 Open Access
Open Access
ARTICLE
The Oxyalkylation of Hydrophilic Black Alder Bark Extractives with Propylene Carbonate with a Focus on Green Polyols Synthesis
Laboratory of Lignin Chemistry, Latvian State Institute of Wood Chemistry, Riga, 1006, Latvia
* Corresponding Author: Alexandr Arshanitsa. Email:
(This article belongs to the Special Issue: Advances in Biorefinery Technologies and Products – 2024)
Journal of Renewable Materials 2024, 12(11), 1927-1948. https://doi.org/10.32604/jrm.2024.056466
Received 23 July 2024; Accepted 23 August 2024; Issue published 22 November 2024
Abstract
The isolated hydrophilic black alder (Alnus glutinosa) bark extractives were characterized in terms of component and functional composition and converted at 150°C–170°C into liquid green polyols using solvent-free and low-toxic base-catalyzed modification with propylene carbonate (PC). FTIR spectroscopy, HP-LC, GC, GPC, and wet chemistry methods were used to characterize the starting constituents, intermediate and final products of the reaction and to monitor the different pathways of PC conversion. The reaction of extractives as well as the model compounds, including catechol, xylose, PEG 400, and benzoic acid, with PC indicated the ability of OH groups of different origins present in the extractives to condense with equivalent amounts of PC. The polyols obtained consist of a copolymer fraction with one oxypropyl unit grafted per OH functionality of extractive components on average and oligo oxypropyl diols with a small number of carbonate linkages in the chain, obtained as a result of remaining PC homopolymerization. The domination of the oxypropylation mechanism vs. transcarbonation for PC ring opening was observed for both copolymerization and homopolymerization processes, making the process of oxypropylation with PC similar to that of conventional oxypropylation. At optimal reaction conditions, including a PC/OH ratio of 3.0 and a 24-h duration at 150°C, uniform polyols with low viscosity of ~900 mPa·s−1, a biomass content of ~27%, and an OHV of ~500 mg KOH·g−1 were obtained. Increasing the temperature of modification allows shortening the process but drastically increases the polyol viscosity. At fixed temperature values, increasing the PC/OH ratio not only decreases the biomass content but also strongly prolongs the processing. The significantly increased duration of the process using PC as an alternative oxyalkylation agent compared to that of oxyalkylation with propylene oxide is a reasonable trade-off for using a safer and more environmentally friendly technology.Graphic Abstract
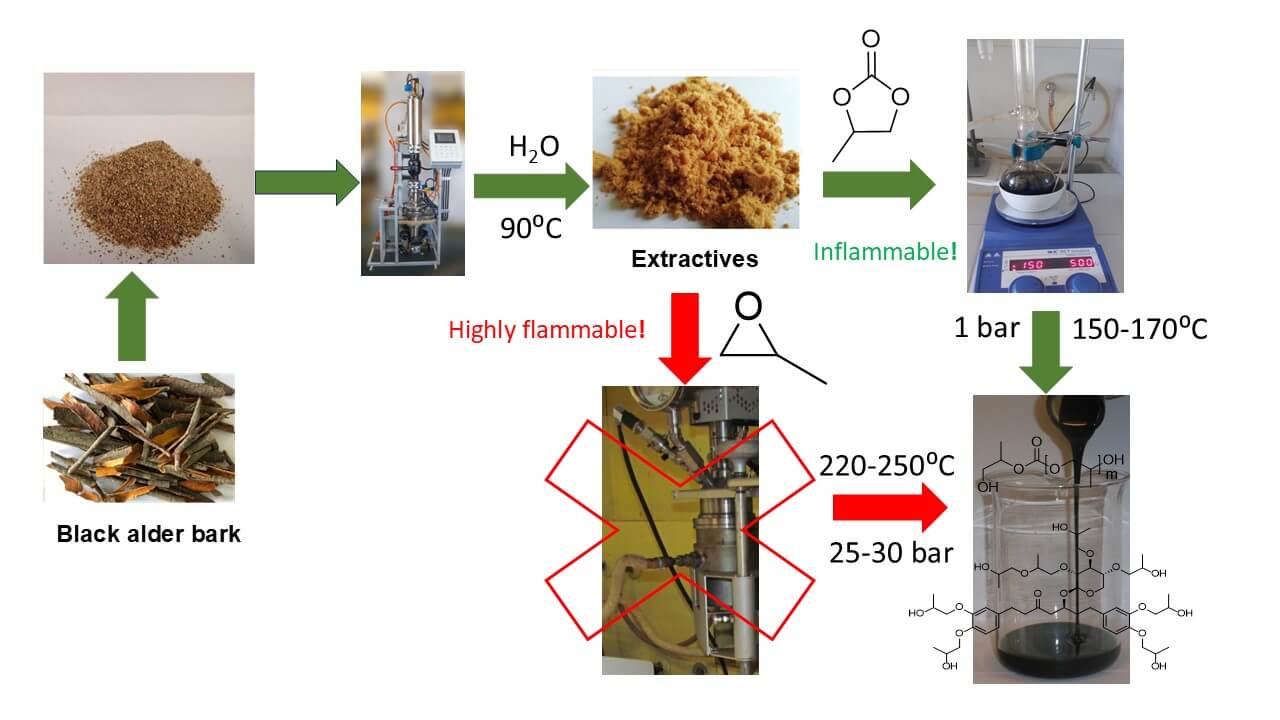
Keywords
Supplementary Material
Supplementary Material FileCite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools