pospiech, D. et al. (2006). Effect of DOP-based compounds on fire retardancy, thermal stability, and mechanical properties of DGEBA cured with 4,4′-dds. Journal of Materials Science, 41(2), 341–353. DOI 10.1007/s10853-005-2720-2. [Google Scholar] [CrossRef]
56. Xu, Y. J., Shi, X. H., Lu, J. H., Qi, M., Guo, D. M. et al. (2020). Novel phosphorus-containing imidazolium as hardener for epoxy resin aiming at controllable latent curing behavior and flame retardancy. Composites Part B: Engineering, 184(32), 107673. DOI 10.1016/j.compositesb.2019.107673. [Google Scholar] [CrossRef]
57. Zhang, Y., Jing, J., Liu, T., Xi, L., Sai, T. et al. (2021). A molecularly engineered bioderived polyphosphate for enhanced flame retardant, UV-blocking and mechanical properties of poly (lactic acid). Chemical Engineering Journal, 411(7), 128493. DOI 10.1016/j.cej.2021.128493. [Google Scholar] [CrossRef]
58. Sai, T., Ran, S., Guo, Z., Yan, H., Zhang, Y. et al. (2021). Transparent, highly thermostable and flame retardant polycarbonate enabled by rod-like phosphorous-containing metal complex aggregates. Chemical Engineering Journal, 409(20), 128223. DOI 10.1016/j.cej.2020.128223. [Google Scholar] [CrossRef]
59. Zhang, D., Jin, S., Wan, J., Wang, J., Li, Y. et al. (2021). A dieugenol-based epoxy monomer with high bio-based content, low viscosity and low flammability. Materials Today Communications, 29(17), 102846. DOI 10.1016/j.mtcomm.2021.102846. [Google Scholar] [CrossRef]
60. Zhang, Y., Xiong, Z., Ge, H., Ni, L., Zhang, T. et al. (2020). Core-shell bioderived flame retardants based on chitosan/alginate coated ammonia polyphosphate for enhancing flame retardancy of polylactic acid. ACS Sustainable Chemistry & Engineering, 8(16), 6402–6412. DOI 10.1021/acssuschemeng.0c00634. [Google Scholar] [CrossRef]
61. Xiong, Z., Zhang, Y., Du, X., Song, P., Fang, Z. (2019). Green and scalable fabrication of core-shell biobased flame retardants for reducing flammability of polylactic acid. ACS Sustainable Chemistry & Engineering, 7(9), 8954–8963. DOI 10.1021/acssuschemeng.9b01016. [Google Scholar] [CrossRef]
62. Jing, J., Zhang, Y., Fang, Z. P., Wang, D. Y. (2018). Core-shell flame retardant/graphene oxide hybrid: A self-assembly strategy towards reducing fire hazard and improving toughness of polylactic acid. Composites Science and Technology, 165, 161–167. DOI 10.1016/j.compscitech.2018.06.024. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |