

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2022.022740
ARTICLE
Self-Blowing Non-Isocyanate Polyurethane Foams Based on Hydrolysable Tannins
1Faculté des Sciences et Technologies, LERMAB, EA 4370-USC INRAE, University of Lorraine, Blvd des Aiguillettes, Nancy, 54506, France
2LERMAB, EA 4370-USC INRAE, University of Lorraine, 27 rue Philippe Seguin, Epinal, 88000, France
3Department of Polymers and Pigments, National Research Center, Cairo, 12622, Egypt
*Corresponding Author: Antonio Pizzi. Email: antonio.pizzi@univ-lorraine.fr
Received: 23 March 2022; Accepted: 23 May 2022
Abstract: Non-isocyanate polyurethane (NIPU) foams using a hydrolysable tannin, also vulgarly called tannic acid, namely here commercial chestnut wood tannin extract was prepared. Compression strength did not appear to depend on the foam apparent density while the formulation composition of the NIPU foams has been shown to be more determinant. These NIPU foams appeared to be self-extinguishing once the high temperature flame is removed. The ignition time gave encouraging results but for improved fire resistance the foams may need some fire-retardant addition. FTIR spectrometry showed the formation of non-isocyanate urethane linkages. Thermogravimetric analysis indicated a good thermal resistance of these foams, with thermal degradation following four phases. First in the interval 25°C–120°C range, mainly evaporation of water occurs with a maximal loss of 10% weight. In the 150°C–450°C temperature range foams mass loss is of almost 70%. In particular in the 125°C–275°C range occurs the degradation of some small molecular weight substances. In the 500°C–790°C temperature range the foams do not present any further large degradation.
Keywords: Non-isocyanate biopolyurethanes (NIPU); biofoams; chestnut tannin; hydrolysable tannins; self-extinguishing
Vegetable tannins have been used for centuries as the agents transforming animal hides into leather. They can loosely be divided into two main broad classes, mainly condensed tannins and hydrolysable tannins. While in the last decades considerable research work for the preparation of new environment friendly biomaterials has been focused on natural polyphenolics, in particular on lignin [1] and condensed tannins for a variety of uses, such as wood adhesives and rigid insulating foams [2], this has not really been the case for hydrolysable tannins. While the literature on hydrolysable tannins for use in leather is extensive, the literature on their use for the preparation of renewable biomaterials is rather scant and limited to some rare papers on their use for wood adhesives [3,4]. This is due to the lower reactivity of their aromatic nuclei towards aldehydes [5] and to their relatively higher cost.
Hydrolysable tannins, of which chestnut wood tannin extract is the most abundantly extracted commercial species, include also several others commercial tannin extracts such as sumach, tara, divi-divi, myrabolans, algarobilla, valonea, oak and several others. They are all composed of gallic and digallic acids esters with a sugar, mainly glucose. Pentagalloyl glucose monomers and oligomers (I) are the main species present but in the extract, they share their importance with their rearrangements to other compounds such as castalagin (II), vescalagin, vescalin (III) and castalin [6,7] (Fig. 1).
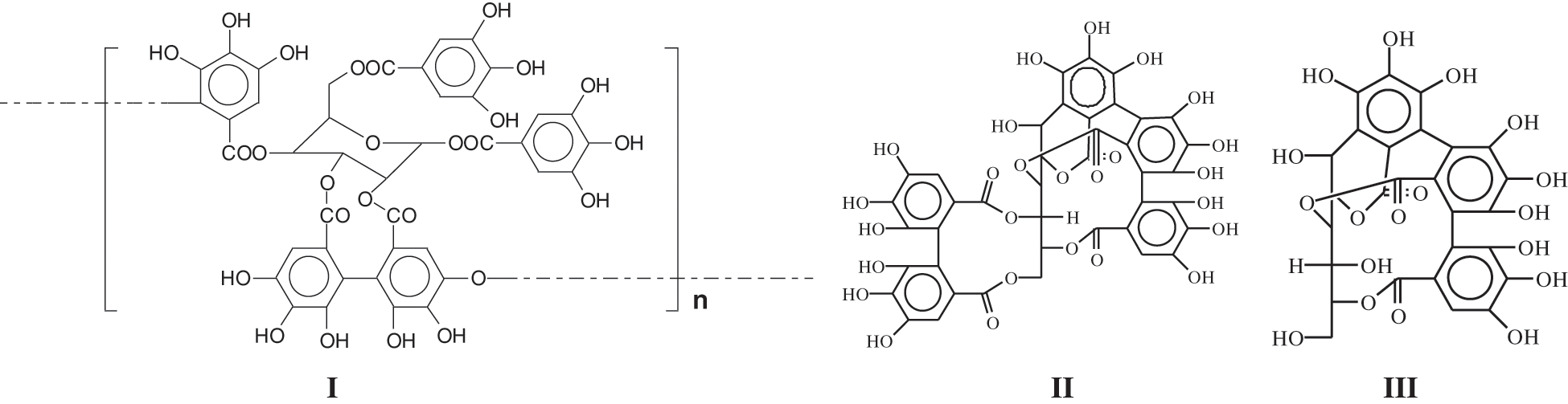
Figure 1: Schematic structural formulas of oligomers of pentagalloyl glucose (I), and its rearrangements to castalagin (II), and vescalin (III)
More recently, due to their structural abundance in hydroxyl groups, hydrolysable tannins, namely chestnut tannin, were found to be ideally suited for the preparation of non-isocyanate biopolyurethanes (NIPU) [8] as the reactions involved did not rely on their aromatic rings’ reactivity. More interestingly both their phenolic and carbohydrate fractions were found to contribute to forming bio-NIPUs [8]. In this previous work the species formed were identified by both 13C NMR and by MALDI ToF mass spectrometry. It was shown that numerous species presenting urethane linkages between gallic acid units (IV) as well as species forming urethane linkages on glucose (V) formed and were identified, such as the species in Figs. 2 and 3.
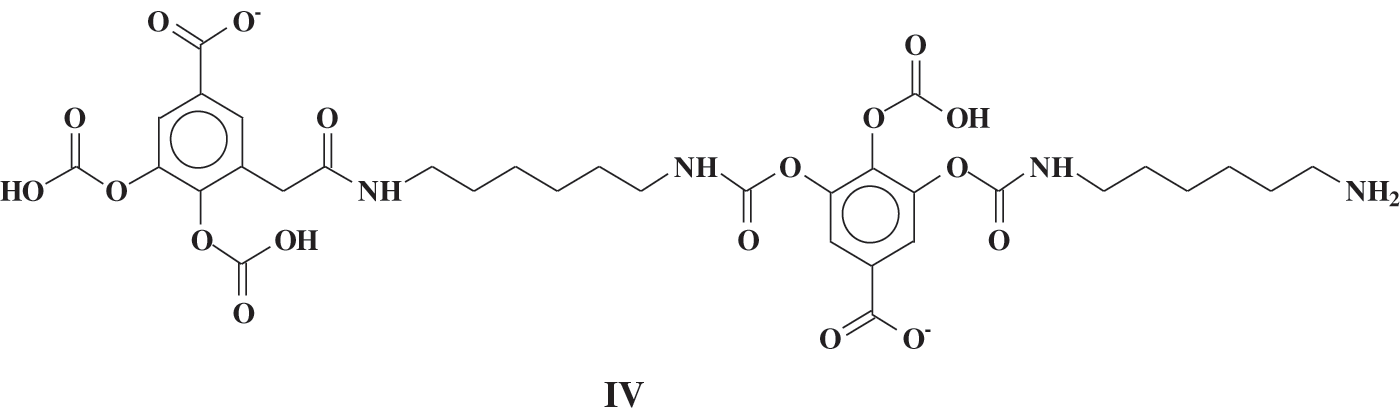
Figure 2: Example of a urethane species isolated by reaction of carbonated gallic acid with hexamethylene diamine in chestnut tannin [8]
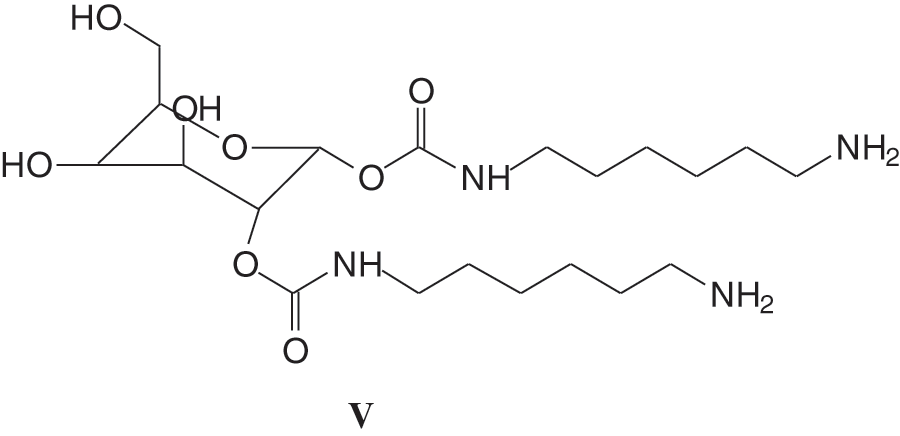
Figure 3: Example of a urethane species formed by glucose present in chestnut extract after carbonation with dimethyl carbonate with hexamethylene diamine
As the focus then shifted to condensed tannins NIPU and on their applications for adhesives and for fire resistant foams [2,5,9–12] the work on the application of hydrolysable tannins NIPU for the preparation of new useful biomaterials did not advance further. However, the presence of gallic acid as the hydrolysable tannins basic structure linked to carbohydrates [6,7,13,14] does instead identify them as an ideal raw material for the preparation of non-isocyanate polyurethanes, whatever their subsequent application. It is then pertinent to try to develop their use for the development of adequate biomaterials. The work presented here then focuses on the development of chestnut tannin-based non-isocyanate polyurethanes (NIPU) foams.
Commercial chestnut tannin extract was obtained from Silva Chimica (S. Michele Mondovi’, Italy). Commercial products, including dimethyl carbonate (DMC, anhydrous, ≥99%), hexamethylenediamine (HDMA, technical grade, 70% water solution), glutaraldehyde, citric acid and hexamethylene tetramine were obtained from Sigma-Aldrich (St.Louis, Missouri, USA). All raw materials in the experiments were employed directly without any pre-treatment.
2.2 Preparation of Chestnut Tannin Extract Non-Isocyanate Polyurethane (NIPU) Resin and Foams
The NIPU foam preparation is based on three phases:
A) Preparation of the NIPU resin;
B) Preparation of a flexible foam or a homogeneous mixture from the NIPU resin;
C) Heat to cure and harden the foam obtained.
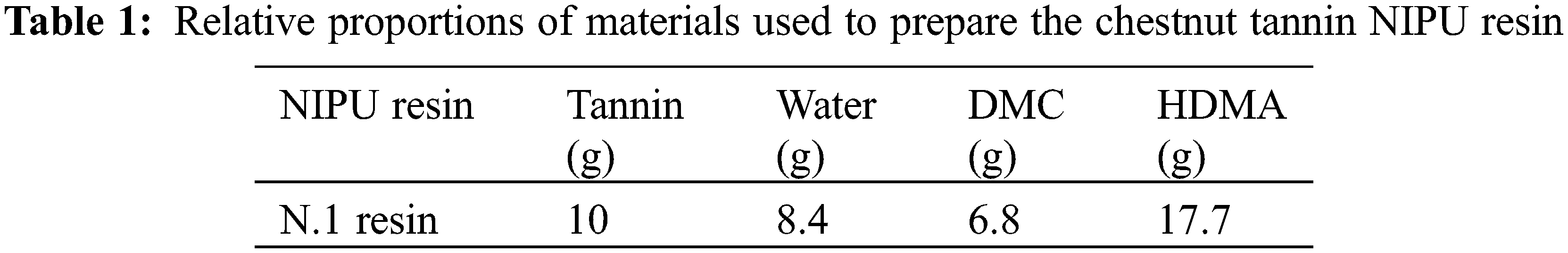
Tannin-based NIPU resins were prepared by the same procedure used and reported previously [8]. Briefly, distilled water was charged into a three-neck round flask with magnetic stirrer and thermometer immersed in an oil bath condition. Then, chestnut tannin extract was added and sufficiently mixed till a homogenous mixture was obtained. Thereafter, DMC was added into the flask and the mixture heated to 65°C and kept at this temperature for 1 h. Finally, HDMA was added under continuous mechanical stirring and maintained at 90°C for 2 h. The reactions were tried by heating them either in an oil bath or without an oil bath. The resin so obtained was cooled down to room temperature and recorded as tannin NIPU. The relative proportions of the reactives used are shown in Table 1.
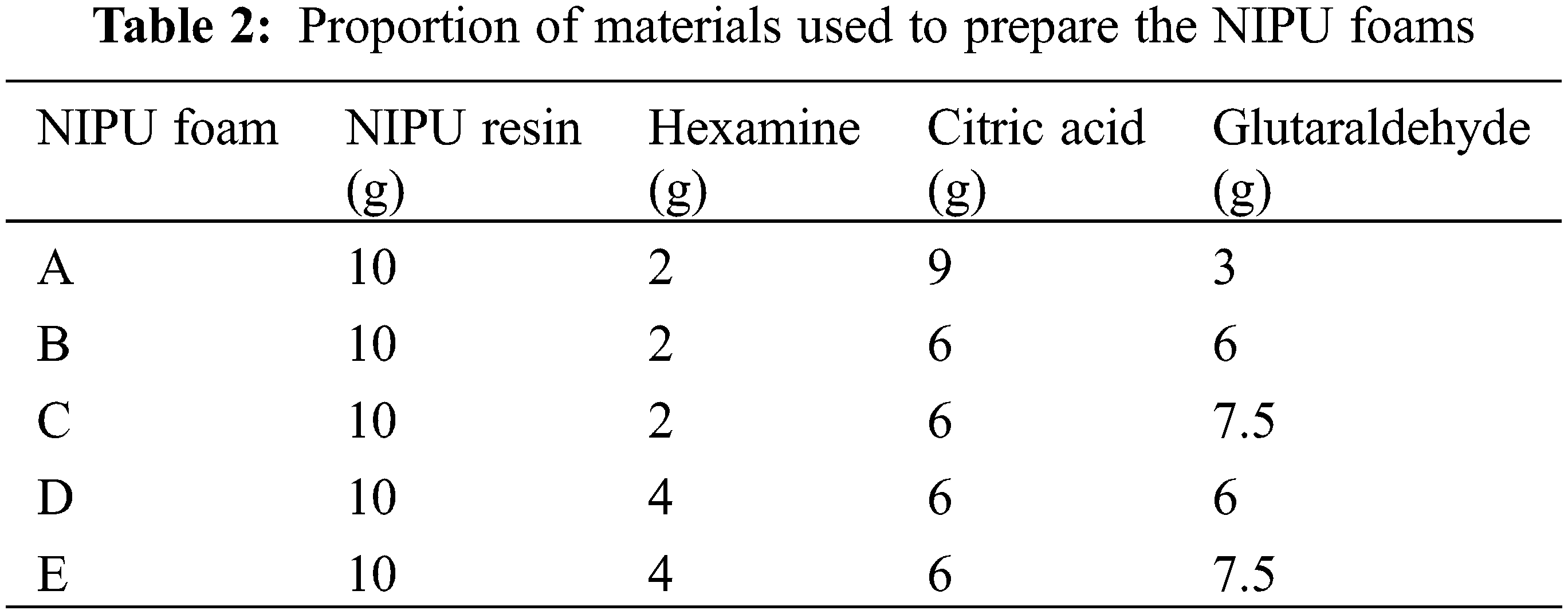
Foams formulations were prepared using the amounts of reagents listed in Table 2. The foams were obtained by mixing several compounds. The first one is a homogeneous acid mixture, the composition of which consists of citric acid (50% in water solution) and glutaraldehyde (50% in water solution). The second compound is a homogeneous tannin NIPU resin, composed of the chestnut tannin based NIPU resin and hexamethylene tetramine (hexamine). Briefly, difference proportions of tannin based NIPU resin and hexamine were mixed in glass beakers, and stirred rapidly, resulting in a homogeneous tannin resin, ready for application. The mixture of citric acid and glutaraldehyde, was then weighed and placed into a foaming module, and the tannin-based NIPU resin and hexamine mix was added to it immediately, stirring manually for an optimal predetermined period of 10–15 s. Subsequently the foams were left to grow at ambient temperature (25°C). When the self-blowing step was finished, a homogeneous dark and red liquid but self-supporting foam was obtained. This was then cured and hardened overnight in an oven at 70 to obtain the final rigid foam. Finally, the hardened foam samples were conditioned for a minimum of 2 days at 25°C and 12% relative humidity before being characterized. Formulation A did not result in a testable foam.
2.3 Apparent Density Determination
The determination of the apparent density for each foam sample was conducted according to the provisions of the standard GB/T 6342-2009 standard [15,16]. The size of the prepared sample was measured, and the following formula was applied for calculating the apparent density:
where m is the mass of the sample (g); V is the volume of the sample (mm3); ρ is the apparent density (kg/m3), and the accuracy limit of the results is around 0.1 kg/m3.
The compression strength of the foams in the direction parallel to that of the foam rise were determined under ambient conditions by using a universal testing machine (Instron 3300, Elancourt, France). The size of the samples was 25 mm × 25 mm × 25 mm, and the crosshead rate was fixed at 2.0 mm min−1 for each sample. At least three samples were tested to determine the average value.
2.5 Fourier Transform Infrared Spectrometry (FTIR)
Samples of the hydrolysable chestnut tannin extract, of the NIPU resin 1 prepared in an oil bath before foaming it, of the NIPU resin 1 prepared without an oil bath before foaming it, and of the NIPU foam after curing, were placed into a diamond eye (1.8 mm) of the PerkinElmer Frontier ATR-FTMIR (PerkinElmer, Villebon-sur-Yvette, France) spectrometer. The scan results of each sample were recorded with 32 scans between the wave range of 600 and 4000 cm−1, with the scan resolution of 4 cm−1.
2.6 Thermogravimetric Analysis (TGA)
The thermal stability of tannin based NIPU foams was investigated with a TGA 5500 analyzer (Mettler Toledo, Guyancourt, France). 5–8 mg of dried powder was put into a platinum pan, and heating was started on the sample to the desired temperature at a heating rate of 10 °C/min, under nitrogen atmosphere with a flow rate of 50 mL/min in the temperature range from 50°C to 790°C.
The direct ignition test was carried out according to a method already described in the literature [17,18]. Foam samples were made to specification size, 30 mm × 30 mm × 30 mm, and then were exposed facially to a stainless-steel frame preheated on a Bunsen burner (around 1000°C), making sure the samples were located in the outer flame area of the Bunsen burner. The ignition time was calculated starting by when the samples were placed on the burner, until the sample started burning. The Bunsen burner flame was then immediately extinguished and the time the sample either continued burning or self-extinguished was determined.
The Chestnut tannin extract FTIR spectrum in Fig. 4a shows the wide hydroxyl groups band between 3000 and 3500 cm−1 as this tannin is particularly rich in –OH groups both on the phenolic part and on its carbohydrate part. The Carboxylic group of the gallic acid and its sugar esters (I) characteristic of this tannin appears at 1719 cm−1. The 1612 cm−1 band is assigned to antisymmetric stretching of C=C groups in the aromatic rings [19]. The band at 1175 cm−1 belonging to the carbohydrate alcoholic –OH or to the ethers that are both abundantly present in the rearranged species castaline and vescaline. The band at 1267 cm−1 is also assigned to the numerous ether bridges present in the rearranged species such as II and III in this tannin extract. The 1089 cm−1 band is the asymmetric stretching of six rings ethers also present in castalagin (II) and vescalin (III). All the ethers presence being confirmed by the small band at 807 cm−1. The aromatic region. All the bands from 870 cm−1 and lower, as well as the two relatively smaller bands around 1500 cm−1 are assigned to the aromatic nuclei of the phenolic part of this tannin.
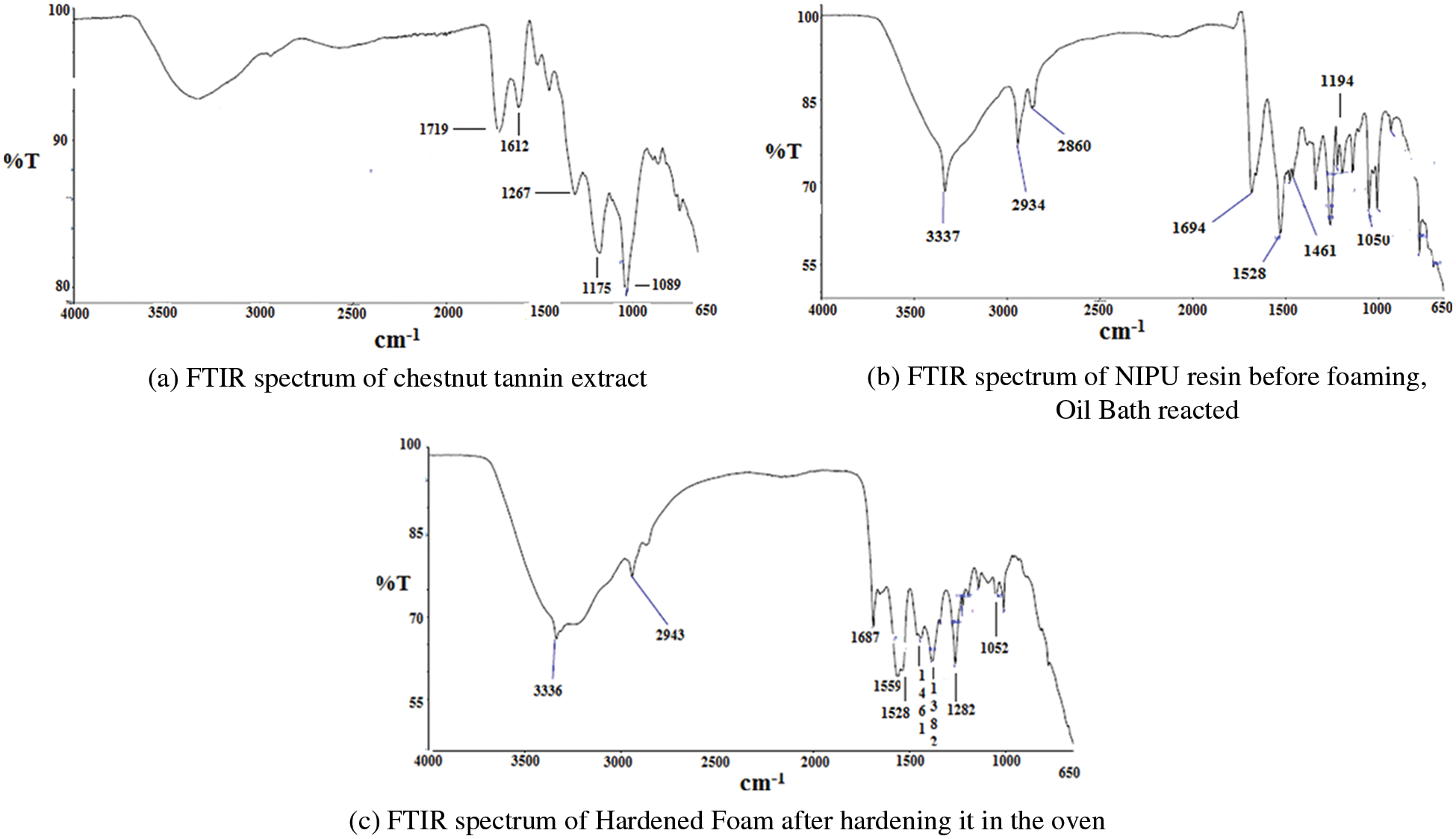
Figure 4: (a) FTIR spectrum of chestnut tannin extract (b) FTIR spectrum of NIPU resin before foaming, Oil Bath reacted (c) FTIR spectrum of Hardened Foam after hardening it in the oven
From the FTIR spectrum of the liquid NIPU resin prepared by heating in an oil bath many peaks more have appeared over the spectrum of the chestnut tannin extract alone (Fig. 4b). Thus, from Fig. 4b the 3337 cm−1 is the asymmetric stretching of the amide bonds of urethane groups. All this shows that urethane linkages have formed. The marked intensity of this peak indicates that urethane linkages are in a relatively high proportion. However, comparing the spectra in Figs. 4a and 4b, the sharpness of this band in Fig. 4b could indicate that the band belongs to carbammic acid (–NH–COOH) groups formed by the reaction of DMC with hexamethylene diamine. The slight change of shift and intensity in Fig. 4c indicating that further urethane linkages (thus –NH–COO–R, esters) have then formed afterwards, on curing. All this shows that urethane linkages and bridges have formed. This is supported in Fig. 4b by the peaks at 1694, and 1687 cm−1 (Fig. 4b) characteristic of the carboxyl group of urethane bridges, as well as of the bands at 1528 cm−1 (also representative of the C–N of a urethane or an amide), 1194, and 1050 cm−1 (urethane C–O–C) all characteristic of urethane bridges. Equally the band at 1282 cm−1 in Fig. 4b confirms the presence of urethane bridges. The peaks at 2934 and 2860 cm−1 are assigned to the –CH2–groups of the hexamethylene diamine, with the 2934 cm−1 band being the C–H symmetric stretching and the 2860 cm−1 band being the C–H asymmetric stretching. These assignments are also supported by the asymmetric stretching bands at 1461 cm−1 of the alkane chain of the hexamethylene diamine. Conversely, the 1333 cm−1 band is characteristic of the stretching of the branched –CH–groups of the glucose chain in (I). The 1382 cm−1 band in Fig. 4b confirms the presence of an aldehyde group from the glutaraldehyde. The spectrum of the NIPU resin in Fig. 4b is much clearer than the spectra being carried out without the oil bath, where urethanes are also formed but in a much lower yield. Once the foam is cured in an oven its spectrum (Fig. 4c) is less defined due to the formation of a cross-linked network, nonetheless that fundamentally the same peaks are in place. The urethane bands have very slightly shifted, an indication that further urethane bridges, thus esters have been formed.
3.2 Thermogravimetric Analysis (TGA)
In order to observe the foams thermal resistance, their characterization by TGA was carried out. Specific degradation up to a temperature at 790°C was used. The thermal decomposition behavior of the series of chestnut tannin based NIPU foams was studied, and the corresponding thermograms as well as relevant DTG are collected. The thermogravimetric analysis (TGA) curves of the four chestnut tannin foams are practically coincidental, thus, to distinguish between them in Fig. 5 are just reported the superimposed the DTG curves pf the four experimental foams. A multi-stage response was encountered for the decomposition behavior as can be seen in all curves.
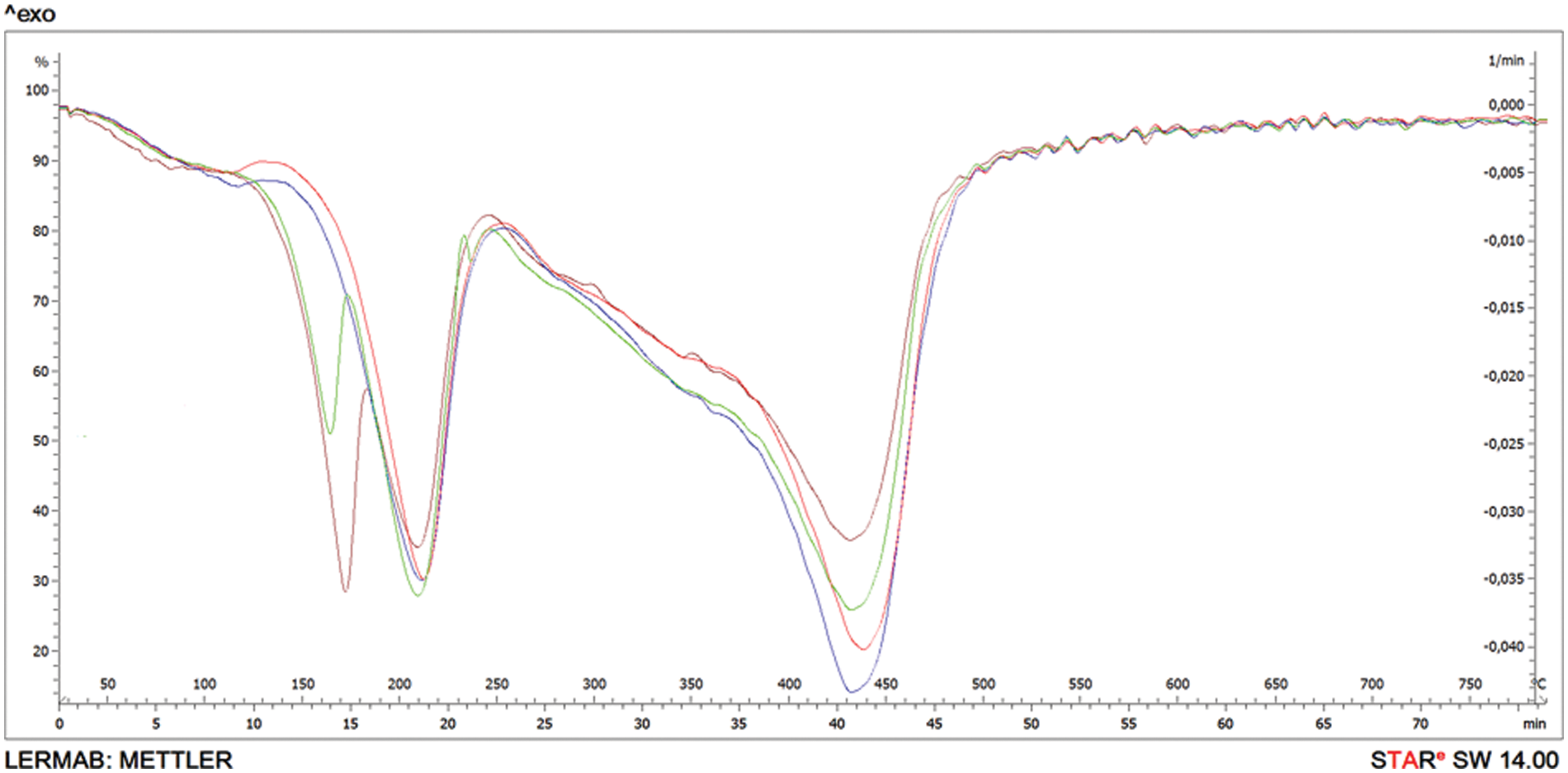
Figure 5: Superimposed DTG curves for the four-chestnut tannin NIPU experimental foams: RED = B; Blue = C; Green = D; Mauve = E
The maximum temperatures are indicated by the peaks of the DTG curves at the different stages of pyrolysis.
Two types of curves are obtained, namely curves with two main DTG peaks (B and C) and curves with three DTG peaks (D and E). The three peaks’ curves, and in particular the DTG peak at 150°C–160°C is probably issued from the depolymerization of the excess of hexamine present in D and E, and probably not due to the glutaraldehyde seen that a higher proportion of this is equally present in C (and this presents only two peaks) and E (with 3 peaks). However, the difference in the DTG 150°C–160°C peak intensity smaller in D than in E appears to indicate that the higher proportion of glutaraldehyde is synergic with the higher proportion of hexamine, thus an excess of hardeners liberating an excess of water as they react.
1) The first stage shows a small weight loss within the temperature interval 25°C–120°C, which belongs to the evaporation of adsorbed water from the foams and with the loss of mass reaching up to 10%.
2) The second stage is between 150°C and 450°C including a loss of mass of the foam of almost 70%. The mass loss in the temperature range 125°C–275°C is assigned to the initial degradation of the samples, typically from some small molecular weight substances (saccharide present in the tannin extract) and unstable chemical linkages such as ether bonds [20]. In addition, the release of excess HDMA can also cause weight loss in this stage because its boiling point is around 190°C.
3) In the temperature range 500°C–790°C the foams do not show any further large degradation.
4) At the temperature of 790°C, only a maximum of 20% of the initial mass of the foam remains.
At the temperature of 185°C, the mass loss reaches 10% for sample B, while for D, which has the same formulation but with twice as much hexamine, 10%mass loss is reached at 167°C while a higher temperature is required for the first degradation. C with a higher proportion of glutaraldehyde than B showed the first degradation at 182°C. E which has a higher proportion of both hexamine and glutaraldehyde than B shows the lowest temperature for the first decomposition stage at 156°C.
The second stage of weight loss appeared within the 275°C–425°C range due to the decomposition of the urethane groups [18]. This is the main degradation step, with a major part of the weight loss occurring in this stage. The second decomposition at the temperature 460°C showed that B lost 70% of its initial mass. The same mass loss occurred for E at the temperature of 442°C. The last weight loss stage occurs in the 425°C–525°C temperature range, which is attributed to covalent C–C bonds breaking. Some pyrolysis residual products coming from the previous few stages can also undergo further decomposition [18,21].
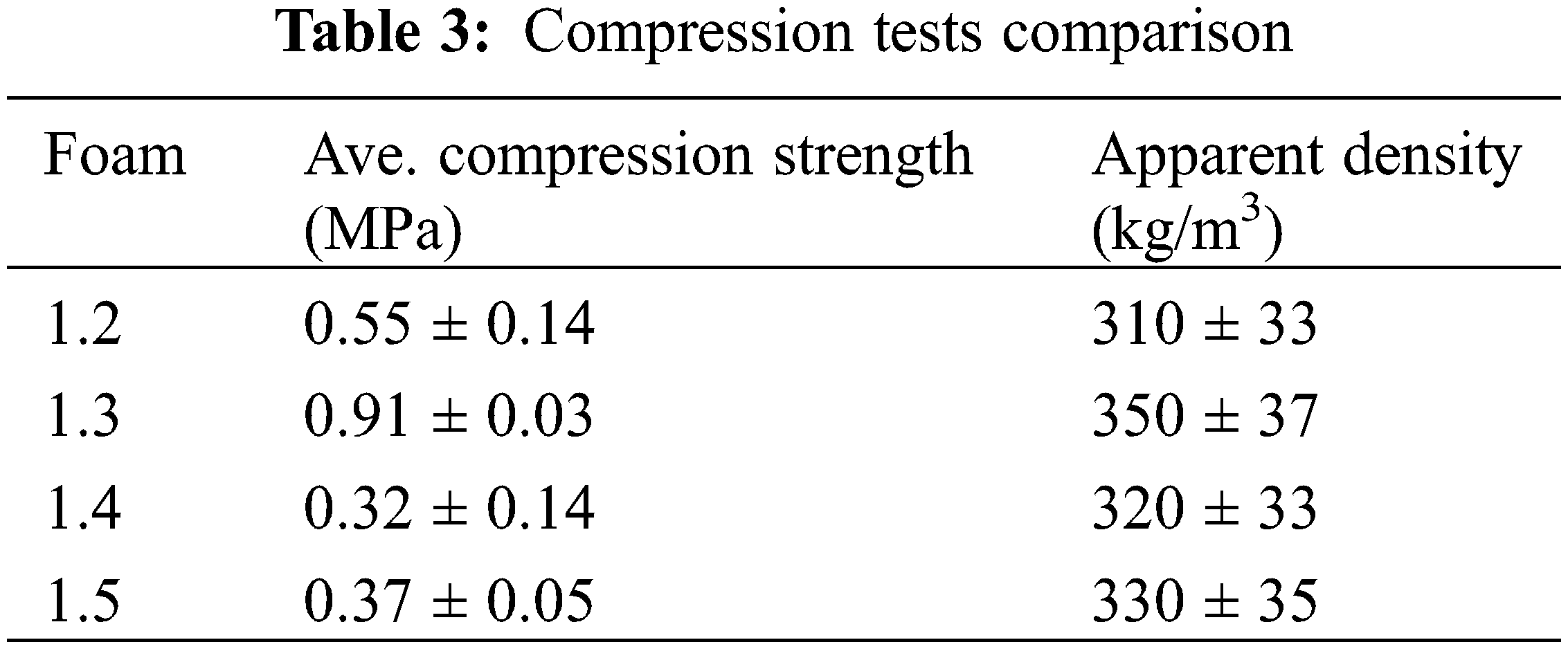
The liquid flexible foams prepared need to be hardened by heating, which was necessary to obtain and measure stable physical properties. According to the relevant literature [22] lower density foam samples have thinner cell walls; thus, they can only provide a rather limited contribution to compression resistance [23]. Thus, to investigate whether any upgrading in mechanical properties is only attributed to the increasing density or is due to the higher strength provided by the difference in formulation, the specific compressive strength of all foams was measured according to the literature [24]. The specific compressive strength of all foam samples is shown in Table 3. From Table 3, it appears that in the case of the chestnut tannin NIPU foams the rule that higher apparent density led to higher compression strength is not respected. This indicates that the differences in formulation, hence of reaction dependent from te relative proportions pf the components have influenced quite strongly the compressive strength of the foams. For foams B, D and E the compression strength differences are not significant seen the standard deviations obtained for them, showing however a certain structural heterogeneity considering the spread of their standard deviations. Foam C however, shows a very significantly different compression strength notwithstanding that its density is only rather slightly higher than that of the other three foams formulations. The main difference noted from Table 2 is its lower level of hexamine and higher level of glutaraldehyde. This infers that for these NIPU foams the relative strength of the cell walls is more upgraded by the reaction of glutaraldehyde than hexamine with the other foam components. Thus, the results show that the strength improvement of the mechanical properties of chestnut tannin NIPU foams is not exclusively attributed to their density alone but related to the contribution of the cell wall and the influence of the relative proportions of the additives contributing to the reaction [24].
Although there are some limitations to the fire resistance evaluation of the ignition tests reported here, they can indicate to a certain extent the combustion resistance of the material. Therefore, this test was designed to investigate the flammability of foams B, C, D and E. The ignition combustion tests were conducted on the foam samples directly. The results are shown in Table 4. The ignition times of the four foams were roughly comparable, with foam E taking a slightly shorter time to ignite and foam D presenting a slightly longer ignition time. Remarkably, foam D had the lower density of all the chestnut tannin NIPU foams. Foams B, D and E self-extinguished immediately as the burner flame was removed. The only different behavior was presented by foam C. While this had the second longest ignition time, possibly due to the difference in composition of its cell walls as shown by its higher compression strength, it was also the only foams that took 40 s to self-extinguish once the burner flame was removed. It is difficult to imagine why it behaved so, because while the density range of its samples was the highest of the four foams, while this might have contributed to the longest ignition time it did not appear to be determinant in causing a 40 s maintenance of burning after the direct burner flame was removed. It also indicates that while an interesting resistance to fire is shown, as already observed for foams obtained from mixed condensed tannin/glucose foams, they need the addition of some fire retardants for achieving a even better resistance to fire. An image of the ignition test for the 4 NIPU foams is shown in Fig. 6, showing the encouraging behavior in fire of foams B, D and E, their instant self-extinguishing, and showing the short afterburning for foam C. The performance of foams D and E are particularly impressive, with hardly any flame been present.

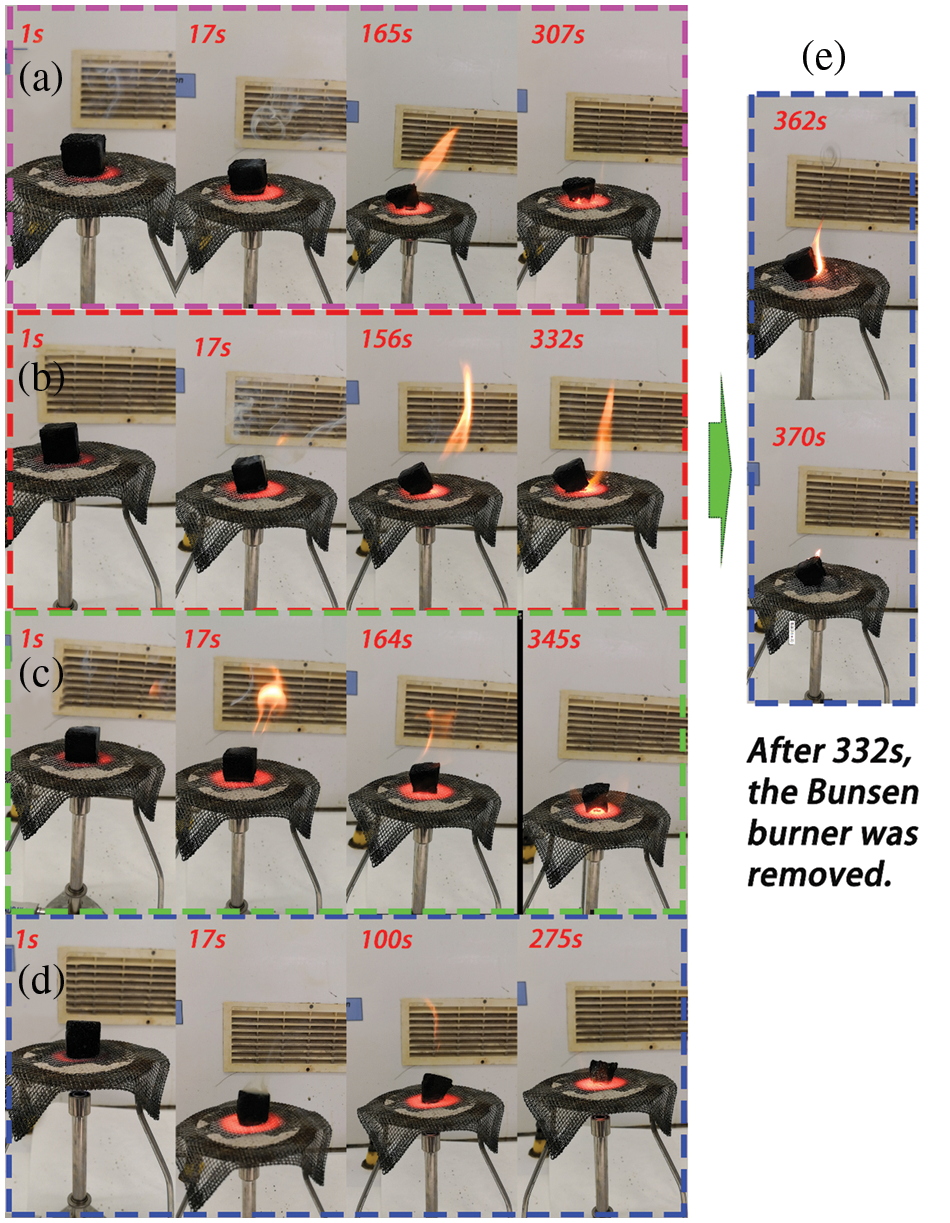
Figure 6: Ignition test images with time scale in seconds for (a) resin B, (b) resin C, (c) resin D, (d) resin E. For C the afterburning before self-extinction is shown
The work presents the preparation of non-isocyanate polyurethane (NIPU) foams using a hydrolysable tannin, namely commercial chestnut wood tannin extract. While NIPU foams have been obtained using condensed flavonoid tannins this is the first time that a hydrolysable tannin extract, also vulgarly called tannic acid, has been used for NIPU foams application. The richness of this extract in phenolic hydroxyl groups and alcoholic hydroxyl groups from the skeletal glucose chain and the rearranged molecular species has shown that it is particularly suitable for NIPU resins and for their rigid foam applications. The formulation of the NIPU foams has shown to be determinant in the apparent density and compression resistance of the foams, rather independently from the foam density. The rigid foam prepared showed to have the interesting property of being self-extinguishing once the high temperature flame is removed. Ignition time was quite encouraging but indicated that for improved fire resistance the foams may be in need of some small proportion of a fire retardant.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Sarazin, J., Pizzi, A., Amirou, S., Schmidt, D., Sernek, M. (2021). Organosolv lignin non-isocyanate based polyurethanes (NIPU) as wood adhesive. Journal of Renewable Materials, 9(5), 881–907. DOI 10.32604/jrm.2021.015047. [Google Scholar] [CrossRef]
2. Pizzi, A. (2019). Tannin-based biofoams. Journal of Renewable Materials, 7(5), 477–492. DOI 10.32604/jrm.2019.06511. [Google Scholar] [CrossRef]
3. Kulvik, E. (1976). Chestnut wood tannin extracts in plywood adhesives. Adhesives Age, 19(3), 19–21. [Google Scholar]
4. Spina, S., Zhou, X., Segovia, C., Pizzi, A., Romagnoli, M. et al. (2013). Phenolic resin adhesives based on chestnut hydrolysable tannins. Journal of Adhesion Science and Technology, 27(18–19), 2103–2111. DOI 10.1080/01694243.2012.697673. [Google Scholar] [CrossRef]
5. Pizzi, A. (1983). Wood adhesives chemistry and technology. New York: Marcel Dekker. [Google Scholar]
6. Pasch, H., Pizzi, A. (2002). On the macromolecular structure of chestnut ellagitannins by MALDI-TOF mass spectrometry. Journal of Applied Polymer Science, 85(2), 429–437. DOI 10.1002/(ISSN)1097-4628. [Google Scholar] [CrossRef]
7. Pizzi, A., Pasch, H., Rode, K., Giovando, S. (2009). Polymer structure of commercial hydrolysable tannins by MALDI-TOF mass spectrometry. Journal of Applied Polymer Science, 113(6), 3847–3859. DOI 10.1002/app.30377. [Google Scholar] [CrossRef]
8. Thebault, M., Pizzi, A., Dumarcay, S., Gerardin, P., Fredon, E. et al. (2014). Polyurethanes from hydrolysable tannins obtained without using isocyanates. Industrial Crops and Products, 59, 329–336. DOI 10.1016/j.indcrop.2014.05.036. [Google Scholar] [CrossRef]
9. Sarazin, J., Poljansek, I., Pizzi, A., Sernek, M. (2022). Curing kinetics of tannin and lignin biobased adhesives by DSC and ABES. Journal of Renewable Materials, 10(8), 2117–2131. DOI 10.32604/jrm.2022.019602. [Google Scholar] [CrossRef]
10. Chen, X., Pizzi, A., Fredon, E., Gerardin, C., Zhou, X. et al. (2022). Low curing temperature tannin-based non-isocyanate polyurethane (NIPU) wood adhesives: Preparation and properties evaluation. International Journal of Adhesion and Adhesives, 112, 103001. DOI 10.1016/j.ijadhadh.2021.103001. [Google Scholar] [CrossRef]
11. Sahimim, W., Boer, F. D., Chapuis, H., Obonou-Akong, F., Pizzi, A. et al. (2022). Feasibility study of the synthesis of isocyanate-free polyurethanes from catechin. Journal of Renewable Materials, 10(5), 1175–1184. DOI 10.32604/jrm.2022.016365. [Google Scholar] [CrossRef]
12. Chen, X., Li, J., Essawy, H., Pizzi, A., Fredon, E. et al. (2022). Flame-retardant and thermally-insulating tannin and soybean protein isolate (SPI) based foams for potential applications in building materials. Construction and Building Materials, 315, 125711. DOI 10.1016/j.conbuildmat.2021.125711. [Google Scholar] [CrossRef]
13. Radebe, N., Rode, K., Pizzi, A., Giovando, S., Pasch, H. (2013). MALDI-TOF-CID for the microstructure elucidation of polymeric hydrolysable tannins. Journal of Applied Polymer Science, 128, 97–107. DOI 10.1002/app.38156. [Google Scholar] [CrossRef]
14. Giovando, S., Pizzi, A., Pasch, H., Pretorius, N. (2013). Structure and oligomers distribution of commercial tara (Caesalpina spinosa) hydrolysable tannin. ProLigno, 9(1), 22–31. [Google Scholar]
15. Liu, B., Zhou, Y., Essawy, H., Feng, S., Li, X. et al. (2022). Formaldehyde free renewable thermosetting foam based on biomass tannin with a lignin additive. Journal of Renewable Materials, 10(8), 2029–2039 DOI 10.32604/jrm.2022.019848. [Google Scholar] [CrossRef]
16. GB/T 6343-2009 standard (2009). Cellular plastics and rubbers. Determination of apparent density. G32 National Standards of People’s Republic of China, Beijing, China. [Google Scholar]
17. Xi, X., Pizzi, A., Gerardin, C., Lei, H., Chen, X. et al. (2019). Preparation and evaluation of glucose based non-isocyanate polyurethane self-blowing rigid foams. Polymers, 11(11), 1802. DOI 10.3390/polym11111802. [Google Scholar] [CrossRef]
18. Chen, X., Li, J., Xi, X., Pizzi, A., Zhou, X. et al. (2020). Condensed glucose-tannin-based NIPU BioFoams of improved fire retardancy. Polymer Degradation and Stability, 175, 109121. DOI 10.1016/j.polymdegradstab.2020.109121. [Google Scholar] [CrossRef]
19. Ricci, A., Lagel, M. C., Parpinello, G. P., Pizzi, A., Kilmartin, P. A. et al. (2016). Spectroscopy analysis of phenolic and sugar patterns in a food grade chestnut tannin. Food Chemistry, 203, 425–429. DOI 10.1016/j.foodchem.2016.02.105. [Google Scholar] [CrossRef]
20. Xu, Y., Han, Y., Shi, S., Gao, Q., Li, J. (2020). Preparation of a moderate viscosity, high performance and adequately-stabilized soy protein-based adhesive via recombination of protein molecules. Journal of Cleaner Production, 255, 120303. DOI 10.1016/j.jclepro.2020.120303. [Google Scholar] [CrossRef]
21. Santos, O. S. H., Coelho, D. S. M., Silva, V. R., Mussel, W. N., Yoshida, M. I. (2017). Polyurethane foam impregnated with lignin as a filler for the removal of crude oil from contaminated water. Journal of Hazardous Materials, 324, 406–413. DOI 10.1016/j.jhazmat.2016.11.004. [Google Scholar] [CrossRef]
22. Li, J., Zhang, A., Zhang, S., Gao, Q., Zhang, W. et al. (2019). Larch tannin-based rigid phenolic foam with high compressive strength, low friability, and low thermal conductivity reinforced by cork powder. Composites B: Engineering, 156, 368–377. DOI 10.1016/j.compositesb.2018.09.005. [Google Scholar] [CrossRef]
23. Tondi, G., Pizzi, A. (2009). Tannin-based rigid foams: Characterization and modification. Industrial Crops and Products, 29(2–3), 356–363. DOI 10.1016/j.indcrop.2008.07.003. [Google Scholar] [CrossRef]
24. Zhou, X., Li, B., Xu, Y., Essawy, H., Wu, Z. et al. (2019). Tannin-furanic resin foam reinforced with cellulose nanofibers (CNF). Industrial Crops and Products, 134, 107–112. DOI 10.1016/j.indcrop.2019.03.052. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |