

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2022.020750
REVIEW
Research Progress of Soybean Protein Adhesive: A Review
1MOE Key Laboratory of Wooden Material Science and Application & Beijing Key Laboratory of Wood Science and Engineering, Beijing Forestry University, Beijing, 100083, China
2College of Materials Science and Engineering, Nanjing Forestry University, Nanjing, 210037, China
3College of Engineering Department of Mechanical and Energy Engineering, University of North Texas, Denton, 76203, USA
4State Forestry and Grassland Administration Key Laboratory of Silviculture in Downstream Areas of the Yellow River, College of Forestry, Shandong Agricultural University, Tai’an, 271000, China
*Corresponding Authors: Qiang Gao. Email: gaoqiang@bjfu.edu.cn; An Mao. Email: dannymaoan@126.com
Received: 12 December 2021; Accepted: 01 March 2022
Abstract: Traditional formaldehyde-based adhesives rely excessively on petrochemical resources, release toxic gases, and pollute the environment. Plant-derived soybean protein adhesives are eco-friendly materials that have the potential to replace the formaldehyde-based adhesives used to fabricate wood-based panels. However, the poor water resistance, high brittleness, and poor mildew resistance of soybean protein adhesives limit their industrial applications. This article reviews recent research progress in the modification of soybean protein adhesives for improving the bonding performance of adhesives used for wood-based panel fabrication. Modification methods were summarized in terms of water resistance, solid content, and mildew resistance. The modification mechanisms and remaining problems were also discussed. Finally, the current industrial applications and the future research direction of soybean protein adhesives are discussed.
Keywords: Soybean protein adhesive; bonding performance; water resistance; solid content; mildew resistance
Wood-based panels play an increasingly important role in the furniture and indoor decoration industries. Due to the rapid development of the wood-based panel manufacturing industry, the consumption of wood adhesives has become an important indicator of the wood-based panel industry development level [1,2]. In the late twentieth century, the wood adhesive market was dominated by formaldehyde-based adhesives, such as urea-formaldehyde (UF) resins, phenol-formaldehyde (PF) resins, and melamine-formaldehyde (MF) resins. These formaldehyde-based adhesives accounted for more than 90% of total wood adhesives, indicating an annual consumption of about seventeen million tons of formaldehyde-based adhesives. Formaldehyde-based resins were extensively used because of their good dry-bonding performance, low price, and high production efficiency [3,4]. However, formaldehyde-based adhesives release toxic substances, such as phenol and formaldehyde, which are first-class carcinogens, according to the World Health Organization (WHO) [5]. Due to increasingly strict environmental protection regulations, petrochemical resource shortages, and improved living standards, non-toxic, renewable, and eco-friendly wood adhesives are demanded. The bio-based adhesive is made of natural polymers, in line with the current sustainable development goals [6]. Therefore, the replacement of harmful formaldehyde-based resins with bio-based wood adhesives has become an important research direction [7].
Soybean protein adhesives use a plant resource, soybean meal, as the raw material and water as the dispersion medium, which are green, non-toxic, and sustainable bio-based adhesives that mainly used in wood-based panel manufacturing [8]. Soybean protein adhesives are completely different from formaldehyde-based adhesives, they use natural soybean protein as the raw material, and completely free of harmful substances such as phenol and formaldehyde. The application of soybean protein adhesives in the wood industry can be traced back to the 1920s. In 1923, Johnson et al. [9] developed an adhesive based on defatted soy flour, but its viscosity was high, and extrusion-type sizing methods were generally used, so it was only suitable for the plywood industry. In later research, the formula added carbon disulfide, lime, sodium silicate and some anti-fungal agents [10]. Until 1942, soy adhesives occupied 85% of the American plywood market. However, due to its short pot life, low solid content, poor biocorrosion resistance, especially poor water resistance, it can only be used indoors [11]. Petroleum-based adhesives entered the wood industry in the 1940s. Compared with traditional soy protein adhesives in terms of quality, cost and bonding performance, they showed obvious advantages. Since then, soy protein adhesives have withdrawn from the dominant market of wood adhesives [12]. However, formaldehyde-based adhesives are overly dependent on petrochemical resources and release toxic gas. In recent years, petrochemical resources are gradually depleted, soybean protein adhesives have shown great development potential because of their wide range of raw materials, renewability, and degradability [13]. Soybean protein adhesives are expected to replace formaldehyde-based adhesives to become the primary adhesive product in the wood-based panel market, which will promote the sustainability of the wood-based panel industry. However, soybean protein adhesives have poor water resistance, high viscosity, brittleness, and poor mildew resistance, which restrict their industrial applications [14]. This is because the curing mechanism of the soybean protein adhesive involves thermal denaturation and dehydration of protein molecules. After curing, the adhesive strength is derived from the entanglement of protein molecules to produce a mechanical locking force and hydrogen bonding between polar groups. Therefore, the curing structure of soybean protein adhesive is easily eroded by water, resulting in poor water resistance, low bonding performance, and slow curing rate, which are different from those of the network structure formed by polycondensation of formaldehyde resin and other thermosetting resins. At the same time, the molecular weight of soybean protein is relatively high and the interaction of side chain groups increases the intermolecular friction. In addition, the soybean meal (SM) raw material also contains large amounts of polysaccharides, resulting in a high viscosity, and easy mildewing.
Many researchers have made various attempts to modify soybean protein adhesives to improve their bonding performance, including water resistance modification, such as physical [15], chemical [16], and enzyme modification [17]; increasing solid content modification; and mildew resistance modification. Modified soybean protein adhesives have been used industrially in veneer-based boards such as blockboard, engineering flooring, and plywood, but the output accounted for less than 0.1% of the total output of wood adhesives due to remaining issues that restrict their industrial applications.
2 Modification Methods to Improve Water Resistance
Physical modification mainly uses mechanical methods to change the aggregate form and polymer structure of soybean protein molecules without changing the primary structure of soybean protein. The main physical modification methods include freezing [18], heating [19], high pressure [20], shear radiation [21], and ultrasonic treatment [22]. These methods use different physical methods to process soybean protein, which essentially denatures the soybean protein molecules [23]. Zhang et al. [24] proposed a simple and clean physical high-pressure homogenization method that used an eco-friendly cross-linking agent to develop a stable, high-performance bio-based adhesive. The results showed that the high-pressure homogenization treatment reduced the soybean meal (SM) particle size by 62%, improved the uniformity of the particle size distribution, and significantly improved the adhesive stability. In addition, high-pressure homogenization exposed many active functional groups in soybean protein and increased their reactivity with cross-linking agents. This helped form a dense covalent network in the adhesive system, which improved the water resistance of the SM adhesive. The wet shear strength of plywood bonded using the modified SM adhesive was 212% higher than that of an untreated one. The principle diagram of high-pressure homogenization modification is shown in Fig. 1. To improve the adhesive properties of soybean protein adhesives, Vnučec et al. [15] proposed a new method to obtain thermally-modified soybean protein adhesives at different temperatures. The results showed that adjusting the pH to 10, increased the viscosity, adhesive penetration, and bonding strength of the adhesive. After thermal modification at 50°C, the wet shear strength was improved, but it decreased after thermal modification at 100°C. Fan et al. [25] combined thermal alkali degradation, thermal acid treatment, and cross-linking to develop a soybean protein wood adhesive with a high bonding strength, good water resistance, and processability. The results showed that thermal alkali degradation improved the processability, thermal acid treatment significantly improved the water resistance, and cross-linking significantly improved the bonding strength and water resistance of the soybean protein adhesive. The type of cross-linking agent, the ratio of hot alkali-degraded soybean protein, and thermal-acid-treated soybean protein also significantly affected the main properties of the soybean protein adhesive. Bacigalupe et al. evaluated four stages of chemical modification of soy protein isolate (SPI): unmodified (U), denatured (D), partially hydrolyzed (PH), and fully hydrolyzed (H). Rheological analysis showed that U behaved as a gel-like substance, while PH and H behaved as viscous substances. Hydrolysis results in a significant reduction in viscosity, even for high solids suspensions. In addition, compared to U and H, the viscosity at high shear strength decreased by 65% and the solids content increased by 219%. The shear strength of the adhesive can be improved by two strategies: crosslinking with epoxy resin and adding different reinforcing agents [26]. Physical modification is an inexpensive and quick modification method that does not produce toxic products. It can increase the solubility of soy protein. By changing the internal structure of soybean protein molecules and exposing internal groups, the bonding performance of soybean protein adhesive is improved. However, hydrophilic groups are exposed during the physical modification process, which will reduce the water resistance of the soy protein adhesive.
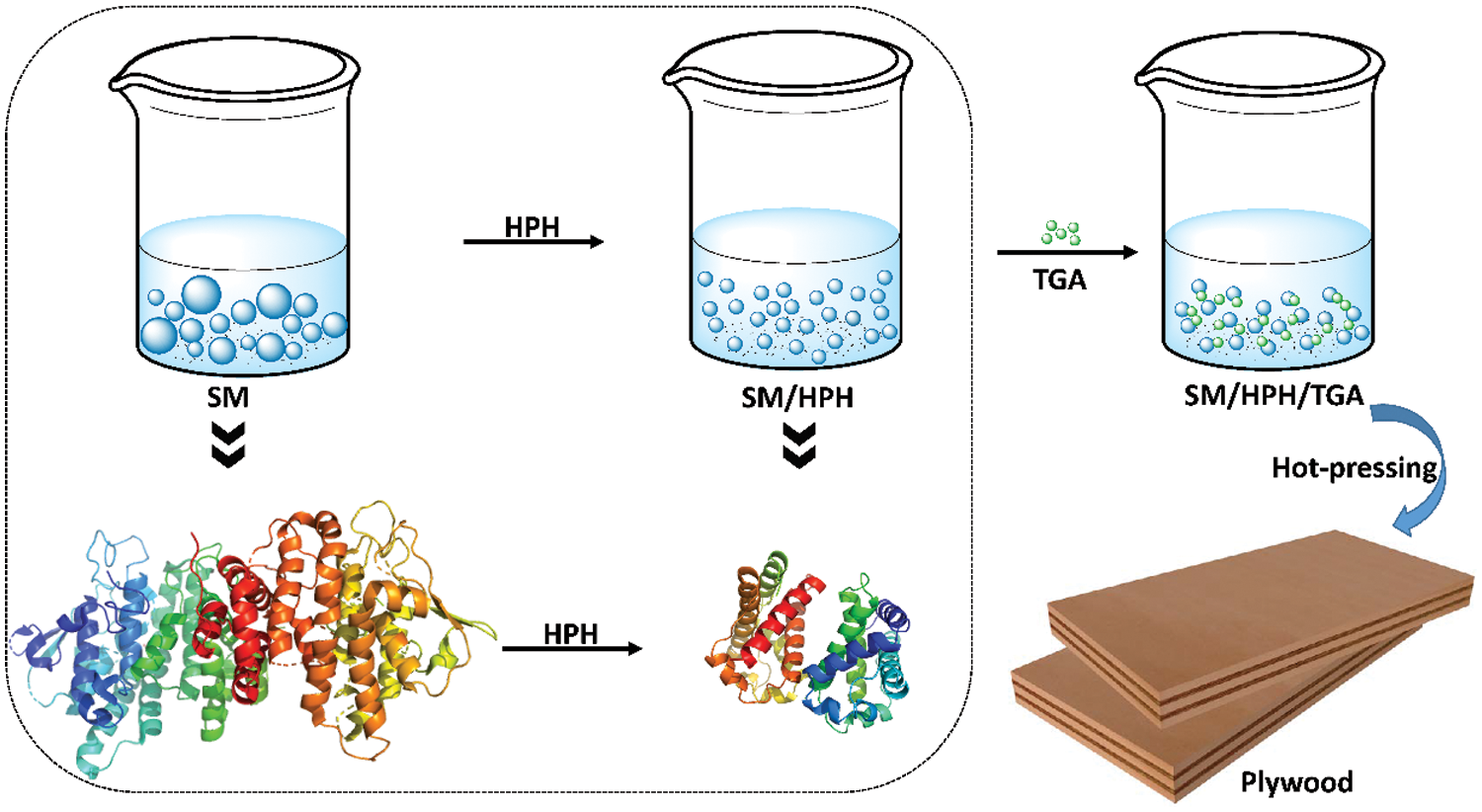
Figure 1: The high-pressure homogenization treatment and preparation process of SM adhesive. Reprinted with permission from [24]
In recent years, chemical modification has been the main method used by researchers to improve the bonding properties of soybean protein adhesives. In chemical modification methods, macromolecular polymers are used to change the functional groups on protein molecules or polypeptide chains via chemical reactions. They can also change bonds between protein molecules and other molecules, thereby changing the molecular structure of soybean protein. Commonly used chemical modification methods include the use of surfactants, acid-base reactions, urea and guanidine hydrochloride, acylation, cross-linking, grafting, bionic, and nanomaterials modification.
Acid-base modification exploits changes in the protein structure when the pH is changed by adding either an acid or alkali. This changes the charge of the soybean protein peptide, which changes the attractive forces between peptide chains. After the soybean protein unfolding, polar and nonpolar groups are exposed [27]. Alkali denaturation occurs when the carboxyl groups (-COOH) in the protein are neutralized and form carboxylate anions under alkaline conditions, generating repulsive forces between the anions. The soybean protein molecular chains are then unfolded, opening the soybean protein spherical structure and exposing polar functional groups. Alkali denaturation can improve the dry bonding strength of adhesive, but it also expands the protein molecular chain and increases the viscosity [28]. Alkali denaturing reagents mainly include NaOH, Ca(OH)2, ammonia, and borax. Chang et al. [29] dissolved waste paper in an alkali-urea system and oxidized sodium periodate to prepare diformaldehyde cellulose (DAC), and produced an SM-based adhesive. The results showed that the wet bonding strength of the SM/DAC/PTGE adhesive reached 1.27 MPa, which was 95% higher than that of unmodified SM/PTGE adhesive. After oxidation, DAC could cross-link with the adhesive matrix through a Schiff base reaction, and hydrogen bonds formed at the interface between the DAC and bonding substrate. A good interface combination and a dense cross-linked network were formed in the SM/DAC/PTGE system. Samson et al. [30] fabricated a Rhizophora spp. particleboard using an SPI-based adhesive modified with sodium hydroxide and itaconic acid-polyamidoamine-epichlorohydrin (IA-PAE) (0, 5, 10, and 15 wt%). The results showed that the SPI-based/NaOH/IA-PAE/Rhizophora spp. particleboard with the addition of 15 wt% IA-PAE had the highest solid content, flexural strength, flexural modulus and internal bond strength, which effectively improved the mechanical properties of the particleboard. The particleboard made with modified adhesive has potential as a suitable tissue-equivalent phantom material for healthcare applications. Acid modification induces protein structure changes and increases the surface hydrophobicity and solubility of soybean protein [31]. Liu et al. [32] developed and characterized undecylenic acid (UA)-modified soybean protein to improve its water resistance. Fourier-transform infrared (FTIR) spectroscopy and ninhydrin tests confirmed that the reaction between the amine group of the protein and the carboxyl group of UA was the main chemical pathway for grafting. Thermogravimetric analysis and differential scanning calorimetric analysis indicated that UA modification reduced the thermal stability due to reduced protein unfolding and protein-protein cross-linking. The wet strength of the modified soybean protein adhesive was significantly increased by 35%−62% compared with the control one. Zheng et al. [33] used 1,2,3,4-butanetetracarboxylic acid (BTCA) as a raw material and sodium hypophosphite (SHP) as a catalyst to develop an environmentally friendly defatted soybean flour bioadhesive. BTCA cross-linked proteins and carbohydrates in defatted soy flour via the formation of esters and amides. The wet shear strength of the modified bioadhesive increased (1.36 MPa), and the sol fraction decreased by 24.8%. The modified bioadhesive displayed better thermal stability with a more uniform surface. The results suggested that BTCA can be used to prepare high-performance environmentally-friendly defatted soybean flour bioadhesives. The schematic diagram of BTCA-modified soybean protein adhesive is shown in Fig. 2. Acid-base modification of soybean protein can improve the bonding strength of soybean protein adhesive, but it is easy to cause problems such as high viscosity and difficult surface sizing. In addition, the acid-base treatment exposed the internal active groups of soybean protein and reduced the water resistance of the adhesive.
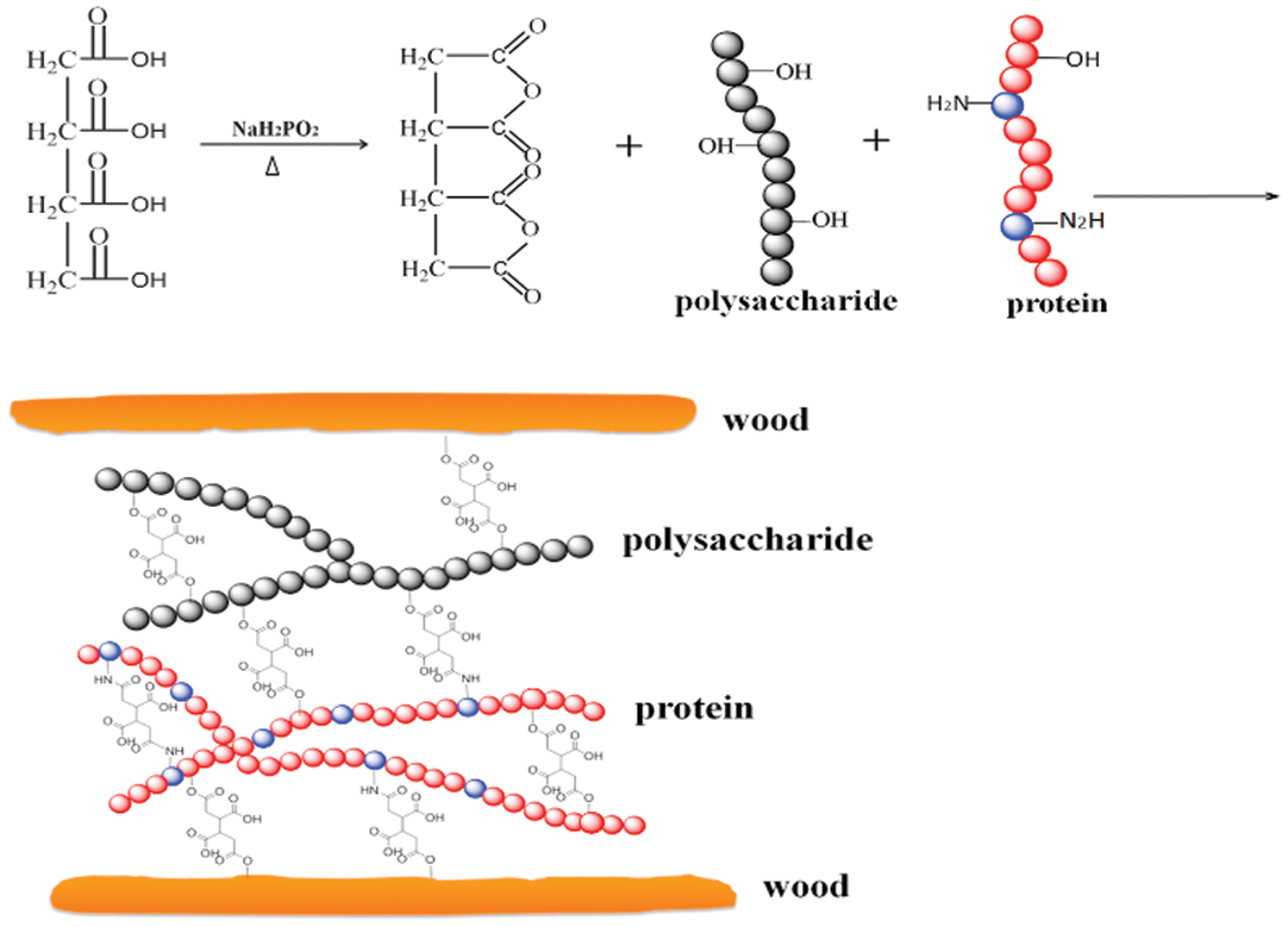
Figure 2: The schematic diagram of 1,2,3,4-butanetetracarboxylic acid-modified soybean protein adhesive. Reprinted with permission from [33]
The molecular chain of soybean protein contains both hydrophilic and hydrophobic functional groups, which have different surface activities. When the surfactant is added, the surfactant combine with the polypeptide chain, destroying the hydrophilic and hydrophobic interactions on the original polypeptide chain. This combination can also break hydrogen bonds, thereby improving the bonding strength and water resistance of the adhesive. Surfactants commonly used in soybean protein include sodium dodecyl sulfate (SDS) and sodium dodecyl sulfonate (SDBS). Zhang et al. [34] studied the effects of different concentrations of sodium sulfate, urea, and sodium dodecyl sulfate (SDS) on the liquefaction properties of soybean protein. The results showed that by adding sodium sulfate, urea, SDS, and sodium hydroxide, soybean protein formed a homogeneous liquefied product with high solid content and low viscosity. Moreover, under the optimal liquefaction conditions (sodium sulfite 1.5 wt%, urea 5 wt%, SDS 1.5 wt%, and sodium hydroxide 3 wt%), the water-resistant bonding strength of the plywood bonded by the adhesive after water treatment reached 1.08 MPa, which met the commercial standard JIS K6806-2003. Xu et al. [35] developed soybean protein-based adhesives by using soybean flour as the main raw material, combining SDS and polyacrylamide (PAM). The results showed that after adding 0.2% SDS and 0.01% PAM, the water resistance of the adhesive increased by 55.4% (from 0.83 to 1.29 MPa). The holes and cracks on the fracture surface of the cured adhesive were reduced, and the plywood bonded with SM/SDS/PAM adhesive met the requirements for indoor plywood usage. SDS and cationic PAM had opposite charges, they formed chemical bonds with oppositely-charged amino acids, which made the adhesive network structure denser. The schematic diagram of a surfactant-modified soybean protein adhesive is shown in Fig. 3. Surfactant modification can effectively improve the adhesive strength and water resistance of the adhesive, but it usually increases the intermolecular force of protein, thus increasing the viscosity of the adhesive.
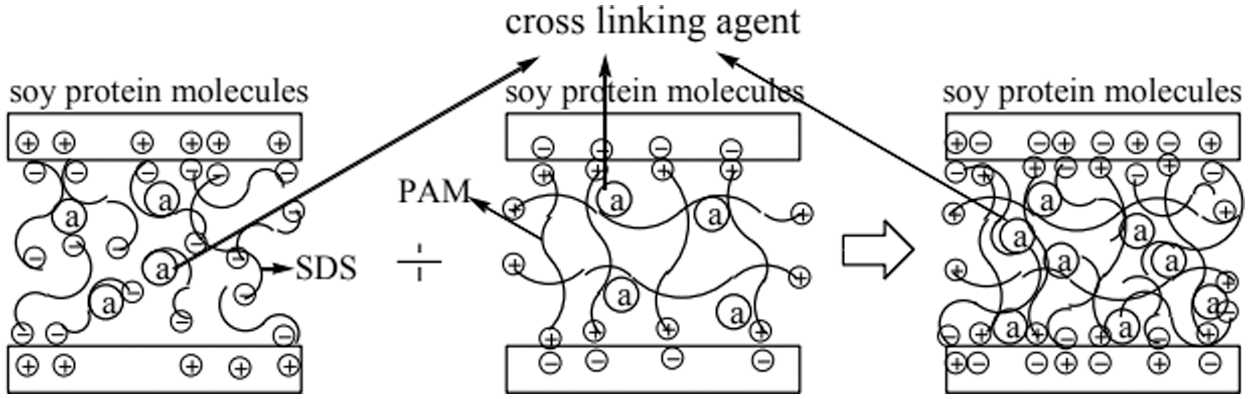
Figure 3: The principle diagram of surfactant-modified soybean protein adhesive. Reprinted with permission from [35]
2.2.3 Urea and Guanidine Hydrochloride (GuHcl) Modification
Urea is a small molecule that can deform proteins, which expands the secondary helix structure of protein molecules. The amino hydrogen and carbonyl oxygen in urea can react with the hydroxyl groups of proteins to destroy internal hydrogen bonding, which causes protein molecules to expand [36,37]. Wu et al. [38] used soybean hydrolyzed protein, melamine, urea, and formaldehyde as raw materials to synthesize a soybean protein-melamine-urea-formaldehyde copolymer resin. The results showed that the thermal stability of the modified adhesive increased, the degree of crosslinking increased, and the wet bonding strength was improved. Pereira et al. [39] studied the application of soybean protein as a natural formaldehyde scavenger in the production of wood particleboard. Soybean protein was incorporated in two forms: a) as a powder during the mixing of wood particles and UF resin; b) as an aqueous solution during the UF resin synthesis. The results showed that soy protein helped reduce the formaldehyde emission from particleboard. When added as a powder or solution during resin polycondensation, the soybean protein had the best formaldehyde reduction effect without significantly affecting the physical properties of the panel.
Guanidine hydrochloride (GuHCl) is a white or slightly yellow solid substance with a structure similar to urea, and it has a similar modification effect on soybean protein [40]. Zhong et al. [41] studied the adhesion properties of GuHCl-modified soybean protein isolate (SPI) on fiberboard, and the results showed that the performance of soybean protein adhesive was affected by its conformation. After modification with GuHCl, the protein conformation loosely expanded, which improved the shear strength of the soybean protein isolate adhesive on the fiberboard. Both urea and GuHCl can break the hydrogen bonds of protein molecules in high concentration aqueous solutions, causing different degrees of denaturation. It can also increase the solubility of hydrophobic residues in water and increase hydrophobic interactions between proteins. When the urea concentration is too high, the degree of expansion of the SPI molecules would beome too large, which adversely affects the bonding strength.
Succinylation and acetylation are commonly used acylation reactions for soybean protein modification [42]. However, because they introduce more hydrophilic groups, modified adhesives usually have unsatisfactory water resistance. Hydrophobic maleic anhydride and octene-1-succinic anhydride are more suitable. The molecular structure of the protein is expanded after acylation, the amount of static charges is reduced, and the isoelectric points are also decreased. Matemu et al. [43] studied the effect of acylation of different saturated fatty acids on the emulsifying properties of soybean protein. The results showed that saturated fatty acids with sufficient chain lengths were ideal options for preparing soybean protein as a functional lipoprotein. Xi et al. [44] used soybean protein isolate to react with maleic anhydride first and then mixed it with hexamethylenediamine to synthesize a soybean protein isolate polyamide adhesive. The results showed that both the dry and wet shear strengths of the bonded panel were improved, and the modified adhesive was a suitable soybean protein-based wood adhesive. Qi et al. [45] studied the adhesion properties of 2-octen-1-ylsuccinic anhydride (OSA)-modified soybean protein adhesive at different concentrations. Through reactions between the amine group, hydroxyl group of the protein, and the anhydride, OSA was grafted onto the soybean protein molecule. When the OSA concentration was 3.5%, the wet strength of the two-layer plywood bonded by the soy protein adhesive was 3.2 MPa. As the OSA concentration further increased, the strength stabilized. The oily and hydrophobic long alkyl chain of OSA is the main reason for the improved bonding strength of soybean protein adhesive. A schematic of the reaction mechanism occurring between soybean protein adhesive and OSA is shown in Fig. 4.
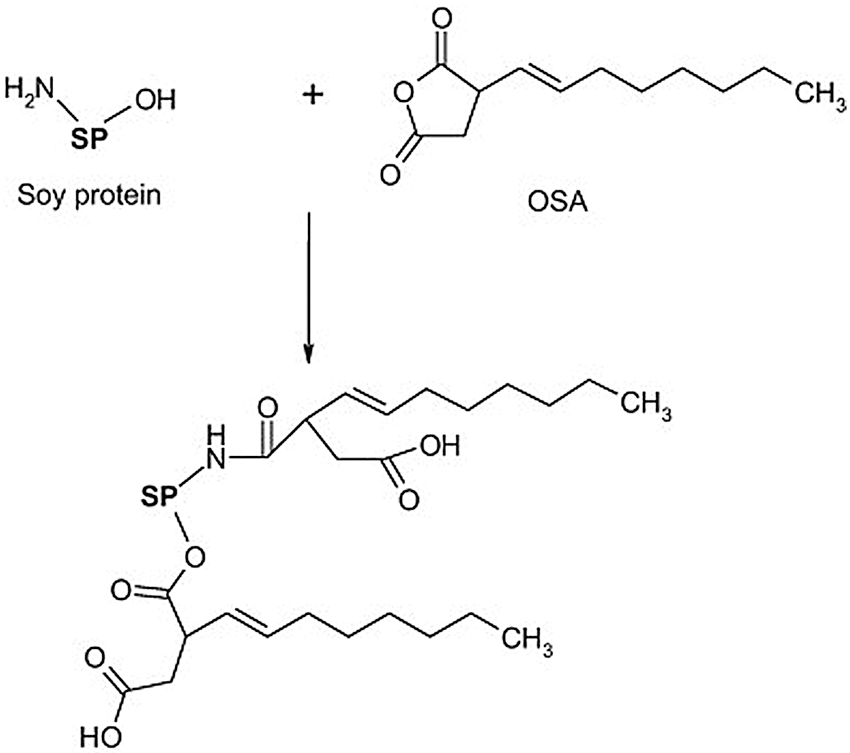
Figure 4: The schematic illustration of the reaction mechanism between soybean protein adhesive and OSA. Reprinted with permission from [45]
Graft modification involves the introduction of active sites onto the soybean protein molecules through chemical reactions. These active sites can react with active groups or monomers with double bonds. When the molecular structure of soybean protein changes, the properties of the adhesive also undergo corresponding changes [46]. Based on the composition and characteristics of soybean protein, many researchers have designed and grafted functional groups and special structures onto soybean protein molecules to improve the performance of soybean protein adhesives. Gu et al. [47] used aramid fiber (AF) with a high surface roughness and grafting degree to develop co-deposited catechol/polyamine and pyrogallic acid/polyamine, which was utilized as a reinforcing agent to improve the performance of SPI-based adhesives. Polyhydrogen covalent bonds between the side chain groups of SPI and functionalized AF greatly improved the bonding strength and water resistance of the adhesive, and the wet shear strength of the plywood prepared using the adhesive was increased by 133.3% to 1.68 MPa. Moreover, the active catechol groups and pyrogallol groups provided the adhesive with enhanced bactericidal activity [47]. The grafting mechanism of functionalized AF is shown in Fig. 5. The graft modification was greatly affected by the molecular chain composition and degree of polymerization of the graft.
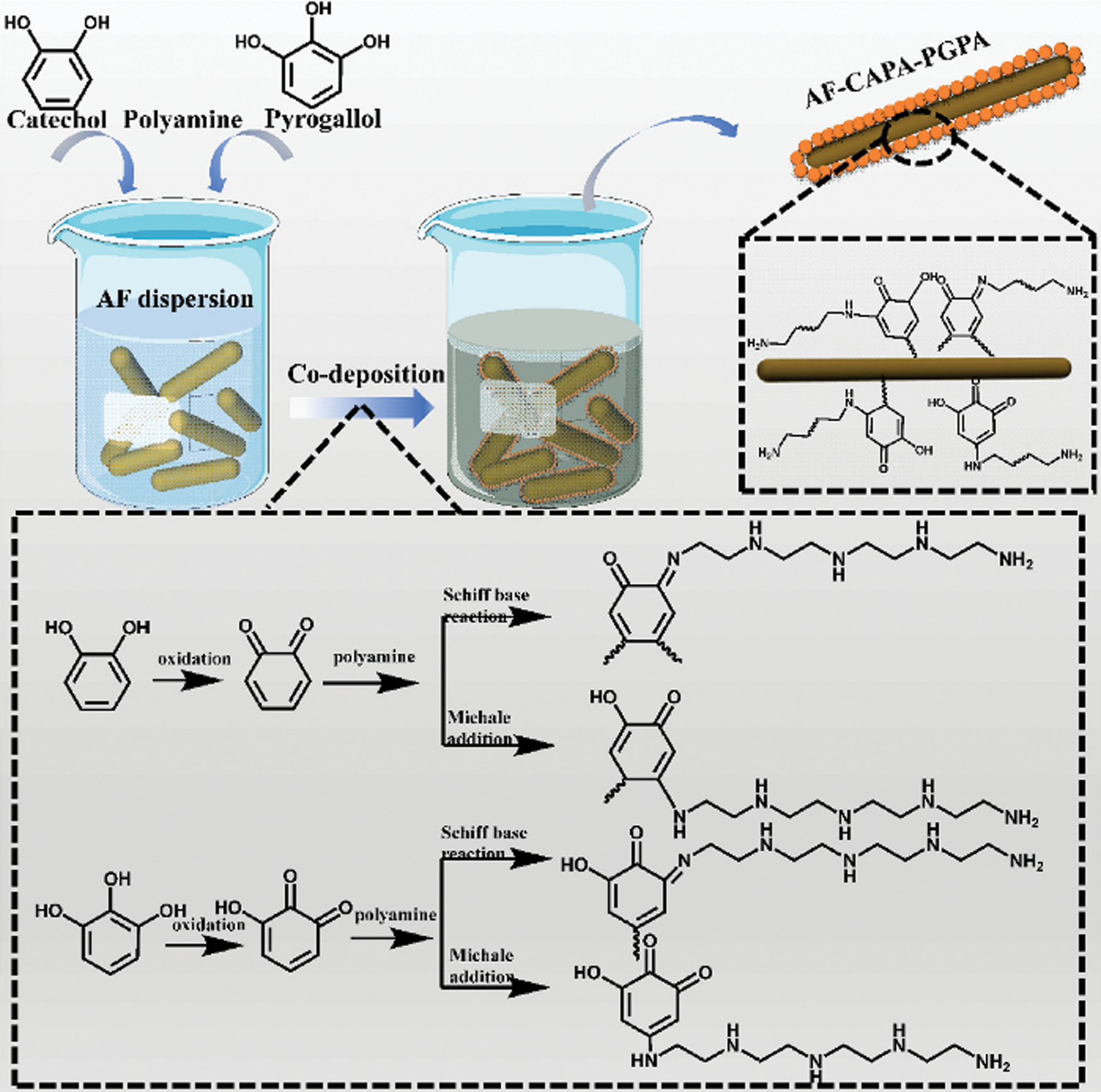
Figure 5: The grafting mechanism of functional AF. Reprinted with permission from [47]
2.2.6 Cross-Linking Modification
Cross-linking agents are substances that react with and connect multiple molecules into a network structure. By adding a highly-reactive cross-linking agent, the active groups on the cross-linking agent, the amino groups/carboxyl groups on the soy protein, and the active groups on the side chains undergo cross-linking reactions [8]. As the crosslink density increases, the bonding strength and water resistance of the adhesive are usually improved. Cross-linking is the most commonly used method to modify soybean protein adhesives, and it can effectively improve the bonding performance. Jin et al. [48] prepared hyperbranched silicone and reacted it with catecholamine-based tannic acid and soybean meal to obtain a strong antibacterial bio-based adhesive with a hyperbranched cross-linked structure. In addition, copper ions are added into the adhesive to form a variety of interface coordination bonds. The results showed that the bonding strength and water resistance of the modified adhesive were improved, and it also showed good antibacterial properties because copper ion was added to the adhesive to form interface coordination bonds. The resulting metal coordination bonds and covalently cross-linked double network in the adhesive system increased the cross-linking density, as well as the cohesion of the adhesive, which improved the bonding strength and water resistance. The maximum wet shear strength of the modified adhesive was 1.27 MPa, which was 309.68% higher than the original soybean protein adhesive. Chen et al. [49] prepared non isocyanate polyurethane (NIPU) thermosetting wood adhesive by reacting soybean protein isolate (SPI) with dimethyl carbonate (DMC) and hexamethylene diamine. Linear and branched oligomers were obtained and identified, these oligomers can be further crosslinked to form hardened networks. The results show that the dry adhesive strength of plywood prepared with SPI-NIPU wood adhesive was improved, which met the requirements of relevant indoor plywood standards. However, it did not meet the requirements of wet bonding strength. Xu et al. [50] synthesized a multifunctional cross-linking agent by reacting soybean daidzein with epichlorohydrin (DDE) and combining it with soybean protein (SPI) to produce a 100% bio-based wood adhesive, with significantly improved water and mildew resistance. The results showed that after adding 6 wt% DDE to the adhesive formulation, compared with the SPI adhesive, the dry and wet shear strengths of the plywood bonded with SPI and DDE adhesives increased by 52.3% and 164.4%, respectively, compared with industrial SPI/PAE (polyamide epichlorohydrin) adhesives, The bonding strength of SPI/DDE-6 wt% were 22.3% and 69.6% higher, respectively. These improvements were attributed to the formation of a double cross-linked network and improved bonding toughness. The cross-linking mechanism of the soybean protein adhesive is shown in Fig. 6. Ghahri et al. [51] studied the feasibility of three natural tannins (quebracho, mimosa and chestnut tannins) as soybean adhesive crosslinking materials. The results showed that tannins formed ionic and covalent bonds with soybean protein at room temperature; however, at higher temperatures, covalent bonds were dominant. The hydrolyzed tannin (chestnut) and condensed flavonoid tannin (mimosa) were used to prepare two types of soybean-based adhesives (soybean powder (SF) and soybean protein isolate) for plywood. Three ply plywood was prepared by adding 300 g/m2 of the adhesive total resin solid content composed of soybean protein adhesive, urea, chestnut and mimosa tannin extract with hexamine as hardener. The results showed that the soybean-based adhesive modified by tannin had good adhesion and water resistance [52]. In addition, hydrolyzed tannin and condensed flavonoid tannin were used together with hexamine and glyoxal to improve the properties of soybean protein adhesive for the preparation of wood particleboard. The results showed that tannin effectively reduce the viscosity of soybean-based adhesive, hydrolyzed tannin extract reacted well with soybean powder and improved the adhesion of soybean powder. In addition, the internal bonding strength of particleboard prepared by tannin modified adhesive was improved, and the thickness expansion and water absorption of particleboard were reduced [53].
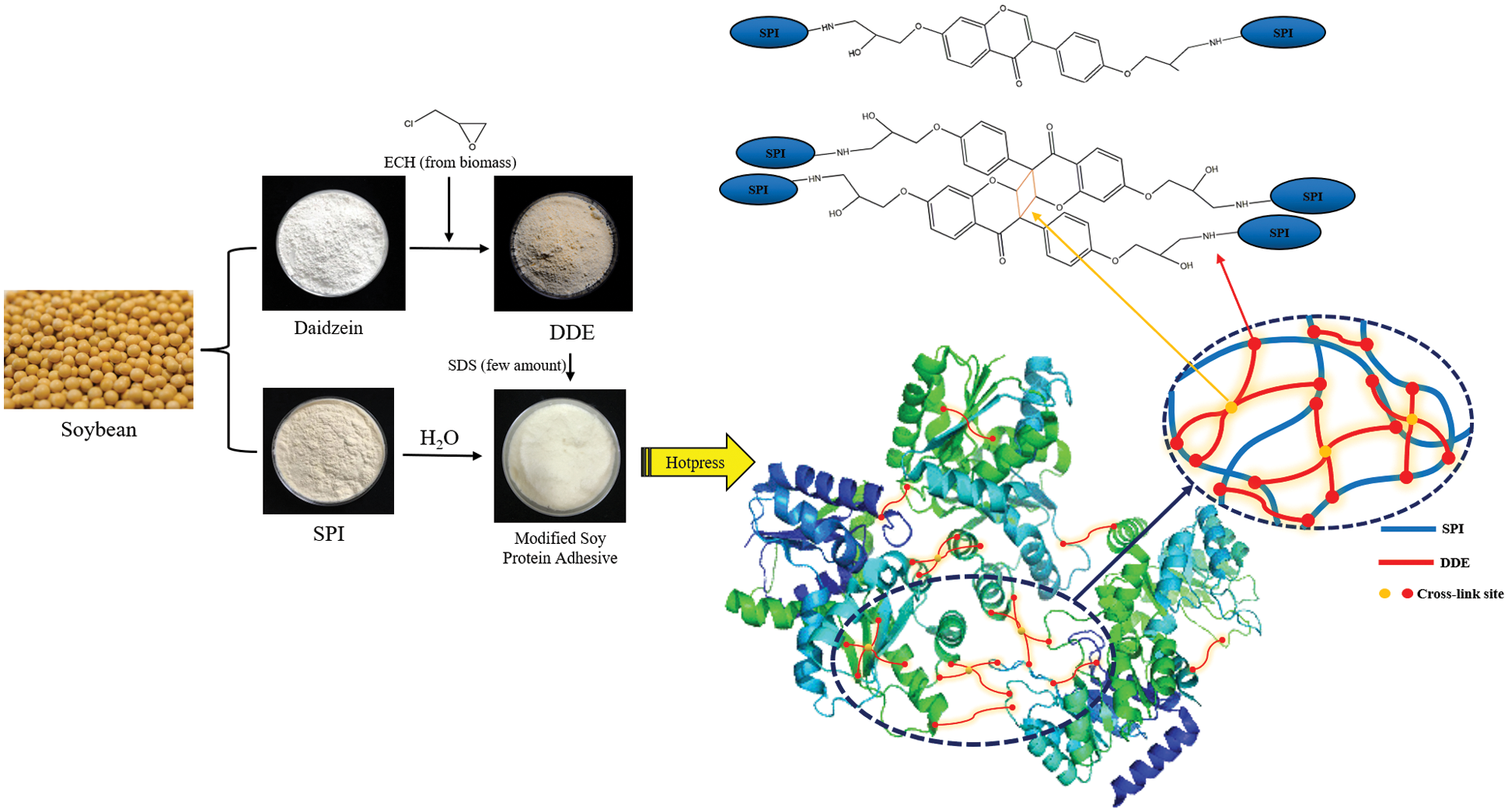
Figure 6: Cross-linked network structure between DDE and SPI molecules. Reprinted with permission from [50]
Cross-linking modification can consume the hydrophilic groups of protein molecules and improve the water resistance of the prepared adhesive. The preparation process of cross-linked modified soybean protein adhesives is also simple, and the performance of the adhesive is controllable, making it one of the most effective modification methods. Epoxy, emulsion, and synthetic resin cross-linking agents are also used to enhance soybean protein adhesives, and they have been applied in industrial production. Cross-linking modification can effectively improve the bonding performance of soybean protein adhesives. However, the increase of crosslinking density of crosslinked modified adhesive leads to the increase of brittleness of adhesive.
The use of bionic technology to modify soybean protein adhesives has become a popular research topic. Shellfish secrete sticky substances that cause them to firmly adhere to objects, such as ships and reefs, so that they can resist displacement by sea waves and seawater. By imitating the bonding mechanism of shellfish viscous substances, soybean protein adhesives were modified to improved water-resistant bonding performance. The main components of mucus secreted by mussels are lysine and 3,4-dihydroxyalanine. 3,4-dihydroxyalanine contains phenolic hydroxyl groups that can chelate with metal ions and form coordination bond. Moreover, the phenolic hydroxyl group is easily oxidized to o-benzoquinone, which can react with the amino group in protein molecules, further improving the water resistance. The amino group of dopamine can react with the carboxyl group of soybean proteins. A structure similar to 3,4-dihydroxyalanine is introduced into soybean protein adhesives, which improves the water-resistant bonding performance. The schematic diagram of the bonding is shown in Fig. 7.
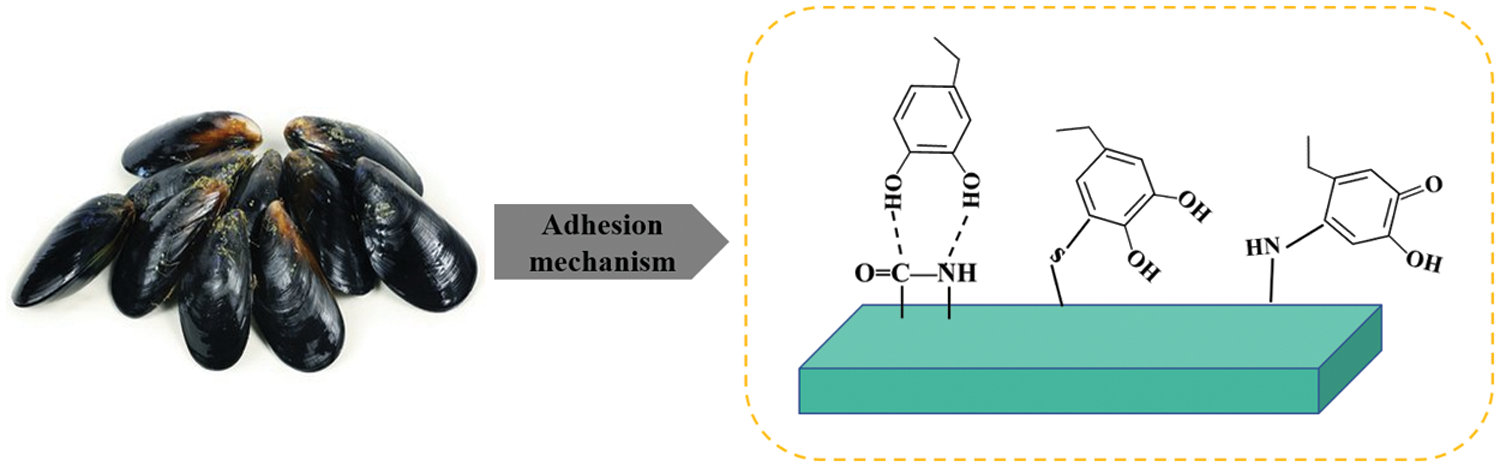
Figure 7: Mussel protein adhesion reaction mechanism
Mussel proteins contain large amounts of mercaptocysteine. Liu et al. [54] imitated the marine mussel protein adhesion mechanism and increased the content of free sulfhydryl in soybean protein, which greatly improved the strength and water resistance of wood composites bonded using modified soybean protein. By embedding modified kenaf fiber (KF), Liu et al. [55] developed a new environmentally-friendly and formaldehyde-free SM-based adhesive with excellent performance. By anchoring thiol-group functional SiO2 onto the fiber surface, KF (a green reinforcing phase) was modified to imitate a gecko-like structure. Then, by introducing pyrogallol groups from plant-derived tannic acid (TA), the surface was modified by simulating mussel adhesive chemistry. Finally, modified KF, TA, and 1,2,3-propanetriol-diglycidyl ether (PTGE) were incorporated into SM to prepare an adhesive. The results showed that the synthetic adhesive had strong mechanical interlocking and a variety of chemically cross-linked structures. Compared with pure SM adhesive, it showed a 270.7% increased wet shear strength and 14.5% increased water resistance [55]. The bionics mechanism is shown in Fig. 8. Imitating natural structure produced bionic-modified soybean protein adhesives with high adhesion performance. However, biomimetic modification processes are complicated, which make their industrialization difficult.
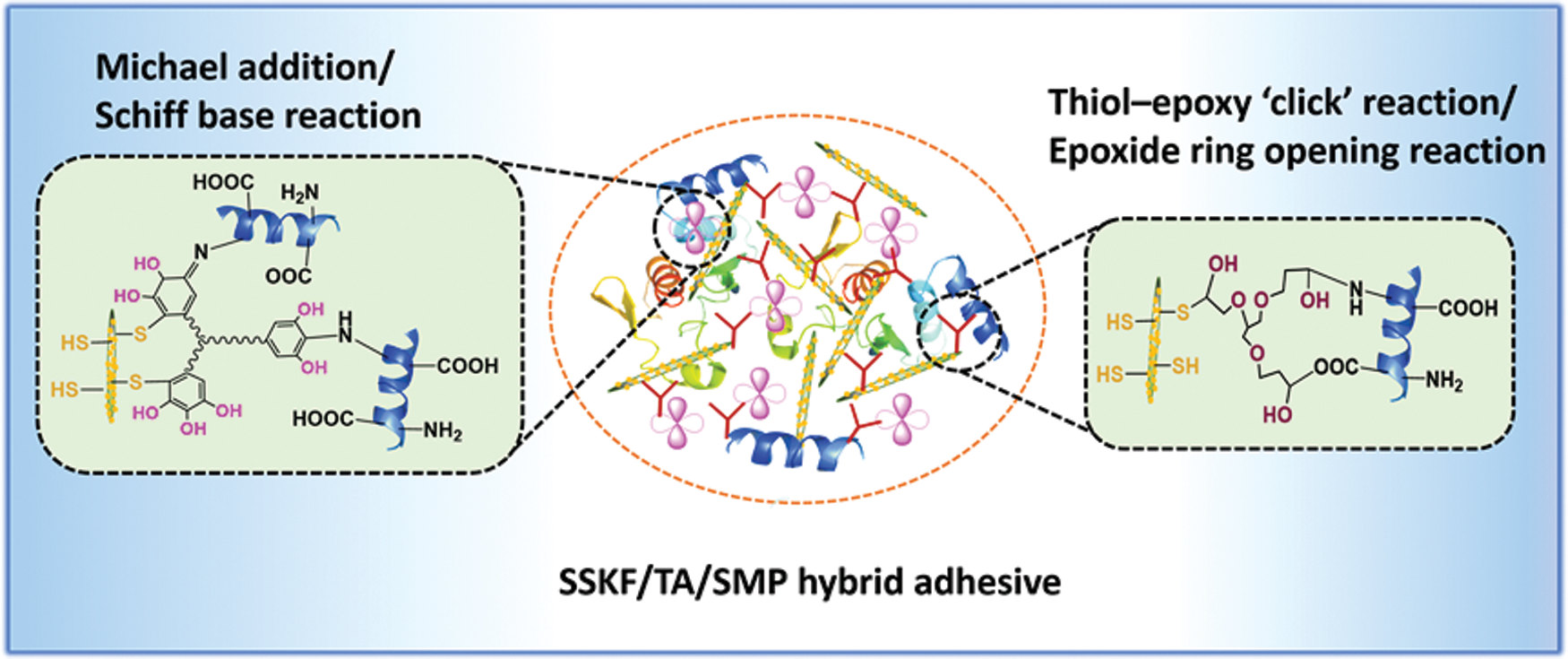
Figure 8: Schematic of possible interactions among SSKF, TA, PTGE, and SPI in the SSKF/TA/SMP adhesive. Reprinted with permission from [55]
2.2.8 Nanomaterial Modification
Nanomaterials have high strengths and specific surface areas, which can enhance soybean protein adhesives. Xu et al. [56] studied the effect of sodium dodecyl sulfonate on the unfolding of soybean protein, and the effect of nano-chitosan on the tensile properties of unfolded soy protein adhesive. The results showed that the tensile strength and water stability of the bioadhesives increased by 157% and 85%, respectively, compared with unmodified soy protein. At the same time, the dry and wet strengths of the slurry/viscose wet-laid nonwoven fabric after bonding increased by more than 43% and 100%, respectively. Sadare et al. [57] investigated the versatility and excellent properties of carbon nanotubes (CNTs) in polymer matrix nanofillers to develop nanocomposite soy-based adhesives with improved shear strength and water resistance. Nanocomposite SPI/CNT adhesives with different CNT loadings were synthesized and evaluated. The results showed that the carbon nanotubes enhanced the shear strength of the SPI/CNT nanocomposite adhesive. The shear strength and water resistance of the nanocomposite SPI/CNTs adhesive were improved by about 100% compared with the alkali-modified adhesive without carbon nanotubes. Zhang et al. [58] used thermal corrosion to degrade soybean protein, and then modified it with polyisocyanate and nano-montmorillonite to develop a new type of water-resistant soybean protein wood adhesive. Polyisocyanate cross-linking increased the molecular weight of soy protein and formed a cross-linked structure, which improved the water resistance of soy protein; however, it also significantly shortened the adhesive’s shelf life. The addition of 3 wt% nano-montmorillonite prolonged the shelf life of modified soybean protein adhesive but reduced the bonding strength. The mechanism by which nano-montmorillonite reduced the reactivity of polyisocyanate-modified soybean protein adhesive is shown in Fig. 9. Podlena et al. [59] used soy protein isolate (SPI) or soy protein flour (SF) with different co-adjuvant polymers to prepare soy protein-based wood adhesives: polyethylene oxide (PEO) with or without added sulfate lignin, hydroxyl propyl methylcellulose (HPMC), cellulose nanofibers (CNF) or polyvinyl alcohol (PVA). The results showed that the strength of the adhesive formulation containing SPI was significantly higher than that of the adhesive formulation containing SF. The dry shear strength of the adhesive depends on the co-adjuvant polymer, wood species and the amount of lignin added [59]. Nano modification can effectively improve the adhesive properties of soybean protein adhesives, but nanomaterials are expensive and increase the cost of adhesives.
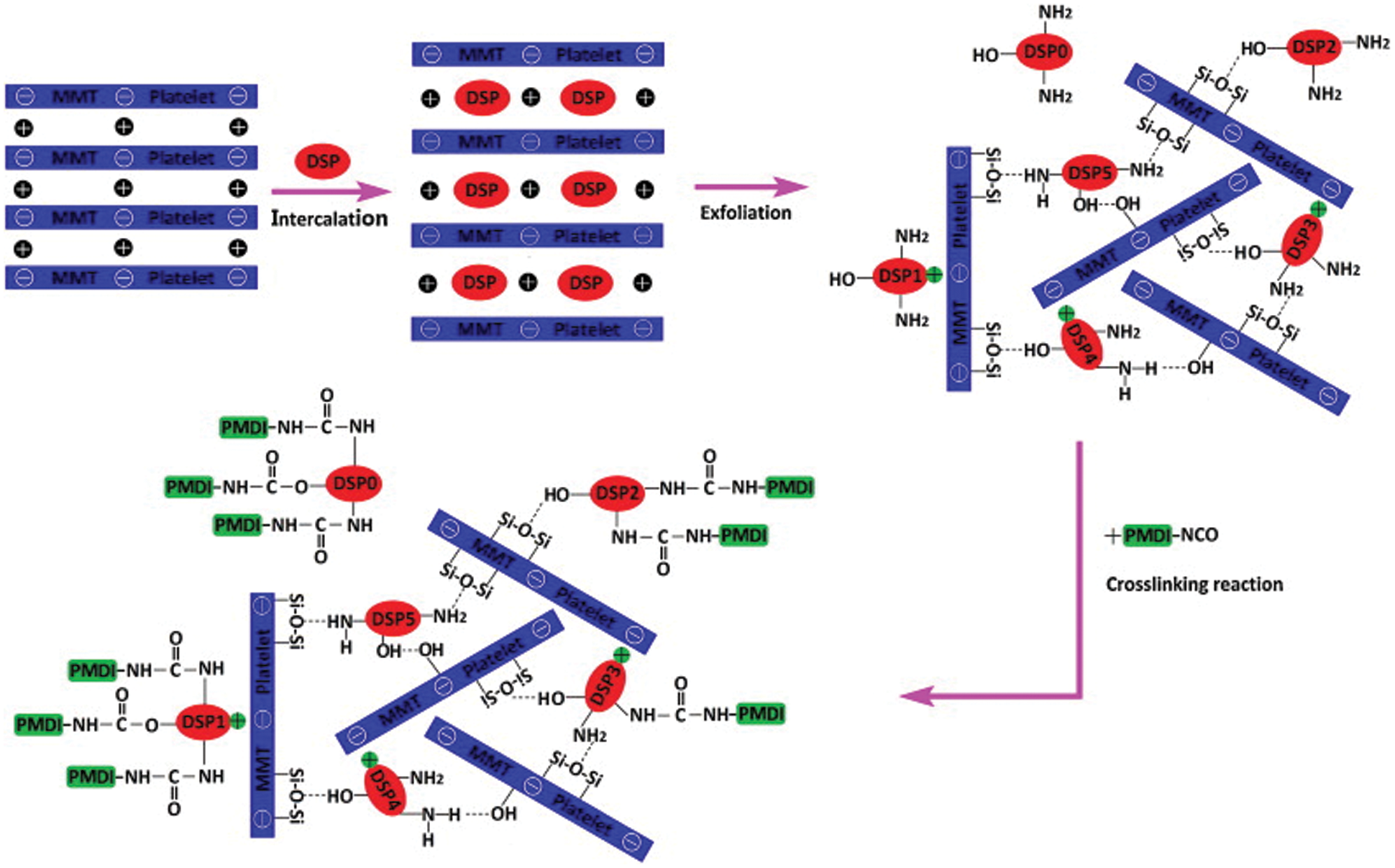
Figure 9: The mechanism by which nano-montmorillonite reduces the reactivity of polyisocyanate-modified soybean protein adhesive. Reprinted with permission from [58]
Oxidant modification is to convert carbohydrates into aldehyde groups through the oxidation of oxidants, and the aldehyde groups are crosslinked with the active groups of soybean protein, so as to improve the bonding performance of the adhesive. Frihart et al. [60] studied that adding oxidants such as periodate, permanganate or iodate to soybean powder could improve the bonding strength of soybean adhesive, especially under wet conditions. Potassium permanganate had a good effect on improving wet strength, iodate also had a certain effect on improving wet strength, while nitric acid, chlorate, perchlorate and bromate had no obvious effect on improving wet strength. Sodium periodate reacted with soybean protein isolate (SPI) and insoluble soybean powder polymer carbohydrates to study the specific oxidation of o-hydroxyl groups. The reaction has been shown to produce one, two or more aldehyde-based carbohydrate oligomer fractions. The reaction of periodate with soy protein isolate could also produce some aldehydes. The in-situ reaction of periodate with soybean powder provided a higher wet strength adhesive for bonding wood products than unmodified soybean powder. This mechanism involved the oxidation of carbohydrates to aldehyde groups, which crosslinked with soybean protein and connected soybean protein together to form a strong waterproof network. The reaction not only increased the bonding strength of original soybean powder, but also increased the bonding strength of heat-treated (modified) soybean powder [61].
Soybean protein is composed of various amino acids connected to each other, and the polypeptide chains fold to form a helix composed of large molecules with secondary, tertiary, and quaternary spatial structures. Protease modification uses biological methods to remove or add groups of the amino acid or polypeptide chain, to change its physical or chemical properties [62]. Enzymatic hydrolysis can effectively change the structure of the protein and interrupt the peptide chain, which reduces the molecular weight of the soybean protein. According to the different sources of proteases, they can be divided into plant proteases, animal proteases, and microbial proteases [63–65]. The enzyme modification of soybean protein can induce the following three changes: (1) the length of the peptide chain can be shortened, and the molecular weight of the soybean protein can be reduced; (2) the number of active groups can increase, reducing the water resistance of the soybean protein, but the soybean protein reaction activity increases after enzymatic modification, making it easier to react with chemical reagents; (3) the molecular structure of soybean protein can change. Compared with other physical and chemical modification methods, enzymatic hydrolysis is specific and produces no toxic products. It is also easy to control, and the rate of enzymatic hydrolysis can be adjusted [66]. Enzymatic hydrolysis is gentle and does not destroy the original protein function and can also be controlled by selecting specific enzyme types.
Xu et al. [67] used bromelain to enzymatically hydrolyze soybean protein molecules into polypeptide chains and added triglycidylamine (TGA) to prepare bioadhesives. The results showed that using 0.1 wt% bromelain increased the content of soybean protein isolate (SPI) from 12 wt% to 18 wt% while keeping the viscosity constant. After adding 9 wt% TGA, the residual rate of SPI/bromelain/TGA adhesive increased by 13.7%, and the wet shear strength of synthetic plywood increased by 681.3% compared with that of SPI/bromelain adhesive. The wet shear strength was 30.2% higher than that of SPI/TGA adhesive, which was attributed to the cleavage of protein molecules into polypeptide chains which allowed the adhesive to form more interlocks with the wood surface during curing. In addition, more hydrophilic groups were exposed and reacted with TGA, forming a denser cross-linked network in the adhesive. The mechanism of the enzyme-modified soybean protein adhesive is shown in Fig. 10. Chen et al. [68] used Viscozyme L to enzymatically hydrolyze polysaccharides in defatted soybean flour to produce reducing sugars and then cross-linked them with defatted soybean flour protein through the Maillard reaction. The synthetic adhesives had enhanced water resistance and bonding strength. Qin et al. [69] studied the adhesion properties of tyrosinase-modified soybean protein adhesives on pig bones. The modification of tyrosinase changed the secondary structure of soybean protein and significantly improved the viscosity, hydrophobicity, and bonding strength of the adhesive. The modification of tyrosinase expanded the protein molecules and increased the viscosity, which significantly increased the bonding strength of soybean protein adhesive to pig bones. Zheng et al. [63] developed a new method for preparing a sustainable, environmentally friendly soybean adhesive. Low-cost Aspergillus niger fermentation broth contains an enzyme complex that can hydrolyze polysaccharides in defatted soybean flour. As the concentration of reducing sugars in the hydrolysate increased, the content of water-insoluble substances decreased, and the rheological properties of the slurry were weakened, proving that the enzyme complex hydrolyzed polysaccharides in defatted soybean flour. The enzymatically treated soybean adhesive has a self-crosslinking structure. Compared with the soy protein adhesive without enzymatic hydrolysis, its adhesive strength and water resistance were significantly improved, and the wet bonding strength of two-layered plywood was increased by more than 30%. A particleboard adhesive based on natural materials was prepared by Balducci et al. [70] Soy flour (38.9 wt%), magnesium oxide (2.8 wt%) and an enzymatic hydrolysate from crops (13.9 wt%) were mixed with water and ground at 44% solids in a ball mill. Compared with urea-formaldehyde resin boards, natural adhesives have poorer internal bond strength and poorer water resistance. The addition of polyamide-epichlorohydrin significantly improved internal bonding and swelling, and for all the combinations these properties were comparable or, in most cases, better than in the urea-formaldehyde controls [70].
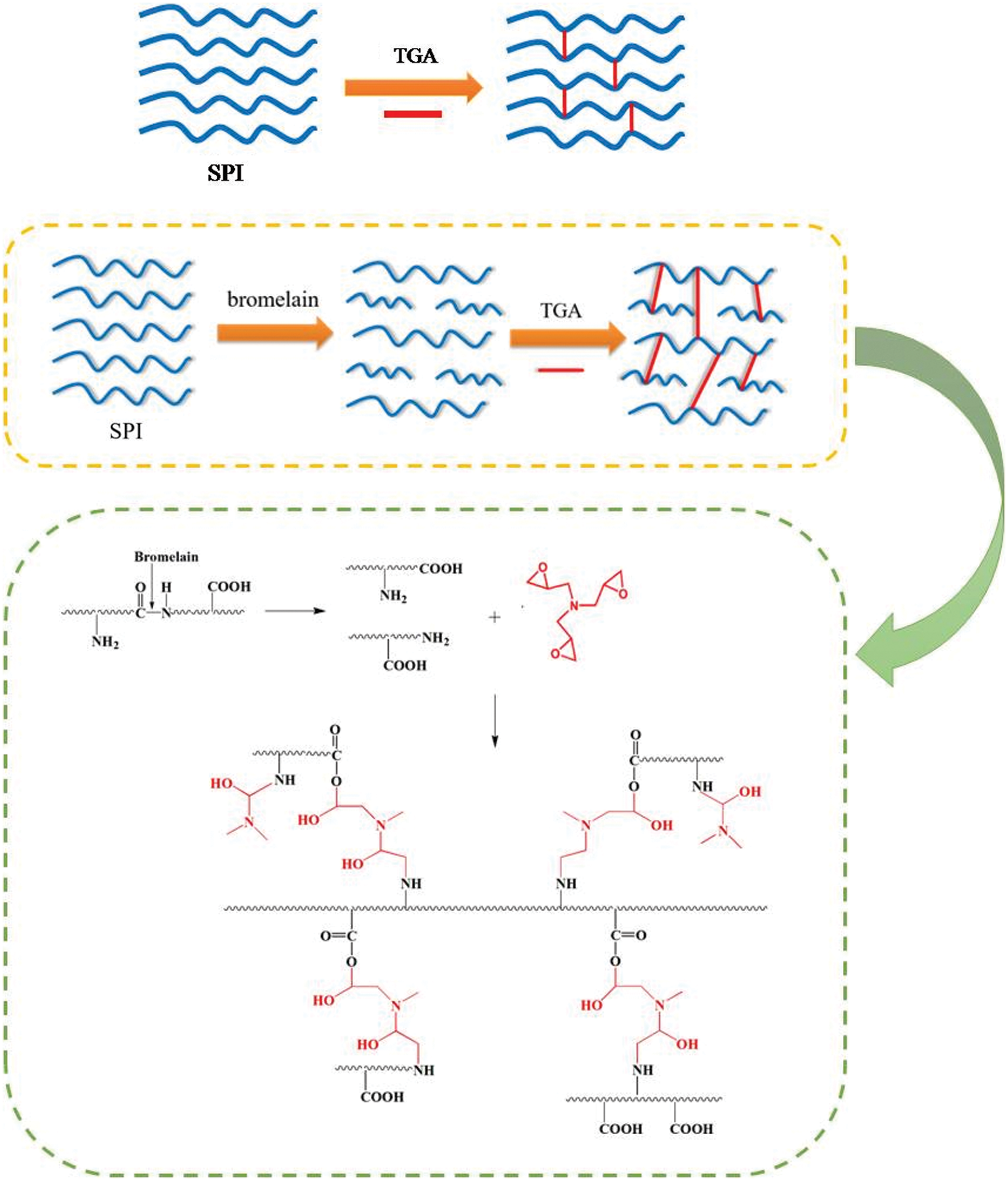
Figure 10: The enzymatic hydrolysis mechanism of soybean protein adhesives. Reprinted with permission from [67]
Enzymes hydrolyze breaks the amide bond of soy protein, so that the soy protein is changed from a large molecule to a small molecule polypeptide chain, and the viscosity is reduced. Since the cohesive force of soy protein adhesives mainly comes from the entanglement of macromolecular proteins, after enzyme modification, the molecular weight decreased and the adhesive properties of soy protein adhesives decreased, the active groups in soy protein are exposed and the water resistance decreased. Enzyme modification is safe and only requires low dosages, but excessive degradation will seriously reduce the molecular weight and bonding strength of soybean protein adhesive, therefore, the protein degradation must be carefully controlled. It is usually used in conjunction with cross-linking agents to reduce the viscosity of an adhesive, increase the solid content, and improve the coating performance and bonding stability.
3 Modification Methods to Increase Solid Content and Reduce Viscosity
Soybean protein is a high molecular weight polypeptide with a complex quaternary structure. Interactions of the side chain groups of soybean protein increase the viscosity and results in a low solid content of the adhesive. During plywood production, a large amount of water is introduced into the wood, which produces unstable composite panels. In addition, the viscosity of soybean protein adhesives is too high, which makes the veneer coating process difficult, and the adhesive layer is not uniformly coated. The suitable viscosity range of the adhesive for plywood is 5000–25000 mPa⋅s [25]. Researchers have improved the adhesion by reducing the viscosity and increasing the solid content of soybean protein adhesives.
Luo et al. [71] introduced egg white (EW) into SM adhesive instead of water, then cross-linked with triglycidylamine (TGA) to develop a high-solid bioadhesive with good performance. The results showed that adding 57.1 wt% EW increased the solid content of SM adhesive to 43.32% while maintaining a suitable viscosity. It also increased the water resistance of the SM adhesive by 36.6% because the high solid content of SM/EW reduced the evaporation of water and prevented degradation of the mechanical properties of plywood. In addition, adhesives with an appropriate viscosity formed more interlocks with wood [71]. Xu et al. [72] used bromelain to degrade soybean protein molecules into small polypeptide chains. A bio-derived cross-linking agent, TGA, was used to reorganize these polypeptide chains to develop a new type of clean soy protein-based adhesive with stable adhesive properties. The results showed that by adding 0.4 wt% bromelain, the molecular weight of soy protein was reduced from 10–170 kDa to less than 25 kDa. Compared with the unmodified adhesive, adding 3 wt% TGA and 0.1 wt% bromelain reduced the viscosity of the resulting adhesive by 95% to 7,316 mPa.s, and its wet shear strength increased by 76.2% to 1.11 MPa. At the same time, the adhesive distribution and bonding stability were significantly improved because the molecular reorganization of soy protein improved the dispersion and structural uniformity of the adhesive [72]. The schematic diagram is shown in Fig. 11. Chen et al. [46] treated defatted soybean flour (DSF) with Viscozyme L, and then adjusted pH to 5.1 with hydrochloric acid containing ferric chloride to unfold the tertiary and/or quaternary structure of soy protein and expose its functional groups. The processed DSF and epichlorohydrin (ECH) were glycinated to obtain a bioadhesive. As the weight percentage of ECH increased from 5% to 25%, the grafting rate decreased to 37.8%, while the viscosity increased by 2889%, and the solid content increased by 10.5%. The wet shear strength of Masson pine plywood prepared using a bioadhesive with an ECH content of more than 10% exceeded 0.93 MPa. Increasing the solid content usually improves the bonding strength of the soybean protein adhesive, but it may also produce an undesirably high viscosity, which makes it difficult to spread the adhesive, resulting in an uneven distribution, reduces the dimensional stability of the products. In addition, high viscosity can also cause difficulties in sizing the adhesive.
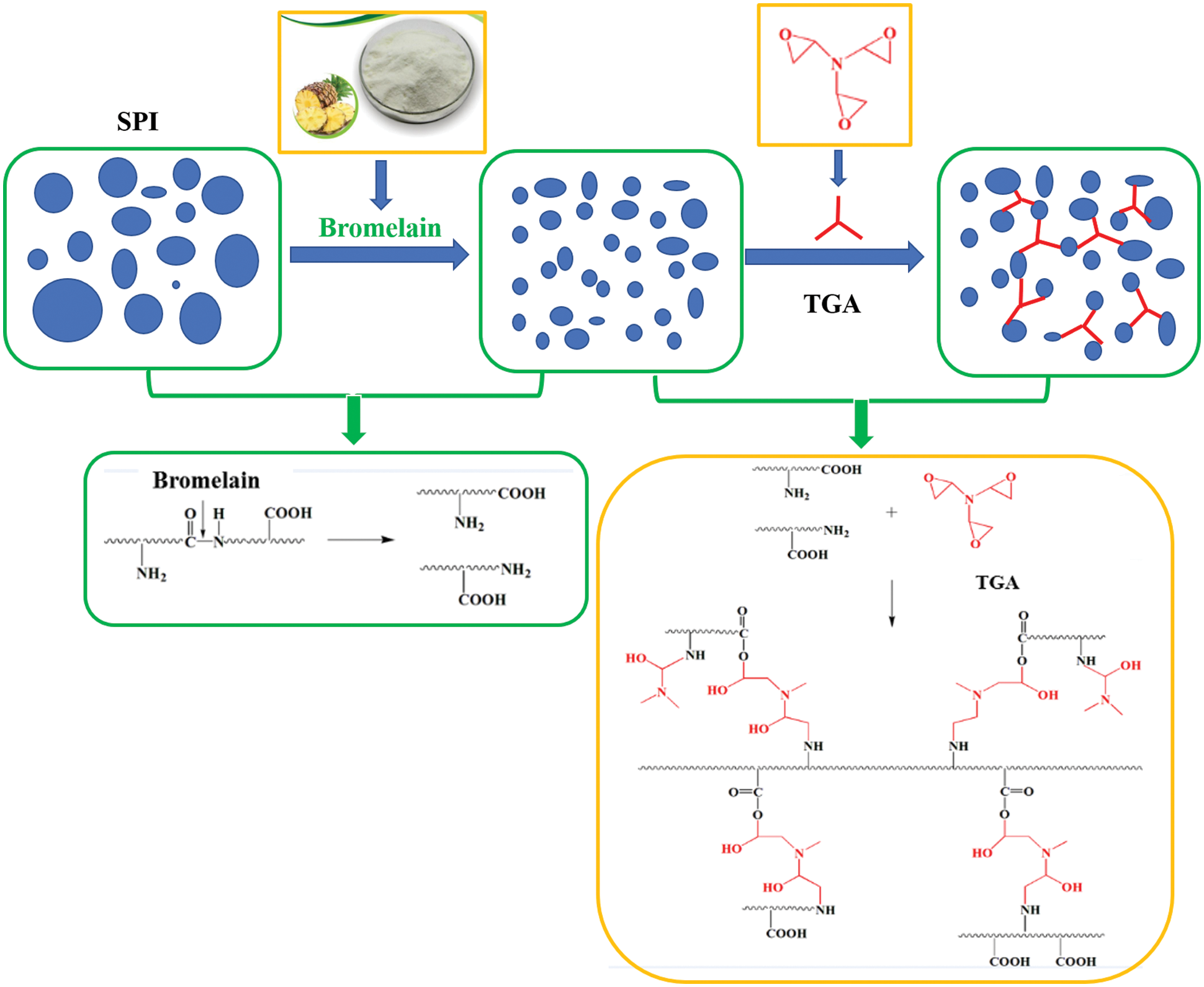
Figure 11: Schematic diagram of soybean protein molecular recombination. Reprinted with permission from [72]
4 Modification Methods to Improve Mildew Resistance
Soybean protein adhesives are mainly derived from biomass materials, which are rich in nutrients and prone to mildew formation, affects the service life of adhesives. As mildew forms, the adhesive strength of plywood gradually decreases and produces unpleasant smells. At the same time, the mildew of the adhesive after curing has a significant impact on the durability of the prepared wood-based panels, especially those used in environments with high temperatures and humidities. Therefore, mildew prevention is of great significance to soybean protein adhesive.
Li et al. [73] used three materials, nano-Ag/TiO2, zinc pyrithione, and 4-cumylphenol, to improve the mildew resistance of soybean meal adhesive by destroying mildew’s cell structure. The results showed that the use of all three materials helped improve the antifungal and mildew resistance of soybean meal adhesive. The composite modified with nano-Ag/TiO2 and zinc pyrithione displayed an antifungal function for soybean meal-based adhesives. The antifungal properties of 4-cumylphenol modified soybean meal were also greatly improved, which might be caused by the reaction between COO- groups of soy protein [73]. The schematic diagram of the anti-mold soybean protein adhesive is shown in Fig. 12. Bai et al. introduced a process to prepare bioadhesives using soybean meal and blood meal. Inspired by marine mussels and brown algae, metal coordination was introduced into protein systems to build multiple chemically-crosslinked networks. The alkali-modified blood powder was mixed with soybean meal, 1,6-hexanediglycidyl ether (HDE), and zinc ions to prepare SM and blood powder-based adhesive. The obtained adhesive had good thermal stability, water resistance (wet shear strength reached 1.1 MPa), and mildew resistance. These excellent properties were attributed to the reaction of 1,6-hexanediol glycidyl ether with proteins to form a pre-gel. Then, the coordination of zinc ions with amino or carboxyl groups made the adhesive tougher. Finally, calcium ions solidified the adhesive and increased the network density [74]. Xing et al. [75] studied the inhibitory effect of p-cumylphenol by observing the mold growth of the preservative-modified soybean flour adhesive and evaluating the bonding strength of poplar plywood and mold growth on the surface. The results showed that p-cumylphenol delayed the initial erosion by microorganisms and reduced the overall degree of microbial erosion. Adding p-cumylphenol to the soybean-based adhesive as a glue line treatment may improve its mildew resistance [75]. Adding an antifungal agent or introducing an active group that has an inhibitory effect against fungi, which increase the pot life of the soybean protein adhesive and the antifungal ability after curing. These will improve the durability of the wood-based panel products.
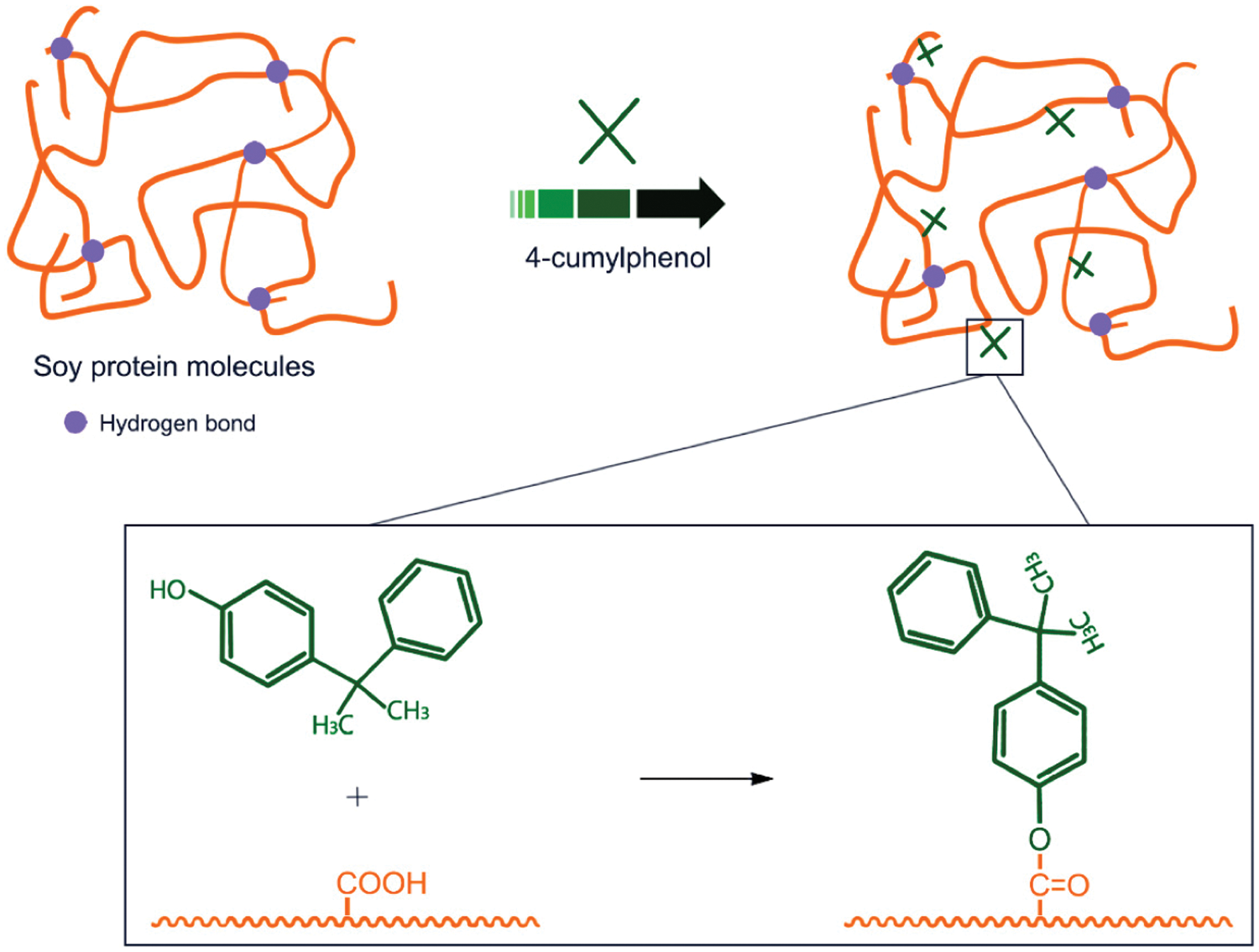
Figure 12: Schematic diagram of anti-mold soy protein adhesive. Reprinted with permission from [73]
Traditional formaldehyde-based adhesives release toxic gases such as formaldehyde and phenol and rely almost exclusively on petrochemical resources. With the implementation of sustainable development strategies, the use of soybean protein adhesives instead of traditional formaldehyde-based adhesives has become a popular research topic. Many researchers have developed modified soybean protein adhesives whose bonding strengths have exceeded commercial UF resins and can be used in the production of plywood, blockboard, and engineering flooring substrates. However, the modified soybean protein adhesive still has some problems in the industrial production process. This article reviewed methods to modify the water resistance, solid content, and mildew resistance of soy protein adhesives and compared their advantages and disadvantages. The results show that the effect of a single modification method is often difficult to meet the production requirements, and the effect of synergistic use of multiple modification methods is better. In addition, the industrial production of soybean protein adhesive faces the problems of complex technology and high cost.
The water-resistant bonding performance of soy protein adhesives has been improved through modification studies, and can be used in the industrial production of plywood, blockboard, and engineering flooring substrates. However, there are still some problems in the production process.
1. Chemical modification is the primary method to modify soybean protein adhesives; however, chemical modification processes often used excessive amount chemical modifiers, making it not sustainable. Moreover, the large dosage of crosslinking modifier would result in an increased cost and brittleness of the adhesive.
2. In addition, in recent years, new soybean protein adhesive systems with high bonding strength, toughness, and mildew resistance have been developed. However, as the natural biomass adhesive, the durability of the resultant board needs to be verified.
3. During actual production, the plywood prepared with soybean protein adhesive has poor precompression performance, so that the prepared plywood slab is easy to crack after pre-compression, which makes it difficult to put veneer stack into the hot press, and affects the production efficiency. Therefore, it is still challenging to develop high-performance soy protein adhesives with good pre-compression properties.
4. More importantly, the modified soybean protein adhesive has the problems of high cost and complicated process. Soybean meal is a bulk commodity, the price fluctuates greatly. The price of soybean meal has a significant upward trend, resulting in high adhesive costs. Therefore, the cost of resultant adhesive is about 1.5 times than that of urea-formaldehyde resin. Also, the productivity of modified soybean protein adhesive during the production process is low because of the low coating ability, low pre-compression ability, easy drying of the resultant adhesive, which increases the productivity cost of board. Therefore, it is very necessary to develop a soybean protein adhesive with low-cost and simple process.
By performing an in-depth study of soy protein adhesives and solving the practical application issues mentioned above, formaldehyde-free soybean protein adhesives have great potential to replace large portion of the formaldehyde-based adhesives to produce wood-based panels. This could help ease reliance on petrochemical resources and promote sustainable development of the wood industry.
Funding Statement: The authors are grateful to the financial support of the National Natural Science Foundation of China (31722011), Beijing Forestry University Outstanding Young Talent Cultivation Project (2019JQ03004), and the Agricultural Science and Technology Fund Project of Shandong Province (Forestry Science and Technology Innovation) (2019LY008).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Wang, N., Maximiuk, L., Fenn, D., Nickerson, M. T., Hou, A. (2020). Development of a method for determining oil absorption capacity in pulse flours and protein materials. Cereal Chemistry, 97(6), 1111–1117. DOI 10.1002/cche.10339. [Google Scholar] [CrossRef]
2. Bliem, P., Fromel-Frybort, S., van Herwijnen, H. W. G., Pinkl, S., Krenke, T. et al. (2020). Engineering of material properties by adhesive selection at the example of a novel structural wood material. Journal of Adhesion, 96(1–4), 144–164. DOI 10.1080/00218464.2019.1664301. [Google Scholar] [CrossRef]
3. Istif, E., Mantione, D., Vallan, L., Hadziioannou, G., Brochon, C. et al. (2020). Thiophene-based aldehyde derivatives for functionalizable and adhesive semiconducting polymers. ACS Applied Materials & Interfaces, 12(7), 8695–8703. DOI 10.1021/acsami.9b21058. [Google Scholar] [CrossRef]
4. Hussin, M. H., Samad, N. A., Abd Latif, N. H., Rozuli, N. A., Yusoff, S. B. et al. (2018). Production of oil palm (Elaeis guineensis) fronds lignin-derived non-toxic aldehyde for eco-friendly wood adhesive. International Journal of Biological Macromolecules, 113, 1266–1272. DOI 10.1016/j.ijbiomac.2018.03.048. [Google Scholar] [CrossRef]
5. Cruz, L. P. S., Luz, S. R., Campos, V. P., Santana, F. O., Alves, R. S. (2020). Determination and risk assessment of formaldehyde and acetaldehyde in the ambient air of gas stations in Salvador, Bahia, Brazil. Journal of the Brazilian Chemical Society, 31(6), 1137–1148. DOI 10.21577/0103-5053.20190278. [Google Scholar] [CrossRef]
6. Bacigalupe, A., Fernandez, M., Eisenberg, P., Escobar, M. M. (2020). Greener adhesives based on UF/soy protein reinforced with montmorillonite clay for wood particleboard. Journal of Applied Polymer Science, 137(37), 10. DOI 10.1002/app.49086. [Google Scholar] [CrossRef]
7. Wang, X. C., Zhu, J. B., Liu, X. H., Zhang, H. J., Zhu, X. (2020). Novel gelatin-based eco-friendly adhesive with a hyperbranched cross-linked structure. Industrial & Engineering Chemistry Research, 59(13), 5500–5511. DOI 10.1021/acs.iecr.9b06822. [Google Scholar] [CrossRef]
8. Wang, Z. T., Chen, Y., Chen, S. Q., Chu, F. X., Zhang, R. et al. (2019). Preparation and characterization of a soy protein based bio-adhesive crosslinked by waterborne epoxy resin and polyacrylamide. RSC Advances, 9(60), 35273–35279. DOI 10.1039/c9ra05931h. [Google Scholar] [CrossRef]
9. Huang, W., Sun, X. (2000). Adhesive properties of soy proteins modified by sodium dodecyl sulfate and sodium dodecylbenzene sulfonate. Journal of the American Oil Chemists Society, 77(7), 705–708. DOI 10.1007/s11746-000-0113-6. [Google Scholar] [CrossRef]
10. Wang, W. H., Li, X. P., Zhang, X. Q. (2008). A soy-based adhesive from basic modification. Pigment & Resin Technology, 37(2), 93–97. DOI 10.1108/03699420810860446. [Google Scholar] [CrossRef]
11. Mousavi, S. Y., Huang, J., Li, K. C. (2021). A cold-set wood adhesive based on soy protein. International Journal of Adhesion and Adhesives, 106, 6. DOI 10.1016/j.ijadhadh.2020.102801. [Google Scholar] [CrossRef]
12. Lamaming, S. Z., Lamaming, J., Rawi, N. F. M., Hashim, R., Kassim, M. H. M. et al. (2021). Improvements and limitation of soy protein-based adhesive: A review. Polymer Engineering and Science, 61(10), 2393–2405. DOI 10.1002/pen.25782. [Google Scholar] [CrossRef]
13. Bacigalupe, A., Escobar, M. M. (2021). Soy protein adhesives for particleboard production-A review. Journal of Polymers and the Environment, 29(7), 2033–2045. DOI 10.1007/s10924-020-02036-8. [Google Scholar] [CrossRef]
14. Luo, H. Y., Yin, Y. Q., Wang, Y., Li, Q. Y., Tang, A. X. et al. (2022). Enhanced properties of a soybean adhesive by modification with a cycloaliphatic epoxy resin. International Journal of Adhesion and Adhesives, 114, 7. DOI 10.1016/j.ijadhadh.2021.103026. [Google Scholar] [CrossRef]
15. Vnučec, D., Gorsek, A., Kutnar, A., Mikuljan, M. (2015). Thermal modification of soy proteins in the vacuum chamber and wood adhesion. Wood Science and Technology, 49(2), 225–239. DOI 10.1007/s00226-014-0685-5. [Google Scholar] [CrossRef]
16. Eslah, F., Jonoobi, M., Faezipour, M., Ashori, A. (2018). Chemical modification of soybean flour-based adhesives using acetylated cellulose nanocrystals. Polymer Composites, 39(10), 3618–3625. DOI 10.1002/pc.24389. [Google Scholar] [CrossRef]
17. Kim, M. J., Sun, X. S. (2015). Correlation between physical properties and shear adhesion strength of enzymatically modified soy protein-based adhesives. Journal of the American Oil Chemists Society, 92(11–12), 1689–1700. DOI 10.1007/s11746-015-2722-4. [Google Scholar] [CrossRef]
18. Bilusic, T., Drvenica, I., Kalusevic, A., Marijanovic, Z., Jerkovic, I. et al. (2020). Influences of freeze- and spray-drying vs. encapsulation with soy and whey proteins on gastrointestinal stability and antioxidant activity of Mediterranean aromatic herbs. International Journal of Food Science and Technology, 56, 1582–1596. DOI 10.1111/ijfs.14774. [Google Scholar] [CrossRef]
19. Wang, J., Burton Navicha, W., Na, X., Ma, W., Xu, X. et al. (2021). Preheat-induced soy protein particles with tunable heat stability. Food Chemistry, 336, 127624. DOI 10.1016/j.foodchem.2020.127624. [Google Scholar] [CrossRef]
20. Shi, R., Liu, Y., Hu, J., Gao, H., Qayum, A. et al. (2020). Combination of high-pressure homogenization and ultrasound improves physiochemical, interfacial and gelation properties of whey protein isolate. Innovative Food Science & Emerging Technologies, 65, 102450. DOI 10.1016/j.ifset.2020.102450. [Google Scholar] [CrossRef]
21. Niemira, B. A., Sommers, C. H., Fan, X. T., Sokorai, K. J. B. (2003). Formulation of soy-based RTE foods influences radiation sensitivity of listeria monocytogenes and postirradiation product sensory properties. Journal of Food Safety, 23(1), 35–46. DOI 10.1111/j.1745-4565.2003.tb00350.x. [Google Scholar] [CrossRef]
22. Cui, Q., Wang, G., Gao, D., Wang, L., Zhang, A. et al. (2020). Improving the gel properties of transgenic microbial transglutaminase cross-linked soybean-whey mixed protein by ultrasonic pretreatment. Process Biochemistry, 91, 104–112. DOI 10.1016/j.procbio.2019.12.001. [Google Scholar] [CrossRef]
23. Wang, Y., Sun, X. S., Wang, D. H. (2007). Effects of preheating treatment on thermal property and adhesion performance of soy protein isolates. Journal of Adhesion Science and Technology, 21(15), 1469–1481. DOI 10.1163/156856107782844756. [Google Scholar] [CrossRef]
24. Zhang, Y., Shi, R. Q., Xu, Y. C., Chen, M. S., Zhang, J. Y. et al. (2020). Developing a stable high-performance soybean meal-based adhesive using a simple high-pressure homogenization technology. Journal of Cleaner Production, 256, 13. DOI 10.1016/j.jclepro.2020.120336. [Google Scholar] [CrossRef]
25. Fan, B., Zhang, L. P., Gao, Z. H., Zhang, Y. H., Shi, J. Y. et al. (2016). Formulation of a novel soybean protein-based wood adhesive with desired water resistance and technological applicability. Journal of Applied Polymer Science, 133(27), 43586. DOI 10.10.1002/app.43586. [Google Scholar] [CrossRef]
26. Bacigalupe, A., Cova, M., Cedrés, J. P., Cancela, G. E., Escobar, M. (2020). Rheological characterization of a wood adhesive based on a hydrolyzed soy protein suspension. Journal of Polymers and the Environment, 28(4), 2490–2497. DOI 10.1007/s10924-020-01784-x. [Google Scholar] [CrossRef]
27. Vnucec, D., Kutnar, A., Gorsek, A. (2017). Soy-based adhesives for wood-bonding-A review. Journal of Adhesion Science and Technology, 31(8), 910–931. DOI 10.1080/01694243.2016.1237278. [Google Scholar] [CrossRef]
28. Bacigalupe, A., Poliszuk, A. K., Eisenberg, P., Escobar, M. M. (2015). Rheological behavior and bonding performance of an alkaline soy protein suspension. International Journal of Adhesion and Adhesives, 62, 1–6. DOI 10.1016/j.ijadhadh.2015.06.004. [Google Scholar] [CrossRef]
29. Chang, Z. W., Mo, L. T., Huang, A. M., Li, J. Z., Zhang, S. F. (2020). Preparation of water-resistant soybean meal-based adhesives with waste paper cellulose via NaOH/urea pretreatment and oxidation. Cellulose, 27(8), 4455–4470. DOI 10.1007/s10570-020-03076-y. [Google Scholar] [CrossRef]
30. Samson, D. O., Shukri, A., Jafri, M. Z. M., Hashim, R., Sulaiman, O. et al. (2021). Characterization of Rhizophora SPP. particle boards with SOY protein isolate modified with NaOH/IA-PAE adhesive for use as phantom material at photon energies of 16.59–25.26 keV. Nuclear Engineering and Technology, 53(1), 216–233. DOI 10.1016/j.net.2020.06.005. [Google Scholar] [CrossRef]
31. Gao, Z. H., Zhang, Y. H., Fang, B., Zhang, L. P., Shi, J. Y. (2015). The effects of thermal-acid treatment and crosslinking on the water resistance of soybean protein. Industrial Crops and Products, 74, 122–131. DOI 10.1016/j.indcrop.2015.04.026. [Google Scholar] [CrossRef]
32. Liu, H. J., Li, C., Sun, X. S. (2015). Improved water resistance in undecylenic acid (UA)-modified soy protein isolate (SPI)-based adhesives. Industrial Crops and Products, 74, 577–584. DOI 10.1016/j.indcrop.2015.05.043. [Google Scholar] [CrossRef]
33. Zheng, P. T., Lin, Q. J., Li, F., Ou, Y. T., Chen, N. R. (2017). Development and characterization of a defatted soy flour-based bio-adhesive crosslinked by 1,2,3,4-butanetetracarboxylic acid. International Journal of Adhesion and Adhesives, 78, 148–154. DOI 10.1016/j.ijadhadh.2017.06.016. [Google Scholar] [CrossRef]
34. Zhang, L. P., Zhang, B. H., Fan, B., Gao, Z. H., Shi, J. Y. (2017). Liquefaction of soybean protein and its effects on the properties of soybean protein adhesive. Pigment & Resin Technology, 46(5), 399–407. DOI 10.1108/prt-07-2016-0074. [Google Scholar] [CrossRef]
35. Xu, H. X., Luo, J., Gao, Q., Zhang, S. F., Li, J. Z. (2014). Improved water resistance of soybean meal-based adhesive with SDS and PAM. Bioresources, 9(3), 4667–4678. DOI 10.15376/biores.9.3.4667-4678. [Google Scholar] [CrossRef]
36. Bacigalupe, A., Fernandez, M., Eisenberg, P., Escobar, M. M. (2020). Greener adhesives based on UF/soy protein reinforced with montmorillonite clay for wood particleboard. Journal of Applied Polymer Science, 137(37), e49086. DOI 10.10.1002/app.49086. [Google Scholar] [CrossRef]
37. Hosseini, S. B., Asadollahzadeh, M., Najfai, S. K., Taherzadeh, M. J. (2019). Partial replacement of urea-formaldehyde adhesive with fungal biomass and soy flour in plywood fabrication. Journal of Adhesion Science and Technology, 14, 1995. DOI 10.1080/01694243.2019.1707948. [Google Scholar] [CrossRef]
38. Wu, Z. G., Zhang, B. G., Zhou, X. J., Li, L. F., Yu, L. P. et al. (2019). Influence of single/collective use of curing agents on the curing behavior and bond strength of soy protein-melamine-urea-formaldehyde (SMUF) resin for plywood assembly. Polymers, 11(12), 14. DOI 10.3390/polym11121995. [Google Scholar] [CrossRef]
39. Pereira, F., Pereira, J., Paiva, N., Ferra, J., Martins, J. M. et al. (2016). Natural additive for reducing formaldehyde emissions in urea-formaldehyde resins. Journal of Renewable Materials, 4(1), 41–46. DOI 10.7569/jrm.2015.634128. [Google Scholar] [CrossRef]
40. Huang, W., Sun, X. (2000). Adhesive properties of soy proteins modified by urea and guanidine hydrochloride. Journal of the American Oil Chemists’ Society, 77(1), 101–104. DOI 10.1007/s11746-000-0016-6. [Google Scholar] [CrossRef]
41. Zhong, Z., Sun, X. S., Fang, X., Ratto, J. A. (2002). Adhesive strength of guanidine hydrochloride—Modified soy protein for fiberboard application. International Journal of Adhesion & Adhesives, 22(4), 267–272. DOI 10.1016/S0143-7496(02)00003-9. [Google Scholar] [CrossRef]
42. Wan, Y. L., Liu, J. Y., Guo, S. T. (2018). Effects of succinylation on the structure and thermal aggregation of soy protein isolate. Food Chemistry, 245, 542–550. DOI 10.1016/j.foodchem.2017.10.137. [Google Scholar] [CrossRef]
43. Matemu, A. O., Kayahara, H., Murasawa, H., Katayama, S., Nakamura, S. (2011). Improved emulsifying properties of soy proteins by acylation with saturated fatty acids. Food Chemistry, 124(2), 596–602. DOI 10.1016/j.foodchem.2010.06.081. [Google Scholar] [CrossRef]
44. Xi, X. D., Pizzi, A., Gerardin, C., Chen, X. Y., Amirou, S. (2020). Soy protein isolate-based polyamides as wood adhesives. Wood Science and Technology, 54(1), 89–102. DOI 10.1007/s00226-019-01141-9. [Google Scholar] [CrossRef]
45. Qi, G. Y., Li, N. B., Wang, D. H., Sun, X. S. (2013). Physicochemical properties of soy protein adhesives modified by 2-octen-1-ylsuccinic anhydride. Industrial Crops and Products, 46, 165–172. DOI 10.1016/j.indcrop.2013.01.024. [Google Scholar] [CrossRef]
46. Chen, N. R., Lin, Q. J., Zheng, P. T., Rao, J. P., Zeng, Q. Z. et al. (2019). A sustainable bio-based adhesive derived from defatted soy flour and epichlorohydrin. Wood Science and Technology, 53(4), 801–817. DOI 10.1007/s00226-019-01102-2. [Google Scholar] [CrossRef]
47. Gu, W. D., Liu, X. R., Ye, Q. Q., Gao, Q., Gong, S. S. et al. (2020). Bio-inspired co-deposition strategy of aramid fibers to improve performance of soy protein isolate-based adhesive. Industrial Crops and Products, 150, 9. DOI 10.1016/j.indcrop.2020.112424. [Google Scholar] [CrossRef]
48. Jin, S. C., Li, K., Gao, Q., Zhang, W., Chen, H. et al. (2020). Multiple crosslinking strategy to achieve high bonding strength and antibacterial properties of double-network soy adhesive. Journal of Cleaner Production, 254, 13. DOI 10.1016/j.jclepro.2020.120143. [Google Scholar] [CrossRef]
49. Chen, X. Y., Pizzi, A., Xi, X. D., Zhou, X. J., Fredon, E. et al. (2021). Soy protein isolate non-isocyanates polyurethanes (NIPU) wood adhesives. Journal of Renewable Materials, 9(6), 1045–1057. DOI 10.32604/jrm.2021.015066. [Google Scholar] [CrossRef]
50. Xu, C. J., Xu, Y. C., Chen, M. S., Zhang, Y., Li, J. Z. et al. (2020). Soy protein adhesive with bio-based epoxidized daidzein for high strength and mildew resistance. Chemical Engineering Journal, 390, 8. DOI 10.1016/j.cej.2020.124622. [Google Scholar] [CrossRef]
51. Ghahri, S., Chen, X. Y., Pizzi, A., Hajihassani, R., Papadopoulos, A. N. (2021). Natural tannins as New cross-linking materials for soy-based adhesives. Polymers, 13(4), 15. DOI 10.3390/polym13040595. [Google Scholar] [CrossRef]
52. Ghahri, S., Pizzi, A., Mohebby, B., Mirshokraie, A., Mansouri, H. R. (2018). Soy-based, tannin-modified plywood adhesives. Journal of Adhesion, 94(3), 218–237. DOI 10.1080/00218464.2016.1258310. [Google Scholar] [CrossRef]
53. Ghahri, S., Pizzi, A. (2018). Improving soy-based adhesives for wood particleboard by tannins addition. Wood Science and Technology, 52(1), 261–279. DOI 10.1007/s00226-017-0957-y. [Google Scholar] [CrossRef]
54. Liu, Y., Li, K. C. (2004). Modification of soy protein for wood adhesives using mussel protein as a model: The influence of a mercapto group. Macromolecular Rapid Communications, 25(21), 1835–1838. DOI 10.1002/marc.200400363. [Google Scholar] [CrossRef]
55. Liu, X. R., Wang, K. L., Gao, Q., Zhang, W., Zhou, W. R. et al. (2019). Bioinspired design by gecko structure and mussel chemistry for bio-based adhesive system through incorporating natural fibers. Journal of Cleaner Production, 236, 10. DOI 10.1016/j.jclepro.2019.07.066. [Google Scholar] [CrossRef]
56. Xu, X. Y., Hu, W. F., Ke, Q. F., Liu, H. G., Li, J. et al. (2020). Bio-adhesives from unfolded soy protein reinforced by nano-chitosan for sustainable textile industry. Textile Research Journal, 90(9–10), 1094–1101. DOI 10.1177/0040517519886560. [Google Scholar] [CrossRef]
57. Sadare, O. O., Daramola, M. O., Afolabi, A. S. (2020). Synthesis and performance evaluation of nanocomposite soy protein isolate/carbon nanotube (SPI/CNTs) adhesive for wood applications. International Journal of Adhesion and Adhesives, 100, 7. DOI 10.1016/j.ijadhadh.2020.102605. [Google Scholar] [CrossRef]
58. Zhang, Y. H., Zhu, W. Q., Lu, Y., Gao, Z. H., Gu, J. Y. (2014). Nano-scale blocking mechanism of MMT and its effects on the properties of polyisocyanate-modified soybean protein adhesive. Industrial Crops and Products, 57, 35–42. DOI 10.1016/j.indcrop.2014.03.027. [Google Scholar] [CrossRef]
59. Podlena, M., Bohm, M., Saloni, D., Velarde, G., Salas, C. (2021). Tuning the adhesive properties of soy protein wood adhesives with different coadjutant polymers, nanocellulose and lignin. Polymers, 13(12), 16. DOI 10.3390/polym13121972. [Google Scholar] [CrossRef]
60. Frihart, C. R., Lorenz, L. F. (2019). Specific oxidants improve the wood bonding strength of soy and other plant flours. Journal of Polymer Science Part A-Polymer Chemistry, 57(9), 1017–1023. DOI 10.1002/pola.29357. [Google Scholar] [CrossRef]
61. Frihart, C. R., Pizzi, A., Xi, X. D., Lorenz, L. E. (2019). Reactions of Soy flour and Soy protein by non-volatile aldehydes generation by specific oxidation. Polymers, 11(9), 18. DOI 10.3390/polym11091478. [Google Scholar] [CrossRef]
62. Chen, N. R., Zeng, Q. Z., Rao, J. P., Lin, Q. J. (2014). Effect of preparation conditions on bonding strength of soy-based adhesives via viscozyme L action on soy flour slurry. Bioresources, 9(4), 7444–7453. DOI 10.15376/biores.9.4.7444-7453. [Google Scholar] [CrossRef]
63. Zheng, P. T., Chen, N. R., Islam, S. M. M., Ju, L. K., Liu, J. et al. (2019). Development of self-cross-linked soy adhesive by enzyme complex from aspergillus Niger for production of All-biomass composite materials. ACS Sustainable Chemistry & Engineering, 7(4), 3909–3916. DOI 10.1021/acssuschemeng.8b04993. [Google Scholar] [CrossRef]
64. Ozturk, O. K., Kaasgaard, S. G., Palmen, L. G., Vidal, B. C., Hamaker, B. R. (2021). Enzyme treatments on corn fiber from wet-milling process for increased starch and protein extraction. Industrial Crops and Products, 168, 11. DOI 10.1016/j.indcrop.2021.113622. [Google Scholar] [CrossRef]
65. Eberhardt, A., Lopez, E. C., Marino, F., Mammarella, E. J., Manzo, R. M. et al. (2021). Whey protein hydrolysis with microbial proteases: Determination of kinetic parameters and bioactive properties for different reaction conditions. International Journal of Dairy Technology, 74(3), 489–504. DOI 10.1111/1471-0307.12795. [Google Scholar] [CrossRef]
66. Huang, J., Zhao, P., Jin, X., Wang, Y., Yuan, H. et al. (2020). Enzymatic biofuel cells based on protein engineering: Recent advances and future prospects. Biomaterials Science, 8(19), 5230–5240. DOI 10.1039/d0bm00925c. [Google Scholar] [CrossRef]
67. Xu, Y. T., Xu, Y. C., Han, Y. F., Chen, M. S., Zhang, W. et al. (2018). The effect of enzymolysis on performance of soy protein-based adhesive. Molecules, 23(11), 12. DOI 10.3390/molecules23112752. [Google Scholar] [CrossRef]
68. Chen, N. R., Zeng, Q. Z., Lin, Q. J., Rao, J. P. (2015). Development of defatted soy flour based bio-adhesives using viscozyme L. Industrial Crops and Products, 76, 198–203. DOI 10.1016/j.indcrop.2015.04.008. [Google Scholar] [CrossRef]
69. Qin, Z. Y., Mo, L. T., Liao, M. R., He, H., Sun, J. P. (2019). Preparation and characterization of soy protein isolate-based nanocomposite films with cellulose nanofibers and nano-silica via silane grafting. Polymers, 11(11), 15. DOI 10.3390/polym11111835. [Google Scholar] [CrossRef]
70. Balducci, F., Adamopoulos, S., Pettinari, C., Canti, E., di Nicola, C. et al. (2020). A formaldehyde-free adhesive for particleboards based on soy flour, magnesium oxide, and a plant-derived enzymatic hydrolysate. Bioresources, 15(2), 3087–3102. DOI 10.15376/biores.15.2.3087-3102. [Google Scholar] [CrossRef]
71. Luo, J. L., Li, L. Y., Luo, J., Li, X. N., Li, K. et al. (2017). A high solid content bioadhesive derived from soybean meal and Egg white: Preparation and properties. Journal of Polymers and the Environment, 25(3), 948–959. DOI 10.1007/s10924-016-0875-3. [Google Scholar] [CrossRef]
72. Xu, Y. T., Han, Y. F., Shi, S. Q., Gao, Q., Li, J. Z. (2020). Preparation of a moderate viscosity, high performance and adequately-stabilized soy protein-based adhesive via recombination of protein molecules. Journal of Cleaner Production, 255, 10. DOI 10.1016/j.jclepro.2020.120303. [Google Scholar] [CrossRef]
73. Li, W. P., Chen, M. S., Li, Y. C., Sun, J. M., Liu, Y. et al. (2020). Improving mildew resistance of soy meal by nano-Ag/TiO2, zinc pyrithione and 4-cumylphenol. Polymers, 12(1), 11. DOI 10.3390/polym12010169. [Google Scholar] [CrossRef]
74. Bai, Y., Liu, X. R., Shi, S. Q., Li, J. Z. (2020). A tough and mildew-proof soybean-based adhesive inspired by mussel and algae. Polymers, 12(4), 14. DOI 10.3390/polym12040756. [Google Scholar] [CrossRef]
75. Xing, F. R., Chen, H., Zhang, S. F., Luo, B., Fang, P. et al. (2015). Effect of p-cumylphenol on the mold resistance of modified soybean flour adhesive and poplar plywood. Bioresources, 10(1), 1543–1552. DOI 10.15376/biores.10.1.1543-1552. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |