

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2022.021163
ARTICLE
Digestibility, Antioxidant and Anti-Inflammatory Activities of Pecan Nutshell (Carya illioinensis) Extracts
1Facultad de Ciencias Agrotecnológicas, Universidad Autónoma de Chihuahua, Chihuahua, México
2Universidad de Sonora, Rosales y Niños Héroes S/N, Hermosillo, Son, México
3Centro de Investigación en Alimentación y Desarrollo, A.C. Unidad Delicias, Delicias, Chihuahua, México
*Corresponding Authors: Carmen Lizette del Toro-Sánchez. Email: carmen.deltoro@unison.mx; María Antonia Flores-Córdova. Email: mariflor_556@hotmail.com
Received: 30 December 2021; Accepted: 11 April 2022
Abstract: Phenolic compounds are related to high biological activity, avoiding oxidation in food and human systems. Nutshells are by-products derived from the pecan nut processing that contain important amounts of phenols which biological activity must be studied. This research aimed to evaluate the antioxidant (DPPH, ABTS, FRAP and hemolysis) and anti-inflammatory activities of shell extracts from pecan nuts harvested during the crop production cycle 2018 and 2019, as well as the in vitro digestibility of their phenolic compounds, including flavonoids. Results showed that extracts from the crop production cycle 2018 obtained the highest yield, while those from 2019 contained the highest concentration of phenolic compounds, flavonoids and antioxidant capacity determined by DPPH (22.96 mmol ET), ABTS (91.55 mmol ET) and inhibition of hemolysis (92.12%). The anti-inflammatory activity exhibited an inhibition of the elastase enzyme up to 50 min and the bioaccessibility of phenolic compounds reached up to 32%. These results showed that pecan nutshell extracts are an important source of biologically active compounds, thus, they are suitable to be used as commodities in different fields such as agricultural, food and pharmaceutical industries. Future studies must be carried out in order to elucidate the activity of nutshell extracts within in vivo systems.
Keywords: Anti-inflammatory activity; antioxidants; digestibility; shell; phenolic compounds
The pecan nut production reaches 171,000 tons in Mexico, representing 52% worldwide [1]. Chihuahua, Coahuila, and Sonora are the main pecan nut producer’s states in Mexico [2]. The first place of economic importance within the agricultural crops in Chihuahua is the pecan nut, with 65% of the national production [3].
Generally, pecan nuts are shelled and marketed as dried fruits. The shell comprises around 50% of the weight and is considered as a waste [4]. In this context, a wide range of by-products derived from crops are generated around the world, inducing adverse consequences in the environment [5]. Taking into consideration the economic growth and the environmental sustainability, it is important to be aware of the production of goods and services, where the by-products including the pecan nutshell, can be considered as an important commodity within the value chain [6].
Additionally, it has been reported that the pecan nutshell contains fiber, proteins, minerals and antioxidants that can be used in a wide range of industries [7]. Among the main antioxidants found in the nutshell, the phenolic compounds (PCs) stand up. Particularly, the shell contains higher concentration of PC as compared with the kernel of the nut, comprising around 60%–80% [8–10]. Authors such as Flores-Córdova et al. [11], reported two principal PCs in the pecan nutshell: gallic acid and catechin. Vazquez-Flores et al. [12] found PCs with both simple (gallic, chlorogenic, vanillic and ellagic acids) and complex structure (hydrolysable tannins composed by units of gallic and ellagic acids and hydroxybiphenyl joined one with others, or with monosaccharides).
It is well known that PCs are synthesized as a response to biotic and abiotic factors [13]. They reduce the cell oxidation by scavenging free radicals (molecules with one or more disappeared electrons). The free radicals naturally occur in the human body in moderate quantities for preserving against bacteria and viruses. However, when the environmental conditions are adverse, the stress conditions increase and the oxidative stress and inflammatory processes are generated. If the human defense system is unable to neutralize free radicals, the cellular damage and the irreversible oxidation of important molecules (i.e., lipids, nucleic acids and proteins) are induced. All these generate a wide range of chronic and degenerative diseases such as diabetes, Alzheimer, cardiovascular diseases and some cancer types [14–16]. Bahadoran et al. [17] reported that the antioxidant capacity of PCs contained in the nutshell diminished the inflammatory process and exerted a protective effect in the human body. Thus, the antioxidant potential of PCs is one of the most important bioactivity and the most widely studied.
It is important to state that once PCs are consumed, their biological activity, bioaccessibility and bioavailability is altered due to the gastrointestinal digestion. The bioaccessibility refers to the liberation of PCs from the food matrix by means of digestive enzymes, allowing the accessibility of these compounds for future absorption. The bioavailability is the quantity of PCs that reach systemic circulation (the fraction of compound that reaches a target cell) [18,19]. In the literature, there are some studies evaluating the hydrolysis of carbohydrates, lipids and peptides from nutshells by means of the in vitro digestion [20,21]. However, studies related with the in vitro digestibility of pecan nutshell compounds are really scarce. For this reason, the purpose of the present research was to evaluate the antioxidant (DPPH, ABTS, FRAP, hemolysis) and anti-inflammatory activities of pecan nutshell harvested during the crop production cycles 2018 and 2019, as well as the in vitro gastrointestinal digestion of their phenolic compounds and flavonoids. The results obtained in this research can be of relevant interest due to the high content of PCs in nutshell, their antioxidant potential and the revalorization of by-products, contributing to the “zero waste” goals from the Food and Agriculture Organization of the United Nations (FAO).
The pecan nut, Western variety, was obtained from producers from Aldama, Chihuahua, Mexico, orchard “El Edén” located in the following coordinates: 28°84’03.8”N, 10°59’41.0”W, altitude 1,262 MASL. Samples were collected in the crop production cycles 2018 and 2019. Afterwards, the samples were weighed, grinded in a mill (Hamilton Beach 80393). Once the sample was pulverized, it was sieved in a mesh number 20 for obtaining a particle size of 0.84 mm and it was again weighed for obtaining the yield. Samples were refrigerated at –20°C until analysis.
For obtaining the extract, 1 g of defatted sample and 5 mL of 80% methanol were mixed and sonicated during 30 min at 40 KHz. After, it was centrifuged at 4000 × g at 4°C for 10 min. The supernatant was recovered and the residue was extracted once again using the same procedure. The supernatants were combined and concentrated in a rotavapor (Buchi, R-100 V) at 45°C/60 rpm and reduced pressure. The extract was weighed for the yield determination. Finally, the concentration of extracts was adjusted to 0.01 g/mL in methanol. Samples were stored at −20°C until analysis.
2.3 Total Phenolic Compounds Determination
This assay was carried out using the methodology of Folin-Ciocalteu [22]. A portion of 10 µL of nutshell extract, 25 µL of 1N Folin-Ciocalteu reagent, 25 µL of Na2CO3 (20%) and 140 µL of MilliQ water were mixed. Samples were placed in darkness for 30 min. After, the absorbance was measured at 765 nm with a spectrophotometer SP2000UV. The results were expressed as mg of gallic acid equivalents for g of sample (mg GAE/g). All the assays were done in triplicate.
2.4 Total Flavonoids Compounds Determination
Total flavonoids were determined using the colorimetric method of Venu et al. [23]. Aliquots of 80 µL of nutshell extract were added to 80 µL of ethanolic solution of aluminum chloride (20 g/L). They were shaken for 30 seconds and placed in the dark during 1 h at 25°C. After, the absorbance at 415 nm was measured. A calibration curve was built with quercetin in methanol. Results were reported as mg equivalents of quercetin per g of sample (mg EQ/g).
2.5 Determination of the Antioxidant Capacity
The antioxidant capacity of nutshell extracts was determined using different methodologies: DPPH (2,2-diphenyl-1-picrylhydrazyl), ABTS (2,2’-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid), FRAP (ferric reducing antioxidant power) and the inhibition of hemolysis caused by AAPH (2,2-azobis(2-methylpropionamidine) dihydrochloridine) as follows:
A portion of 2.5 mg of DPPH radical was dissolved in 100 mL of methanol. After, 200 µL of this solution was added to 20 µL of the sample. They were mixed and stored for 30 min and the absorbance was measured at 515 nm. A calibration curve was built with Trolox, and the results were reported in millimoles of Trolox equivalents per g of sample (mmol ET/g) [24].
This assay was carried out mixing 19.3 mg of ABTS with 5 mL of distilled water. In another container, 0.0378 g of K2S2O8 were added to 1 mL of water. A portion of 88 µL from the latest solution was added to that containing ABTS and they were mixed and stored in darkness for 12 to 16 h at room temperature until reaching an intense blue color. A portion of 270 µL of this solution was mixed with 20 µL of sample and stored for 30 min, the absorbance was measured at 374 nm. The 80% methanol was considered as a control. Results were expressed in mmol ET/g [25].
First, stock solutions were prepared as follows: NaCH3COO buffer (300 mmol/L, pH 3.6), FeCl3 (20 mmol) and TPTZ solution (2,4,6-Tripridil-s-triazine, 10 mM) in HCl (40 mmol). The FRAP solution was prepared in the proportion 10:1:1 v/v/v for the buffer, FeCl3 and TPTZ, respectively. After, 20 µL of the sample were combined with 280 µL of FRAP solution and placed in a microplate container (Thermo Fisher Scientific Inc. Multiskan GO, Waltham, MA, EE. UU.). After 30 min of reaction, the absorbance at 638 nm was read. The FRAP solution was considered as the control. The results were reported as mmol ET/g of sample.
2.5.4 Inhibition of Hemolysis Induced by AAPH
Blood (5 mL) from a human healthy volunteer was extracted under informed consent. The platelets were separated from the plasma through centrifugation at 1500 rpm for 10 min at 4°C. The erythrocytes were washed threefold with PBS (phosphate-buffered saline) at pH 7.4. After, a suspension containing erythrocytes and PBS in the proportion 5:95 (v/v) was prepared. A mixture containing 100 µL of erythrocytes, 100 µL of AAPH radical and 100 µL of nutshell extract were incubated at 37°C for 3 h with agitation (30 rpm). Later, 1 mL of PBS was added to the mixture and was centrifuged at 1500 rpm for 10 min at 4°C. A suspension containing erythrocytes and AAPH, without the nutshell extract, was also prepared as a control. The supernatant was placed in a microplate and the absorbance was read at 540 nm. The percentage of inhibition was determined with the following Eq. (1):
where AAPH1 = the absorbance of the hemolysis induced by AAPH in the control; HS = the absorbance from the nutshell extract.
2.5.5 Anti-Inflammatory Capacity
For this assay, a technique of the porcine pancreatic elastase (PPE, Sigma, type IV) was used following the methodology of Lee et al. [26] with some modifications. The enzyme PPE hydrolyzes the substrate N-succinyl-(ala)-3-p-nitroanilide, delivering p-nitroanilide. This reaction was controlled during 58 min at 28°C. The concentration for each extract in the reaction system was 66.66 µg/mL. The sample was prepared at the concentration of 1.015 mM in a solution of 0.1 M of biologic buffer Tris-HCl pH 8. The PPE was dissolved in 0.2 M Tris-HCl (pH 8) using a concentration of 1.376 U/ml. The reaction was carried out in plates of 96 wells by addition of 10 µL of extract, 40 µL of enzyme (PPE) and 100 µL of substrate, obtaining a volume of 150 µL. The reaction began when the enzyme and substrate were in contact. The control was obtained using the same procedure without nutshell extract. The assay was done in triplicate.
The extracts from nutshell were digested following the in vitro gastrointestinal model reported by van-Campen et al. [27] and Tarko et al. [28]. For this, the pecan nutshell was placed in contact with digestive enzymes (amylase, pepsin and pancreatin) evaluating the content of total phenolic compounds, total flavonoids, and antioxidant capacity. A healthy volunteer contributed to the oral phase, previous to the assay, he washed his teeth with toothpaste and he fasted for 90 min. He chewed 15 g of nutshell, 15 times during 15 seconds. After, this sample was homogenized with 10 mL of purified water. After, the samples were acidified with 6M HCl until reaching a pH = 2. A portion of 22.5 mL of pepsin (315 U/mL) (Sigma, P7012-5G) and 22.5 mL of distilled water were added. The sample was placed in a water bath at 37°C/80 rpm for 2 h. Later, the samples were neutralized (pH = 7) with 1.25 M NaHCO3 and 5.625 mL of pancreatin (4 mg/mL) (Sigma, P1750-100G) were added to each flask. Samples were homogenized and placed into a shaking water bath (80 rpm) at 37°C during 4 h. Afterwards, the analysis compounds (PCs, flavonoids and antioxidant capacity) were carried out.
An analysis of variance (ANOVA) of the results followed by a Tukey test with a significance level from 5% (p < 0.05) was carried out. The statistical package used for this analysis was Infostat (version 2008). The results were expressed as the mean ± standard deviation with three determinations in each analysis.
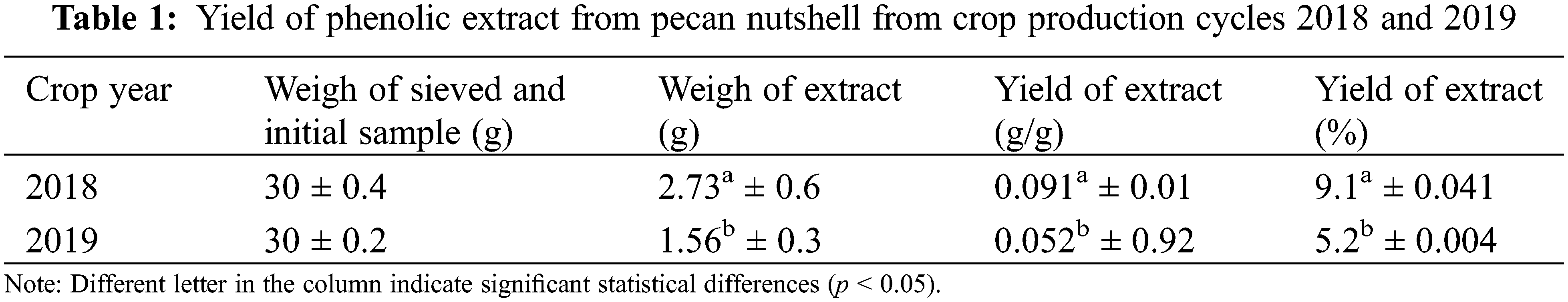
Table 1 shows the yield of phenolic extract from pecan nutshell, western variety, from the crop production cycles 2018 and 2019. Significant differences between samples (p < 0.05) were found. The highest percentage of yield from the nutshell extract corresponded to the year 2018, with a difference of 75% as compared with that obtained in 2019. Yang et al. [29] obtained a yield between 0.42% and 4.54% using methanol with a similar procedure. However, this range is lower than the values obtained in this research where 80% methanol was used. On the other hand, Alarcón et al. [30] obtained bigger yields in nutshell extracts, between 23.3% and 66.40%, using different proportions of ethanolic solvent (50% and 100%, respectively).
The activity of extracts is related to the extraction procedure, where the solvents play an important role and depending on the solvent polarity, the extract will be solubilized to a greater or lesser extent. Thus, differences in the results reported in the literature and that obtained in the present research could be attributed to differences in the extraction procedures as the time, temperature, relationship between sample and solvents, physicochemical composition of samples, among others. For this, it is important to evaluate the extent to which a solvent can improve the yield of sample extracts, such as the nutshell that is a by-product with a high content of compounds that can be extracted and used as ingredients in the food, agriculture, cosmetic and pharmaceutical industries [31].
3.2 Total Phenolic Compounds and Flavonoids
The content of total phenolic compounds and flavonoids from the nutshell extracts from the crop production cycles 2018 and 2019 is shown in the Fig. 1, obtaining significant differences between the samples (p < 0.05). Particularly, samples from the year 2019 had a higher PC and flavonoid content, being 28.51 mg of GAE/g and 3.8 mg of EQ/g, respectively. Flavonoids are classified within the phenolic compounds group due to their chemical structure, which could explain why in the same crop year (2019) both compounds (flavonoids and PCs) obtained the highest concentration.
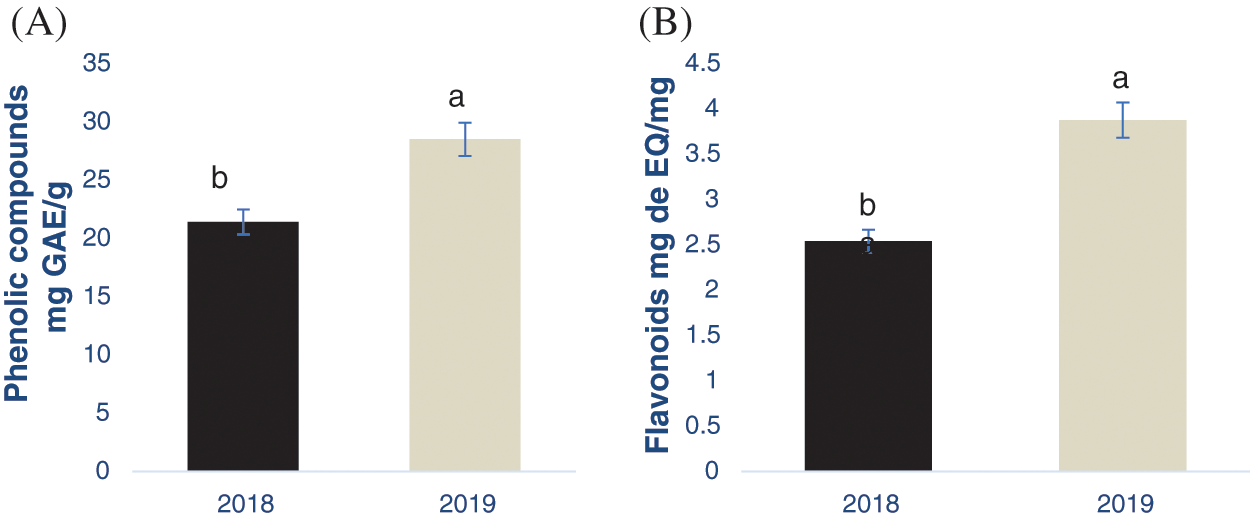
Figure 1: Determination of phenolic compounds (A) and flavonoids (B) in the pecan nutshell from the crop production year 2018 and 2019. Different letter in each figure indicates significant statistical differences (p < 0.05). The bars in the top of each column represents the standard deviation. mg GAE/g = mg equivalents of gallic acid per g of sample. mg EQ/g = mg equivalents of quercetin per g of sample
In this context, Flores-Córdova et al. [32] obtained 147.28 mg/g using a similar extraction procedure, including the solvent type. However, these authors obtained the samples from the crop production cycles 2013 and 2014, while in the present study were from 2018 and 2019. These results showed the great influence that the crop production cycle has on the phenolic and flavonoid content as their biosynthesis depends on biotic and abiotic conditions. Flores-Estrada et al. [33] also extracted phenolic compounds from pecan nutshell, obtaining a concentration of 27.36 mg GAE/g which is similar to the results obtained herein. In the literature there are other reports analyzing the concentration of PCs and flavonoids in the nutshell, however, it is difficult to be compared with this study due to the differences in the extraction procedures. For example, Pinheiro et al. [34] reported a phenolic compound concentration of 117 mg of GAE/g using infusion in water, while in the present study 80% methanol was used. The extrusion and fermentation procedures were used by Xavier [35] in nutshell, obtained from 8.44 to 79 mg GAE/g.
Regarding the flavonoid concentration, Flores- Estrada et al. (2019) reported higher values of 23.37 mg CE/g than those obtained in this research despite the extraction procedures were similar. These differences could be also attributed to the extraction procedure and the place where the samples were collected, bearing in mind that the flavonoid concentration is influenced by environmental conditions as previously stated. Additionally, Flores-Córdova et al. [10] showed that pecan trees produce fruits in alternate cycles, inducing differences in the phenolic compounds composition. As similar as PCs, there are a widespread variety of studies evaluating the flavonoids in nutshells. They differ in the extraction technique, as well as the reporting units, making the comparison of the results difficult. As an example, Xavier [35] showed a range from 2.55 to 8.16 mg ER/ g. Alarcón et al. [30] reported 33.28 mg CE/g, while Yang et al. [29] found 98.85 mg REs/g. Finally, Fernández-Agulló et al. [36] showed a concentration of 81.50 mg/g. However, it is important to highlight the antioxidant effect of flavonoids from nutshells and their impact on health [9]. Flavonoids protect the human body against oxidative stress and they were bioavailable after their consumption [36]. Both flavonoids and phenolic acids were biologically active compounds, decreasing the oxidation process by means of scavenging free radicals and inducing the formation of stable molecules [37].
All these results showed that the nutshell is a low cost by-product with a high phenolic content [38] with antioxidant capacity, despite the differences found in the procedures of extraction.
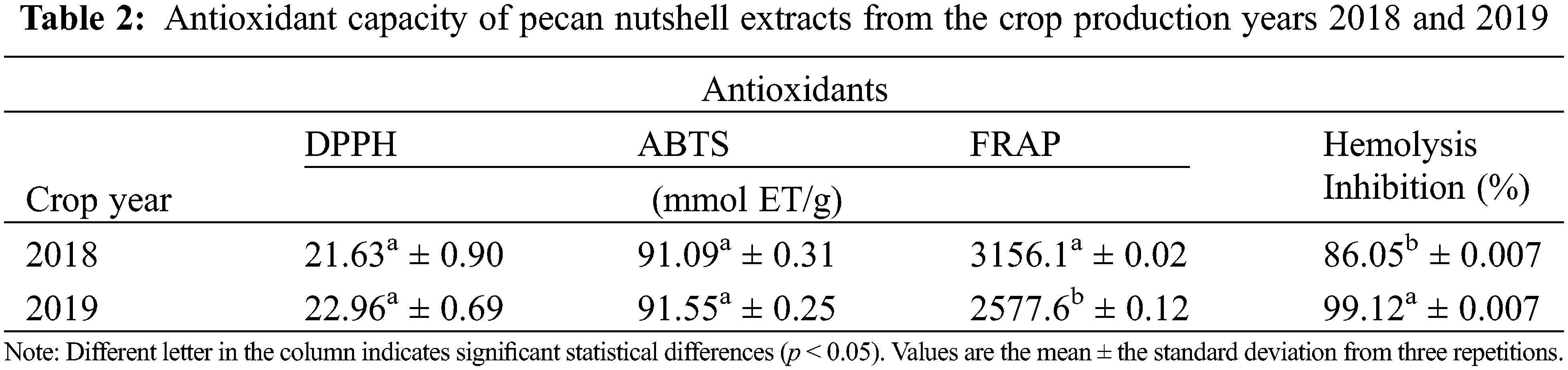
According to the obtained results, there was antioxidant capacity in the nutshell extracts (Table 2). However, no significant differences were found in the antioxidant capacity with the DPPH and ABTS assays between the crop production year 2018 and 2019 (p > 0.05). On the contrary, there were observed significant differences (p < 0.05) in the antioxidant capacity with the FRAP assay, as well as with the inhibition of hemolysis induced by AAPH between the crop production year 2018 and 2019. As stated in Table 2, the extract from the year 2019 showed lesser FRAP content (2577.6 mmol ET/g) but higher percentage of inhibition of hemolysis (99.12%) as compared with the year 2018. This could indicate that extracts from the cycle 2019 had less reducing power by electron transfer (FRAP mechanism) and, therefore, their mechanism of action could be by proton transfer as that of the AAPH assay. On the contrary, the extracts from the year 2018 seem to have a mechanism of action by means of electron transfer (3156 mmol ET/g) instead of by protons (86.05%). Some authors, such as Pinheiro et al. [34] obtained values in the DPPH assay from 305 to 488 mg TEAC/g and Fernández-Agulló et al. [36] showed results from 334.86 μmol ET/g in nutshells, being lower than those obtained in this research. Flores-Estrada et al. [33] reported a concentration of 955.69 µmol ET/g from DPPH assay and 631.09 µmol ET/g from ABTS. Xavier [35] found values from 2.39 trolox/g in the content of ABTS and 159.90 trolox/g in FRAP using a fermentation process.
In addition, the hemolytic activity of human erythrocytes was evaluated. The assay is based on the susceptibility to the membrane of erythrocytes to degradation due to lipid peroxidation by peroxyl radicals [39]. Results obtained in this research indicate that nutshells contain antioxidant compounds, independently of the year of production. Thus, they can be used as low-cost foodstuff for obtaining natural antioxidants which for industrial applications in foods, pharmacist, and cosmetics.
3.4 Anti-Inflammatory Activity
The results from the anti-inflammatory activity are presented in Fig. 2. It was observed that samples from the crop production year 2018 exhibited the highest inhibition of the elastase enzyme until the minute 50, approximately. This means that the inflammatory process was inhibited due to the presence of important anti-inflammatory compounds, such as phenolic compounds. On the other hand, it was also observed that samples from 2019 inhibited the elastase enzyme until minute 28. Bahadoran et al. [17] showed that the antioxidant activity of compounds contained in the extract from Carya illinoinensis, such as proanthocyanidins, exerted a protective effect against pain, inflammation and reduced the incidence of cardiovascular disease in diabetic patients. As similar as the anti-inflammatory process, the mechanism in which these compounds improved the cardiovascular condition in diabetic patients was related to their antioxidants properties as they diminished the oxidative damage generated by lipidic oxidation through scavenging free radicals [17].
Some studies have identified different phenolic compounds from pecan nutshell extracts with anti-inflammatory properties, such as pro-anthocyanins, chlorogenic acid, catechins, ellagic acid, gallic acid, among others [40,41]. Other authors reported that gallic acid was the main compound contained in the nutshell with photoprotective effect in the skin by inducing the synthesis of pro-collagen and inhibiting pro-inflammatory molecules that induce the aging (i.e., interleukin (IL)-6). The IL-6 promotes the production of matrix metalloproteinases (MMP-1) which degrades collagen, a protein that form part of the skin structure [40].
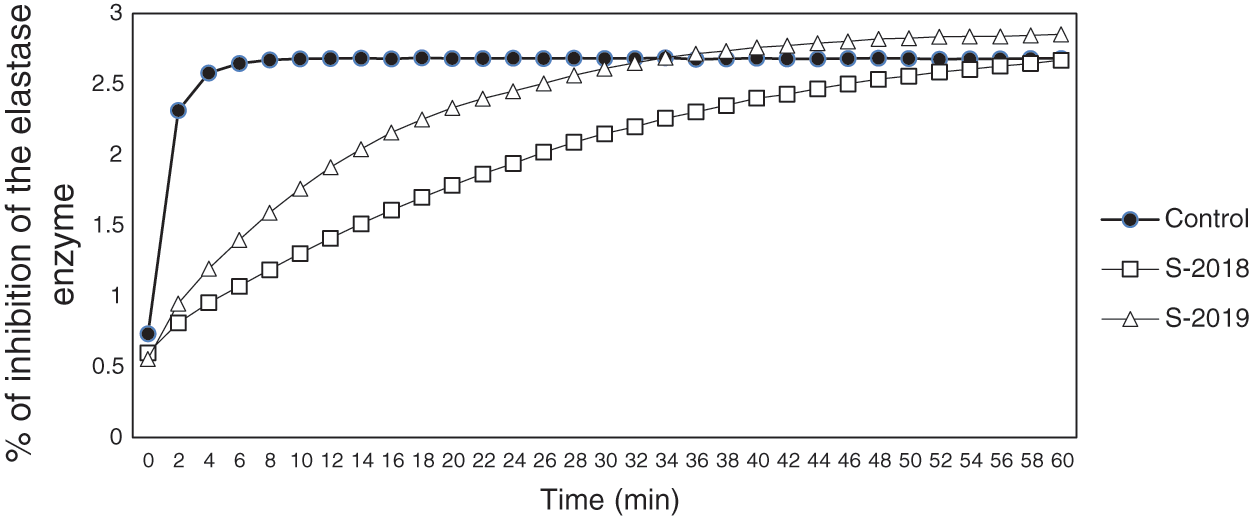
Figure 2: Percentage of inhibition of the elastase enzyme (related with the anti-inflammatory effect) of pecan nutshell extracts from the crop production years 2018 and 2019. The control was obtained using the procedure described in Section 2.5.5 and did not contained the nutshell extract
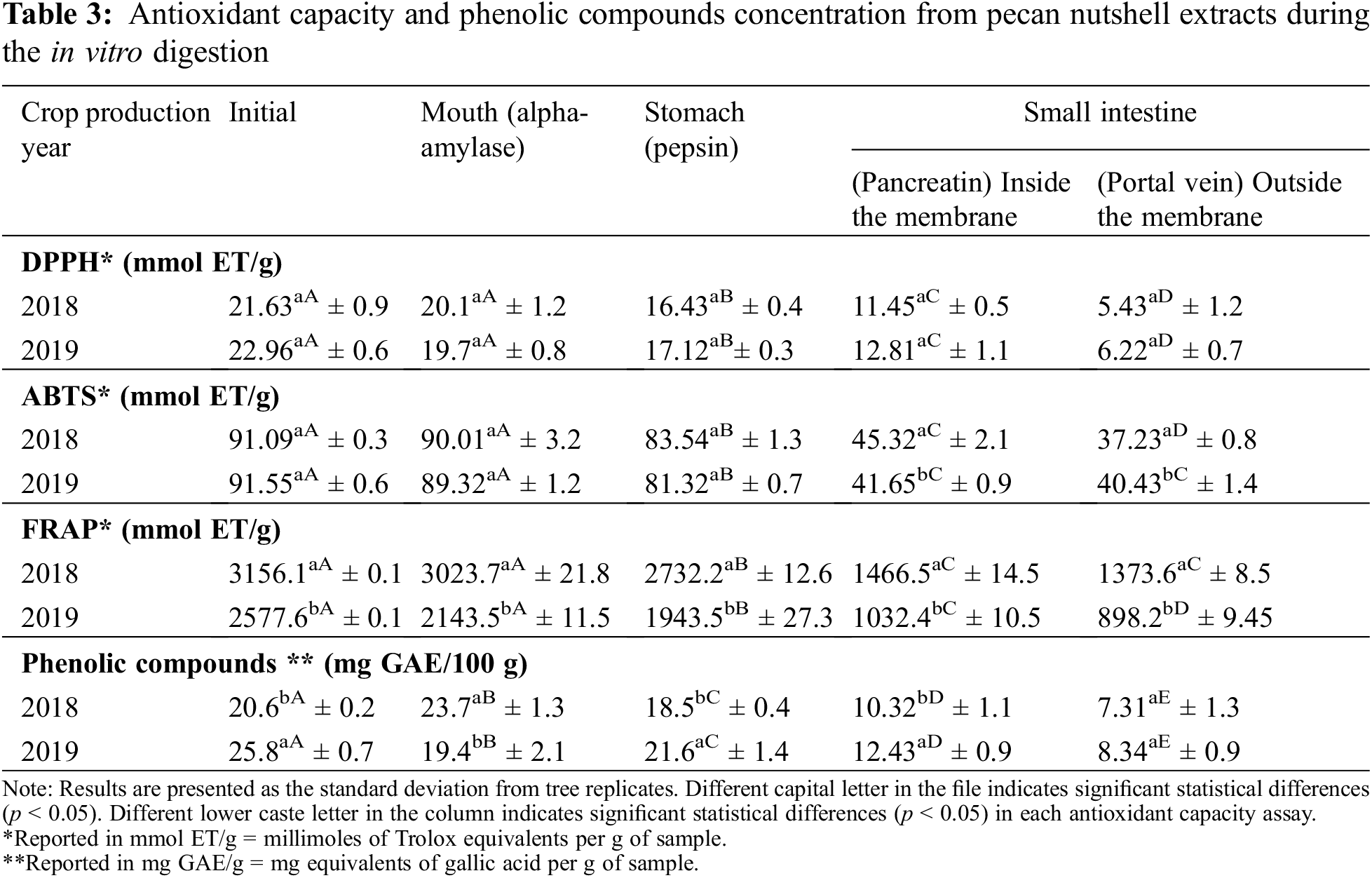
In Table 3 was reported the phenolic content of pecan nutshell extracts through an in vitro gastrointestinal digestion, as well as their antioxidant capacity. Overall, significant differences were found between the crop production years 2018 and 2019. The phenolic compounds diminished as it passed through the mouth, stomach and until reaching the small intestine. In the pancreatic digestion (small intestine, inside the membrane) around 50% of PCs were bioaccessible with respect to the initial concentration. However, outside the membrane (simulation of plasmatic circulation) the bioaccessibility of PCs decreased to 32%. Mateo et al. [42] and Hemery et al. [43] reported that phenolic compound bioaccessibility depends on several factors during the in vitro digestion, such as the pH, temperature, enzyme activity, time of each digestive stage. At the same time, processing modifies the bioaccessibility of phenolic compounds by inducing changes in the food matrix (such as in the pH) and food microstructure (such as the release of bounded PCs to the food matrix or changes in the solubilization) [44].
Regarding the antioxidant capacity evaluated by means of DPPH assay, 55% of the initial antioxidant capacity was recovered from the small intestine (inside the membrane), while outside showed 27%. The FRAP assay displayed a 40% and 32% of antioxidant capacity in the small intestine, inside and outside the membrane, respectively. A similar % was found in ABTS assay, obtaining a 45% in the pancreatic digestion (inside the membrane) and 44% outside the membrane. As can be seen, the results of antioxidant capacity were similar during the in vitro gastrointestinal digestion, indicating that an enough proportion of compounds preserved their antioxidant capacity during the in vitro digestion and thus, they were bioaccessible for exerting their function. Factors from the extraction procedure influenced the antioxidant capacity of extracts in addition to the biochemical and structural changes under the food matrix during the digestive process. Thus, the bioaccessibility is in function of the phenolic compounds contained in samples or extracts, their release from the food matrix and conditions during the digestive process [45,46].
The pecan nutshell presented higher yield in the crop production year 2018 as compared with the crop production year 2019. The greatest phenolic and flavonoid content was obtained in the crop production year 2019. However, the nutshell extracts from 2018 showed the highest anti-inflammatory power, mainly at the minute 50. Additionally, the in vitro gastrointestinal digestion displayed that phenolic compounds were 50% bioaccessible after pancreatic digestion and that around 32% can reach the portal vein and exert their biological function. Thus, the pecan nutshell is a by-product that can be used by the agri-food and pharmaceutical industries as it is an important source of biologically active compounds with antioxidant and anti-inflammatory potential. However, future studies must be carried out for obtaining additional information related to the bioactive compounds contained in the nutshell and their functions. The scientific knowledge on pecan nutshell, its compounds and applications will help to properly revalorize this by-product for contributing to the sustainable development goals.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Servicio de Información Agroalimentaria y Pesquera SIAP (2021). Producción agraria. https://nube.siap.gob.mx/cierreagricola/. [Google Scholar]
2. Romero-Arenas, O., Damián, M. A., Hernández, I., Parraguire, C., Márquez, M. et al. (2013). Evaluación económica de cáscara de nuez como sustrato para producción de plántulas de Pinus patula Schl. Et Cham. En vivero. Avances en Investigación Agropecuaria, 17(2), 23–40. [Google Scholar]
3. Fernández-Chávez, M., Guerrero-Morales, S., Palacios-Monárrez, A., Uranga-Valencia, L. P., Escalera-Ochoa, L. et al. (2021). Análisis de diversos aspectos económicos de la producción en huertas de nogales de alta y baja densidad. Estudio de caso. Cultivos Tropicales, 42(2), 1–3. [Google Scholar]
4. Bello-Huitle, V., Atenco-Fernández, P., Reyes-Mazzoco, R. (2010). Adsorption studies of methylene blue and phenol onto pecan and castile nutshells prepared by chemical activation. Revista Mexicana de Investigación Química, 9(3), 313–332. [Google Scholar]
5. Vargas, C. Y. A., Pérez, P. L. (2018). Aprovechamiento de residuos agroindustriales para el mejoramiento de la calidad del ambiente. Revista Facultad de Ciencias Básicas, 14(1), 59–72. DOI 10.18359/rfcb.3108. [Google Scholar] [CrossRef]
6. Gustavsson, J., Cederberg, C., Sonesson, U., Otterdijk, R. V., Meybeck, A. (2012). Pérdidas y desperdicio de alimentos en el mundo. Alcance, causas y prevención. FAO, Italia: AGS Press. [Google Scholar]
7. Medina-Morales, M. A., Aguilera-Carbo, A. F., Rodríguez-Herrera, R., Aguilar, C. N. (2007). Cáscara de nuez fermentada para la producción de potentes antioxidantes fenólicos. XII Congreso Nacional de Biotecnología y Bioingeniería, México: Sociedad Mexicana de Biotecnología y Bioingeniería Press. [Google Scholar]
8. Villarreal-Lozoya, J. E., Lombardini, L., Cisneros-Zevallos, L. (2007). Phytochemicalconstituents and antioxidant capacity of different pecan [Carya illinoinensis (Wangenh.) K. Koch] cultivars. Food Chemistry, 102(4), 1241–1249. DOI 10.1016/j.foodchem.2006.07.024. [Google Scholar] [CrossRef]
9. de La Rosa, L., Alvarez, E., Shahidi, F. (2011). Phenolic compounds and antioxidant activity of kernels and shells of Mexican pecan (Carya illinoinensis). Journal Agriculture Food Chemistry, 59(1), 152–162. DOI 10.1021/jf1034306. [Google Scholar] [CrossRef]
10. Flores-Córdova, M. A., Berzoza-Vasquez, P., Sánchez-Chávez, E., Sáenz Solís, J. I., Guerrero-Morales, S. et al. (2016). Composición fisicoquímica y capacidad antioxidante del fruto del pecanero en condiciones de año de elevada producción (on) y de año de baja producción (off). Instituto Tecnológico de Educación Avanzada, 112(3), 255–270. DOI 10.12706/itea.2016.016. [Google Scholar] [CrossRef]
11. Flores-Córdova, M. A., Sanchez, C. E., Muñoz-Márquez, E., Ojeda-Barrios, D., Soto-Parra, J. et al. (2017). Phytochemical composition and antioxidant capacity in Mexican pecan nut. Emirates Journal of Food and Agriculture, 29(5), 346–350. DOI 10.9755/ejfa.EJFA-2016-08-1075. [Google Scholar] [CrossRef]
12. Vázquez-Flores, A. A., Álvarez-Parrilla, E., Rodrigo-García, J., de la Rosa, L. A. (2018). Cáscara de nuez pecanera en Alimentos vegetales autóctonos iberoamericanos subutilizados. In: Sáyago, A. S., Álvarez, P. E. (Eds.Alimentos vegetales autóctonos iberoamericanos subtuilizados. México: Fabro Press. [Google Scholar]
13. Feng, J., Zhang, X. L., Li, Y. Y., Cui, Y. Y., Chen, Y. H. (2016). Pinus massoniana Bark Extract: Structure-activity relationship and biomedical potentials. American Journal of Chinese Medicine, 44(8), 1559–1577. DOI 10.1142/S0192415X16500877. [Google Scholar] [CrossRef]
14. López-Alarcona, E., De Nicola, A. (2013). Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Analítica Chimica Acta, 763(Suppl. 1), 1–10. DOI 10.1016/j.aca.2012.11.051. [Google Scholar] [CrossRef]
15. Carvajal, C. C. (2019). Especies reactivas del oxígeno: Formación, función y estrés oxidativo. Medicina legal de Costa Rica, 36(1), 91–100. [Google Scholar]
16. Mital, M., Siddiqui, M. R., Tran, K., Reddy, S., Malik, A. B. (2014). Reactive oxygen species in inflammation and tissue injury. Antioxidants and Redox Signaling, 20(7), 1126–1167. DOI 10.1089/ars.2012.5149. [Google Scholar] [CrossRef]
17. Bahadoran, Z., Mirmiran, P., Azizi, F. (2013). Dietary polyphenols as potential nutraceuticals in management of diabetes: A review. Journal of Diabetes & Metabolic Disorders, 12(1), 1–9. DOI 10.1186/2251-6581-12-43. [Google Scholar] [CrossRef]
18. Özdemir, K. S., Yılmaz, C., Durmaz, G., Gökmen, V. (2014). Hazelnut skin powder: A new brown colored functional ingredient. Food Research International, 65(13), 1–7. DOI 10.1016/j.foodres.2014.01.060. [Google Scholar] [CrossRef]
19. Pérez-Perez, L. M., Arnebta-Villegas, L., Santacruz-Ortega, H., Gutiérrez-Lomelí, M., Aguilar, J. A. et al. (2017). Characterization of Anemopsis califórnica essential oil- β-cyclodextrin inclusion complex as antioxidant prolonged-release system. Chemical Papers, 71(7), 1331–1342. DOI 10.1007/s11696-016-0125-0. [Google Scholar] [CrossRef]
20. Vázquez-Flores, A. A., Wong-Paz, J. E., Lerma-Herrera, M. A., Martinez-Gonzalez, A. I., Olivas-guirre, F. J. et al. (2017). Proanthocyanins from the kernel and shell of pecan (Carya illinoinensisAverage degree of polymerization and effects on carbohydrate, lipid, and peptide hydrolysis in a simulated human digestive system. Journal of Funtional Foods, 28, 227–234. DOI 10.1016/j.jff.2016.11.003. [Google Scholar] [CrossRef]
21. Sun, Y. Y., Li, S. S., Zeng, F. H., Qi, J. Y., We, Q. et al. (2019). Functional components, antioxidant activity and hypoglycemic ability following simulated gastro-intestinal digestion of pigments from walnut brown shell and green husk. Antioxidants, 8(573), 1–14. DOI 10.3390/antiox8120573. [Google Scholar] [CrossRef]
22. Prior, R. L., Wu, X., Schaich, K. (2005). Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. Journal of Agricultural and Food Chemistry, 53(10), 4290–4302. DOI 10.1021/jf0502698. [Google Scholar] [CrossRef]
23. Venu, P., Holm, D. G., Jayanty, S. S. (2012). Effects of cooking methods on polyphenols, pigments and antioxidant activity in potato tubers. Food Science and Technology, 45(2), 161–171. DOI 10.1016/j.lwt.2011.08.005. [Google Scholar] [CrossRef]
24. Molyneux, P. (2004). The use of the stable radical dipheylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin Journal of Science Technology, 26(2), 211–219. [Google Scholar]
25. Re, R., Pellegrini, N., Protoggente, A., Pannala, A., Yang, M. et al. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine, 26(9–10), 1231–1237. DOI 10.1016/S0891-5849(98)00315-3. [Google Scholar] [CrossRef]
26. Lee, K. K., Kim, J. H., Cho, J. J., Choi, D. (1999). Inhibitory effects of 150 plant extracts on elastase activity, and their anti-inflammatory effects. International Journal of Cosmetic Science, 21(2), 71–82. DOI 10.1046/j.1467-2494.1999.181638.x. [Google Scholar] [CrossRef]
27. van-Campen, D. R., Glahn, R. P. (1999). Micronutrient bioavailability techniques: Accuracy, problems and limitations. Field Crops Research, 60(1–2), 93–113. DOI 10.1016/S0378-4290(98)00135-X. [Google Scholar] [CrossRef]
28. Tarko, T., DudaChodak, A., Sroka, P., Satora, P., Michalik, J. (2009). Tranformations of phenolic compounds in an in vitro model simulating the human alimentary tract. Food Technology and Biotechnology, 47(4), 456–463. [Google Scholar]
29. Yang, J., Chen, C., Zhao, S., Feng, G., Liu, D. (2014). Effect of solvents on the antioxidant activity of walnut (Juglans regia L.) shell extracts. Journal of Food and Nutrition Research, 2(9), 621–626. DOI 10.12691/jfnr-2-9-15. [Google Scholar] [CrossRef]
30. Alarcón, H. W., Jiménez, G. V., Ponce, S. H., Obregón, C. E., García, J. A. et al. (2016). Extracción de fitocompuestos de la cáscara de nuez pecanera del estado de chihuahua. Revista del centro de graduados e investigación. Instituto Tecnológico de Mérida, 31(63), 174–175. [Google Scholar]
31. Chandrasekaran, M. (2012). Valorization of food processing by-products, (1st ed.836. Boca de Raton, FL: CRC Press.DOI 10.1201/b12816. [Google Scholar] [CrossRef]
32. Flores-Córdova, M. A., Sánchez, C. E. (2016). Fitoquímicos y nutrientes en almendra y cáscara de nuez pecanera. Revista Internacional de Investigación e Innovación Tecnológica, 3(18), 1–10. [Google Scholar]
33. Flores-Estrada, R. A., Gámez-Meza, N., Medina-Juárez, L. A., Castillón-Campaña, L. G., Molina-Domínguez, C. C. et al. (2019). Chemical composition, antioxidant, antimicrobial and antiproliferative activities of wastes from pecan nut [Carya illinoinensis (Wagenh) K. Koch]. Waste and Biomass Valorization, 11(7), 3419–3432. DOI 10.1007/s12649-019-00681-2. [Google Scholar] [CrossRef]
34. Pinheiro do Prado, A. C., Monalise, A. A., Fett, R., Mara, B. J. (2009). Antioxidant properties of pecan nut [Carya illinoinensis (Wangenh.) C. Koch] Shell infusion. Grasas y Aceites, 60(4), 330–335. DOI 10.3989/gya.107708. [Google Scholar] [CrossRef]
35. Javier, R. A. (2019). Estudio de la cáscara de nuez pecana extruida y fermentada (Tesis de licenciatura). Universidad Politécnica de Catalunya, España. [Google Scholar]
36. Fernández-Agulló, A., Pereira, E., Freire, M. S., Valentão, P., Andrade, P. B. et al. (2013). Infuence of solvent on the antioxidant and antimicrobial properties of walnut (Juglans regia L.) green husk extracts. Industrial Crops and Products., 42(4), 126–132. DOI 10.1016/j.indcrop.2012.05.021. [Google Scholar] [CrossRef]
37. Girardi, M. L., Simonetti, P., Reckziegelb, R., Barcelosb, C. S., Boufleura, N. et al. (2013). Hepatoprotective effects of pecan nut shells on ethanol-induced liver damage. Experimental and Toxicologic Pathology, 65(1-2), 165–171. DOI 10.1016/j.etp.2011.08.002. [Google Scholar] [CrossRef]
38. Blomhoff, R., Carlsen, M. H., Frost, A. L., Jacobs, D. R. (2006). Health benefits of nuts, potential role of antioxidants. British Journal of Nutrition, 96(S2), 52–60. DOI 10.1017/bjn20061864. [Google Scholar] [CrossRef]
39. García-Romo, J. S., Noguera-Artiaga, L., Galvez-Iriqui, A. C., Galvez-Iriqui, A. C., Hernandez-Zazueta, M. et al. (2020). Antioxidant, antihemolysis, and retinoprotective potentials of bioctive lipidic compunds from wild shrimp (Litopenaeus stylirostris) muscle. CyTA–Journal of Food, 18(1), 153–163. DOI 10.1080/19476337.2020.1719210. [Google Scholar] [CrossRef]
40. Hwang, E., Park, S. Y., Lee, H. J., Lee, T. Y., Sun, Z. W. et al. (2014). Gallic acid regulates skin photoaging in UVB-exposed fibroblast and hairless mice. Phytotherapy Research, 28(12), 1778–1788. DOI 10.1002/ptr.5198. [Google Scholar] [CrossRef]
41. Trevisan, G., Rossato, M. F., Hoffmeister, C., Muller, L. G., Pase, G. et al. (2014). Antinociceptive and antiedematogenic effect of pecan (Carya illinoensis) nut shell extract in mice: A possible beneficial use for a by-product of the nut industry. Journal of Basic and Clinical Physiology and Pharmacology, 25(4), 1–10. DOI 10.1515/jbcpp-2013-0137. [Google Scholar] [CrossRef]
42. Mateo, N., Berg, R., van, D., Havenaar, R., Bast, A. et al. (2009). Bioavailability of ferulic acid is determined by its bioaccessibility. Journal Cereal Science, 49(2), 296–300. DOI 10.1016/j.jcs.2008.12.001. [Google Scholar] [CrossRef]
43. Hemery, Y., Mateo, N., Havenaar, R., Haenen, G., Noort, M. et al. (2010). Dry fractionation of wheat bran increases the bioaccessibility of phenolic acids in breads made from processed bran fractions. Food Research International, 43(5), 1429–1438. DOI 10.1016/j.foodres.2010.04.013. [Google Scholar] [CrossRef]
44. Rodríguez-Roque, M. J., de Ancos, B., Sánchez-Vega, R., Sánchez-Moreno, C., Elez-Martínez, P. et al. (2020). In vitro bioaccessibility of isoflavones from a soymilk-based beverage as affected by thermal and non-thermal processing. Innovative Food Science and Emerging Technologies, 66(17), 1–8. DOI 10.1016/j.ifset.2020.102504. [Google Scholar] [CrossRef]
45. Herbello-Hermelo, P., Lamas, J. P., Lores, M., Domínguez-González, R., Bermejo-Barrera, P. et al. (2018). Polyphenol bioavailability in nuts and seeds by an in vitro dialyzability approach. Food Chemistry, 254(15), 20–25. DOI 10.1016/j.foodchem.2018.01.183. [Google Scholar] [CrossRef]
46. Lafarga, T., Rodríguez-Roque, M. J., Bobo, G., Villaró, S., Aguiló-Aguayo, I. (2019). Effect of ultrasound processing on the bioaccessibility of phenolic compounds and antioxidant capacity of selected vegetables. Food Science and Biotechnology, 28(6), 1713–1721. DOI 10.1007/s10068-019-00618-4. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |