 Open Access
Open Access
ARTICLE
Characterization of Hydroxyapatite Extracted from Crab Shell Using the Hydrothermal Method with Varying Holding Times
1 Department of Mechanical Engineering, Faculty of Engineering, Diponegoro University, Semarang, Jawa Tengah, 50275, Indonesia
2 Department of Mechanical Engineering, Faculty of Engineering, Universitas Negeri Semarang, Gunung Pati, Semarang, 50229, Indonesia
3 Center for Biomechanics, Biomaterial, Biomechatronics, and Biosignal Processing (CBIOM3s), Diponegoro University, Semarang, 50275, Indonesia
4 Automotive Engineering Center (AEC), Universiti Malaysia Pahang Al-Sultan Abdullah (UMPSA), Pahang, Malaysia
5 Faculty of Mechanical & Automotive Engineering Technology, Universiti Malaysia Pahang Al-Sultan Abdullah, Pekan, 26600, Malaysia
6 Faculty of Engineering and Quantity Surveying, INTI International University, Nilai, Malaysia
* Corresponding Authors: Deni Fajar Fitriyana. Email: ; Rifky Ismail. Email:
(This article belongs to the Special Issue: Recent Advances on Renewable Materials)
Journal of Renewable Materials 2024, 12(6), 1145-1163. https://doi.org/10.32604/jrm.2024.052165
Received 25 March 2024; Accepted 29 April 2024; Issue published 02 August 2024
Abstract
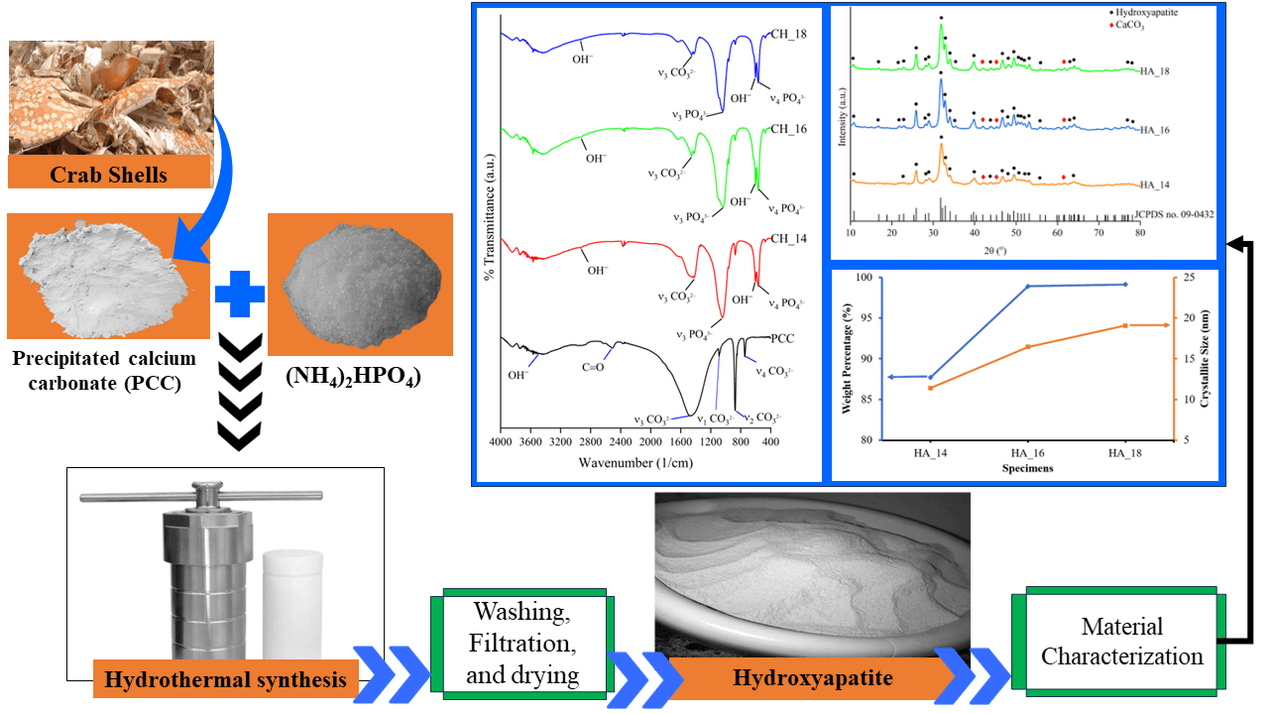
Hydroxyapatite (HA) is a bio ceramic commonly utilized in bone tissue engineering due to its bioactive and osteoconductive properties. Crab shells are usually disregarded as waste material despite their significant CaCO content, and have not been widely utilized in the synthesis of HA. This study aims to synthesize and analyze HA derived from crab shells using the hydrothermal method with different durations of holding time. This study utilized precipitated calcium carbonate (PCC) derived from crab shells. With a hydrothermal reactor set at 160°C and varying holding times of 14 (HA_14), 16 (HA_16), and 18 (HA_18) h, a PCC and (NH)HPO mixture was used to synthesize HA. The synthesis results were analyzed using scanning electron microscopy (SEM), fourier transform infrared spectroscopy (FTIR), and X-ray diffraction (XRD) tests. This study has accomplished the synthesis of HA from crab shells. Nonetheless, the final product of synthesis still contained CaCO as an impurity. The prolonged hydrothermal holding time of 14 to 18 h resulted in a reduction of impurities while increasing the percentage of crystal weight and crystallite size of HA. Specimen CH_18 is the best-quality product generated in this study. This specimen produced HA with the highest percentage of crystal weight and crystallite size compared to the other specimens. Furthermore, specimen CH_18 exhibited the lowest concentration of impurities. The Ca/P ratio in this specimen was also the closest to 1.67. The Ca/P ratio, crystallite size, and crystal weight percentage of this specimen are 1.54, 19.06 nm, and 99.1%, respectively.Graphic Abstract
Keywords
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools