

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2022.021401
ARTICLE
Effect of Active Zeolite in the Pyrolysis of Polypropylene and Low Density Polyethylene Types of Plastic Waste
1Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Negeri Malang, Malang, 65145, Indonesia
2Department of Chemistry, Faculty of Science and Technology, Universiti Kebangsaan Malaysia UKM Bangi, Selangor, 43600, Malaysia
3Graduate School of Engineering and Science, Shibaura Institute of Technology, Saitama, 3378570, Japan
*Corresponding Author: Aman Santoso. Email: aman.santoso.fmipa@um.ac.id
Received: 12 January 2022; Accepted: 29 March 2022
Abstract: Plastic is a basic need for humans, but it has also caused big problems for the environment. Then, the purpose of this study was to determine the effect of the type of plastic and the addition of a zeolite catalyst on the oil yield from the pyrolysis of plastic waste. The research stages were natural zeolite activation, pyrolysis reactor settings, pyrolysis of plastic waste (PP and LDPE types), and characterization. The results showed that the used natural zeolite had a mordenite phase and activated natural zeolite had a higher Si/Al ratio than the inactivated one. The addition of a zeolite catalyst had an effect on the produced yield. The yields of oil from plastic waste pyrolysis with zeolite catalyst for PP and LDPE plastics were about 75.9 and 76.9 w/w, respectively. The results of the GC-MS analysis showed that the compounds of the pyrolysis oil were thought to be from the alkanes, cycloalkanes, alkenes, carboxylic acids with aromatic rings, and ketones. The results of the GC-MS test showed that the uncatalyzed pyrolysis product consists of compounds with a range of C5-C11 carbon chains. Meanwhile, the ranges of pyrolysis products with active zeolite catalyst were C6-C24 carbon chains.
Keywords: Zeolite; catalyst; plastic; pyrolysis; reactor
Nomenclature
| PP | Polypropylene |
| LDPE | Low Density Polyethylene |
| HDPE | High Density Polyethylene |
Plastic waste is a threat to the environment [1]. Plastics as carbon compounds can be converted into fuel substitutes for petroleum, such as pyrolysis, pyrolysis-gasification, solvolysis, hydrocracking, hydrogenolysis and the light/electrochemical-induced processes [2]. The pyrolysis process is a process of decomposition of materials by heating at high temperatures without oxygen to produce liquid, gas, and charcoal products [3,4]. Based on several previous studies concluded that the quality of the plastic waste pyrolysis oil is below kerosene and above the quality of diesel oil. Pyrolysis oil or bio-oil has the advantage of low water content, so that the calorific value is high and it does not cause corrosion [5].
The addition of a catalyst in pyrolysis can optimize the pyrolysis process. Zeolite is one of the catalysts commonly used in the pyrolysis process. It has a hollow structure that can filter ions, molecules, or atoms selectively [6]. Modified natural zeolite increases the surface area and acidity to support the work of catalytic activity of natural zeolite. Pyrolysis of HDPE plastic waste with and without natural zeolite catalyst showed that pyrolysis of HDPE plastic waste producing Gasoline fractionated oil and Kerosene fractionated oil, respectively [7].
In this study, research was conducted on how the effect of activated natural zeolite catalysts on bio-oil resulting from the pyrolysis of various plastics. Therefore, the purpose of this study was to determine the character of natural zeolite before and after activation, and determine the effect of activated natural zeolite catalyst on bio-oil resulting from plastic pyrolysis with activated natural zeolite catalyst. The benefit of this research is superior bio-oil can be made from plastic waste that is abundant in Indonesia through the pyrolysis process with natural zeolite catalysts.
Pyrolysis is a cracking process of polymer chains into simpler compounds through heating or combustion in the absence of oxygen. The pyrolysis process can take place at a minimum temperature of 200°C. The combustion process in pyrolysis encourages oxidation so that most of the decomposed carbon molecules become charcoal. Generally, the pyrolysis products consist of three, namely gas (H2, CO, CO2, H2O and CH4), tar (bio-oil), and charcoal (char). The results of the pyrolysis process are highly dependent on the operating conditions. The pyrolysis process is influenced by several things, namely the type of fuel, temperature, pressure, heating rate, and reaction time [1,8]. Pyrolysis with the added catalyst can support to cracking of plastic into oil at a lower temperature than without a catalyst. The catalytic process in plastic pyrolysis with an acid catalyst involves cracking, oligomerization, cyclization, aromatization, and isomerization reactions [9].
Catalysts are involved in chemical bonds with reactants in a reaction and will produce products. Zeolite has a porous structure that can be used as a catalyst and filter ions or molecules. Zeolite activation can be performed physically or chemically. Physical activation of zeolite is carried out by heating at high temperatures, so that the water molecules contained in the pores of the zeolite evaporate [10]. The chemical activation of zeolite is carried out by dissolving the cations that close the zeolite pores with an acid or alkaline solution, so that the zeolite is more porous [3,11].
Generally, zeolites are used as catalysts in various natural catalytic reactions. This is because zeolite has a high hydrocarbon acid catalytic activity so it is often used in the hydrocarbon cracking process. In the pyrolysis process, the ratio of SiO2/Al2O3 determines the effectiveness of the zeolite catalyst [12]. The zeolite used as a catalyst can be used to reduce oxygen levels in bio-oil and help the formation of aromatic bonds. Zeolite can also help crack long hydrocarbon chains into short hydrocarbons due to the presence of acidic sites in the zeolite. Decomposition through the use of catalysts is more attractive than thermal degradation because the process is faster and requires lower temperatures to reduce energy use [13,14].
2.1 Natural Zeolite Preparation and Activation
Zeolite was ground and sieved through a 60 mesh sieve. Then it soaked in distilled water and stirred constantly for one hour at room temperature. Furthermore, the clean precipitate was filtered and dried in an oven at a temperature of 100°C for 1 h. The physical activation of zeolite was carried out by calcination in a furnace at a temperature of 500°C for 4 h. The activated and inactivated natural zeolite were identified crystallization by X-Ray Diffraction (XRD). The XRD patterns of both that zeolites were recorded on a PANalytical X’Pert Pro diffractometer (PANalytical B.V., the Netherlands). The XRD conditions were CuKα1 radiation, 40 keV and 30 mA 2ϴ range 10°–50°, step 0.02, counting time of 300 s, using an X’Celator Detector. The quantitative analysis of the XRD patterns were analyzed using Origin software to determine the crystallization and type of natural zeolite phase using Origin and Maud software, respectively. The zeolite samples were then prepared with double-sided copper tape and sputter coated with gold/palladium (Au/Pd).
2.2 Pyrolysis of PP and LDPE Plastic
PP plastic was weighed about 1 kilogram and put in a pyrolysis reactor. The pyrolysis process was then carried out at a temperature of 500°C until finish (there was no product formed). The pyrolysis process was repeated 5 times with the following variations: (1) Pyrolysis of PP plastic with the addition of 10% activated zeolite catalyst; (2) PP, LDPE, HDPE plastics pyrolysis without catalyst; (3) Pyrolysis of LDPE plastic with the addition of 10% activated zeolite catalyst; (4) Pyrolysis of HDPE plastic with the addition of 10% activated zeolite catalyst.
2.3 Characterization and Identification of Pyrolysis Plastic Waste
The characterization of pyrolysis bio-oil was carried out by determining the yield, density, and viscosity. The yield of bio-oil produced in each pyrolysis process was determined by comparing the produced oil with samples of pyrolyzed plastic waste. The density of the pyrolysis oil was determined by comparing the mass per unit volume of the oil. The viscosity of the oil was determined using an Oswald viscometer. Identification of the constituent components of the pyrolysis oil used gas chromatography-mass spectroscopy. Spectrum analysis of GC-MS was to determine the compounds resulting from pyrolysis.
Natural zeolite preparation was prepared by washing and grinding natural zeolite. Zeolite was washed with distilled water, filtered, and dried in an oven at a temperature of 100°C for 1 h. Physical activation was carried out by reducing the grain size, sieving, and calcining the zeolite at a temperature of 500°C for 4 h. Zeolite activation was performed to maximize the efficiency of the zeolite as a catalyst which is influenced by several factors, namely surface area, acidity, crystallinity, and the ratio of Si/Al zeolite. Some researchers reported that natural zeolite had an acidity of 1.576 g/mol and Si/Al ratio of 1.576 g/mol, range of surface area from 20.8 to 23.2 m2/g [6,15,16]. The pore size was in the range from 7.73 to 11.4 Å by SEM-EDS and BET, and detected mineralogy was mordenite phase and the lattice parameter are a = 18.115(8) Å, b = 20.520(9) Å and c = 7.515(2) Å by XRD [17]. Based on that research, the clearly purpose of physical activation is to remove organic impurities, enlarge pores and expand the surface [18,19]. Identification through XRD is used to determine the type of crystal and crystallinity of natural zeolite. XRD analysis of activated natural zeolite with acid treatment showed that activated natural zeolite has a mordenite phase. The activation of natural zeolite increases the crystallinity of solids because the natural zeolite’s tetrahedral bonds become relatively more uniform as shown by the increase in intensity shown in Fig. 1. The crystallization of the catalyst greatly affects the effectiveness of the cracking process because the crystals in the catalyst only have an active phase that facilitates the cracking process. Based on the diffractogram of XRD test results on activated and inactivated natural zeolite is known that activated natural zeolite has a higher peak intensity than inactivated natural zeolite. The high intensity of the composition shows the SiO2 zeolite is also high. This shows that the active site on the surface of activated natural zeolite is better than inactivated natural zeolite so that activated natural zeolite has better catalytic properties in the pyrolysis process. In addition, other new investigations model such as SEM and BET analysis before/after reaction should be applied in the natural zeolite as catalyst in the future.

Figure 1: XRD results of activated natural zeolite and inactivated natural zeolite
Based on the Fig. 1, the type of natural zeolite phase used in this study is also known through XRD data processing with Maud software. The XRD results of the natural zeolite used were 100% similar to the standard mordenite zeolite [18,20].
In this research, pyrolysis of polypropylene (PP) and Low-Density Polyethylene (PE) plastics was carried out. The plastic pyrolysis process for each type was carried out twice, namely pyrolysis without a catalyst and pyrolysis with an activated natural zeolite catalyst. The mass of used plastic in each pyrolysis process is 1 kg and the mass of catalyst in each type of plastic is 10% of the mass of plastic. The pyrolysis process caused degradation of the plastic polymer into monomers and shorter carbon fractions. The gas formed during pyrolysis was condensed in a condenser, and liquid oil was produced as a distillate. The result of the pyrolysis of waste plastic either with catalyst or without catalyst is shown in Fig. 2.

Figure 2: PP plastic pyrolysis liquid product: (a) without catalyst and (b) with activated natural zeolite catalyst
Pyrolysis of PP plastic was carried out with a plastic mass of 1 kg and a catalyst mass of 10% of the plastic mass. Table 1 shows the amount of yield produced with a temperature range of 50°C. As listed in Table 1, it can be seen that the higher temperature in the pyrolysis process causing the higher yield of the liquid product, because the higher temperature caused the more chemical components that undergo thermal degradation [3,5,18].

The components of the pyrolysis oil were analyzed by GC-MS and adjusted according to existing references and the summary of the results is shown in Table 2. The constituent components are alkanes, alkenes, phenols, ketones, acids, amides, ethers, and esters. The largest composition is aromatic compounds with a long range of carbon atoms C4–C24.

Pyrolysis of LDPE plastic was carried out in two variations, namely without catalyst and with activated natural zeolite catalyst. The pyrolysis process was carried out until no liquid product is formed. The catalyst used in the pyrolysis process is 10% of the mass of the plastic. The yield of bio-oil is shown in Fig. 3.

Figure 3: LDPE plastic pyrolysis liquid product: (a) without catalyst and (b) with activated natural zeolite catalyst
The amount of yield produced is classified based on the temperature range of 50°C which is shown in Table 3. As listed in Table 3, it is known that the pyrolysis of LDPE plastic with natural zeolite catalyst results in higher yields of products with higher temperatures. The pyrolysis of LDPE plastic without a catalyst at a temperature of 150°C–200°C yields less yield than the yield at a temperature of 100°C–150°C. This is because the temperature in the heating process is not stable revealing the number of condensed components is also unstable [9,18,21].

The components of the pyrolysis oil were analyzed by GC-MS and adjusted according to existing references and the summary of the results is shown in Table 4. The components of the bio-oil produced by the pyrolysis of LDPE plastics were alkanes, alkenes, acids, phenols, and aromatics. The largest component is aliphatic hydrocarbon compounds with a long range of carbon atoms C13-C29.

3.4 Characterization and Identification of Compounds Compounding Pyrolysis Bio-Oil
The characterization of the pyrolysis oil was carried out by calculating the yield, density, and viscosity. The amount of oil yield from pyrolysis with activated natural zeolite catalyst (Table 5) is expected to be higher than the yield of bio-oil without catalyst [22]. This is because the catalyst can increase the decomposition reaction so that more long-chain hydrocarbons are split into short ones which cause more gas to be formed and condensed into bio-oil [23,24].

The density or viscosity of the oil is influenced by the molecular weight of the compounds contained therein. The density and viscosity of the oil produced in this study are shown in Table 6.
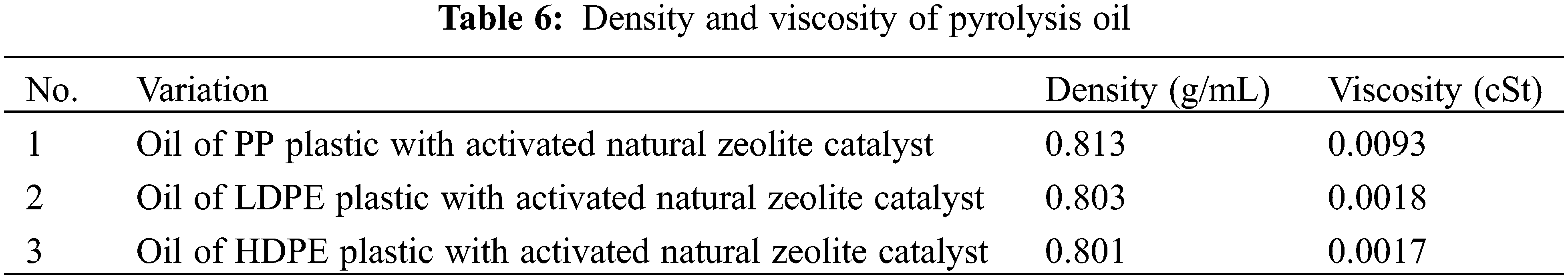
The smaller density value is better to use as fuel because it is getting closer to the density of the diesel oil range, which is 0.81–0.89 g/mL [11,25]. The density value is directly proportional to the viscosity value, where the higher density value following the higher viscosity value. This study showed that the production of bio-oil using the zeolite catalyst producing oil with a lower viscosity than oil production without the use of the zeolite catalyst
The following conclusions are: (1) activation of natural zeolite affects the crystallinity of natural zeolite which can increase the effectiveness of natural zeolite as a catalyst in the plastic pyrolysis process, (2) in pyrolysis of waste plastic types PP and LPDE, the addition of the zeolite catalyst can improve efficiency of the pyrolysis process and yield of the reaction, (3) based on the results of GC-MS, the constituent components of PP plastic pyrolysis oil are alkanes, alkenes, phenols, ketones, acids, amides, ethers, and esters. Meanwhile, the constituent components of LDPE plastic pyrolysis oil are alkanes, alkenes, acids, phenols, and aromatics, and (4) oil of plastic pyrolysis with activated natural zeolite catalyst has a higher yield than oil of plastic pyrolysis without zeolite catalyst.
Acknowledgement: The authors would like to thank the Chancellor and Chair of LP2 M UM, who provided PNBP research funds.
Funding Statement: The funding that have supported the work, namely: PNBP No. 4.3.13/UN32/KP/2021 by Assoc. Prof. Aman Santoso.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Miandad, R., Barakat, M. A., Aburiazaiza, A. S., Rehan, M., Nizami, A. S. (2016). Catalytic pyrolysis of plastic waste: A review. Process Safety and Environmental Protection, 102, 822–838. DOI 10.1016/j.psep.2016.06.022. [Google Scholar] [CrossRef]
2. Wang, C., Han, H., Wu, Y., Astruc, D. (2022). Nanocatalyzed upcycling of the plastic wastes for a circular economy. Coordination Chemistry Reviews, 458, 214422. DOI 10.1016/j.ccr.2022.214422. [Google Scholar] [CrossRef]
3. Al-Salem, S. M., Antelava, A., Constantinou, A., Manos, G., Dutta, A. (2017). A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). Journal of Environmental Management, 197, 177–198. DOI 10.1016/j.jenvman.2017.03.084. [Google Scholar] [CrossRef]
4. Zhang, H., Wu, J., Qin, Z., Luo, Y. (2022). The effect of bio-oil on high-temperature performance of bio-oil recycled asphalt binders. Journal of Renewable Materials, 10(4), 1025–1037. DOI 10.32604/jrm.2022.017483. [Google Scholar] [CrossRef]
5. Demirbas, A. (2004). Pyrolysis of municipal plastic wastes for recovery of gasoline-range hydrocarbons. Journal of Analytical and Applied Pyrolysis, 72(1), 97–102. DOI 10.1016/j.jaap.2004.03.001. [Google Scholar] [CrossRef]
6. Santoso, A., Sumari, S., Safitri, N. N., Wijaya, A. R., Putri, D. E. (2020). Activation of zeolite from malang as catalyst for plastic waste conversion to fuel. Key Engineering Materials, 851, 212–219. DOI 10.4028/www.scientific.net/KEM.851.212. [Google Scholar] [CrossRef]
7. Anuar Sharuddin, S. D., Abnisa, F., Wan Daud, W. M. A., Aroua, M. K. (2016). A review on pyrolysis of plastic wastes. Energy Conversion and Management, 115, 308–326. DOI 10.1016/j.enconman.2016.02.037. [Google Scholar] [CrossRef]
8. Veksha, A., Giannis, A., Oh, W. D., Chang, V. W. C., Lisak, G. (2018). Upgrading of non-condensable pyrolysis gas from mixed plastics through catalytic decomposition and dechlorination. Fuel Processing Technology, 170, 13–20. DOI 10.1016/j.fuproc.2017.10.019. [Google Scholar] [CrossRef]
9. Budsaereechai, S., Hunt, A. J., Ngernyen, Y. (2019). Catalytic pyrolysis of plastic waste for the production of liquid fuels for engines. RSC Advances, 9(10), 5844–5857. DOI 10.1039/C8RA10058F. [Google Scholar] [CrossRef]
10. Sumari, Fajaroh, F., Santoso, A., Wardani, R. K. (2018). Performance of activated natural zeolite/Cu as a catalyst on degradation of glycerol into ethanol assisted by ultrasonic. Journal of Physics: Conference Series, 1093, 12036. DOI 10.1088/1742-6596/1093/1/012036. [Google Scholar] [CrossRef]
11. Hwang, K. R., Choi, S. A., Choi, I. H., Lee, K. H. (2021). Catalytic cracking of chlorinated heavy wax from pyrolysis of plastic wastes to low carbon-range fuels: Catalyst effect on properties of liquid products and dechlorination. Journal of Analytical and Applied Pyrolysis, 155, 105090. DOI 10.1016/j.jaap.2021.105090. [Google Scholar] [CrossRef]
12. Obeid, F., Zeaiter, J., Al-Muhtaseb, A. H., Bouhadir, K. (2014). Thermo-catalytic pyrolysis of waste polyethylene bottles in a packed bed reactor with different bed materials and catalysts. Energy Conversion and Management, 85, 1–6. DOI 10.1016/j.enconman.2014.05.075. [Google Scholar] [CrossRef]
13. Sharuddin, S. D. A., Abnisa, F., Daud, W. M. A. W., Aroua, M. K. (2018). Pyrolysis of plastic waste for liquid fuel production as prospective energy resource. IOP Conference Series: Materials Science and Engineering, 334, 12001. DOI 10.1088/1757-899X/334/1/012001. [Google Scholar] [CrossRef]
14. Sriningsih, W., Saerodji, M. G., Trisunaryanti, W., Triyono, Armunanto, R. et al. (2014). Fuel production from LDPE plastic waste over natural zeolite supported Ni, Ni-mo, Co and Co-mo metals. Procedia Environmental Sciences, 20, 215–224. DOI 10.1016/j.proenv.2014.03.028. [Google Scholar] [CrossRef]
15. Pamungkas, D. P. W., Amalia, S., Khalifah, S. N. (2015). Utilization of natural zeolite catalyst impregnated Sn metal in glucose isomerization with temperature variations. Alchemy, 4(1), 79–87. DOI 10.18860/al.v4i1.3151. [Google Scholar] [CrossRef]
16. Cahyono, E., Muchalal, M., Triyono, T., Pranowo, H. D. (2014). Catalytic activities of Fe3+- and Zn2+-Natural zeolite on the direct cyclisation-acetylation of (R)-(+)-Citronellal. Bulletin of Chemical Reaction Engineering & Catalysis, 9(2), 128–135. DOI 10.9767/bcrec.9.2.5936.128-135. [Google Scholar] [CrossRef]
17. Suminta, S. (2006). Characterization of natural zeolite by X-ray diffractometer. Jurnal Zeolit Indonesia, 5(2), 52–68. [Google Scholar]
18. Marcilla, A., Beltrán, M. I., Navarro, R. (2009). Thermal and catalytic pyrolysis of polyethylene over HZSM5 and HUSY zeolites in a batch reactor under dynamic conditions. Applied Catalysis B: Environmental, 86(1), 78–86. DOI 10.1016/j.apcatb.2008.07.026. [Google Scholar] [CrossRef]
19. Sumari, S., Fajaroh, F., Yahmin, Sholihah, N., Santoso, A. et al. (2019). Effect of temperature synthesis on structural behaviours of NaY zeolite using local sand as a silica source. IOP Conference Series: Materials Science and Engineering, 515, 12036. DOI 10.1088/1757-899X/515/1/012036. [Google Scholar] [CrossRef]
20. Li, X., San, X., Zhang, Y., Ichii, T., Meng, M. et al. (2010). Direct synthesis of ethanol from dimethyl ether and syngas over combined H-mordenite and Cu/ZnO catalysts. ChemSusChem, 3(10), 1192–1199. DOI 10.1002/cssc.201000109. [Google Scholar] [CrossRef]
21. FakhrHoseini, S. M., Dastanian, M. (2013). Predicting pyrolysis products of PE, PP, and PET using NRTL activity coefficient model. Journal of Chemistry, 2013, 487676. DOI 10.1155/2013/487676. [Google Scholar] [CrossRef]
22. Tulashie, S. K., Boadu, E. K., Dapaah, S. (2019). Plastic waste to fuel via pyrolysis: A key way to solving the severe plastic waste problem in Ghana. Thermal Science and Engineering Progress, 11, 417–424. DOI 10.1016/j.tsep.2019.05.002. [Google Scholar] [CrossRef]
23. Sakata, Y., Uddin, M. A., Muto, A. (1999). Degradation of polyethylene and polypropylene into fuel oil by using solid acid and non-acid catalysts. Journal of Analytical and Applied Pyrolysis, 51(1), 135–155. DOI 10.1016/S0165-2370(99)00013-3. [Google Scholar] [CrossRef]
24. Syamsiro, M., Saptoadi, H., Norsujianto, T., Noviasri, P., Cheng, S. et al. (2014). Fuel oil production from municipal plastic wastes in sequential pyrolysis and catalytic reforming reactors. Energy Procedia, 47, 180–188. DOI 10.1016/j.egypro.2014.01.212. [Google Scholar] [CrossRef]
25. Xue, Y., Zhou, S., Brown, R. C., Kelkar, A., Bai, X. (2015). Fast pyrolysis of biomass and waste plastic in a fluidized bed reactor. Fuel, 156, 40–46. DOI 10.1016/j.fuel.2015.04.033. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |