

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2022.023122
ARTICLE
Grain Boundary Passivation Modulated by Molecular Doping for High-Performance Perovskite Solar Cells
College of Chemical and Engineering, Qingdao University of Science and Technology, Qingdao, 266042, China
*Corresponding Authors: Guorui Cao. Email: caoguorui@qust.edu.cn; Yue Liu. Email: liuyuequst@163.com
Received: 11 April 2022; Accepted: 12 May 2022
Abstract: Aiming to reduce the defects of perovskite film and improve carrier transport, an organic small molecule, benzo[d]isothiazol-3(2H)-one 1,1-dioxide (OBS), is introduced as an additive in the solution-processing of perovskite and prepare uniform perovskite films with a continuous distribution of OBS at grain boundaries. Fourier transform infrared spectroscopy and X-ray photoelectron spectroscopy are conducted to reveal the interactions of hydrogen bonding and coordination bonding between OBS and perovskite. Various characterizations (including X-ray diffraction, UV-vis spectroscopy, electrochemical impedance spectroscopy, etc.) are conducted to uncover the effect of OBS on device performance. Consequently, high efficiency of 23.26% is obtained for the OBS-treated device, while the control device shows only a companion efficiency of 21.60%.
Keywords: Perovskite solar cells; organic small molecule; grain boundary; hydrogen bonding; coordination bonding
Owing to its excellent photophysical properties, the continuous development of thin films fabrication technology, and the implementations of various device engineering, the perovskite-based solar cells (PSCs) have been developing rapidly with the efficiency rising from the initial 3.8% to a record efficiency of 25.7% [1–5]. However, the commercialization of PSCs still faces significant challenges. On the one hand, there is a large space to attain the Shockley-Queisser limit theoretical prediction in a single-junction device [6–10]. On the other hand, there is a long way from the 20 years required for commercialization for stability [11–15]. These issues depend largely on defects at grain boundaries caused by the film preparation process, such as non-stoichiometric components [16–20], loss of organic components [21,22], migration of halogen ions [23–25], etc. These defects can trap carriers, resulting in charge non-irradiation recombination, which reduces device efficiency and stability [26–30]. Therefore, effective regulation of grain boundaries and passivation of defects is vital for achieving efficient and stable devices.
Chemical doping is a frequently-used strategy for grain boundary adjusting and defects passivation [31–36]. Na+ has a similar size as methylamine ion (MA+) and has been reported to be incorporated into the perovskite lattice to passivate negatively charged defects at grain boundaries by ionic bonding [37]. The introduction of Na+ can enlarge the grain size of perovskite and improve the morphology of thin films. F− can passivate the halogen vacancy in perovskite and bind with uncoordinated Pb2+ through an ionic bond [38]. At the same time, F− can immobilize organic components by hydrogen bonding. Chen et al. introduced poly (bithiophene imide) (PBTI) to improve the open-circuit voltage (VOC) and efficiency of the devices [39]. PBTI can passivate defects at grain boundaries via S binding with Pb2+, and S and N binding with I vacancy. The introduction of PBTI can inhibit ion migration and promote charge transfer. Wen et al. introduced an organic small molecule-1-(4-bromophenyl)-6,7-diphenylimidazo [5,1,2-c, d] indolizine (PDPII), into the precursor solution [40]. The π electrons in PDPII can passivate the defects through π-Pb2+ interactions, which reduces the defect density and inhibits non-radiative recombination, leading to improved efficiency and stability. In summary, it is a meaningful strategy to utilize abundant organic small molecules to passivate defects in perovskite films, which can reduce nonradiative recombination and increase carrier transport [41–43]. Nonetheless, behind defect passivation are various interactions that are responsible for reducing/inhibiting defects and thus improving device performance and stability [44].
Herein, we introduce an organic small molecule, benzo[d]isothiazol-3(2H)-one 1,1-dioxide (OBS), to modulate the grain boundaries and passivate defects of perovskite films. We discovered that the perovskite grains were surrounded by OBS, which passivate defects via coordination and hydrogen bonding interactions. The introduction of OBS reduces both the hole and electron defects density and decreases both the series resistance and charge transfer resistance. As a result, the efficiency improved significantly from 21.60% to 23.26%.
All chemicals were purchased from commercial suppliers and used without further processing. The lead (II) iodide (PbI2, 99.99%, TCI), cesium Iodide (CsI, >99.0%, TCI) and lead (II) bromide (PbBr2, >98%, TCI) were purchased from Beijing Innochem Science & Technology Co., Ltd., China. The methylammonium bromide (MABr), methylamine chloride (MACl) and formamidine iodide (FAI) were purchased from Xi’an Polymer Light Technology Corp., China. Superdehydrated dimethylformamide (DMF, Acros), dimethyl sulfoxide (DMSO, Acros), anisole (Acros), chlorobenzene (CB, Acros), benzo[d]isothiazol-3(2H)-one 1,1-dioxide (OBS, Bide) and isopropanol (IPA, Acros) were purchased from Beijing Innochem Science & Technology Co., Ltd., China.
The fluorine doped tin oxide (FTO) conductive substrate glass that has been laser etched needs to be cleaned with alkaline cleaning solution, deionized water, acetone, and isopropanol in an ultrasonic cleaning machine for several times for 20 min. Then the cleaned substrate was treated in an O2 plasma cleaning machine for 5 min before use. A compact layer of TiO2 was deposited atop FTO by atomic layer deposition and then sintered at 500°C for 30 min in ambient air. SnO2 colloid precursor was synthesized by SnCl4 hydrolysis according to previously published work. SnO2 electron transport layer was obtained by spin-coating on the FTO/TiO2 glass at 3000 rpm for 30 s, and then annealing for 30 min in an air atmosphere at 180°C. The perovskite solution was prepared by mixing 1.53 M PbI2, 1.33 M FAI, 0.5 M MACl, 0.038 M MABr, 0.070 M CsI and 0.038 M PbBr2 in mixed DMF/DMSO solvent system (v:v = 9:1). The precursor solution was firstly spin-coated at 1000 rpm for 10 s, then at 4000 rpm for 30 s, and 300 μL of the anti-solvent anisole was poured slowly at 20 s before the end of the second step. When preparing OBS-treated devices, different concentrations of OBS were pre-dissolved in the perovskite precursor solution. After the anti-solvent treatment, the devices were placed on a hot plate at 100°C annealing for 40 min. Later, 40 μL of 1 mg/mL PTABr (phenyltrimethylammonium bromide) was evenly spread on the surface of the perovskite film and was spin-coated at 4000 rpm for 20 s. The Spiro-OMeTAD solution, containing 72.3 mg Spiro-OMeTAD, 28.8 μL 4-tert-butyl pyridine, and 17.5 μL Li-TFSI solution (520 mg Li-TSFI in 1 mL Acetonitrile) in 1 mL CB, was deposited on the perovskite film at a speed of 4000 rpm for 20 s after the perovskite film cooling to room temperature. Finally, the high-purity gold particles were vapor-deposited onto the surface of the Spiro-OMeTAD at an evaporation rate of 0.1–0.7 Å/s using a vacuum evaporation apparatus, and the thickness of the gold electrode was 80 nm.
Top-view scanning electron microscopy (SEM) images were obtained by field emission scanning electron microscopy (S-4800, Hitachi). XRD spectra were measured by Ultima IV of Rigaku with Cu Kα radiation (1.5406 Å). Steady PL was recorded on a fluorometer (Ocean Optics) excited at 460 nm. Fourier transform infrared (FTIR) spectroscopic characterization was performed by a Nicolet iS50 (Thermo Scientific) under nitrogen purge. Silicon substrates are used for FTIR measurements. X-ray photoelectron spectroscopy (XPS) spectra analysis of films on ITO was performed in the air using a Thermo Fisher ESCALAB 250 Xi. Curve fitting was performed using the Thermo Avantage software. The curves were corrected based on the C1s peak at 284.6 eV. Electrochemical impedance spectroscopy (EIS) curves in the dark. TPV curves were made using a homemade laser pulse oscilloscope. The UV-vis absorbance was measured by UV/Vis spectrometer (Ocean Optics). J-V characteristics of the device (voltage sweep rate 10 mV/30 ms) under AM 1.5G illumination at 100 mW cm–2 using a solar simulator (Sumitomo Heavy Industries Advanced Machinery) in ambient condition. The J-V curves of all devices were measured by masking the devices using a metal mold with a hole area of 0.09 cm2. The light intensity of the solar simulator is calibrated using standard silicon solar cells certified by the National Renewable Energy Laboratory.
Fig. 1 shows the top-view SEM images of perovskite films with/without OBS. Fig. 1a is the top-view SEM images of pristine perovskite films, where the grain boundaries and pinholes can be observed. After the introduction of OBS, the grain boundaries become more visible as shown in Fig. 1b, where OBS dispersed at grain boundaries of perovskite, forming continuous coating along all of the grain boundaries. Additionally, there are no pinholes in OBS-treated perovskite film, which may reduce leakage current and facilitate carrier transport.

Figure 1: Top-view SEM images of perovskite films without (a) and with OBS (b)
Fig. 2 shows the XRD patterns that correspond to the SEM images in Fig. 1. We can observe the same diffraction peaks in Fig. 2, indicating that the addition of OBS has no effect on the phase composition of perovskite film and does not change the structure of the perovskite. It is speculated that most OBS molecules are distributed in grain boundaries or attached to the film surface. The intensity of the main diffraction peaks (at ~14° and ~28°) increases with the addition of OBS, which indicates that the addition of OBS can enhance the crystallinity of the perovskite film and is expected to improve the photovoltaic performance of the device.

Figure 2: XRD patterns of perovskite films without/with OBS
To observe the distribution of OBS in perovskite, we conducted the PL spectra of perovskite films with/without OBS on glass obtained from the perovskite side and the glass side as shown in Fig. 3, respectively. We can see that there is a blue shift after the introduction of OBS, and the PL peaks obtained from both sides of the pristine perovskite film and the OBS-treated film remain basically unchanged. From the PL measurements, we speculate that the OBS is distributed at the grain boundaries throughout the perovskite film, rather than on the surface.

Figure 3: PL spectra of perovskite films with/without OBS on glass obtained from both perovskite side and glass side
The interactions between OBS and perovskite components were then to be investigated. FTIR measurements were firstly conducted and the results are shown in Fig. 4. As shown in Fig. 4a, the peak around 3348 cm−1 in FAI belongs to the N-H stretching vibration and it shifts to 3360 cm−1 in FAI+OBS film, indicating the formation of hydrogen bonding between FAI and OBS. In OBS film, the peak around 1337 cm−1 belongs to the S=O absorption, and it moves to 1317 cm−1 in PbI2+OBS film as shown in Fig. 4b. The C=O absorption peak around 1724 cm−1 in OBS remains unchanged in PbI2+OBS film, which is 1724 cm−1 (Fig. 4c). This demonstrates that the coordination interaction between OBS and PbI2 is S=O⋯Pb, not C=O.

Figure 4: FTIR spectrum of (a) FAI films with/without OBS, (b) and (c) PbI2 films with/without OBS
The molecular interactions between perovskite and OBS were further investigated by XPS measurements. The binding energies of 400.35 eV in pristine perovskite belonging to N 1s orbit shift to 400.40 eV after the introduction of OBS (Fig. 5a). As shown in Fig. 5b, the binding energies of 143.0 and 138.15 eV of the pristine perovskite film belong to Pb 4f5/2 and Pb 4f7/2 orbits, respectively. They shift to 143.15 and 138.30 eV in perovskite film with OBS, respectively. We also observed that the binding energy of the O 1s peak shifts from 532.45 to 532.35 eV after the addition of OBS (Fig. 5c). The XPS measurements show consistent results as FTIR.

Figure 5: XPS patterns of perovskite films with/without OBS
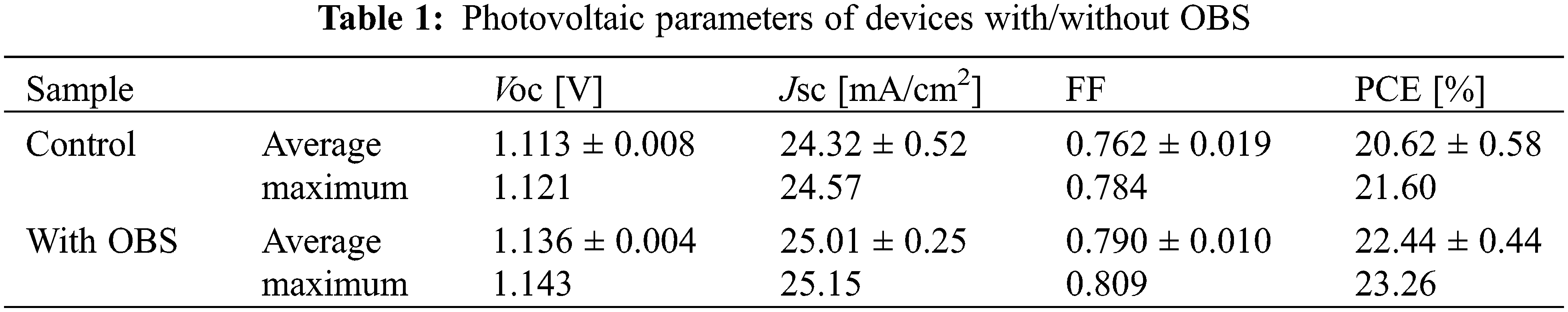
Then we assembled devices with the configuration of FTO/compact TiO2 (c-TiO2)/SnO2/Perovskite/Spiro-OMeTAD/Au to assess the effect of OBS on devices’ performance. The device’s performance was used to confirm the optimal amount of OBS, and the results are shown in Fig. 6a, where the optimal OBS doping concentration is 0.6 mg/ml. Fig. 6a exhibits the current density–voltage (J–V) curves of devices with different OBS concentrations. The control device shows an open-circuit voltage (VOC) of 1.121 V, a short-circuit current density (JSC) of 24.57 mA cm−2, a fill factor (FF) of 0.784, and a PCE of 21.60%. The OBS-treated companion device shows a VOC of 1.143 V, a JSC of 25.15 mA cm−2, an FF of 0.809, and a PCE of 23.26%. The structure of additive OBS is shown in Fig. 6b.

Figure 6: (a) J–V characteristics of devices based on different concentrations of OBS (b) Chemical structure of OBS
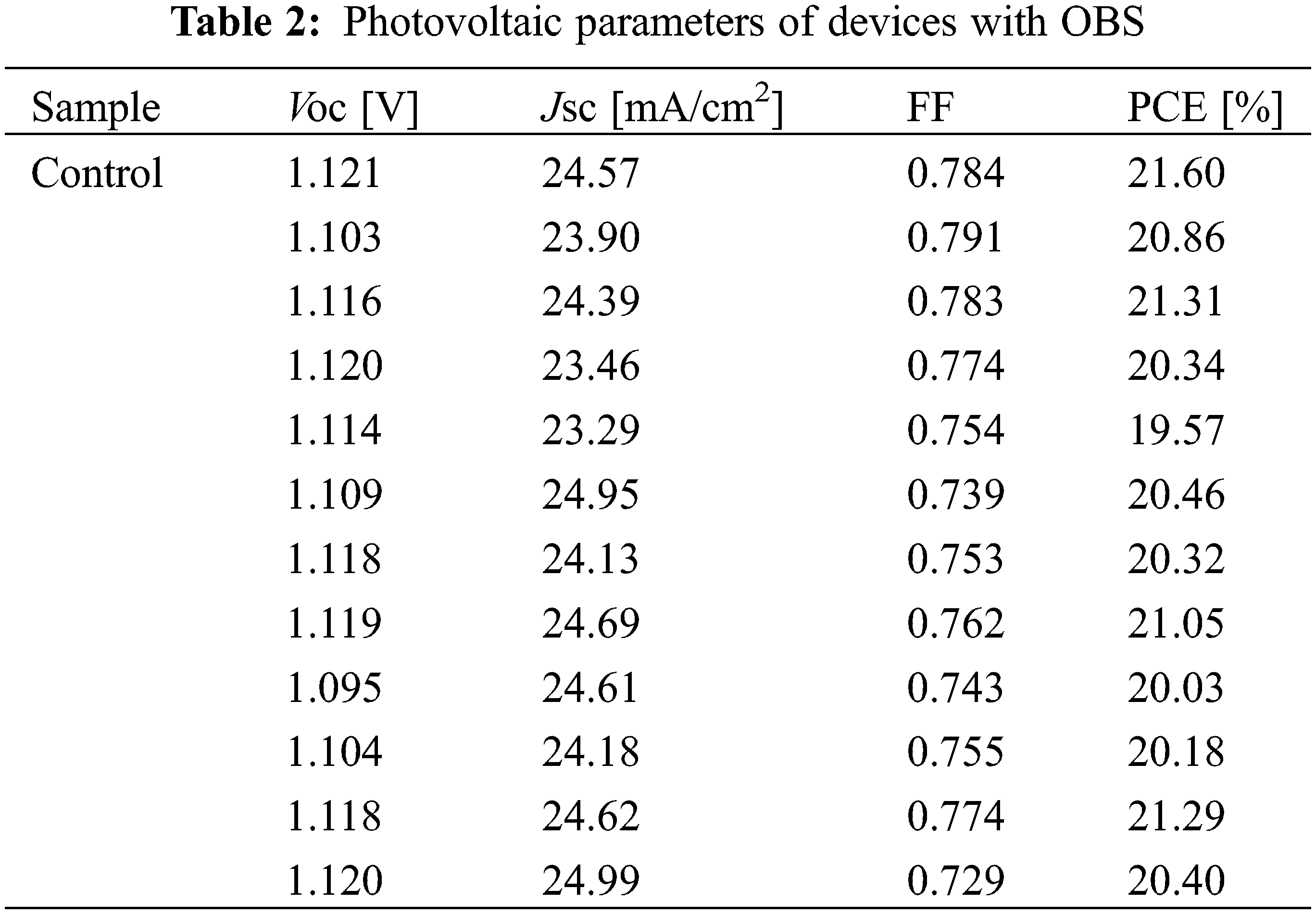
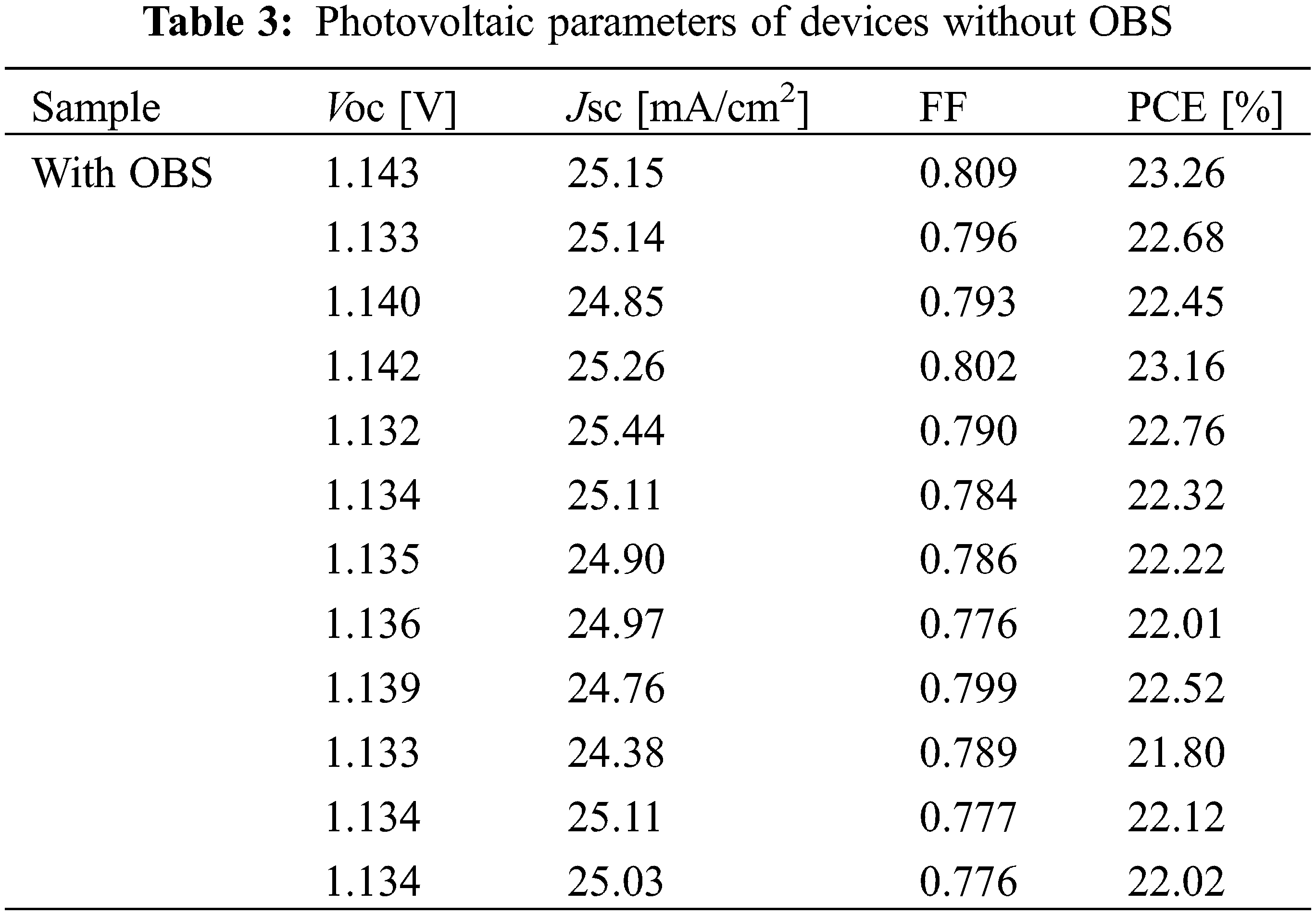
Fig. 7 and Table 1 show the performance statistics of the devices with/without OBS treatment. All the average performance parameters of OBS-treated devices show improvement compared with those of control devices, which is attributed to the effective passivation of OBS. The performance parameters for devices with/without OBS treatment are summarized in Tables 2 and 3, separately.

Figure 7: Performance statistics of the parameters of devices based on perovskite films with/without OBS
The optical absorption of perovskite films with/without OBS was investigated by UV-vis absorption spectroscopy. As shown in Fig. 8a, the light absorption intensity of the samples is significantly enhanced after the addition of OBS, which may be attributed to the high crystallinity and low-density grain boundaries in that film. The Urbach energy (Eu) can be obtained according to the formula [45]:
where α is the absorption coefficient, α0 is a constant, and hυ is the photon energy. It can be seen from Fig. 8b that Eu derived from the absorption tails decreases from 30.2 to 23.5 meV after OBS doping, indicating the low defect density in OBS-treated film.

Figure 8: (a) UV–vis spectra of perovskite thin films with/without OBS. (b) The estimation of the Urbach energy for both perovskite films
Carrier dynamics can be studied by steady-state photoluminescence (PL) spectroscopy and transient photovoltage (TPV) spectroscopy. It can be seen from Fig. 9 that the OBS-treated perovskite film exhibits stronger peak intensities than the pristine perovskite film, and the OBS-treated device has a longer photovoltage decay time. The results show that the addition of OBS can improve the crystallinity, and the generated carriers have a longer lifetime, realizing the separation of electrons and holes in the device, and effectively suppressing the non-radiative charge recombination.

Figure 9: (a) PL spectra of perovskite films with/without OBS. (b) TPV spectra of perovskite devices with/without OBS
To investigate the trap density (ntrap) in PSCs with/without OBS doping, space charge limited current (SCLC) measurements were performed on electron-only and hole-only devices under dark conditions. The J–V characteristics of the device consist of two regions: the ohmic region (low bias) and the trap fill limiting region (TFL). The intersection of the tangent lines of two different regions is the defect filling limit voltage (VTFL). As shown in Figs. 10a and 10b, after doping with OBS, the VTFLs of electron-only device and hole-only device decrease from 0.86 to 0.76 V and from 0.25 to 0.20 V, respectively. The trap states are linearly related to the onset voltage of the VTFL, and the defect density nt can be calculated by the equation below [46,47]:
where q is the fundamental charge, L is the thickness of the perovskite film, and εr is the relative permittivity of the perovskite film (29 in this system). The calculated nt of the electron-only device decreases from 1.72 × 1016 cm−3 to 1.52 × 1016 cm−3, while the nt of the hole-only device decreases from 5.01 × 1015 cm−3 to 4.01 × 1015 cm−3. Therefore, the introduction of OBS significantly reduces both hole and electron trap densities, which is responsible for the improvement of device performance.

Figure 10: Dark current–voltage responses of electron-only (a) and hole-only (b) Devices based on perovskite devices with/without OBS
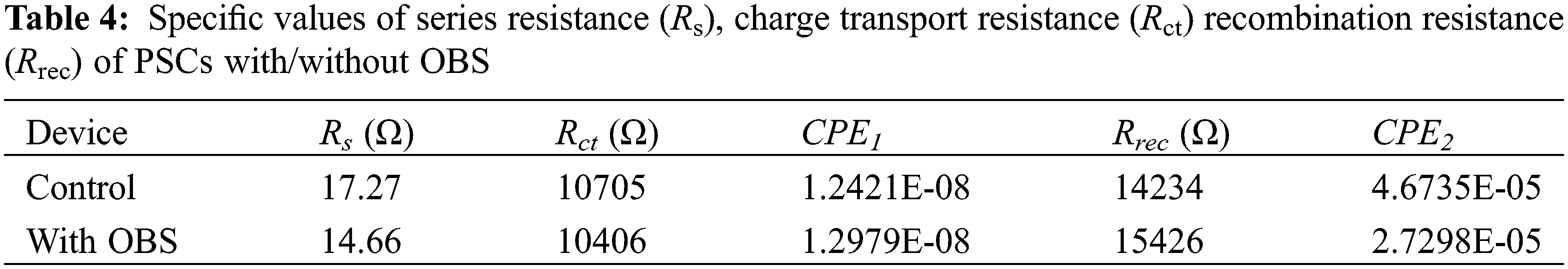
As shown in Fig. 11, the charge recombination and transfer of the devices with/without OBS were evaluated by EIS [48]. The EIS data can be fitted with the equivalent circuit (in the inset in Fig. 11) within a certain error range, and the results are shown in Table 4. Where Rs is the series resistance, and Rct and Rrec are the charge transfer resistance and recombination resistance, respectively. The Nyquist plots of the devices with/without doping show two semicircular, high-frequency arcing and low-frequency arcing, which are related to charge transfer and charge recombination, respectively. It can be seen from Table 4 that after doping OBS, both the Rs and Rct show a decreasing trend, while the Rrec increases, indicating that the charge transfer process is effectively enhanced, and the charge recombination loss is suppressed.

Figure 11: Nyquist plots of the devices based on perovskite films with/without OBS
As shown in Fig. 12a, the OBS-treated device shows lower dark current. It can be attributed to the reduction of pinholes, indicating an enhanced charge transfer capability at the interface. The external quantum efficiency (EQE) spectrum of OBS-treated device is shown in Fig. 12b. The integrated JSC is 24.08 mA/cm2 for the OBS-treated device, which is almost consistent with the J-V result, indicating the high reliability of the OBS-treated devices.
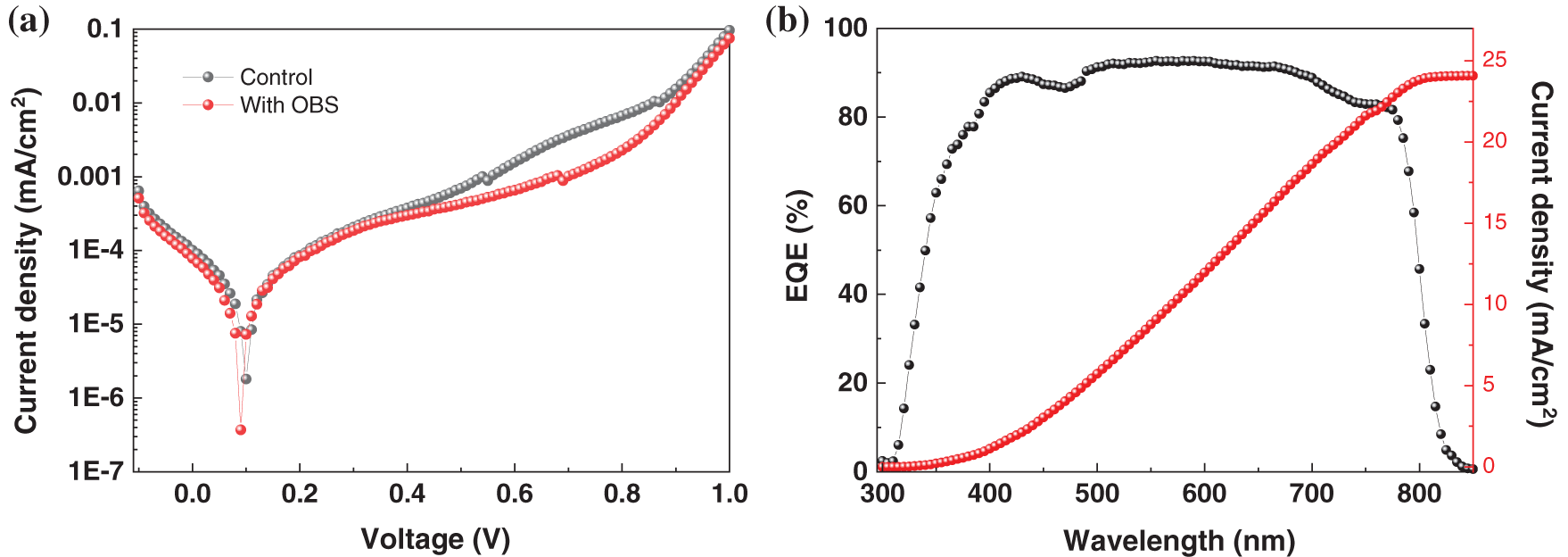
Figure 12: (a) Dark J–V curves (b) EQE spectrum of the devices based on perovskite films with/without OBS
Fig. 13 shows the stability results of devices with/without OBS. Both unencapsulated devises were placed at room temperature in the dark with the relative humidity 20 ± 5%. As we can see from Fig. 13a that the PCE of the control device keeps less than 70% after 600 h storage. However, the OBS-treated device still maintains more than 80% of its initial PCE after 700 h storage at the same condition. Fig. 13b is the thermal stability test of unencapsulated devices in a glovebox at 20 ± 5% relative humidity. The PCE of the control device dropped below 70% at 500 h, while it still maintained about 90% of the initial PCE after OBS treatment. The enhanced stability can be attributed to the effect of grain boundary passivation by OBS.

Figure 13: (a) Storage stability (b) Thermal stability of the devices based on perovskite films with/without OBS
To sum up, we have demonstrated successfully grain-boundary engineering using an organic small molecule (OBS) to regulate perovskite grains and passivate defects of perovskite films via hydrogen bonding and coordination interactions between OBS and perovskite. On the one hand, it reduced both electron and hole defects density. On the other hand, it facilitated charge transfer. As a result, an OBS-treated device achieved a significantly improved PCE of 23.26% compared with that of 21.60% for the control device, along with improved storage stability. This work provides an effective chemical strategy to improve the performance of PSCs.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Mishra, S., Ghosh, S., Singh, T. (2020). Progress in materials development for flexible perovskite solar cells and future prospects. ChemSusChem, 14(2), 512–538. DOI 10.1002/cssc.202002095. [Google Scholar] [CrossRef]
2. He, R., Ren, S., Chen, C., Yi, Z., Luo, Y. et al. (2021). Wide-bandgap organic-inorganic hybrid and all-inorganic perovskite solar cells and their application in all-perovskite tandem solar cells. Energy & Environmental Science, 14(11), 5723–5759. DOI 10.1039/D1EE01562A. [Google Scholar] [CrossRef]
3. Cao, Q., Li, Y., Zhang, H., Yang, J., Han, J. et al. (2021). Efficient and stable inverted perovskite solar cells with very high fill factors via incorporation of star-shaped polymer. Science Advances, 7(28), eabg0633. DOI 10.1126/sciadv.abg0633. [Google Scholar] [CrossRef]
4. Wei, Y., Zhao, Y., Liu, C., Wang, Z., Jiang, F. et al. (2021). Constructing all-inorganic perovskite/fluoride nanocomposites for efficient and ultra-stable perovskite solar cells. Advanced Functional Materials, 31(47), 2106386. DOI 10.1002/adfm.202106386. [Google Scholar] [CrossRef]
5. Chao, L., Niu, T., Gao, W., Ran, C., Song, L. et al. (2021). Solvent engineering of the precursor solution toward large-area production of perovskite solar cells. Advanced Materials, 33(14), 2005410. DOI 10.1002/adma.202005410. [Google Scholar] [CrossRef]
6. Luo, D., Su, R., Zhang, W., Gong, Q., Zhu, R. (2020). Minimizing non-radiative recombination losses in perovskite solar cells. Nature Reviews Materials, 5(1), 44–60. DOI 10.1038/s41578-019-0151-y. [Google Scholar] [CrossRef]
7. Zhu, Z., Mao, K., Xu, J. (2021). Perovskite tandem solar cells with improved efficiency and stability. Journal of Energy Chemistry, 58, 219–232. DOI 10.1016/j.jechem.2020.09.022. [Google Scholar] [CrossRef]
8. Luo, X., Wu, T., Wang, Y., Lin, X., Su, H. et al. (2021). Progress of all-perovskite tandem solar cells: The role of narrow-bandgap absorbers. Science China Chemistry, 64(2), 218–227. DOI 10.1007/s11426-020-9870-4. [Google Scholar] [CrossRef]
9. Rajagopal, A., Yang, Z., Jo, S. B., Braly, I. L., Liang, P. et al. (2017). Highly efficient perovskite-perovskite tandem solar cells reaching 80% of the theoretical limit in photovoltage. Advanced Materials, 29(34), 1702140. DOI 10.1002/adma.201702140. [Google Scholar] [CrossRef]
10. Peng, J., Kremer, F., Walter, D., Wu, Y., Ji, Y. et al. (2022). Centimetre-scale perovskite solar cells with fill factors of more than 86 percent. Nature, 601(7894), 573–578. DOI 10.1038/s41586-021-04216-5. [Google Scholar] [CrossRef]
11. Hu, Y., Song, W., Wang, X., Shi, X., Jia, X. et al. (2022). A holistic sunscreen interface strategy to effectively improve the performance of perovskite solar cells and prevent lead leakage. Chemical Engineering Journal, 433, 134566. DOI 10.1016/j.cej.2022.134566. [Google Scholar] [CrossRef]
12. Dong, C., Chen, J., Chen, C. H., Shi, Y. R., Yang, W. F. et al. (2022). Annealing-free perovskite films by EDOT-assisted anti-solvent strategy for flexible indoor and outdoor photovoltaics. Nano Energy, 94, 106866. DOI 10.1016/j.nanoen.2021.106866. [Google Scholar] [CrossRef]
13. Chen, W., Han, B., Hu, Q., Gu, M., Zhu, Y. et al. (2021). Interfacial stabilization for inverted perovskite solar cells with long-term stability. Science Bulletin, 66(10), 991–1002. DOI 10.1016/j.scib.2021.02.029. [Google Scholar] [CrossRef]
14. Zhang, H., Li, K., Sun, M., Wang, F., Wang, H. et al. (2021). Design of superhydrophobic surfaces for stable perovskite solar cells with reducing lead leakage. Advanced Energy Materials, 11(41), 2102281. DOI 10.1002/aenm.202102281. [Google Scholar] [CrossRef]
15. Dong, Y., Zhu, H., Cao, X., Han, Y. P., Zhang, H. Y. et al. (2020). Simple 9,10-dihydrophenanthrene based hole-transporting materials for efficient perovskite solar cells. Chemical Engineering Journal, 402, 126298. DOI 10.1016/j.cej.2020.126298. [Google Scholar] [CrossRef]
16. Jia, P., Qin, L., Zhao, D., Tang, Y., Song, B. et al. (2021). The trapped charges at grain boundaries in perovskite solar cells. Advanced Functional Materials, 31(49), 2107125. DOI 10.1002/adfm.202107125. [Google Scholar] [CrossRef]
17. Xi, J., Xi, K., Sadhanala, A., Zhang, K. H. L., Li, G. et al. (2019). Chemical sintering reduced grain boundary defects for stable planar perovskite solar cells. Nano Energy, 56, 741–750. DOI 10.1016/j.nanoen.2018.11.021. [Google Scholar] [CrossRef]
18. Xiao, K., Han, Q., Gao, Y., Gu, S., Luo, X. et al. (2021). Simultaneously enhanced moisture tolerance and defect passivation of perovskite solar cells with cross-linked grain encapsulation. Journal of Energy Chemistry, 56, 455–462. DOI 10.1016/j.jechem.2020.08.020. [Google Scholar] [CrossRef]
19. Zhou, X., Qi, W., Li, J., Cheng, J., Li, Y. et al. (2020). Toward efficient and stable perovskite solar cells: Choosing appropriate passivator to specific defects. Solar RRL, 4(10), 2000308. DOI 10.1002/solr.202000308. [Google Scholar] [CrossRef]
20. Yang, Y., Wu, J., Wang, X., Guo, Q., Liu, X. et al. (2019). Suppressing vacancy defects and grain boundaries via ostwald ripening for high-performance and stable perovskite solar cells. Advanced Materials, 32(7), 1904347. DOI 10.1002/adma.201904347. [Google Scholar] [CrossRef]
21. Zhang, J., Yu, H. (2021). Multifunctional dopamine-assisted preparation of efficient and stable perovskite solar cells. Journal of Energy Chemistry, 54, 291–300. DOI 10.1016/j.jechem.2020.05.061. [Google Scholar] [CrossRef]
22. Pham, H. D., Yang, T. C. J., Jain, S. M., Wilson, G. J., Sonar, P. (2020). Development of dopant-free organic hole transporting materials for perovskite solar cells. Advanced Energy Materials, 10(13), 1903326. DOI 10.1002/aenm.201903326. [Google Scholar] [CrossRef]
23. Zhang, Z., Jiang, J., Liu, X., Wang, X., Wang, L. et al. (2021). Surface-anchored acetylcholine regulates band-edge states and suppresses ion migration in a 21%-efficient quadruple-cation perovskite solar cell. Small, 18(6), 2105184. DOI 10.1002/smll.202105184. [Google Scholar] [CrossRef]
24. Zai, H., Ma, Y., Chen, Q., Zhou, H. (2021). Ion migration in halide perovskite solar cells: Mechanism, characterization, impact and suppression. Journal of Energy Chemistry, 63, 528–549. DOI 10.1016/j.jechem.2021.08.006. [Google Scholar] [CrossRef]
25. Tan, S., Yavuz, I., Marco, N. D., Huang, T., Lee, S. J. et al. (2020). Steric impediment of ion migration contributes to improved operational stability of perovskite solar cells. Advanced Materials, 32(11), 1906995. DOI 10.1002/adma.201906995. [Google Scholar] [CrossRef]
26. Yun, S. C., Ma, S., Kwon, H. C., Kim, K., Jang, J. et al. (2019). Amino acid salt-driven planar hybrid perovskite solar cells with enhanced humidity stability. Nano Energy, 59, 481–491. DOI 10.1016/j.nanoen.2019.02.064. [Google Scholar] [CrossRef]
27. Zhang, Y., Gao, L., Wei, X., Zhao, W., Wang, W. et al. (2021). Spectroscopic perception of trap states on the performance of methylammonium and formamidinium lead iodide perovskite solar cells. Advanced Materials, 33(38), 2102241. DOI 10.1002/adma.202102241. [Google Scholar] [CrossRef]
28. Hao, M. Y., Wang, H. Y., Wang, Y., Qin, Y., Zhang, J. P. et al. (2020). Effect of energetic distribution of trap states on fill factor in perovskite solar cells. Journal of Power Sources, 479(15), 229077. DOI 10.1016/j.jpowsour.2020.229077. [Google Scholar] [CrossRef]
29. He, J., Chu, Y., Sun, Y., Zhang, R., Li, J. et al. (2021). Beyond the limit of goldschmidt tolerance factor: Crystal surface engineering to boost the α-phase stability of formamidinium-only hybrid inorganic-organic perovskites. Solar RRL, 5(8), 2100188. DOI 10.1002/solr.202100188. [Google Scholar] [CrossRef]
30. Yu, S., Liu, H., Wang, S., Zhu, H., Dong, X. et al. (2021). Hydrazinium cation mixed FAPbI3-based perovskite with 1D/3D hybrid dimension structure for efficient and stable solar cells. Chemical Engineering Journal, 403(1), 125724. DOI 10.1016/j.cej.2020.125724. [Google Scholar] [CrossRef]
31. Chen, C., Li, F., Zhu, L., Shen, Z., Weng, Y. et al. (2020). Efficient and stable perovskite solar cells thanks to dual functions of oleyl amine-coated PbSO4(PbO)4 quantum dots: Defect passivation and moisture/oxygen blocking. Nano Energy, 68, 104313. DOI 10.1016/j.nanoen.2019.104313. [Google Scholar] [CrossRef]
32. Du, J., Duan, J., Yang, X., Zhou, Q., Duan, Y. et al. (2021). Reducing defect of inorganic perovskite film by sulphur-containing lewis base for robust photodetectors. Journal of Energy Chemistry, 61, 163–169. DOI 10.1016/j.jechem.2021.02.004. [Google Scholar] [CrossRef]
33. Guo, Y., Lei, H., Wang, C., Ma, J., Chen, C. et al. (2019). Reconfiguration of interfacial and bulk energy band structure for high-performance organic and thermal-stability enhanced perovskite solar cells. Solar RRL, 4(4), 1900482. DOI 10.1002/solr.201900482. [Google Scholar] [CrossRef]
34. Li, T., Wang, S., Yang, J., Pu, X., Gao, B. et al. (2021). Multiple functional groups synergistically improve the performance of inverted planar perovskite solar cells. Nano Energy, 82, 105742. DOI 10.1016/j.nanoen.2021.105742. [Google Scholar] [CrossRef]
35. Zhong, M., Chai, L., Wang, Y., Di, J. (2021). Enhanced efficiency and stability of perovskite solar cell by adding polymer mixture in perovskite photoactive layer. Journal of Alloys and Compounds, 864(25), 158793. DOI 10.1016/j.jallcom.2021.158793. [Google Scholar] [CrossRef]
36. Li, S., Zhu, L., Kan, Z., Hua, Y., Wu, F. et al. (2020). A multifunctional additive of scandium trifluoromethanesulfonate to achieve efficient inverted perovskite solar cells with a high fill factor of 83.80%. Journal of Materials Chemistry A, 8(37), 19555–19560. DOI 10.1039/D0TA07567A. [Google Scholar] [CrossRef]
37. Bi, C., Zheng, X., Chen, B., Wei, H., Huang, J. et al. (2017). Spontaneous passivation of hybrid perovskite by sodium ions from glass substrates: Mysterious enhancement of device efficiency revealed. ACS Energy Letters, 2(6), 1400–1406. DOI 10.1021/acsenergylett.7b00356. [Google Scholar] [CrossRef]
38. Li, N., Tao, S., Chen, Y., Niu, X., Onwudinanti, C. K. et al. (2019). Cation and anion immobilization through chemical bonding enhancement with fluorides for stable halide perovskite solar cells. Nature Energy, 4(5), 408–415. DOI 10.1038/s41560-019-0382-6. [Google Scholar] [CrossRef]
39. Wu, Y., Tu, B., Liu, F., Chen, R., Woo, H. Y. et al. (2019). Conjugated polymer-assisted grain boundary passivation for efficient inverted planar perovskite solar cells. Advanced Functional Materials, 29, 1808855. DOI 10.1002/adfm.201808855. [Google Scholar] [CrossRef]
40. Wen, L., Rao, Y., Zhu, M., Li, R., Zhan, J. et al. (2021). Reducing defects density and enhancing hole extraction for efficient perovskite solar cells enabled by π-Pb2+ interactions. Angewandte Chemie International Edition, 60(32), 17356–17361. DOI 10.1002/anie.202102096. [Google Scholar] [CrossRef]
41. Sun, Y., Zhang, J., Yu, H., Huang, C., Huang, J. (2022). Several triazine-based small molecules assisted in the preparation of high-performance and stable perovskite solar cells by trap passivation and heterojunction engineering. ACS Applied Materials & Interfaces, 14(5), 6625–6637. DOI 10.1021/acsami.1c21081. [Google Scholar] [CrossRef]
42. Wang, R., Sun, T., Wu, T., Zhu, Z., Shao, Y. J. et al. (2022). Hydrophobic π-conjugated organic small molecule as a multi-functional interface material enables efficient and stable perovskite solar cells. Chemical Engineering Journal, 430, 133065. DOI 10.1016/j.cej.2021.133065. [Google Scholar] [CrossRef]
43. Ma, Y., Zhang, S., Yi, Y., Zhang, L., Hu, R. et al. (2022). Deep level defects passivated by small molecules for the enhanced efficiency and stability of inverted perovskite solar cells. Journal of Materials Chemistry C, 10(15), 5922–5928. DOI 10.1039/D2TC00283C. [Google Scholar] [CrossRef]
44. Zhu, M., Li, C., Li, B., Zhang, J., Sun, Y. et al. (2020). Interaction engineering in organic-inorganic hybrid perovskite solar cells. Materials Horizons, 7(9), 2208–2236. DOI 10.1039/d0mh00745e. [Google Scholar] [CrossRef]
45. Subedi, B., Li, C., Junda, M. M., Song, Z., Yan, Y. et al. (2020). Effects of intrinsic and atmospherically induced defects in narrow bandgap (FASnI3)x(MAPbI3)1-x perovskite films and solar cells. The Journal of Chemical Physics, 152, 064705. DOI 10.1063/1.5126867. [Google Scholar] [CrossRef]
46. Kang, D. H., Kim, S. Y., Lee, J. W., Park, N. G. (2021). Efficient surface passivation of perovskite films by a post-treatment method with a minimal dose. Journal of Materials Chemistry A, 9(6), 3441–3450. DOI 10.1039/d0ta10581c. [Google Scholar] [CrossRef]
47. Zhou, Z., Qiang, Z., Sakamaki, T., Takei, I., Shang, R. (2019). Organic/inorganic hybrid p-type semiconductor doping affords hole transporting layer free thin-film perovskite solar cells with high stability. ACS Applied Materials & Interfaces, 11(25), 22603–22611. DOI 10.1021/acsami.9b06513. [Google Scholar] [CrossRef]
48. Liu, B., Bi, H., He, D., Bai, L., Wang, W. et al. (2021). Interfacial defect passivation and stress release via multi-active-site ligand anchoring enables efficient and stable methylammonium-free perovskite solar cells. ACS Energy Letters, 6(7), 2526–2538. DOI 10.1021/acsenergylett.1c00794. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |