 Open Access
Open Access
ARTICLE
Evaluating the Potential of Birch Bark Suberinic Acids for Solid Wood Impregnation
1 Latvian State Institute of Wood Chemistry, Biorefinery Laboratory, Riga, LV-1006, Latvia
2 Norwegian Institute of Bioeconomy Research, Department Wood Technology, Høgskoleveien 8, Ås, 1433, Norway
* Corresponding Author: Daniela Godina. Email:
(This article belongs to the Special Issue: Advances in Biorefinery Technologies and Products – 2024)
Journal of Renewable Materials 2025, 13(1), 147-161. https://doi.org/10.32604/jrm.2024.056822
Received 31 July 2024; Accepted 24 September 2024; Issue published 20 January 2025
Abstract
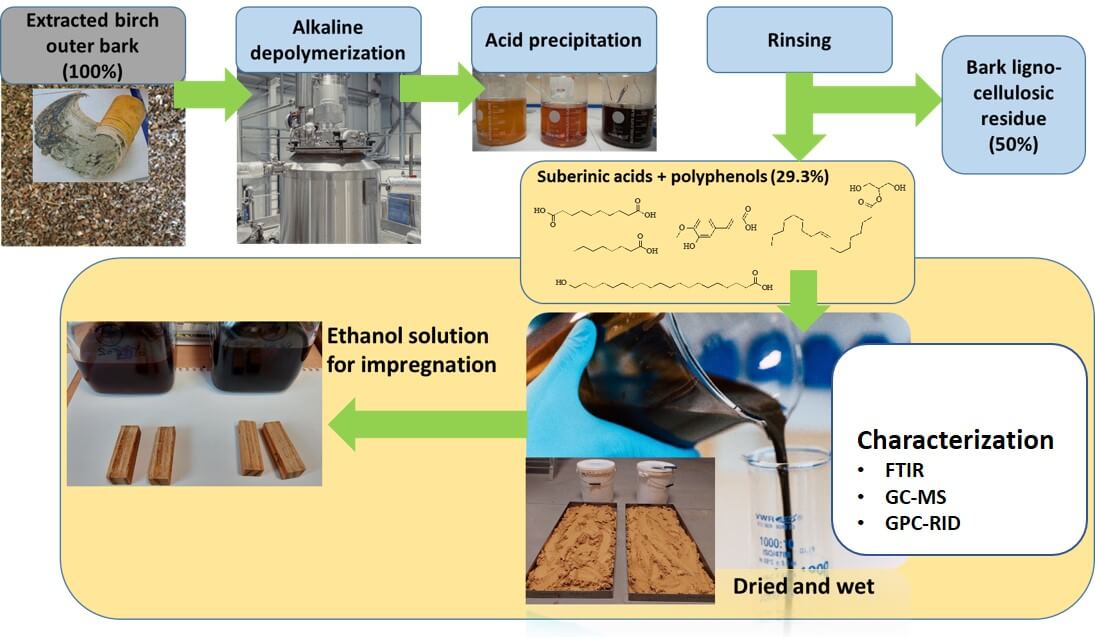
Instead of the traditional linear model of taking, making, and disposing, the circular bio-economy promotes a regenerative approach. Although there is potential to create valuable products like betulin, lupeol, and suberinic acids (SA) from outer birch bark, many industries, such as plywood and pulp, often choose to incinerate substantial amounts of leftover birch bark to meet their energy needs. This highlights the importance of obtaining valuable products from wood. The objective of this study was to examine various fractions of SA and assess their potential for wood impregnation. The fractions included SA potassium salts in ethanol (SAK-EtOH) and water (SAK-H2O), SA suspension in water (SAS-H2O) and dried SA, which was subsequently diluted in ethanol (DSA-EtOH). There is significant potential for utilizing SA in wood treatment formulations as a sustainable alternative to harmful petroleum-derived chemicals. This approach not only addresses environmental concerns but also enhances the functionality of wood in construction applications, such as improving impregnation for moisture and fungal protection. Among the solutions tested, the ethanol solution of SA, specifically DSA-EtOH, showed the highest weight percent gain (WPG) and the greatest leaching resistance. GPC analysis showed that SA salts in ethanol (SAK-EtOH) and water (SAK-H2O) predominantly consist of low molecular fractions and each process (acidification and drying) reduces the low molecular content in the sample. This suggests that SA polymerizes after drying, making it necessary to dissolve it in ethanol to meet the requirements for impregnation. Further optimization, including adjustments in the concentration of the SA ethanol solution and the curing temperature, is essential to identify the optimal conditions for more in-depth impregnation studies.Graphic Abstract
Keywords
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools