

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2022.018147
ARTICLE
Borate-Modified, Flame-Retardant Paper Packaging Materials for Archive Conservation
1Engineering Research Center of Historical and Cultural Heritage Protection, Ministry of Education, School of Materials Science and Engineering, Shaanxi Normal University, Xi’an, 710119, China
2Ningbo University of Finance & Economics, Ningbo, 315175, China
*Corresponding Authors: Yuhu Li. Email: liyuhu@snnu.edu.cn; Jing Cao. Email: jingcao@snnu.edu.cn
Received: 02 July 2021; Accepted: 31 August 2021
Abstract: Paper packaging materials like cardboards are widely used to protect archives which are a major kind of cultural relics. Unfortunately, paper is a combustible material, and thus exploring environment-friendly flame retardant for paper-based archive packaging material plays an important role. Herein, boric acid, borax and disodium octaborate are used to modify the craft paper-based packaging materials for archive conservation to improve fire safety. The modified craft paper exhibits much higher flame retardancy than the pristine one dose based on vertical burning tests, without much influence on mechanical properties such as tensile strength and elongation at break. Thermogravimetric analysis (TGA), scanning electron microscope (SEM), and X-ray photoelectron spectroscopy (XPS) reveal that porous glass structure is formed during the combustion, because thermal decomposition of boric acid, borax and disodium octaborate will produce porous glassy matter as B2O3. The porous glass covers the paper surface as an insulating layer which retards the further pyrolysis and combustion, resulting in a denser carbon layer. Our study provides a robust way to reduce the fire hazard of the archive packaging material by applying environment-friendly boron-based fire retardants.
Keywords: Paper packaging materials; archive conservation; flame retardant
Paper-based archives are cultural heritages which carry valuable cultural information. Paper consists of two-dimensional self-bonding cellulose fiber network with a small amount of lignin and hemicellulose [1]. Paper-based archives suffer from a series of erosion such as temperature, humidity, air pollution, dust, light and microorganisms [2–6]. The preservation environment greatly impacts upon their longevity of archives. For this reason, much efforts have been made to resolve the issues related to the aging of archives due to physical and chemical reasons in many museums [7–9]. In particular, paper-based boxes for packaging archives have been recognized as an important tool to protect precious cultural relics.
Archive box is the smallest unit to save paper documents and convenient to classify and store paper documents. Their production materials greatly affect the security and durability of stored archives. Most archives boxes are made of paper-based materials which is easy to mildew, vermiculate and acidizing. The acidification is the main reason for paper aging, yellowing, and brittleness [10–12]. Due to acid-catalyzed hydrolysis effect, the main component cellulose of paper undergoes chain breaking reactions. In order to prevent the deterioration processes, deacidification is the main method to solve the problem of paper acidification. Deacidification treatment is to neutralize the acidic species of paper with alkaline species to prevent or delay further acidification during natural aging. Most of the paper deacidification agents reported in literature are alkaline oxides, hydroxides and amines [13–21].
On basis of this fundamental mechanism, many researchers devote to the studies of acid-free paper. Acid-free paper does not contain active acid species and the pH of paper is 7.0∼8.5. Since the 1960s and 1970s, many international cultural relic collection institutions have gradually used acid-free paper to replace the old paper packaging. With the in-depth research on the application of acid-free paper, acid-free paper has been widely recognized for its advantages in the preservation of paper relics, fabric and negative films. In recent decades, archivists mainly focus on using of the acid-free paper archival packing materials [22–27] to fabricate archive box.
However, paper is flammable because the large surface area and its many constituents, i.e., cellulous fiber. Paper can catch fire easily. Once a fire occurs, it is extremely difficult to control of high flame propagation speed and the smoke production. After the fire, it is generally difficult for the archives to survive, and even some of the surviving archives that are less damaged by the fire are also extremely difficult to recover. According to statistics, up to now, there have been nearly a hundred direct fires in all kinds of archives at all levels in China. There are destroyed more than 3 million volumes of archives and caused huge losses. Therefore, it is imperative to develop flame retardant archive packaging materials. At present, there is no report on flame retardant and acid-free paper packaging materials. Therefore, developing a new type of acid-free flame retardant file packaging material has great application potential.
In order to endow paper-based materials with good flame retardancy, it is necessary to use various flame retardants to treat them. With increasingly strict environmental regulations, halogen-free flame retardants have attracted increased attention. Boron compounds as boric acid and borax express their ability to improve the flame retardancy of different polymer matrices, and function as a kind of efficient halogen-free flame retardant with low toxicity and high thermal stability [28–33]. They have been widely applied as a flame-retardant additive to biomass polymeric systems (e.g., wood, cotton, natural fibers, and sawdust) [34–36]. The flame retardant mechanisms of boron compound involved the molten boron oxide evolved by thermal decomposition of boron compounds rapidly resembles into numerous glassy cages in situ during a combustion process, accelerating the creation of a carbonized layer on the substances, resulting in a greater char yield as physical barrier [37–39]. Meanwhile, many borates are basic and high buffering capacity, which are helpful to increase basicity of paper. In this work, the boron-based fire retardants are permeated into bovine cardboard to increase a flame retardancy of archives packaging boxes. By means such as SEM, TG and XPS, the thermal stability and char residues microstructure of untreated and treated craft paper were characterized. The mechanical properties and flame retardant properties of craft paper were systematically studies with flame retardant mechanisms highlighted.
H3BO3, Na2B4O7⋅10H2O, Na2B8O7⋅10H2O were obtained from Shanghai Su Yi Chemical Reagent Co., Ltd., China. Craft paper (70 cm × 50 cm) was purchased from Pingxiang County Qunfeng Archive Supplies Fittings Factory, China.
H3BO3, Na2B4O7⋅10H2O, Na2B8O7⋅10H2O were dissolved in the H2O with stirring at the mass ratio of 2:3:16. Then, the craft paper was soaked into the above slat solution with ultrasonication for 5 min at 30°C. Moreover, the craft paper was dried for 3 h. Finally, the craft paper is dried in a microwave dryer for 8 min.
2.3 Performance Testing and Characterization
Vertical burning test was carried out using a vertical burning tester (Suzhou Testech Testing Instrument Technology Co., Ltd., China) in accordance with GB/T 14656−2009 and the sample size was 210 mm × 70 mm × 0.41 mm [40–42].
2.3.2 Thermogravimetric Analysis (TGA)
The thermal stability of pristine and modified craft paper was analyzed by using a TGA (SDTQ 600, TA Instruments) under nitrogen and air atmosphere (100 mL/min). About 5 mg sample was placed in the Al2O3 crucible and heated from 25 to 800°C at 20 °C/min. The temperatures of decomposition for 5% weight loss (Td,5%), initial decomposition (Tonset) and maximum loss rate (Tmax) and the residual weight at 750°C were used for comparison.
2.3.3 Scanning Electronic Microscopy (SEM-EDX)
The micromorphology of the surface of untreated and treated sample and char residues were observed by a Hitachi SU3500 scanning electron micro-scope (SEM) with a conductive gold coating under a voltage of 10 KV. The equipped energy dispersive spectrometry (EDS) was used to analyze the elements.
2.3.4 X-ray Photoelectron Spectroscopy (XPS)
The X-ray photoelectron spectroscopy was performed (ESCALAB Xi+, Thermo Fisher Scientific Co., Ltd., USA) to analyze the brunt residues of samples.
Mechanical properties of untreated and treated craft paper were measured according ISO 1924-2:1994 by using a universal testing machine (QT-1136, Dongguan Gaotai Testing Instrument Co., Ltd., China) at room temperature. Tensile speed was 5 mm/min and 10 specimens were tested in parallel.
pH values of the untreated and treated craft paper samples were measured with a Mettler Toledo Seven CompactTM pH S210 Ph-meter equipped with flat electrode (HI1413).
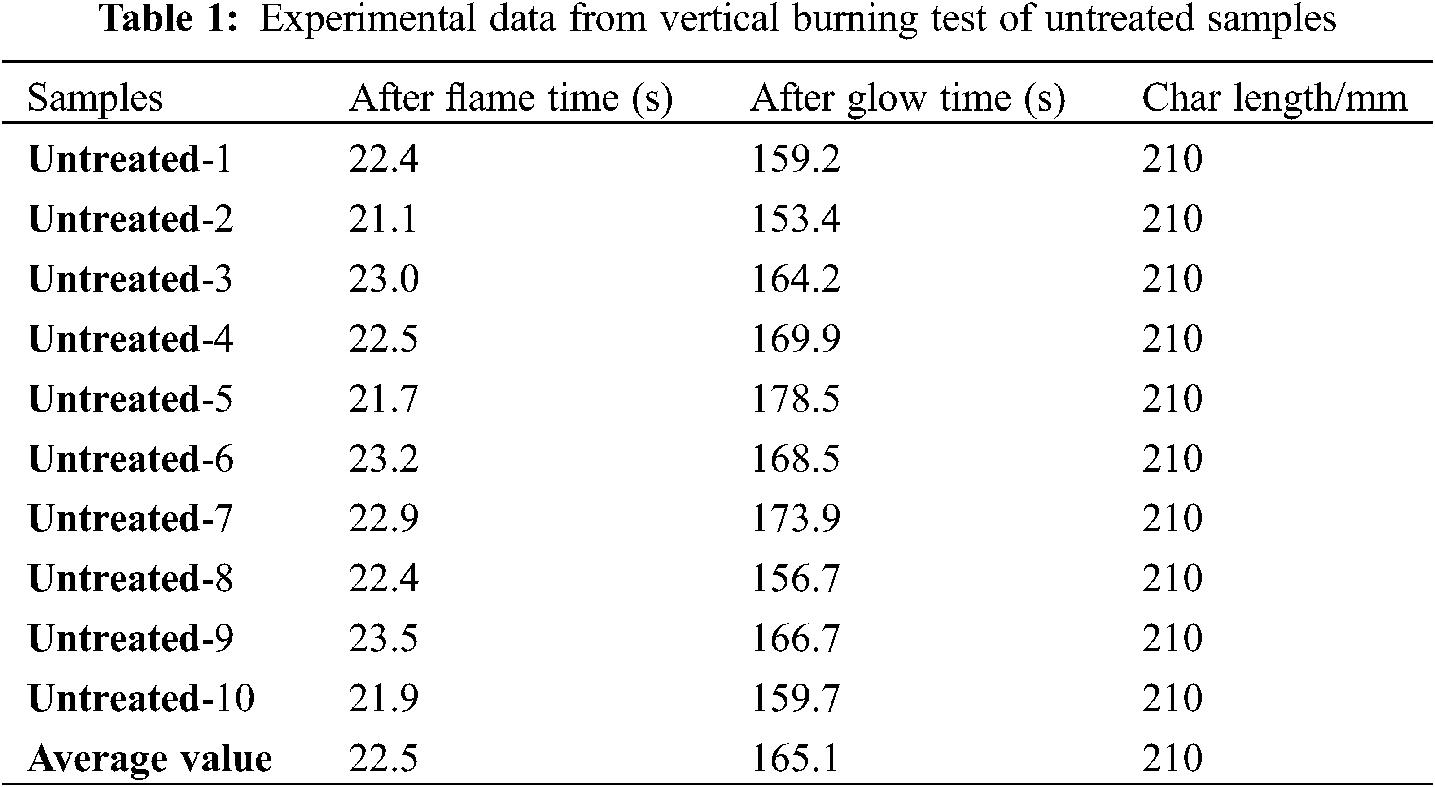
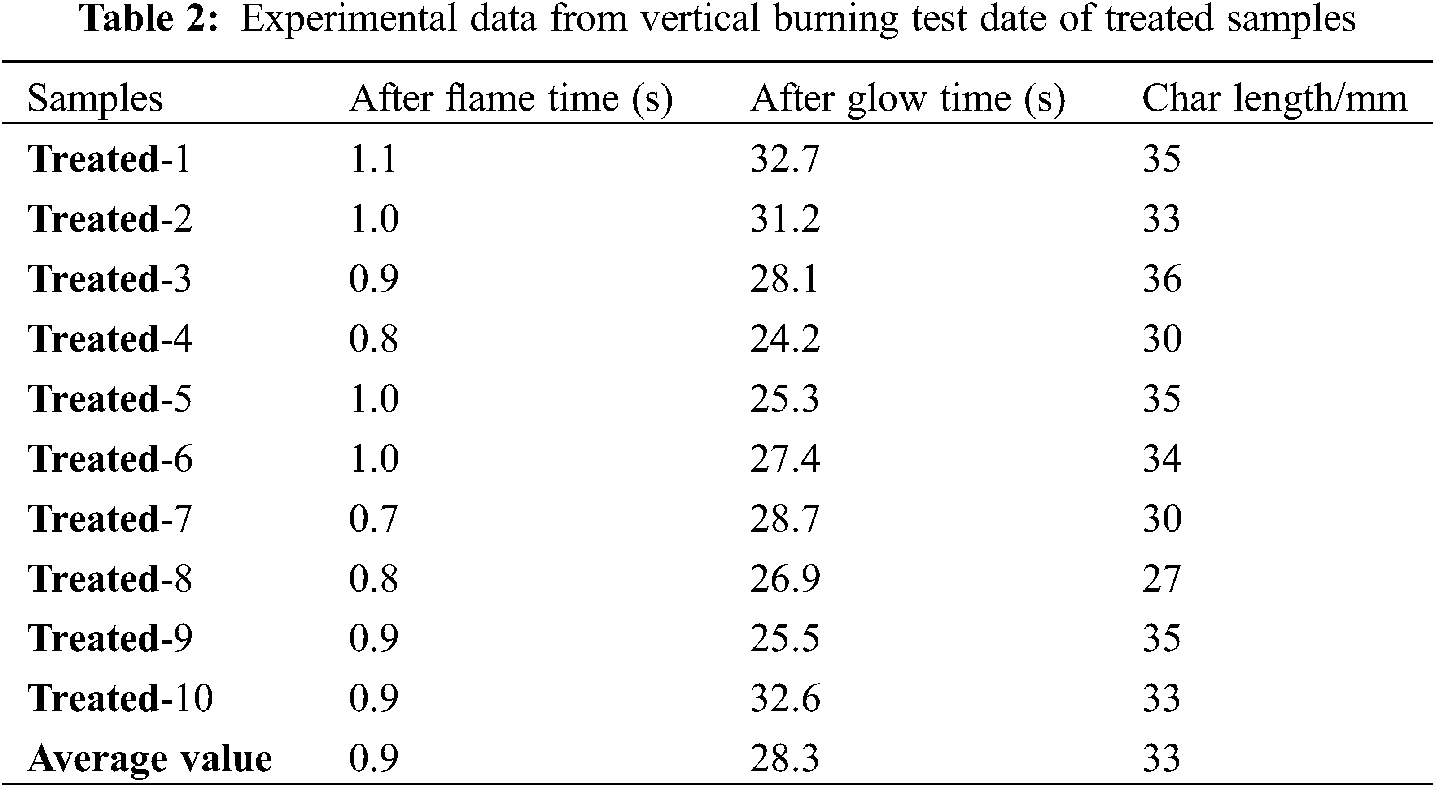
3.1 Flame-Retardant Properties
To characterize flame-retardant properties of the untreated and treated samples, vertical burning test were carried out according to national standard of China (GB/T 14656–2009), and results are listed in Tables 1 and 2. The flame-retardant performance test results of cardboard in the standard shall be up to the following requirements. 1) Average after-flame time ≤ 5 s; 2) Average after-glow time ≤ 60 s; 3) average char-length 115 mm or less. See the Table 1, the result shows that after flame time and after glow time of untreated craft paper were 22.5 and 165.1 s, respectively. Meanwhile, the char length was 210 mm, which indicates that the untreated craft paper had a high flammability. In contrast, the after-flame time and after-glow time of treated craft paper were only 0.9 s and 28.3 s, respectively, affirming good flame retardancy. To illustrate, Fig. 1 compares the images of the untreated and treated sample at the different burning time. When exposed to the flame, the untreated sample was immediately ignited and quickly burnt out until extinguish at 22 s. In contrast, after ignition, the treated craft paper self-extinguishes in a very short time (∼1 s), showing a significantly enhanced resistance to the fire propagation. Thus, the boron-based flame retardants greatly reduced the inflammability of their modified craft paper, because treated sample is easy to carbonize during the pyrolysis.
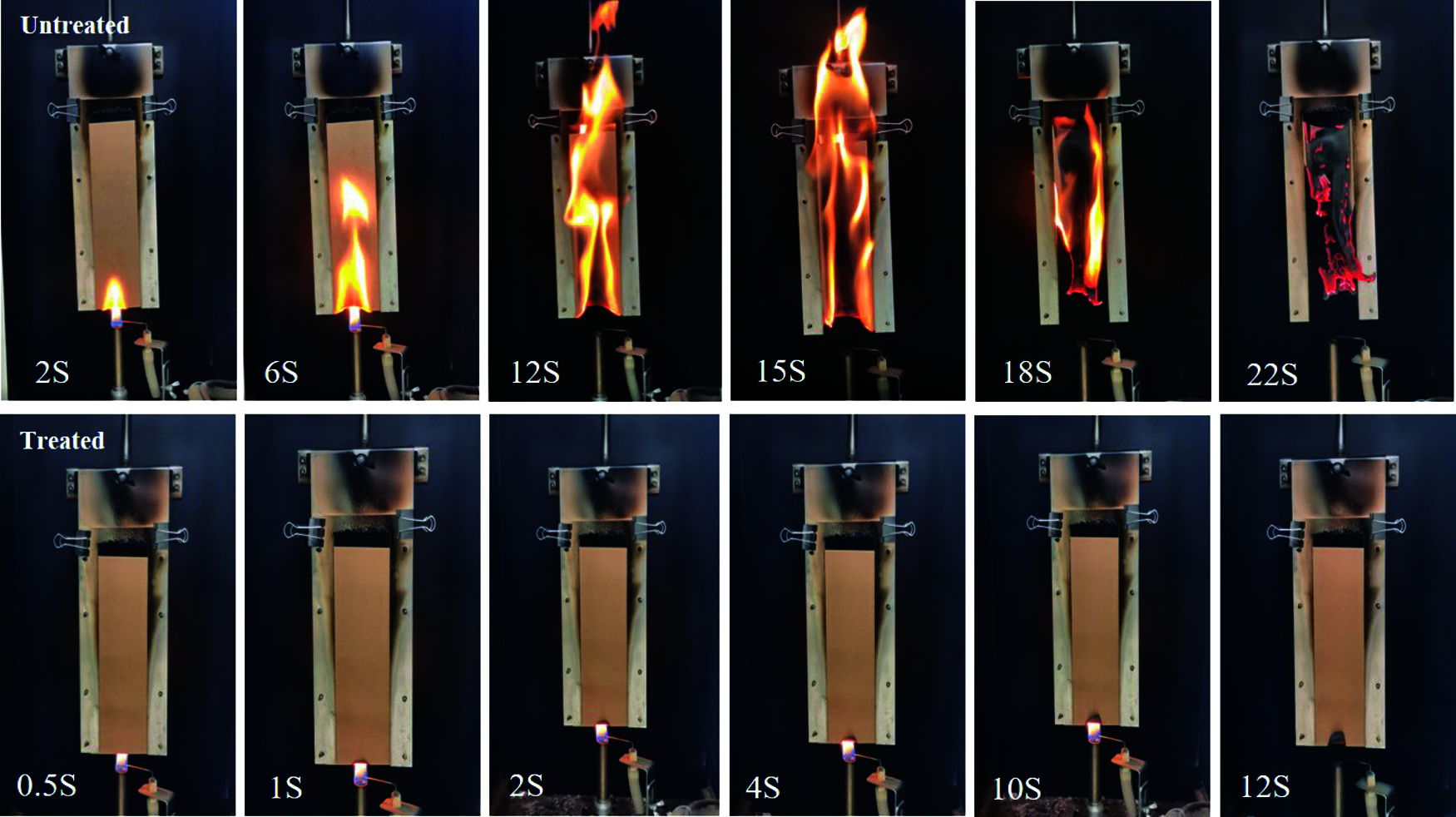
Figure 1: Combustion of untreated and treated paper samples by boron-based flame retardants during the vertical burning test. The different time is recorded to give the image
3.2 Thermal Analysis of Untreated and Treated Samples
The decomposition behaviors of materials are closely related to their flammability. Here TG measurements were conducted to exam the decomposition of the paper samples in nitrogen and air atmosphere. The recorded characteristic temperatures, Td,5%, Tonset, Tmax, Dmax and char yield are compared in Fig. 2 and Table 3. In nitrogen, Td,5% of untreated and treated samples is 70 and 75°C, respectively, due largely to the liberation of absorbed water, see Fig. 2a. The initial decomposition temperature (Tonset) of untreated samples is 325°C, owing likely to the decomposition of the cellulose amorphous region, producing a small amount of water and carbon dioxide. The major decomposition is found at 325–425°C (the mass loss up to 70%), with the maximum (Tmax) at 355°C. This finding lies in that the cellulose crystalline region is depolymerized and produces many volatiles. The formation of the chars mainly occurred over 360°C, during which process the paper fibers are gradually carbonized by removing some small molecules. Finally, for untreated paper sample only 18.18% residues at 750°C were found.

Figure 2: TGA (a) and DTG (b) curves of untreated and treated samples in nitrogen. TGA (c) and DTG (d)curves of untreated and treated samples in air
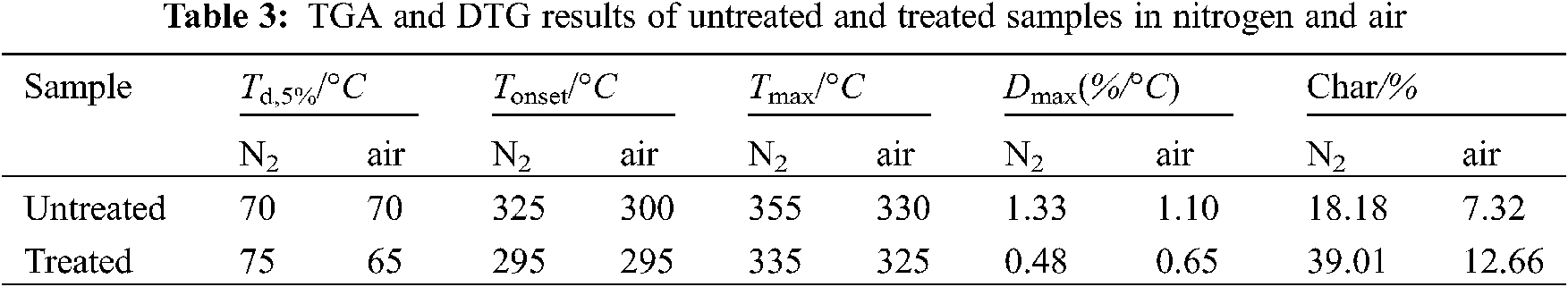
The Tonset and Tmax of the treated sample were 295 and 335°C, respectively, and are shifted to lower temperature regions compared with the untreated samples. As revealed in Table 3, Dmax (rate at Tmax) of treated sample are lower than that the untreated sample. This phenomenon indicates that boron compounds decreased the thermal decomposition of fibers, which might be caused that boron compounds promoted the formation of carbonized layer on the surface fibers [43–47]. Meanwhile, the heat would be absorbed by vaporization of their crystal water during the decomposition of the borates, thereby playing a dual function: dilution and cooling [48–50]. With further increased temperature, the thermal decomposition products B2O3 from boric acid, borax and disodium octaborate will form glassy melt, covering the sample surface and promoting the charring process. Furthermore, the glassy matter also insulating air and combustibles preventing further pyrolysis (combustion). The char residue of treated is as high as 39.01% at 750°C, which almost 2 times of the untreated.
Figs. 2c, 2d compared decomposition of the untreated and treated samples in an air atmosphere, with three apparent thermal decompositions observed. The treated samples show a higher degradation temperature than untreated samples dose during this decomposition stage (250–400°C). With the increased temperature, the treated samples display reduced degradation and improved thermal stability during the second and third degradation stages. As a result, the residual of the treated samples at 750°C increases by 70% compared with the untreated samples. Such thermal degradation behaviors of the treated samples can be attributed to the earlier decomposition of boron-based flame retardants that could promote the thermal degradation of the paper fiber to form more stable char residual to protect the underlying fiber, and thus improve the thermal stability.
To further investigate the flame retardant mechanisms of boron-based flame retardants on the craft paper, the morphologies of the untreated and treated samples are examined with SEM. Fig. 3 displays the SEM images of the char residues of the untreated and treated paper samples after the vertical burning test. The untreated craft paper fibers are seriously damaged after burning with the fiber residues being thin and broken, and their char residues are cracked with cavities. In contrast, the treated craft paper fibers retain their original morphology (Fig. 3d) with many glass cages formed and evenly coated the surface. So, it is advantageous for boron-based flame retardants to endow strong charring ability and physical barrier against fire with the treated paper. Moreover, formation of protective layer effectively inhibited the depolymerization of the cellulose fiber. This finding agrees with the other reports [51–55]. Furthermore, the boron-containing layer could effectively the underlying fiber from further combustion, producing the strong the self-extinguishing action of the treated sample (Fig. 1).

Figure 3: SEM micrographs of the original samples (a: untreated; b: treated) and char residues samples obtained from vertical flammability test (c: untreated; d: treated)
Elemental composition of char residues of the untreated and treated samples was analyzed by using XPS spectra (Fig. 4). The obtained atomic percentage of char was listed in Table 4. Fig. 4 shows that the treated sample has the additional signals at 191.44 eV(B1 s) which confirms the presence of borate oxide. The treated sample shows B atom of as high as 20.7%. The formed B2O3 from thermal decomposition of boric acid, borax and disodium octaborate could improve the barrier property to hold back the heat, air and flammable gases.
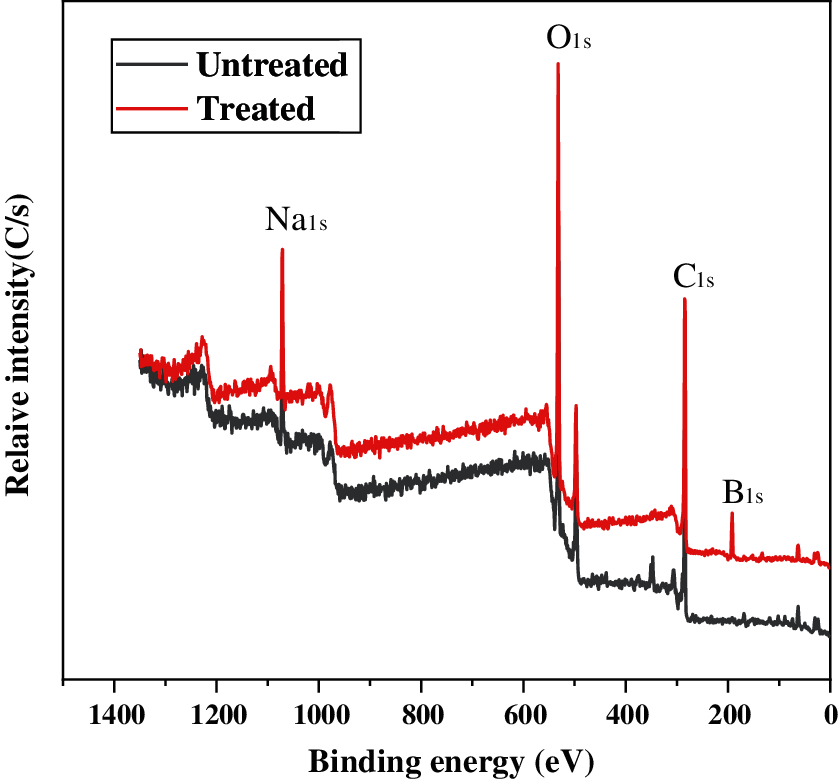
Figure 4: XPS spectra of char residues of untreated and treated samples after vertical burning tests
3.5 Mechanical Properties of Untreated and Treated Samples
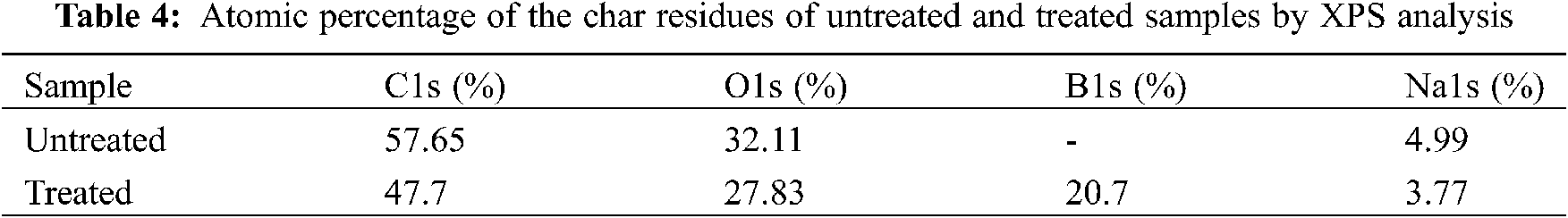
Introducing flame retardants will bring negative influence on mechanical performances of materials sometimes. For kraft paper, tensile properties are an important consideration for applications. As compared in Fig. 5, the tensile strength and breaking elongation of the untreated and treated paper samples are 9.41 MPa and 5.87%, respectively. Compared with the untreated sample, the tensile strength and breaking elongation of the treated sample reduced by only 2.5% and 3.9%, respectively. Therefore boron-based flame retardants does not much affect the mechanical properties of the treated paper, because only the small loading needed to achieve desired flame retardancy.
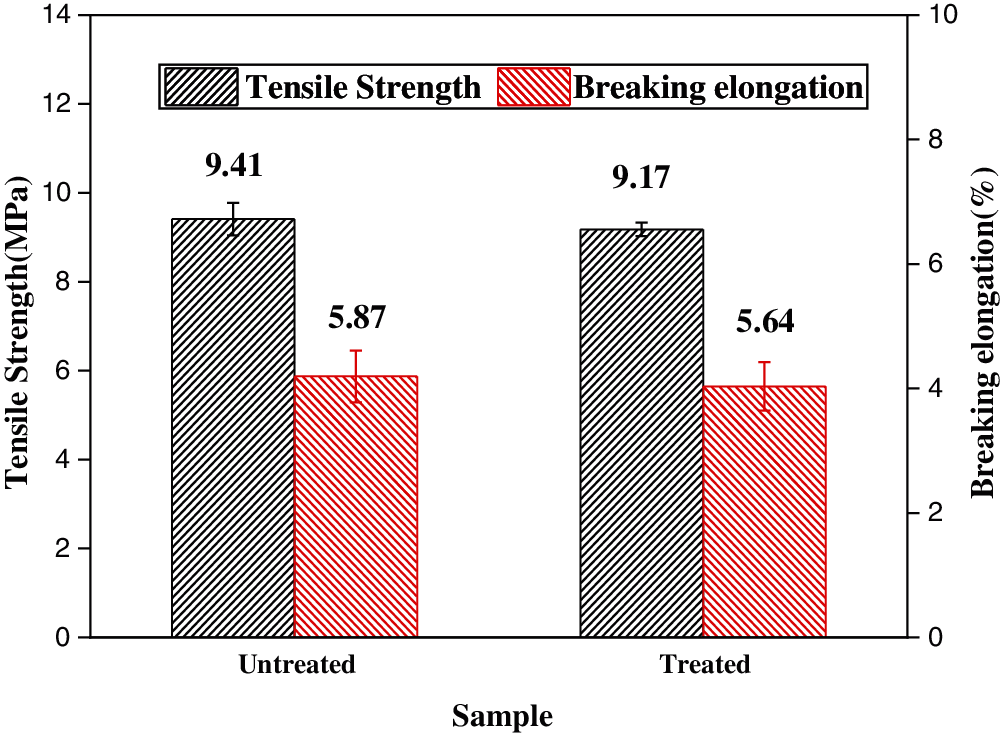
Figure 5: Tensile properties of untreated and treated
pH value is a very important factor to indicate the aging process of papers, because acidic compounds will form during this process and will accelerate the further degradation. In this study, to examine change of pH values of the paper samples, a large piece of craft paper was cut into equal halves, with one half treated by boron-based flame retardants and the other untreated, and dried at room temperature naturally. The pH values are measured on the any five points of sample and the results are shown in the Fig. 6. Compared with the untreated paper, pH value of treated the craft paper increases markedly, implicating that the treated paper can provide moderate alkaline environment to retard paper aging, which is benefit to the long-term preservation of paper-based archives.
Figure 6: pH values of untreated and treated craft papers
A facile method was developed to improve the flame retardancy of paper packaging material for archive conservation by soaking the craft paper with borates. The flame retardancy of the modified craft paper was evaluated from vertical burning tests. The after-flame time and after-glow time of the modified craft paper were shortened to 0.9 and 28.3 s, respectively, indicating the good flame retardancy. The flame retardant mechanisms of boron compounds were analyzed by TG, SEM and XPS. The experimental results show that the flame-retardancy effect of the boron compound on craft paper is a chemical as well as a physical phenomenon. The thermogravimetric analysis showed that borates introduced could catalyze and promote the thermal degradation of paper fiber to form the stable residue char to protect the underlying fiber, thus improving the thermal stability and flame retardancy. The residual of the modified craft paper was increased by 114.5%. The microstructure and flame retardant mechanisms were studied from analyzing residual char after combustion by SEM and XPS. The results showed that borates could promote the formation of a continuous and porous B2O3 glass layer on the surface, thus providing an effective physical barrier against fire and promoting charring. The mechanical strength and elongation at break of the modified craft paper were little changed. Meanwhile, the modified craft paper was slight alkaline (pH = 8), which is conducive to the long-term preservation of archives. In summary, this work provides an effective and scalable means to produce paper-based flame-retardant packaging materials to increase fire safety of archives.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Area, M. C., Cheradame, H. (2011). Paper aging and degradation: Recent findings and research methods. Bioresources, 64(4), 5307–5337. DOI 10.1515/HF.2010.121. [Google Scholar] [CrossRef]
2. Zervos, S., Moropoulou, A. (2006). Methodology and criteria for the evaluation of paper conservation interventions: A literature review. Restaurator, 27(4), 219–274. DOI 10.1515/REST.2006.219. [Google Scholar] [CrossRef]
3. Proniewicz, L. M., Paluszkiewicz, C., Wesełucha-Birczyńska, A., Majcherczyk, H., Barański, A. et al. (2001). FT-Ir and FT-Raman study of hydrothermally degradatedcellulose. Journal of Molecular Structure, 596, 163–169. DOI 10.1016/S0022-2860(01)00706-2. [Google Scholar] [CrossRef]
4. Castillo, I. F., Matteis L. D., Marquina, C., Guillén, E. G., de La Fuente, J. M. et al. (2019). Protection of 18th century paper using antimicrobial nano-magnesium oxide. International Biodeterioration & Biodegradation, 141, 79–86. DOI 10.1016/j.ibiod.2018.04.004. [Google Scholar] [CrossRef]
5. Rakotonirainy, M. S., Lavédrine, B. (2005). Screening for antifungal activity of essential oils and related compounds to control the biocontamination in libraries and archives storage areas. International Biodeterioration & Biodegradation, 55, 141–147. DOI 10.1016/j.ibiod.2004.10.02. [Google Scholar] [CrossRef]
6. Ganzerla, R., Gambaro, A., Cappelletto, E., Fantin, M., Montalbani, S. et al. (2009). Characterization of selected paper documents from the archives of palazzo ducale (VeniceItaly using various analytical techniques. Microchemical Journal, 91, 70–77. DOI 10.1016/j.microc.2008.08.003.7 [Google Scholar] [CrossRef]
7. Krupińska, B., van Grieken, R., de Wael, K. (2013). Air quality monitoring in a museum for preventive conservation: Results of a three-year study in the Plantin-Moretus Museum in Antwerp, Belgium. Microchemical Journal, 110, 350–360. DOI 10.1016/j.microc.2013.05.006. [Google Scholar] [CrossRef]
8. Krüger, E. L., Diniz, W. (2011). Relationship between indoor thermal comfort conditions and the time weighted preservation index (TWPI) in three Brazilian archives. Applied Energy, 88, 712–723. DOI 10.1016/j.apenergy.2010.09.011. [Google Scholar] [CrossRef]
9. Afsharpour, M., Hadadi, M. (2014). Titanium dioxide thin film: Environmental control for preservation of paper-art-works. Journal of Cultural Heritage, 15, 569–574. DOI 10.1016/j.culher.2013.10.008. [Google Scholar] [CrossRef]
10. Weng, J., Zhang, X., Jia, M., Zhang, J. (2019). Deacidification of aged papers using dispersion of Ca(OH)2 nanoparticles in subcritical 1, 1, 1, 2-tetrafluoroethane (R134a). Journal of Cultural Heritage, 37, 137–147. DOI 10.1016/j.culher.2018.12.001. [Google Scholar] [CrossRef]
11. Poggi, G., Giorgi, R., Mirabile, A., Xing, H. P., Baglioni, P. (2017). A stabilizer-free non-polar dispersion for the deacidifification of contemporary art on paper. Journal of Cultural Heritage, 26, 44–52. DOI 10.1016/j.culher.2017.02.006. [Google Scholar] [CrossRef]
12. Wang, Y., Fang, Y., Tan, W., Liu, C. (2013). Preservation of aged paper using borax in alcohols and the supercritical carbon dioxide system. Journal of Cultural Heritage, 14, 16–22. DOI 10.1016/j.culher.2012.02.010. [Google Scholar] [CrossRef]
13. Daniels, V. D. (1996). The chemistry of paper conservation. Chemical Society Reviews, 25, 179–186, DOI 10.1039/CS9962500179. [Google Scholar] [CrossRef]
14. Zumbühl, S., Wuelfert, S. (2001). Chemical aspects of the bookkeeper deacidification of cellulosic materials: The influence of surfactants. Studiesin Conservation, 46, 169–180. DOI 10.1179/sic.2001.46.3.169. [Google Scholar] [CrossRef]
15. Giorgi, R., Bozzi, C., Dei, L., Gabbiani, C., Ninham, B. et al. (2005). Nanoparticles of Mg(OH)2: Synthesis and application to paper conservation. Langmuir, 21, 8495–8501. DOI 10.1021/la050564 m. [Google Scholar] [CrossRef]
16. Amornkitbamrung, L., Mohan, T., Hribernik, S., Reichel, V., Faivre, D. et al. (2015). Polysaccharide stabilized nanoparticles for deacidification and strengthening of paper. Royal Society of Chemistry, 5, 32950–32961. DOI 10.1039/C4RA15153D. [Google Scholar] [CrossRef]
17. Poggi, G., Giorgi, R., Toccafondi, N., Katzur, V., Baglioni, P. (2010). Hydroxide nanoparticles for deacidification and concomitant inhibition of iron-gall ink corrosion of paper. Langmuir, 26, 19084–19090. DOI 10.1021/la1030944. [Google Scholar] [CrossRef]
18. Stefanis, E., Panayiotou, C. (2008). Study of the photochemical stability of paper deacidifified with dispersions of Ca(OH)2 and Mg(OH)2 nanoparticles in alcohols. Restaurator, 29, 125–138. DOI 10.1515/rest.2008.007. [Google Scholar] [CrossRef]
19. Sequeira, S., Casanova, C., Cabrita, E. J. (2006). Deacidifification of paper using dispersions of Ca(OH)2 nanoparticles in isopropanol. Study of Effificiency. Journal of Cultural Heritage, 7, 264–272. DOI 10.1016/j.culher.2006.04.004. [Google Scholar] [CrossRef]
20. Ipert, S., Dupont, A. L., Lavedrine, B., Begin, P., Rousset, E. et al. (2006). Mass deacidification of papers and books. IV–A study of papers treated with aminoalkylalkoxysilanes and their resistance to ageing. Polymer Degradation and Stability, 91(12), 3448–3455. DOI 10.1016/j.polymdegradstab.2006.04.033. [Google Scholar] [CrossRef]
21. Dupont, A. L., Lavedrine, B., Cheradame, H. (2010). Mass deacidification and reinforcement of papers and books VI-study of aminopropylmethyldiethoxysilane treated papers. Polymer Degradation and Stability, 95, 2300–2308. DOI 10.1016/j.polymdegradstab.2010.09.002. [Google Scholar] [CrossRef]
22. Wang, Z. Y., Wei, X. X., Dai, Y., Li, Y. L., Wang, Y. L. (2021). Research progress of acid-free paper for cultural relic protection. Papermaking Equipment & Materials, 1, 8–10. DOI 10.3969/j.issn.1672-3066.2021.01.004. [Google Scholar] [CrossRef]
23. Wang, Y. F. (2020). The impact of new-type acid-free paper materials on the conservation of ancient books and its evaluation. Hunan Provincial Museum, 14, 593–610. DOI CNKI:SUN:HNBW.0.2018-00-067. [Google Scholar]
24. Xu, W. J., Wu, L. M., Xie, Y. L., Dai, H. Q. (2009). Development and application of acid-free paper in conservation of cultural relics. Sciences of Conservation and Archaeology, 21(Supplement), 76–78. DOI 10.16334/j.cnki.cn31-1652/k.2009.s1.005 [Google Scholar] [CrossRef]
25. Xu, W. J., Wu, L. M., DaiI, H. Q. (2014). Studies on acid-free paper for packaging and storage of cultural relics. Sciences of Conservation and Archaeology, 26(4), 25–29. DOI 10.3969/j.issn.1005-1538.2014.04.004 [Google Scholar] [CrossRef]
26. Hanus, J., Komorníková, M., Mináriková, J. (1995). Influence of boxing materials on the properties of different paper items stored inside. Restaurator, 16(4), 194–208. DOI 10.1515/rest.1995.16.4.194 [Google Scholar] [CrossRef]
27. Li, W., Yang, J., Han, J., Zheng, L., Jiang, Q. et al. (2014). The manufacture of acid-free paper boxes for storage of cultural relics. Sciences of Conservation and Archaeology, 26(2), 104–108. DOI 10.16334/j.cnki.cn31-1652/k.2014.02.021 [Google Scholar] [CrossRef]
28. Kundu, C. K., Wang, X., Song, L., Hu, Y. (2018). Borate cross-linked layer-by-layer assembly of green polyelectrolytes on polyamide 66 fabrics for flame-retardant treatment. Progress in Organic Coatings, 121, 173–181. DOI 10.1016/j.porgcoat.2018.04.031. [Google Scholar] [CrossRef]
29. Davis, R., Li, Y. C., Gervasio, M., Luu, J., Kim, Y. S. (2015). One-pot, bioinspired coatings to reduce the flammability of flexible polyurethane foams. ACS Applied Materials & Interfaces, 7(11), 6082–6092. DOI 10.1021/acsami.5b01105. [Google Scholar] [CrossRef]
30. Wang, X. S., Li, L., Tong, Y. J., Dai, Y., Chen, W. D. (2021). Synthesis of core/Shell structured zinc borate/Silica and Its surface charring for enhanced flame retardant properties. Polymer Degradation and Stability, 183, 109432. DOI 10.1016/j.Polymdegradstab.2020.109432. [Google Scholar] [CrossRef]
31. Attia, N., Ahmed, H., Yehia, D., Hassan, M., Zaddin, Y. (2017). Novel synthesis of nanoparticles based back coating flame-retardant materials for historic textile fabrics conservation. Journal of Industrial Textiles, 46(6), 1379–1392. DOI 10.1177/1528083715619957. [Google Scholar] [CrossRef]
32. Pang, H. C., Wang, X. S., Zhu, X. K., Tian, P., Ning, G. L. (2015). Nanoengineering of brucite@SiO2 for enhanced mechanical properties and flame retardant behaviors. Polymer Degradation and Stability, 120, 410–418. DOI 10.1016/j.Polymdegradstab.2015.08.002. [Google Scholar] [CrossRef]
33. Kundu, C. K., Wang, X., Liu, L. X., Song, L., Hu, Y. (2019). Few layers deposition and sol-gel finishing of organic-inorganic compounds for improved flame retardant and hydrophilic properties of polyamide 66 textiles: A hybrid approach. Progress in Organic Coatings, 129, 318–326. DOI 10.1016/j.porgcoat.2019.01.010.28. [Google Scholar] [CrossRef]
34. Marney, D., Russell, L. (2008). Combined fire retardant and wood preservative treatments for outdoor wood applications–A review of the literature. Fire Technology, 44(1), 1–14. DOI:10.1007/s10694-007-0016-6 [Google Scholar] [CrossRef]
35. Shah, A. U. R., Prabhakar, M., Song, J. I. (2017). Current advances in the fire retardancy of natural fiber and bio-based composites–A review. International Journal of Precision Engineering and Manufacturing-Green Technology, 4(2), 247–62. DOI 10.1007/s40684-017-0030-1. [Google Scholar] [CrossRef]
36. Mngomezulu, M., John, M. J., Jacobs, V., Luyt, A. S. (2014). Review on flammability of biofibres and biocomposites. Carbohydrate Polymer, 111, 149–82. DOI 10.1016/j.carbpol.2014.03.071. [Google Scholar] [CrossRef]
37. Wang, F., Liu, J., Lv, W. (2017). Thermal degradation and fire performance of wood treated with PMUF resin and boron compounds. Fire and Materials, 41(8), 1051–1057. DOI:10.1002/fam.2445. [Google Scholar] [CrossRef]
38. Griffin, G. J. (2011). The effect of fire retardants on combustion and pyrolysis of sugar-cane bagasse. Bioresource Technology, 102(17), 8199–8204. DOI 10.1016/j.biortech.2011.05.051. [Google Scholar] [CrossRef]
39. Cavdar, A. D., Mengeloǧlu, F., Karakus, K. (2015). Effect of boric acid and borax on mechanical, fire and thermal properties of wood flour filled high density polyethylene composites. Measurment, 60, 6–12. DOI 10.1016/j.measurement.2014.09.078. [Google Scholar] [CrossRef]
40. Wang, N., Liu, Y. S., Liu, Y., Wang, Q. (2017). Properties and mechanisms of different guanidine flame retardant wood pulp paper. Journal of Analytical and Applied Pyrolysis, 128, 224–231. DOI 10.1016/j.jaap.2017.10.007. [Google Scholar] [CrossRef]
41. Kundu, C. K., Wang, X., Song, L., Hu, Y. (2018). Borate cross-linked layer-by-layer assembly of green polyelectrolytes on polyamide 66 fabrics for flame-retardant treatment. Progress in Organic Coatings, 121, 173–181. DOI 10.1016/j.porgcoat.2018.04.031. [Google Scholar] [CrossRef]
42. Li, P., Wang, B., Liu, Y. Y., Xu, Y. J., Jiang, Z. M. (2020). Fully bio-based coating from chitosan and phytate for fire-safety and antibacterial cotton fabrics. Carbohydrate Polymers, 237, 116173. DOI 10.1016/j.carbpol.2020.116173. [Google Scholar] [CrossRef]
43. Mostashari, S., Fayyaz, F. (2008). TG of a cotton fabric impregnated by sodium borate decahydrate (Na2 B4O7⋅10H2O) as a flame-retardant. Journal of Thermal Analysis and Calorimetry, 93(3), 933–936. DOI 10.1007/s10973-007-8933-7. [Google Scholar] [CrossRef]
44. Zhang, Y., Mu, J., Li, S. J., Zhao, Y. (2015). The effect of boric acid-borax on the pyrolysis characteristics of poplar-oriented strand board. Journal of Beijing Forestry University, 37(1), 127–133. DOI 10.13332/j.cnki.jhfu.2015.01.003. [Google Scholar] [CrossRef]
45. Dogan, Mehmet. (2014). Thermal stability and flame retardancy of guanidinium and imidazolium borate finished cotton fabrics. Journal of Thermal Analysis and Calorimetry, 118, 93–98. DOI 10.1007/s10973-014-3950-9. [Google Scholar] [CrossRef]
46. Li, H., Lyu, H. F., Chen, M. L., Liu, R., Fei, B. H. (2018). Pyrolysis and combustion characteristics of fire retardant treated decorative bamboo filament. Journal of Forestry Engineering, 3(3), 12–17. DOI 10.13360/j.issn.2096-1359.2018.03.002. [Google Scholar] [CrossRef]
47. Donmez, C. A., Mengeloǧlu, F., Karakus, K. (2015). Effect of boric acid and borax on mechanical, fire and thermal properties of wood flour filled high density polyethylene composites. Measurement, 60, 6–12. DOI 10.1016/j.measurement.2014.09.078. [Google Scholar] [CrossRef]
48. Intharapat, P., Nakason, C., Kongnoo, A. (2016). Preparation of boric acid supported natural rubber as a reactive flame retardant and its properties. Polymer Degradation and Stability, 128, 217–227. DOI 10.1016/j.polymdegradstab.2016.03.004. [Google Scholar] [CrossRef]
49. Suharty, N. S., Dihardjo, K., Handayani, D. S., Firdaus, M. (2016). Effect of single flame retardant aluminum tri-hydroxide and boric acid against inflammability and biode-gradability of recycled PP/KF composites. AIP Conference Proceedings, 1717, 40026(1–6). DOI 10.1063/1.4943469 [Google Scholar] [CrossRef]
50. Uddin, K., Ago, M., Rojas, O. J. (2017). Hybrid films of chitosan, cellulose nanofibrils and boric acid: Flame retardancy, optical and thermo-mechanical properties. Carbohydrate Polymer, 177, 13–21. DOI 10.1016/j.carbpol.2017.08.116. [Google Scholar] [CrossRef]
51. Xu, W. Z., Wang, X. L., Wu, Y., Wu, L., Chen, C. Y. (2019). Functionalized graphene with Co-zIF adsorbed borate ions as an effective flame retardant and smoke suppression agent for epoxy resin. Journal of Hazardous Materials, 363, 138–151. DOI 10.1016/j.jhazmat.2018.09.086. [Google Scholar] [CrossRef]
52. Li, H., Ma, X., Gu, Z., Wang, X., Li, J. et al. (2020). Pyrolysis and combustion characteristics of boric acid and borax treated decorative bamboo filaments. Bioresources, 15(4), 8146–8160. DOI 10.15376/biores.15.4.8146-8160 [Google Scholar] [CrossRef]
53. Wang, F., Liu, J. L., Lyu, W. H. (2019). Wang effect of boron compounds on properties of Chinese Fir wood treated with PMUF resin. Journal of Bioresources and Bioproducts, 4(1), 60–66. DOI 10.21967/jbb.v4i1.182. [Google Scholar] [CrossRef]
54. Kizilcan, N., Dincer, P. (2013). In situ modification of cyclohexanone formaldehyde resin with boric acid for high-performance applications. Journal of Applied Polymer Science, 129(5), 2813–2820. DOI 10.1002/app.39951. [Google Scholar] [CrossRef]
55. Dogan, M., Dogan, S. D., Savas, L. A., Ozcelik, G., Tayfun, U. (2021). Flame retardant effect of boron compounds in polymeric materials. Composites Part B, 222, 109088DOI 10.1016/j.compositesb.2021.109088. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |