 Open Access
Open Access
ARTICLE
Experimental Study of Selective Batch Bio-Adsorption for the Removal of Dyes in Industrial Textile Effluents
1 Laboratory of Environmental Process Engineering (LIPE), Department of Environmental Engineering, Faculty of Engineering Process, University Salah Boubnider-Constantine 3, New City Ali Mendjeli, Constantine, 25000, Algeria
2 Laboratory of Process Engineering for Sustainable Development and Health Products (GPDDPS), Department of Process Engineering, National Polytechnic School of Constantine, Constantine, 25000, Algeria
3 Physics of Matter and Radiation Laboratory (LPMR), Department of Process Engineering, Faculty of Science and Technology, University Mohamed Cherif Messaadia, Souk Ahras, 41000, Algeria
4 Higher Normal School of Constantine, Ali Mendjeli Nouvelle Ville, Constantine, 25000, Algeria
5 Laboratoire d’Etude et Recherche sur le Matériau Bois (LERMAB), Ecole Nationale Supérieure des Technologies et Industries du Bois (ENSTIB), University of Lorraine, Epinal, 88000, France
* Corresponding Authors: Zakaria Laggoun. Email: ; Kerroum Derbal. Email:
Journal of Renewable Materials 2025, 13(1), 127-146. https://doi.org/10.32604/jrm.2024.056970
Received 04 August 2024; Accepted 25 October 2024; Issue published 20 January 2025
Abstract
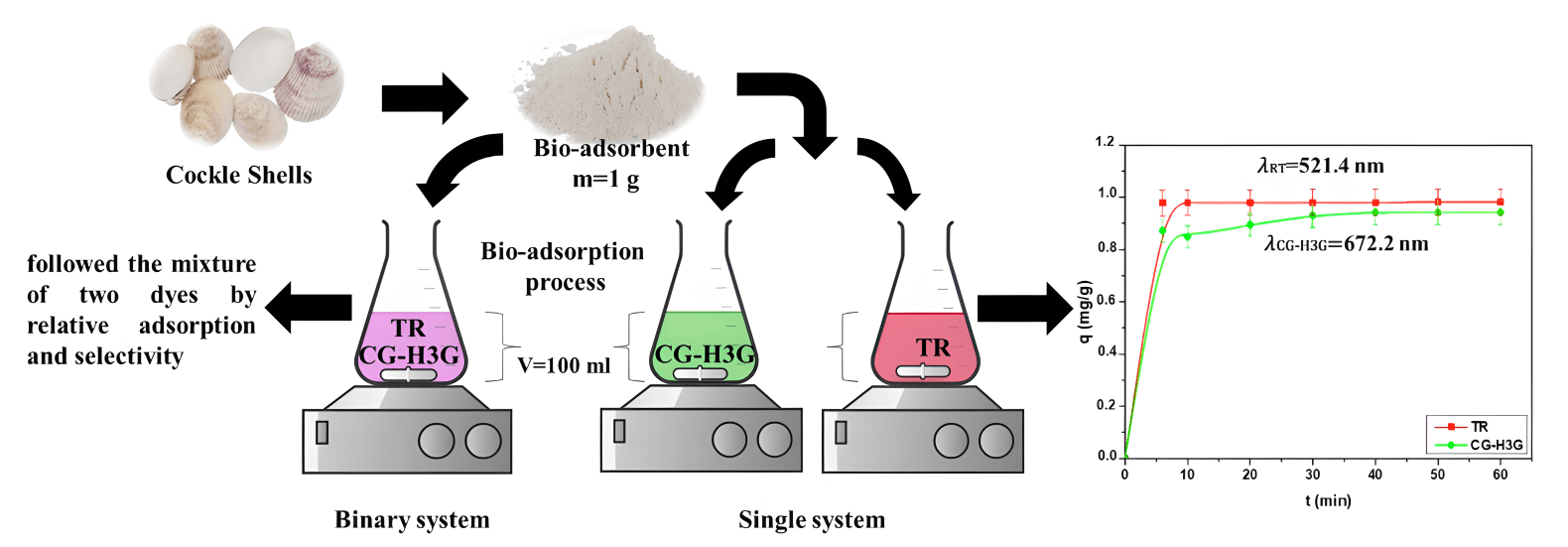
This research aims to study the bio-adsorption process of two dyes, Cibacron Green H3G (CG-H3G) and Terasil Red (TR), in a single system and to bring them closer to the industrial textile discharge by a binary mixture of two dyes (TR+CG-H3G). The Cockle Shell (CS) was used as a natural bio-adsorbent. The characterizations of CS were investigated by Fourier transform infrared (FTIR), X-ray diffraction (XRD), scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDX) and Brunauer–Emmett–Teller (BET). The adsorption potential of Cockle Shells was tested in two cases (single and binary system) and determined by: contact time (0–60 min), bio-adsorption dose (3–15 g/L), initial concentration (10–300 mg/L), temperature (22–61°C) and pH solution (2–12). The study of bio-adsorption (equilibrium and kinetics) was conducted at 22°C. The kinetic studies demonstrated that a pseudo-second-order adsorption mechanism had a good correlation coefficient (R2 ≥ 0.999). The Langmuir isotherm modeling provided a well-defined description of TR and CG-H3G bio-adsorption on cockle shells, exhibiting maximum capacities of 29.41 and 3.69 mg/g respectively at 22°C. The thermodynamic study shows that the reaction between the TR, CG-H3G dyes molecules and the bio-adsorbent is exothermic, spontaneous in the range of 22–31°C with the aleatory character decrease at the solid-liquid interface. The study of selectivity in single and binary systems has been performed under optimal operating conditions using the industrial textile rejection pH (pH = 6.04). CG-H3G dye is found to have a higher selectivity than TR in single (0–60 min) and binary systems with a range of 6–45 min, as shown by the selectivity measurement. It was discovered that CS has the capability to remove both CG-H3G and TR dyes in both simple and binary systems, making it a superior bio-adsorbent.Graphic Abstract
Keywords
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools