 Open Access
Open Access
ARTICLE
Study on Coconut Shell Activated Carbon Temperature Swing Adsorption of Benzene and Formaldehyde
1
Henan Province Key Laboratory of Water Pollution Control and Rehabilitation Technology, Henan University of Urban
Construction, Pingdingshan, 467036, China
2
College of Resources and Environment, University of Chinese Academy of Sciences, Beijing, 100085, China
3
College of Basic Medical Sciences, Xinxiang Medical University, Xinxiang, 453003, China
* Corresponding Authors: Zhiguang Yang. Email: ; Linlin Shan. Email:
(This article belongs to the Special Issue: Biochar Based Materials for a Green Future)
Journal of Renewable Materials 2022, 10(12), 3573-3585. https://doi.org/10.32604/jrm.2022.022031
Received 17 February 2022; Accepted 15 April 2022; Issue published 14 July 2022
Abstract
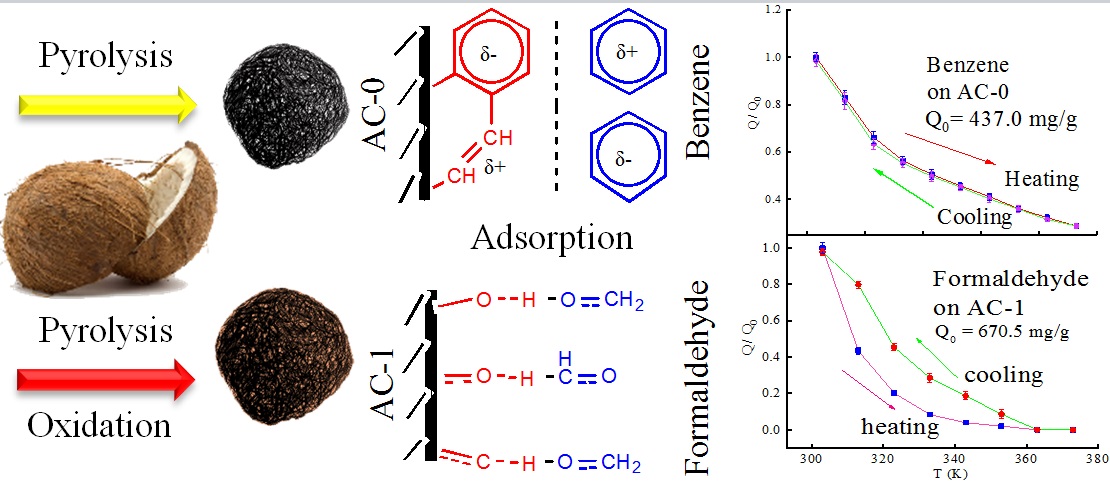
Adsorption can be used to recover effectively the volatile organic gases (VOCs) in the exhaust gas from factories through using an appropriate adsorption bed. Due to form a physical or chemical bond, adsorption occurs between the porous solid medium and the liquid or gas multi-component fluid mixture. The regeneration capacity of the adsorbent is as important as the adsorption capacity and it determines the economics of the adsorption system. The regeneration of adsorbent can be realized through changing the pressure or temperature of the system. Here, activated carbon samples from coconut shell were prepared and characterized. Benzene or formaldehyde in the mixed air was used as the adsorption object, and the adsorption experiment was carried out in a U-shaped bed. Discussed how adsorption was affected by activated carbon type, adsorbate and temperature. The results show that oxidation modified activated carbon can increase the adsorption effect of formaldehyde, but will reduce the adsorption effect of benzene, because their adsorption mechanism is different. At 30°C, the saturated adsorption capacity of AC-0 for benzene is 437.0 mg/g, and that of AC-1 for formaldehyde is 670.5 mg/g. In the experimental range, it is found that the adsorption capacity increases with the decrease of temperature, and their changes are very consistent with the fitted ExpDecay1 function.Graphic Abstract
Keywords
Cite This Article
 Copyright © 2022 The Author(s). Published by Tech Science Press.
Copyright © 2022 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools