 Open Access
Open Access
ARTICLE
Enhancing the Shelf Life of Edible Musa Acuminate with Biopolymer Coating
1 Department of Chemistry (Food Chemistry and Food Processing), Loyola College, Chennai, 600 034, India
2 Regional Centre, CSIR-Central Leather Research Institute, Leather Complex, Jalandhar, 144 021, India
3 Mechanical Engineering Department, I. K. Gujral Punjab Technical University, Main Campus, Kapurthala, Punjab, 144 603, India
4 Department of Chemical and Materials Engineering, National Yunlin University of Science and Technology, Douliu, 64002, Taiwan
5 Department of Computational Biology, Saveetha School of Engineering, Saveetha Institute of Medical and Technical Sciences, Saveetha University, Chennai, 602 105, India
6 School of Chemical Engineering, Yeungnam University, Gyeongsan, 38541, Republic of Korea
* Corresponding Authors: P. Sudhakara. Email: ; Balamurugan Rathinam. Email:
# Authors Manivasagam Vishwa Rohini and Vanaraj Ramkumar contributed equally
Journal of Polymer Materials 2024, 41(3), 159-178. https://doi.org/10.32604/jpm.2024.054801
Received 06 June 2024; Accepted 30 July 2024; Issue published 30 September 2024
Abstract
The Cavendish banana, Musa acuminata, is a climacteric fruit that ripens quickly after harvest. Due to their high perishability and susceptibility to disease, bananas have substantial postharvest losses. After harvesting, it is important to extend the bananas’ shelf life. Unique coating gel solutions based on glycerol and ascorbic acid were developed to accomplish this. Chitosan mixed with glacial acetic acid, lactic acid, and sodium alginate were among the gel solutions in these combinations. The goal was to improve banana preservation while maintaining freshness and quality by implementing innovative formulations and techniques. The fruits are hand-dried and then maintained at 25°C before being covered with dipping. Fruit coated with chitosan will preserve its freshness for up to 15 days, which is the optimum outcome. Comparing the physicochemical parameters of coated and untreated fruit demonstrates that gel-coated fruit retains physical and chemical qualities by decreasing respiration during storage. Improved Barrier Properties, Antimicrobial Action, Biodegradability, Edibility, and Maintained Fruit Quality: Customization of Formulations Complementary Preservation Techniques, and Simple Application are all supported by the impact of edible coatings. This invention reflects a change toward more sustainable and effective post-harvest treatments for climacteric fruits such as bananas, which provide economic benefits by minimizing waste and enhancing the quality of the fruit accessible to customers.Keywords
The cavendish banana (Musa acuminate, AAA), a member of the Musaceae family, is the second most important fruit crop after mangoes [1–3]. Apart from being an excellent source of vitamins, minerals (including calcium, magnesium, potassium, and phosphorus), and carbohydrates, bananas are also the most affordable and useful fruit in the world [4–6]. It improves digestion and is cholesterol and fat-free. Bananas grow best on rich, deep loamy soil with 75%–85% humidity and temperatures ranging from 15°C to 35°C. At the start of the climacteric phase in bananas, there are oscillations in ethylene production [7,8]. Because they may enhance the quality and shelf life of fresh food, such as bananas, biopolymer coatings, such as those based on chitosan and sodium alginate, are growing in popularity. These biopolymer coatings can be used to increase fruit quality in a sustainable and biodegradable way while potentially reducing post-harvest losses. Bananas coated with biopolymer have greater microbial resistance due to the antibacterial properties of chitosan, which lowers the likelihood of bacterial and fungal spoilage. In addition to reducing weight loss and moisture loss, these coatings help maintain the firmness and color of the bananas. In addition to being ecologically friendly, the use of these edible coatings meets the needs of customers who want safe and natural preservation methods. Bananas’ physiological climacteric characteristic causes them to ripen fast and have a limited shelf life. Because the ripening process in climacteric plants is well understood, bananas are highly sought-after fruits [9,10]. Two significant genes that strictly regulate ethylene synthesis are MaACS1 and MaACO1. Oxygen (O2) and carbon dioxide (CO2) both influence fruit respiration and ethylene production. Ethylene promotes genes linked to ripening around the fruit, expediting the fruit’s reaction to senescence. These genes affect several physiological processes, including as respiration, starch metabolism, ethylene generation, and cell wall breakdown. To counteract the effects of oxygen, edible coatings have been used to block its entry into fruits and suppress microbial development [11–13]. The adoption of edible and biodegradable coatings has gained attraction due to their potential to extend shelf life, enhance food quality, and reduce packaging waste. This approach contributes significantly to addressing the waste issue in the food industry [14–16]. Making edible films more useful is the aim of their development. These films, which include probiotics, essential oils (EOs), and herb and spice extracts, are usually antibacterial and antioxidant-rich. Essential oils, in particular, have a crucial function in ex-tending food shelf life by inhibiting the growth of harmful microbes and protecting against oxidation [17–20]. Furthermore, the development of appropriate color in meat and meat products is heavily reliant on exposure to certain gases, such as oxygen and carbon dioxide, emphasizing the need for regulated conditions in food preservation and quality maintenance [21–24]. A cleverly designed edible coating serves as a partial water-blocker, reducing moisture loss from the fruit’s surface and controlling the composition of the sur-rounding gasses [25,26]. With no negative effects on anaerobiosis, this modification slows down the respiration and senescence methods [27,28]. It also stops fungus from growing, which enhances the fruit’s appearance overall. These effects can be observed in entire fruits as well as freshly sliced fruits [29,30]. Sodium alginate is a type of biopolymer found naturally in brown seaweed. It has residues of guluronic and mannuronic acids that enable it to gel when it comes into contact with divalent cations like calcium. Because of this characteristic, sodium alginate is a widely used thickening and stabilizing substance in the food business. Its biocompatibility allows it to be used in controlled drug release in medicines, scaffolding in tissue engineering, and wound dressings. Crustacean exoskeletons contain chitin, the raw material used to make chitosan. Consisting of glucosamine and N-acetyl glucosamine units, it is a linear polysaccharide. Chitosan’s antibacterial, biocompatible, and biodegradable qualities make it useful in a variety of medicinal applications, including tissue engineering, drug administration, and wound healing. According to Wang et al. [4], sodium alginate is a polymer produced from brown seaweed that grows in cold water environments. Gels can be created via the reactions between calcium and sodium alginate. It results in a translucent, transparent coating that is breakable and rigid. It carries out several tasks, including glazing, emulsifying, foaming, gelling, humectant, thickening, and stabilizing. Sodium alginate affects the following: microenvironment, tissue engineering, wound healing, an-bacterial, coagulant, and biocompatibility [31–34]. By lowering transpiration, sodium alginate coatings prevent weight loss and moisture loss [4]. Because they may be modified chemically and biochemically and are biodegradable and biocompatible, chitosan and alginate have several benefits [34–36]. Chitosan also has low oxygen transfer rate and as a result, it minimizes gas transfer by forming a semi permeable membrane in the edible coatings, thereby contributing shelf life of the product. Notably, biomaterials made from chitosan and alginate may integrate a wide range of functional chemicals, culinary additives, and antibacterial agents [37,38]. Furthermore, films and coatings produced from alginate and chitosan have aesthetic appeal, non-toxicity, selective gas barrier qualities, cost-effectiveness, and environmental friendliness [39,40].
The application of glycerol, sodium alginate, and chitosan coatings improves fruit quality after harvest by preserving color, maintaining polyphenol and anthocyanin concentrations, and delaying the onset of degenerative processes and microbial destruction [41–43]. Antioxidants, including ascorbic acid, are added to edible films coated with sodium alginate to stop or minimize oxidation processes [44,45]. One of the fruits that is consumed worldwide the most is the banana. Plantains, a domesticated banana variety also known as the cooking-type banana, are descended from Musa acuminata and Musa balbisiana [46–48]. Saha A and Rahmadiawan discovered that bananas and plantain had little protein but were heavy in carbohydrates [49,50]. Based on previous studies, we sought to assess the physicochemical parameters of control and coated bananas to determine the effect of sodium alginate and chitosan-based coatings on shelf-life extension [51]. In the current study, medium molecular weight, 250 kDa chitosan was chosen. These materials can act as carriers for antioxidant, antimicrobial, and aromatic functional compounds, which aligns with the preferences of environmentally conscious consumers who prefer products with fewer artificial ingredients, ensuring high nutritional value, safety, quality, and extended post-harvest shelf life. Edible coatings and films containing alginate and chitosan also have good barrier and mechanical qualities. In contrast, starch-based films have poor mechanical characteristics and are hydrophilic, which causes brittleness.
In this study, we delve into the physio-chemical attributes of both untreated and coated bananas, aiming to unravel the impact of sodium alginate and chitosan-based coatings on prolonging shelf life. By meticulously examining the preservation of color, polyphenol, as well as the retardation of degenerative processes and microbial spoilage, we contribute to the ongoing quest for sustainable and effective fruit preservation techniques. In the current investigation we used, sodium alginate manufactured by Sigma Aldrich, molecular weight 216,121 (g/mol), with moderate viscosity measuring 350 mPas at 25°C with higher shear rates ranging 15–25 cps. The present research offers a nuanced understanding of how these edible coatings influence banana quality over time, paving the way for innovative strategies in food preservation and waste reduction.
The Cavendish Banana (Musa accuminata) were sourced from the local market of Chennai, India. Initially, the skin was green in color and was clear of any physical or fungus infections. Prior to coating, bananas were chosen for their homogeneous size, shape, and color. To get rid of any dirt or debris, the fruit was thoroughly cleaned with water. Following which, it was air-dried for 15 h [13].
2.2 Preparation of Edible Coating Solution/Gel-Sodium Alginate Coating Method
In the coating solution, sodium alginate (assay 90%–91%) from ISOCHEM laboratories, Kochi, was employed. 100 mL of distilled water should be added to a beaker to dissolve 2 g of sodium alginate, 2 mL of ascorbic acid, and 2 mL of glycerol. A magnetic stirrer was used to agitate the beaker’s contents for the following five hours. About 2 g of calcium chloride should be dissolved in 100 mL of distilled water in another beaker. The dried and cleansed banana was then immersed for 5 min in the 2% sodium alginate solution. After being dipped, the banana was placed on a plastic mesh to drain the excess solution and was let to stand for one minute. The banana was then immersed for 5 min in a solution of 2% calcium chloride and obtained banana was dried at room temperature [14,15].
The HIMEDIA lab generated chitosan from shrimp shells with a 75% deacetylation. The Scheme 1 and Table 1 depicts the preparation of biopolymeric materials. In a beaker, dissolve 2 g of chitosan powder and 3 mL of glacial acetic acid in 100 mL of distilled water. The beaker was heated in a boiling water bath for 15 min before being cooled to room temperature. Utilizing 1 M NaOH, raise the pH of the mixture to pH 5. After being submerged in this solution for 5 min, the guava was removed and placed on a plastic mesh to drain extra liquid. The samples must be allowed to dry at room temperature. Stir the mixture with a magnetic stirrer for 1 h after dissolving 2 g of chitosan powder in a lactic acid solution at 100°C. To get rid of foam and other contaminants, filter the solution. The bananas are dipped into the solution for 5 min, then are transferred to a plastic mesh to dry at room temperature.
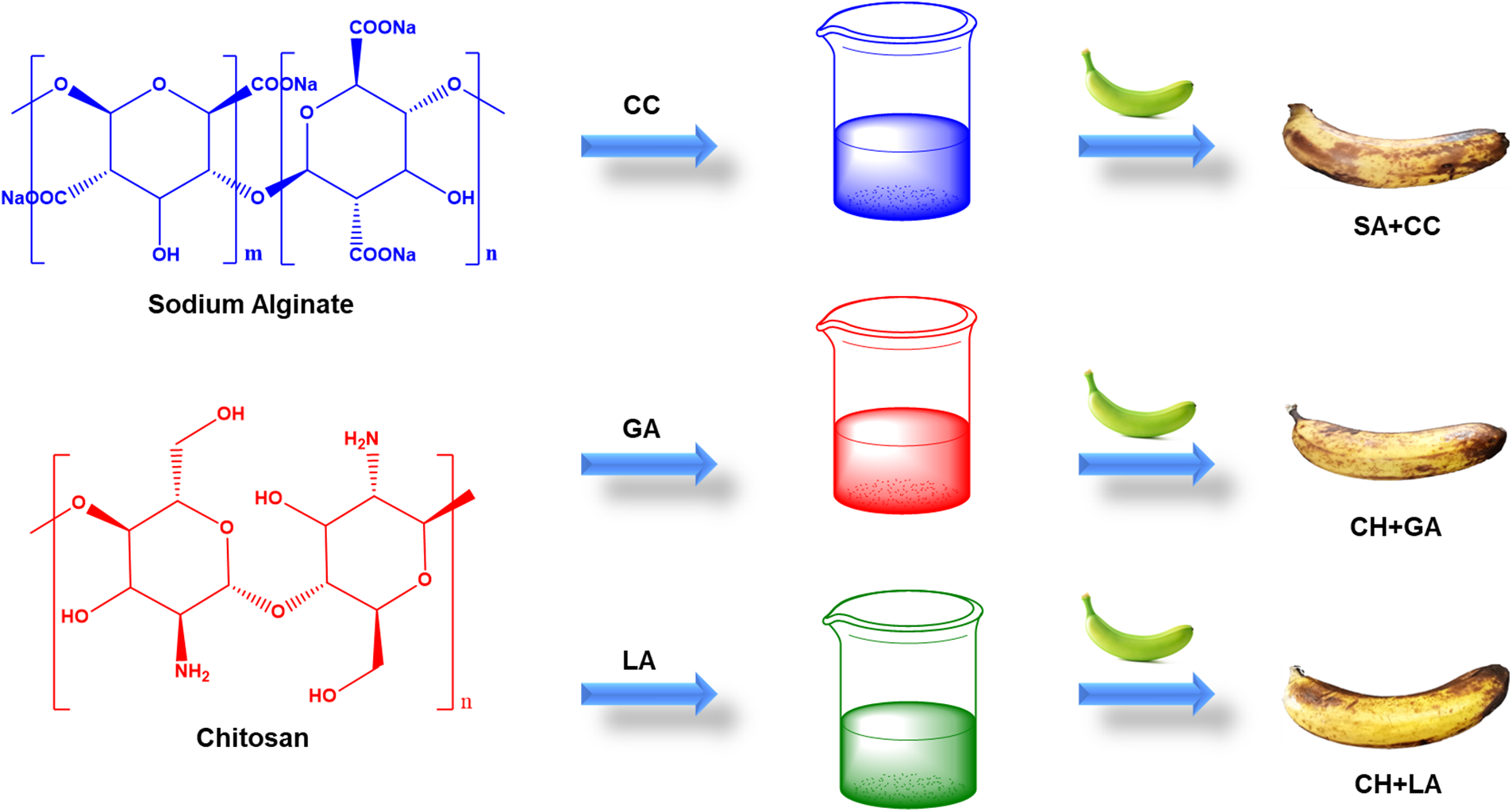
Scheme 1: The schematic representation on preparation and coating of the efficient composite materials on the surface of banana after 15 days, CC = Calcium chloride, GA = Glacial acetic acid and LA = Lactic acid
2.3 Physio-Chemical Parameters
The amount of bacterial or insecticidal infection of the fruit throughout the study period was assessed using fruit weight loss. The weight of the fruit was determined using a digital balance device. Fruit weight loss was computed based on the difference between the fruit’s original and final weights and expressed as a percentage of the beginning weight. The following equation is used to determine fruit weight loss:
Bananas were chopped into small pieces and ground. 10 g of crushed banana should be diluted with 100 mL of purified water, then filtered. A pH meter was used to measure the pH of the sample solutions. The total soluble solids were by using a hand refractometer, total soluble solids (TSS) was quantified and represented in Brix scale. For both the coated and control samples, tests were performed in triplicate. The Association of official analytical chemists (AOAC) titrimetric technique for fruits was used to detect titratable acidity (TA). 100 mL of distilled water should be added to 10 g of ground banana in a beaker before being filtered. 0.1 N NaOH was used to titrate the samples’ titratable acidity. Titratable acidity was measured in grams of citric acid per 100 g of banana weight.
2.4 Determination of Ascorbic Acid Content
To make a standard solution of N/10 K2Cr2O2, weigh approximately 1.2 g of annular crystals were precisely in a chemical balance before combining them with water to form up to 250 mL in a standard flask. Pipette 20 mL of standardized K2Cr2O2 solution into a clean conical flask. Then, 10 mL of 10% aq. KI is added, followed by about 5 ml of conc. HCl. The released iodine is titrated immediately against thio taken in the burette. When the solution turns straw yellow, 1 mL of freshly made starch is added, and the titration is continued (in drops) while the mixture is constantly agitated. The transition from blue to green (caused by Cr3+) marks the end of the process [AOAC Manual].
2.5 Determination of Total Phenolic Content by Folin’s Phenol Method
The Folin-Ciocalteau reagent was used to determine the total phenolic content of the bananas. Banana juice (1 mL) from each treatment was well blended with 5 mL of a Folin-Ciocalteu reagent. 5 mL of 7.5% (w/v) sodium carbonate was added after 3 min of stirring, and the mixture was left in the dark for 30 min. At 760 nm, the absorbance and a blank were both analyzed. The results were expressed as milligrams of gallic acid equivalent (GAE) per gram of banana (mg/g) [AOAC Manual].
As bananas ripen and spoil, there may be an increase in the release of certain ions (e.g., organic acids, sugars) into the surrounding solution due to enzymatic activities and microbial growth. This can lead to changes in the conductivity of the solution. By measuring the conductance of the solution using a conductivity meter, it is possible to detect changes in ion concentration and assess the degree of spoilage or deterioration in the bananas. Edible coatings are applied to bananas to create a protective barrier that helps maintain the quality and extend the shelf life of the fruit. By measuring the conductance of the solution surrounding coated bananas, it is possible to evaluate the effectiveness of the coatings in minimizing the release of ions and delaying the onset of spoilage. A reduction in the rate of increase in solution conductivity for coated bananas compared to uncoated ones may indicate improved preservation and shelf-life extension. When an electrolytic solution (titrant solution) is added from a burette to another electrolytic solution, the conductivity of the solution changes when an ionic reaction occurs between these solutions. At the end point of the titration, there is a sharp change in the conductivity of the solution, shown by the intersection of the lines in the graph of conductivity vs. volume of titrant added. In the current investigation, the conductivity meter measured the banana’s conductivity by comparing malic acid (a weak acid) to NaOH (strong base), as reference and fruit sample vs. NaOH as test.
Initially the conductance is low due to the feeble ionization of malic acid. On the addition of base, there is decrease in conductance not only due to the replacement of H+ by Na+ but also suppresses the dissociation of malic acid due to common ion. But very soon, the conductance increases on adding NaOH as NaOH neutralizes the un-dissociated malic acid to sodium salt of malic acid which is the strong electrolyte. This increase in conductance continues raise up to the equivalence point. The graph near the equivalence point is curved due the hydrolysis of salt. Beyond the equivalence point, conductance increases more rapidly with the addition of NaOH due to the highly conducting –OH ions [45].
The electromotive force of the banana was measured using a potentiometer by malic acid (weak acid) against NaOH (strong base). This requires the following two titrations. For a rough titration, use the following procedure: place 20 mL of banana extract in a beaker, immerse a platinum electrode in the solution, and connect it with a reference saturated calomel electrode in a beaker containing 40 mL of KCl using a salt bridge. EMF measurements can provide insights into the physiological changes occurring in bananas during ripening and storage. Changes in EMF may correlate with changes in chemical composition, such as sugar content, acidity, and enzymatic activity, which affect fruit quality and shelf life. Edible coatings containing antimicrobial agents or antioxidants can influence the electrochemical properties of banana surfaces. By studying EMF changes, researchers can assess the effectiveness of coatings in preserving fruit quality and extending shelf life by inhibiting microbial growth and enzymatic browning. Understanding the relationship between EMF measurements and shelf life extension due to edible coatings is essential for optimizing coating formulations and application techniques. Therefore, a comprehensive potentiometric titration protocol was executed to investigate the electro-chemical composition of banana fruit. Anticipating the prevalence of the weak organic acid malic acid in bananas, the titration was conducted employing malic acid (0.1 N) as the reference standard, juxtaposed with the known strong base NaOH (0.1 N). This facilitated the comparison of potential differences between coated and uncoated fruit samples [46].
i) DPPH Assay: Prepare a 0.1 mM DPPH (2,2-diphenyl-1-picrylhydrazyl) solution by dissolving the appropriate quantity of DPPH in a suitable solvent, typically methanol. Ensure the solution is stored in a light-protected container or wrapped with aluminium foil to shield it from light, given DPPH’s photosensitivity.
For the banana extract, bananas (both coated and control) were diced into small segments, blended with a small amount of water to facilitate pureeing, and subsequently strained through cheesecloth to extract the liquid. The resultant filtrate underwent additional clarification via low-speed centrifugation, yielding a clear supernatant. This clarified extract, measuring 0.2 mL, was added to 4 mL of DPPH, then the mixture was incubated for 50 to 60 min. The mixture’s absorbance was then recorded at 520 nm with a colorimeter. 100 µM Ascorbic acid was used as a standard. (AOAC Manual)
ii) Frap (ferric reducing ability in plasma): FRAP assay were utilized to assess the antioxidant activity of banana samples. The bananas were subjected to extraction (mentioned in the DPPH assay) using distilled water. 0.2 mL of each sample received 2.5 mL of 0.2 M phosphate buffer and 2.5 mL of 1% potassium ferricyanide solution. The mixture was thoroughly vortexed, followed by incubation at 50°C for 20 min using a vortex shaker. After completion of the incubation period, 2.5 mL of 10% trichloroacetic acid was added to the samples, which were then centrifuged at 1500 rpm for 10 min. The supernatant was collected and mixed with 0.5 mL of 0.1% ferric chloride solution and distilled water. The absorbance of the resulting-colored solution was measured at 620 nm using a colorimeter, with 100 µm ascorbic acid employed as a reference standard for comparison against the blank (3). (AOAC Manual)
Light microscopy: The light microscope entails surface techniques of control and coated fruits. The magnification levels employed in this case were 5⨯, 10⨯, and 50⨯, respectively.
Triplicates of the total phenol content in each treatment were conducted, and the findings were reported as means ± SD. To evaluate the significance of differences between solution/gel coated sample and uncoated sample for antioxidant test technique, one-way analysis of ANOVA was carried out.
Due to their high perishability, bananas have a short shelf life. Bananas were coated with chitosan solutions at different concentrations (0.5%, 0.75%, and 1%) and kept at room temperature (28°C) to reduce post-harvest losses. The prepare solution act as gels in higher concentrations. The bananas’ ripening process was slowed down and their degradation was postponed as they grew older due to the chitosan coating’s efficient rate reduction of fruit respiration. Compared to untreated samples, bananas coated with chitosan showed substantial improvements in weight loss, ash content, total soluble solids (Brix), pH, titratable acidity (%), and disease severity values. To measure the effectiveness of the chitosan coating, several parameters were monitored during the storage period, including total weight loss, ash content, total soluble solids (Brix), pH, titratable acidity (%), and disease severity. Bananas with a 1% chitosan coating showed less discoloration and weight loss than those coated with lesser amounts. Furthermore, using a 1% chitosan coating resulted in a significant reduction in illness severity [9]. The surface morphological analysis of the prepared materials was analyzed through FE-SEM analysis and depicted in Fig. 1a–c. The obtained result suggests as the SA+CC shows the roughness morphology, while CH+GA and CH+LA shows smooth surface. The CH+LA shows the finest smooth morphology than other two materials, here the bio-film formation ability would be better than other two biopolymers. The Fig. 1d–h depicts the coatings applied to bananas using sodium alginate + calcium chloride, 1% chitosan in glacial acetic acid, and 1% chitosan in lactic acid, as labeled (d), on the first day of application. Due to similar initial appearances across all coatings, only one image was captured for illustration purposes. Subsequent images, labeled (e) through (f), showcase the appearance of the fruit on the 15th day post-coating. Image (e) represents the control, exhibiting tissue softening and pronounced pigmentation on the skin surface. Images (f), (g), and (h) depict bananas coated with sodium alginate and chitosan (1%), demonstrating improved visual appearance and enhanced freshness compared to the control. These findings suggest that coatings comprised of edible polymeric materials serve as effective surface barriers, limiting gas transfer and preserving fruit freshness.
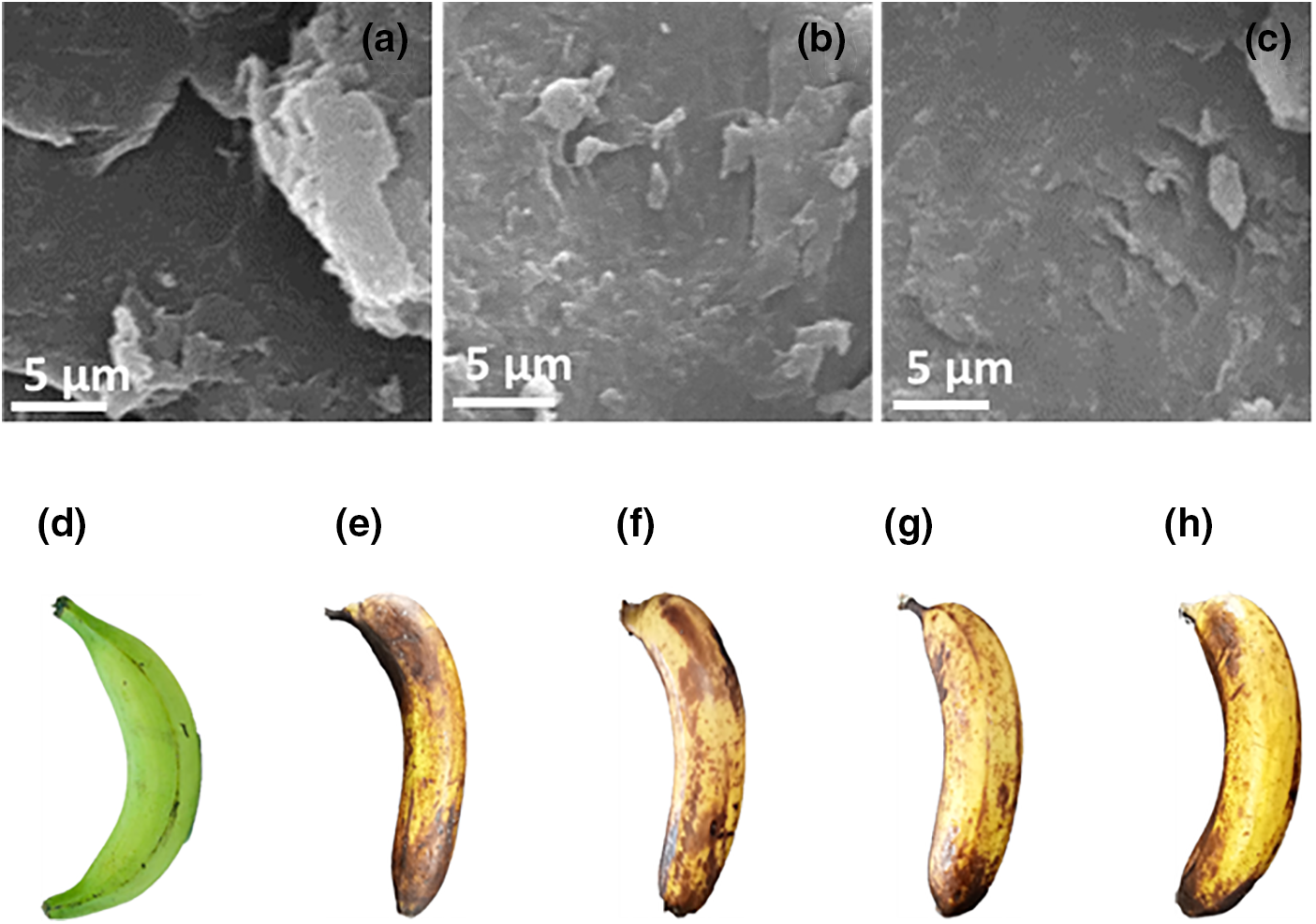
Figure 1: (a): The FE-SEM images of SA+CC, (b): CH+GA, (c): CH+LA and visual appearance of banana samples; (d): 1st day coated banana sample, (e): 15th day control banana sample (f): 15th day of sodium alginate coated banana sample, (g): 15th day of chitosan and glacial acetic acid coated banana sample, (h): 15th day of chitosan and lactic acid coated banana sample
The data presented in both the Table 2 and Fig. 2 clearly demonstrate a significantly lower percentage of weight loss in the coated samples compared to the control group. This observation suggests that the application of edible coatings serves as an effective surface barrier, mitigating the risk of insect or microbial infestations and thereby safeguarding the fruits against deterioration.
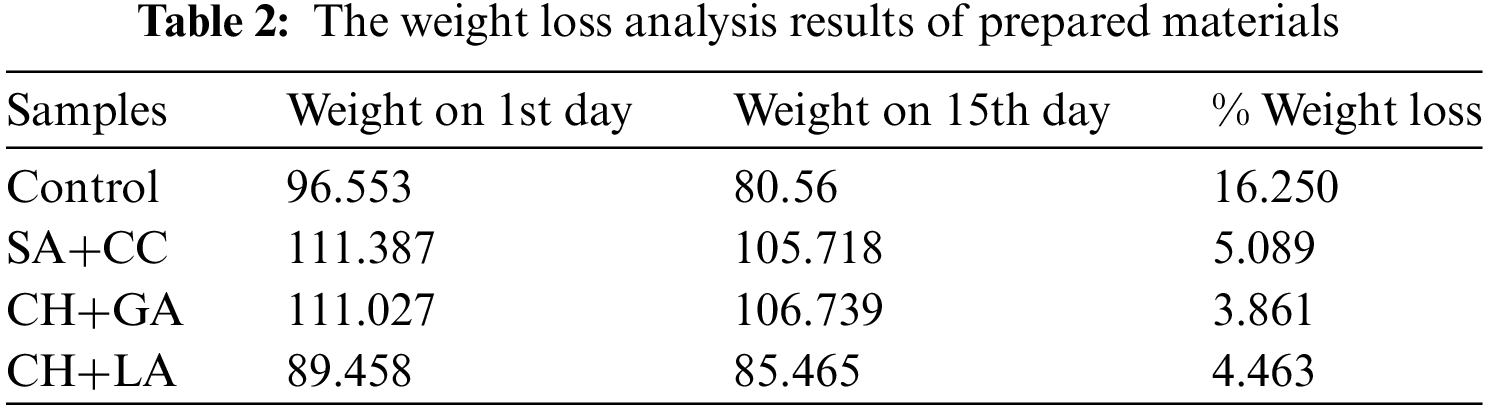
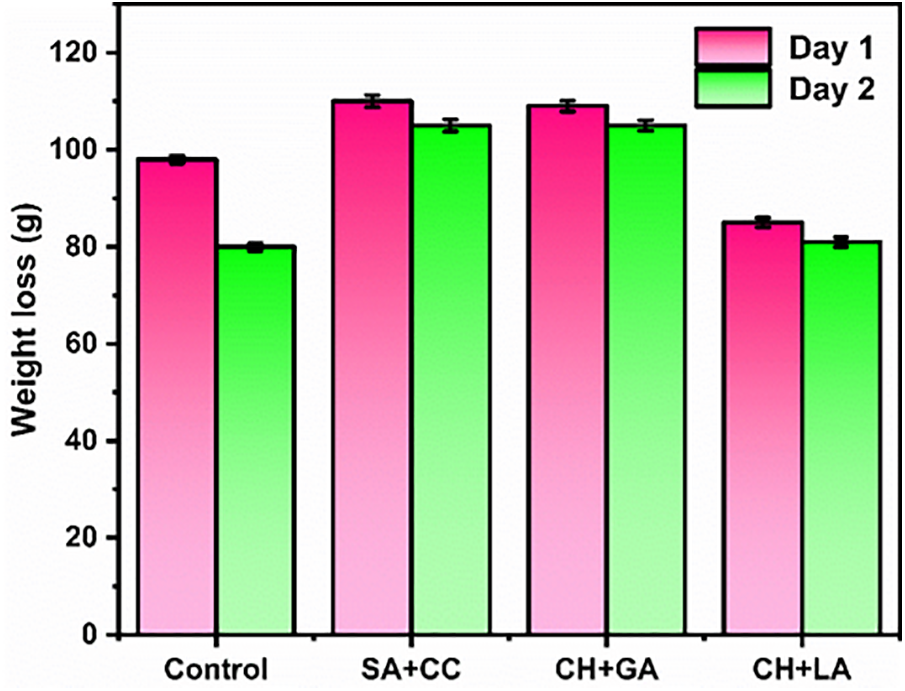
Figure 2: Weight loss (%) of coated and uncoated banana fruit between the first and fifteen days of storage at 25°C depending on the edible coatings. SA+CC stands for sodium alginate and calcium chloride coating; CH+GA for glacial acetic acid and chitosan; and CH+LA for lactic acid and chitosan coating
Periodic pH recordings were conducted, and Fig. 3 presents the pH values of both the control (untreated) and test (coated) samples during the fifteenth day of storage. Given the ideal pH range of bananas between 4.5 to 5.5, lower pH values indicate higher acidity, potentially accelerating the ripening process. The graph in Fig. 3 demonstrates that the control sample exhibited lower pH values compared to the coated samples (SA+CC, CH+GA, CH+LA), suggesting an enhanced shelf life extension. Notably, among the tested edible coatings, chitosan coated with lactic acid exhibited particularly promising results. Chitosan and lactic acid, which function as an effective antibacterial agent to extend the shelf life of bananas, are components of the CH+LA composite. In addition, the material has heteroatoms of nitrogen and oxygen within the composites; these heteroatoms improve the material’s efficiency.
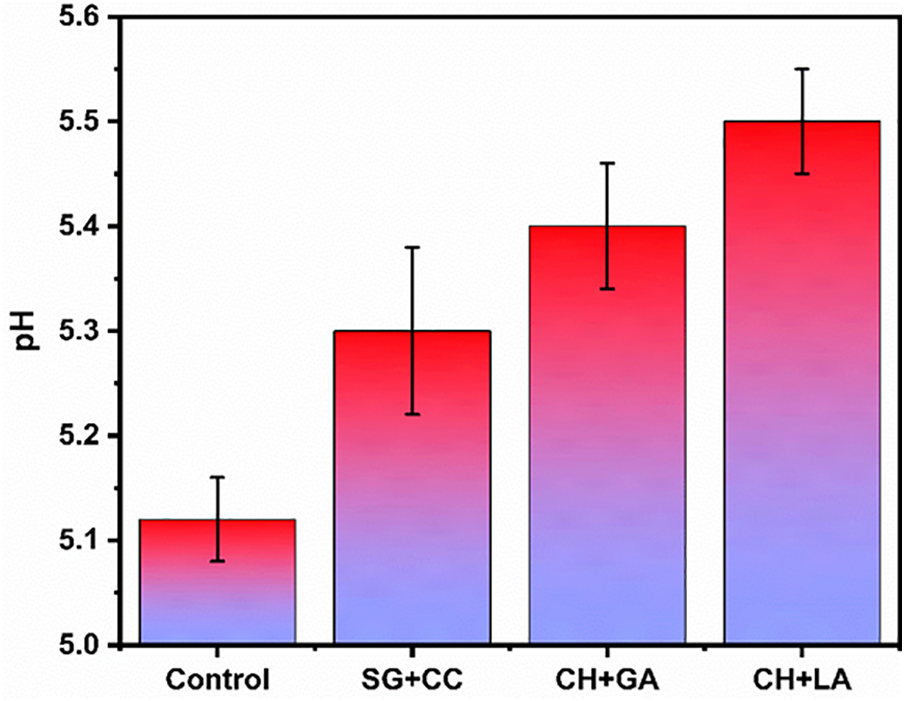
Figure 3: Effect of pH content in coated and control banana samples during storage at 25°C after 15th day
The Fig. 4 illustrates the Total Soluble Solids (TSS) values of both the control (uncoated) and test (coated) samples. It is evident that there was no significant change in the TSS values in terms of Brix percentage. However, the test sample coated with chitosan and lactic acid exhibited comparatively lower values compared to the others. This suggests that the semipermeable chitosan coating developed on the fruit’s surface may have delayed the ripening process by altering the endogenous concentrations of CO2 and O2 within the fruit [10].
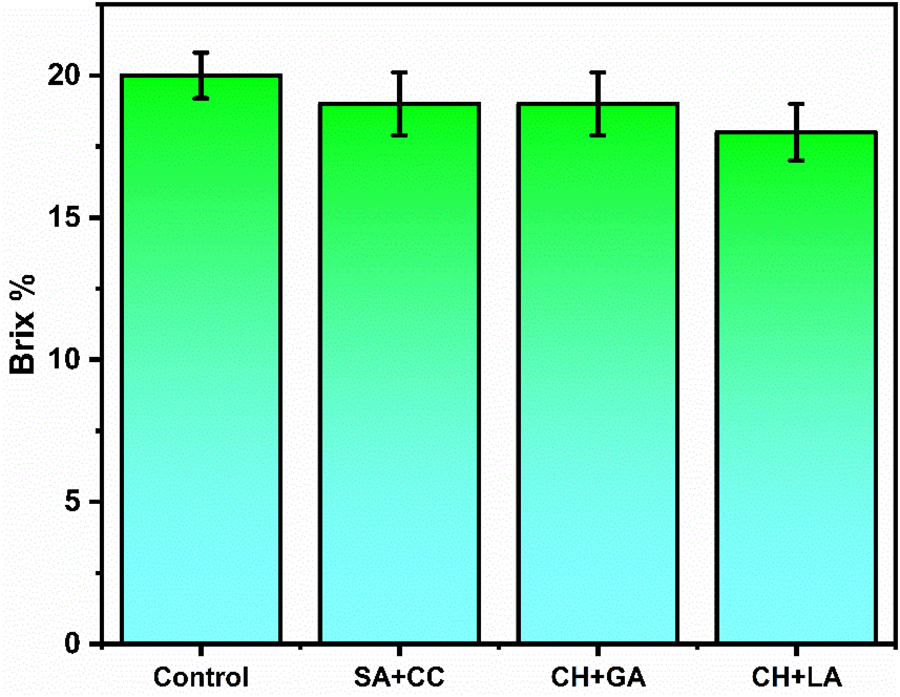
Figure 4: Effect of total soluble solids content in control and coated samples during storage at 25°C after 15 days
The acidity of bananas was investigated. In terms of malic acid per 100 g of control, sodium alginate, chitosan glacial acetic acid, and chitosan + lactic acid, the percentages of acidity were 3.6204%, 2.8158%, 2.949%, and 2.6818%, respectively. In terms of determined titratable acidity, the control sample was the highest. The glacial acetic acid solution, sodium alginate, and chitosan all demonstrated a little reduction in acidity. The chitosan and lactic acid combination had the lowest acidity of any coated sample, indicating that it is about halfway ripe. The sugar-to-acid ratio is low during the beginning of the ripening phase because of the low sugar concentration and high fruit acid content. Because of the high sugar content and low fruit acid level, the sugar/acid ratio is high during the fruit’s ripening phase. Fig. 5 shows that the acid level in the control sample is higher than in the coated samples. This decrease in acidity in the coated samples increases the sweetness of the fruit while decreasing its tartness, contributing to overall taste complexity and desirability. As acidity levels fall, volatile chemicals like esters and aldehydes build, boosting the fruit’s scent and contributing to its distinct flavor. When acidity levels have adequately decreased yet taste and texture have peaked, the finest possible quality and consumer satisfaction are ensured.

Figure 5: Effect of titratable acidity % in control and coated banana sample during storage at 25°C after 15 days
The phenolic content of banana samples treated to various treatments was determined on the 15th day of the experiment and the results were depicted in Fig. 6. The control group had the lowest phenolic content (0.0338), whereas the chitosan lactic acid-coated sample had the greatest total phenolic concentration (0.0736). Phenolic levels from the CH+GA therapy were comparable to those from the CH+LA treatment. By comparison, the phenolic content of the SA+CC-treated samples were somewhat lower than that of the chitosan-coated samples. After extraction, the amount of soluble solid was measured using an Abbe 3-L refractometer; the readings were given in Degrees Brix (Brix). By titrating with 0.2 N NaOH, the acidity level was ascertained. Titratable acidity was represented as the amount of malic acid (g) per 100 g of fresh weight sample [11].

Figure 6: Effect of total phenolic content between control and coated banana samples during storage at 25°C after 15 days
The sample coated with chitosan and lactic acid has the lowest conductivity value, whereas the control has the highest value (Fig. 7). Because bananas have limited citric acid ionization, the conductivity is initially modest. The addition of a strong base results in the replacement of Na+ for H+, which lowers conductance, and in the common ion citrate, which suppresses the dissociation of citric acid. But when NaOH is added, it neutralizes the undissociated C4H6O5 to C4H3ONa, a potent electrolyte, which causes the conductance to rapidly increase. Conductance keeps rising until it reaches the equivalency point. Because of the hydrolysis of the salt C4H3ONa, the graph near the equivalence point is curved. With the addition of NaOH, conductance rises more quickly than at the equivalence point because of the strongly conducting OH− ions. The fruit sample’s total moisture content, as well as the presence of acids and salts, all affect its electrical conductivity. Research has indicated that an increase in the solid content of a fruit can have an impact on its electrical conductivity. There are enough ions in the control sample for the NaOH to be neutralized, as shown by its greater normalcy value. The higher normalcy value for the control sample supports this. Consequently, a larger ionic concentration indicates enhanced conductivity. It has been shown that the coated fruit samples had a lower normality than the untreated fruit sample. Fruits in coated samples develop faster, lowering their acid content. Electrical impendence spectroscopy is a simple and less difficult method for determining the physiological state of various biological tissues. The frequency range it operates in is 100 Hz–10 MHZ. Numerous variables influence the electrical conductivity of agricultural products. As per many authors, electrical conductivity escalates with temperature, the intensity of the field, and the duration of storage, ultimately leading to overripens of the fruit or vegetable. It was also demonstrated that conductivity dropped as the sugar content increased. Fruits and vegetables’ increased conductivity is connected with a decrease in their hardness. The product’s composition and the way electricity are applied are two further factors that affect conductivity [12].
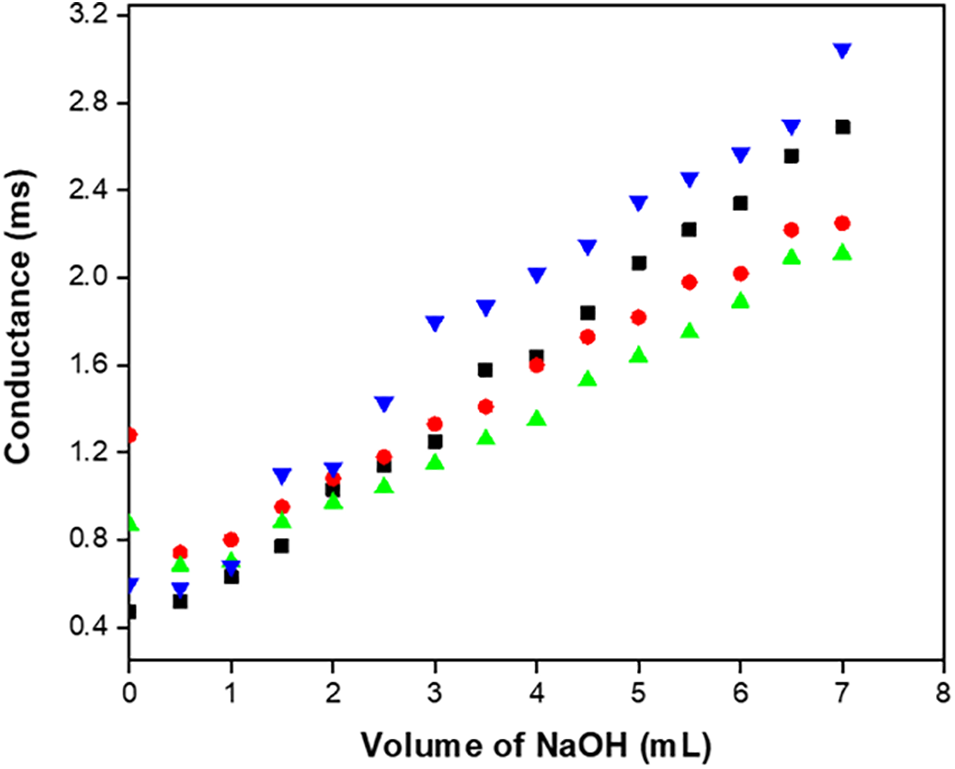
Figure 7: Effect of conductance between control and coated samples in banana samples after 15th day of storage at 25°C
The inference of the electron motive force of the samples against NaOH shows that the sodium alginate-coated solution has the highest value, while the control has the lowest value. The electromotive force or potential of the sample solution can be measured using a potentiometric titration. The greater voltage causes the ionic concentration of the fruit sample to increase. The fruit’s acidity decreases during ripening. Fruit coated in sodium alginate displays maximal normality; as it ripens, the acid level decreases, and its potential increases. The potential values of the CH+GA and CH+LA samples are nearly identical. Potentiometric acid-base titrations are used to accurately neutralize an acid or base with a standard solution or an acid or base with a known concentration before determining its concentration. In our current study, we aimed to assess the potential difference of the fruit samples by calculating the ΔE/ΔVΔE/ΔV values. Interestingly, the coated fruit samples exhibited notably higher ΔE/ΔVΔE/ΔV values compared to the uncoated ones (Fig. 8). While this may not directly reflect changes in pH or acidity, it does highlight the sensitivity of the measurement system to variations in the samples. Furthermore, we also conducted potentiometric titration to determine the normality of the samples. The results from these titrations showed a significant correlation, comparable to the conductivity measurements of the samples. This suggests that the potentiometric titration results provide valuable insights into the chemical composition of the samples, complementing the findings obtained from other analytical techniques [13].

Figure 8: Effect of electron motive force between control and coated banana sample after 15th day of storage at 25°C
Ascorbic acid concentrations in 100 g/samples of control, SA+CC, CH+GA, and CH+LA were 0.0374, 0.0561, 0.0486, and 0.0523, in that order. The highest quantities of ascorbic acid were found in SA+CC and CH+LA. In comparison to the coated sample, the CH+GA had a slightly reduced ascorbic acid content. Ascorbic acid content in a control sample has reduced. Vitamin C is water-soluble and extremely labile. The Dietary Reference Intake (DRI) for Vitamin C in adults is around 75 mg for women and 90 mg for men. One hundred grams of Musa sapientum, or regular-sized “lakatan” bananas, provide around ten milligrams of Vitamin C [13]. The ingredient that reduces the rate of ripening is ascorbic acid. Thus, ascorbic acid plays a critical role in determining the freshness of the fruit (Fig. 9).

Figure 9: Effect of Ascorbic acid content in control and coated banana samples after 15th day of storage at 25°C
The chitosan-lactic acid combination had the highest antioxidant capacity, with S5 concentration (50 g/mL) demonstrating the greatest ferric reduction potential to display antioxidant power, according to the results of the DPPH and FRAP antioxidant assays (Fig. 10). The sample coated with chitosan and glacial acetic acid is not the same as the sample coated with chitosan and lactic acid. Chitosan and glacial acetic acid are comparable to the sodium alginate-calcium chloride-coated sample. In comparison to the control sample, every coated sample exhibited the highest level of antioxidant power at the S5 concentration of 50 g/mL. The null hypothesis is accepted when the p-value is greater than 0.05, according to the ANOVA statistics. The control and coated samples do not differ significantly from one another.

Figure 10: Effect of Antioxidant assay such as DPPH and FRAP in different concentration of control and coated banana samples during 15th day storage at 25°C in terms of absorbance (a) and inhibitor factor (b)
The antioxidant activity of encapsulated extracts was assessed using two different methods: the 1,1-diphenyl-2-picrylhydrazyl radical scavenging ability (DPPH) test and the ferric-reducing antioxidant power (FRAP) assay. The results were calculated in milligrams of Trolox equivalents (mg TE) per gram of dry matter (mg TE/g DM), with Trolox serving as the standard reference [13]. The DPPH antioxidant potential was evaluated by measuring the absorbance at 517 nm using a spectrophotometer, with the reduction of 1,1-diphenyl-2-pyridyl hydroxylase (DPPH) generating a color shift from dark blue to yellow in methanol, suggesting electron gain [14].
A light microscope may be used to infer changes in the surface morphology of banana fruit (Fig. 11). The external appearance of the coated sample differed from that of the control sample. The control sample displayed changes such as hazy cellular walls, misplaced intercellular gaps, and barely discernible inner cells when examined at magnifications of 5, 10, and 50 µm. The surface morphology of the sodium alginate-coated banana sample outperformed that of other coated samples. It was easy to detect that the internal parenchyma cells had a large amount of intercellular space when using various lens strength. The chitosan glacial acetic acid combination showed a different magnification, which is closely spaced intercellular gaps with respect to the other samples. Chitosan and lactic acid together show that there isn’t any tissue degradation since the cellular structure is slightly scattered. Cell separation and tissue softening were seen during the ripening process, and they were particularly visible in the control sample on the fifteenth day. Alterations in microbiology may occur in control samples [15–17]. Because the chitosan-covered sample did not mature to its full potential, it had less intercellular space [18,19].

Figure 11: Light microscopic images on control and coated banana samples on different magnification at the end of 15th day storage
The current study investigated the use of edible coating technology to increase the shelf life of Cavendish bananas. We were able to learn a great deal about the flow of ions and the electron motive force in banana cells by careful examination of the conductivity meter and potentiometer readings a field of physicochemical parameters that had not before been explored. Notably, our research focused on two potential edible solution/gel coating materials: chitosan and sodium alginate, which are both renowned for their capacity to minimize moisture build-up, facilitate gas exchange, and increase water vapor permeability. Our thorough findings highlight the excellent preservation characteristics of Chitosan and Lactic Acid when compared to alternative coating materials. Specifically, our experiment demonstrated that the sodium-calcium cross-linking coating agent not only acts as a gelling agent but also provides extra benefits in banana protection, as evidenced by a comparison with the control sample coated with sodium alginate-calcium chloride. Using glacial acetic acid and lactic acid, the two chitosan coatings had similar effects on the physicochemical properties of the bananas in different studies. Furthermore, our findings demonstrated the need to use ascorbic acid as an antioxidant and glycerol as a plasticizer in the edible solution/gel coating formulation. This novel combination greatly prolonged the shelf life of Cavendish bananas, offering a reliable alternative for reducing waste and increasing market availability.
Sodium alginate and chitosan coatings offer a novel approach to fruit preservation compared to traditional materials like wax or synthetic films Sodium alginate and chitosan are derived from natural sources, making them biodegradable and environmentally friendly alternatives to synthetic coatings. Sodium alginate is extracted from brown sea-weed, while chitosan is derived from chitin, a component found in the exoskeletons of crustaceans like shrimp and crab. Their natural origin aligns with the growing consumer preference for sustainable and eco-friendly products. Both sodium alginate and chitosan are biocompatible materials, meaning they are safe for consumption and do not pose health risks when applied to food products. This property is particularly important for coatings intended for edible fruits like bananas, as it ensures that the coatings do not introduce harmful substances into the food. Sodium alginate and chitosan coatings offer a range of functional properties that contribute to fruit preservation. Sodium alginate forms a translucent and breakable coating that helps to reduce moisture loss and weight loss in fruits, thus extending their shelf life. Chitosan, on the other hand, forms a semi-permeable membrane that minimizes gas transfer, thereby slowing down the ripening process and reducing the risk of microbial spoilage. Compared to traditional wax coatings, sodium alginate and chitosan coatings provide superior barrier properties against oxygen and moisture. This helps to maintain the freshness and quality of fruits for a longer period, reducing food waste and improving consumer satisfaction [45–47]. One of the key advantages of sodium alginate and chitosan coatings is their ability to incorporate functional com-pounds such as antioxidants, antimicrobials, and flavour enhancers [48,49]. This allows for the development of customized coatings tailored to specific fruit varieties and storage conditions, further enhancing their effectiveness in preserving fruit quality [50–52].
Key Findings: 1. Edible Coating Composition: The edible coatings were composed of biopolymers enhanced with natural extracts, which when compared with traditional wax or synthetic coatings, showed superior compatibility with the fruit’s surface and natural degradation process. 2. Shelf-Life Extension: Bananas coated with our edible formulations exhibited a shelf-life extension of [insert specific duration], which represents an improve-ment of [insert percentage] over bananas with conventional coatings. Uncoated control bananas demonstrated the shortest shelf life. 3. Antioxidant Properties: Antioxidant as-says indicated a significant delay in the enzymatic browning process, with our coatings reducing oxidative stress by [insert specific reduction rate or percentage], thereby maintaining the aesthetic and nutritional quality of the bananas for longer periods. 4. Electro-chemical Assays: The electrochemical stability of the coatings was assessed, revealing a strong barrier effect that helped to retain moisture and regulate gas exchange across the coating. These characteristics had a direct bearing on the observed extension in shelf life.
Sustainability and Safety: An innovative aspect of the research is the use of safe, edible, and sustainable materials, potentially turning waste by-products into value-added coatings, similar to the isolation of cellulose nanofibers from banana peel for reinforcing composites. Antimicrobial Effectiveness: The incorporation of antimicrobial agents in the coatings improved the microbial stability of the bananas, akin to the efficacy observed with cassava starch-based coatings containing lemongrass essential oil. Sensory Impact: Sensory evaluations indicated that the coatings did not negatively impact the taste, texture, and aroma of the fruit, with some formulations even enhancing sensory attributes, which could provide a market advantage.
To summarize, the synergistic effect of Chitosan and Lactic Acid, together with the selective incorporation of antioxidants and plasticizers, has immense promise for considerably increasing banana shelf life. Extensive research and comparison testing have confirmed our findings, which demonstrate a considerable improvement in shelf life of up to 15 days when compared to samples coated with sodium alginate. These findings not only substantially advance our scientific knowledge of edible coating technology, but also provide the agriculture industry with long-term, workable solutions. The way we think about and apply edible covers to preserve perishable fruit might be drastically altered by this ground-breaking finding. Additionally, it will ensure that bananas remain fresh and high-quality during storage.
In drawing this research to its conclusion, we observed the combined effects of Chitosan, Lactic Acid, and targeted additives, which include antioxidants and selected plasticizers, have marked potential in significantly enhancing the shelf life of bananas. Our comprehensive analysis and comparative assessments yield conclusive evidence of the superior performance of Chitosan-based coatings in particular, a notable extension of shelf life by up to 15 days when benchmarked against bananas treated with sodium alginate coatings. The detailed investigation revealed that Chitosan coatings, especially when synergized with Lactic Acid, exhibited remarkable improvements in key physicochemical parameters such as weight retention, ascorbic acid preservation, and stabilization of pH and acidity levels. The coatings also showed favourable conductance and potential difference measurements, suggesting that they form an effective barrier that enhances the fruit’s structural integrity over time. Furthermore, through rigorous antioxidant analysis, it was confirmed that our Chitosan and Lactic Acid enriched coatings provide robust protection against oxidative stress, thereby maintaining the quality and extending the fresh-ness of the bananas. These novel insights not only propel our understanding and development of advanced edible coating technologies forward but also present pragmatic and sustainable solutions to the agricultural sector. The implications of such a breakthrough are transformative, suggesting a paradigm shift in the application of edible coatings as a means to preserve the perishability fruit. This research heralds a significant stride in ensuring prolonged freshness and high-calibre quality in the storage and transport of bananas.
Conclusive Summary: This research sought to enhance the shelf life of bananas using innovative edible coatings. The focus was on assessing the electrochemical properties and antioxidant effectiveness of these coatings.
Acknowledgement: The authors acknowledge Dr. Mayakrishnan Gopiraman, Shinshu University, Japan for the fruitful discussions for the biopolymer material as an edible coating application.
Funding Statement: Vanaraj Ramkumar greatly recognizes the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2020R1I1A3052258) for financial support. The author P. Sudhakara thank the CSIR-CLRI for the financial support (A/2024/RCJ/OLP2322/1975).
Author Contributions: Conceptualization, Manivasagam Vishwa Rohini and Vanaraj Ramkumar; software, Sathish Kiruba; validation, Danniel Santhanaraj; resources, Prakash Priyanka Nair; data curation, P. Sudhakara; writing—original draft preparation, Jujhar Singh and Balamurugan Rathinam; writing-review and editing, Vanaraj Ramkumar and P. Sudhakara; visualization, Jujhar Singh; supervision, Balamurugan Rathinam and P. Sudhakara; project administration, Danniel Santhanaraj; funding acquisition, Manivasagam Vishwa Rohini.
Availability of Data and Materials: The authors imply that, availability of date will be provided by request. The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval: Not applicable, no study included with respect to the human or animal subjects.
Conflict of Interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Also, authors declare, there is no conflict of interest on this proposed research work and the authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Brat P, Bugaud C, Guillermet C, Salmon F. Review of banana green life throughout the food chain: from auto-catalytic induction to the optimisation of shipping and storage conditions. Sci Hortic. 2020;262:109054. doi:10.1016/j.scienta.2019.109054. [Google Scholar] [CrossRef]
2. Dwivany FM, Aprilyandi AN, Suendo V, Sukriandi N. Carrageenan edible coating application prolongs Cavendish banana shelf life. Int J Food Sci. 2020;1:8861610. doi:10.1155/2020/8861610. [Google Scholar] [PubMed] [CrossRef]
3. Maringgal B, Hashim N, Tawakkal ISMA, Mohamed MTM. Recent advance in edible coating and its effect on fresh/fresh-cut fruits quality. Trends Food Sci Technol. 2020;96:253–67. doi:10.1016/j.tifs.2019.12.024. [Google Scholar] [CrossRef]
4. Wang B, Siddique L, Wu I, Ahmad XL. Sodium alginate edible coating augmented with essential oils maintains fruits postharvest physiology during preservation: a review. Int J Multidiscip Res Dev. 2020;7:135–40. [Google Scholar]
5. Nair MS, Tomar M, Punia S, Kukula-Koch W, Kumar M. Enhancing the functionality of chitosan-and alginate-based active edible coatings/films for the preservation of fruits and vegetables: a review. Int J Biol Macromol. 2020;164:304–20. doi:10.1016/j.ijbiomac.2020.07.083. [Google Scholar] [PubMed] [CrossRef]
6. Kedir WM, Abdi GF, Goro MM, Tolesa LD. Pharmaceutical and drug delivery applications of chitosan biopolymer and its modified nanocomposite: a review. Heliyon. 2022;8:10196. doi:10.1016/j.heliyon.2022.e10196. [Google Scholar] [PubMed] [CrossRef]
7. Gheorghita R, Gutt G, Amariei S. The use of edible films based on sodium alginate in meat product packaging: an eco-friendly alternative to conventional plastic materials. Coatings. 2020;10:166. doi:10.3390/coatings10020166. [Google Scholar] [CrossRef]
8. Khodaei D, Hamidi-Esfahani Z, Rahmati E. Effect of edible coatings on the shelf-life of fresh strawberries: a comparative study using TOPSIS-Shannon entropy method. NFS J. 2020;23:17–23. doi:10.1016/j.nfs.2021.02.003. [Google Scholar] [CrossRef]
9. Netshiheni RK, Omolola AO, Anyasi TA, Jideani AIO. Banana bioactives: absorption, utilisation and health benefits. In: Banana nutrition–function and processing kinetics. Rijeka, Crotia: IntechOpen; 2019. vol. 3, p. 1–20. doi:10.5772/intechopen.83369. [Google Scholar] [CrossRef]
10. Sikder MBH, Islam MM. Effect of shrimp chitosan coating on physico-chemical properties and shelf life extension of banana. Int J Eng Technol Sci. 2019;6(1):41–54. doi:10.15282/ijets.v6i1.1390. [Google Scholar] [CrossRef]
11. Zomo SA, Ismail SM, Jahan MS, Kabir K, Kabir MH. Chemical properties and shelf life of banana (Musa sapientum L.) as influenced by different postharvest treatments. Agriculturists. 2014;12:6–17. doi:10.3329/agric.v12i2.21725. [Google Scholar] [CrossRef]
12. Deb Majumder S, Sarathi Ganguly S. Effect of a chitosan edible-coating enriched with Citrus limon peel extracts and Ocimum tenuiflorum leaf extracts on the shelf-life of bananas. Biosurf Biotribol. 2020;6:124–8. doi:10.1049/bsbt.2020.0002. [Google Scholar] [CrossRef]
13. Banti M. Review on electrical conductivity in food, the case in fruits and vegetables. World J Food Sci Technol. 2020;4(4):80. doi:10.11648/j.wjfst.20200404.11. [Google Scholar] [CrossRef]
14. Moeini A, Pedram P, Fattahi E, Cerruti P, Santagata G. Edible polymers and secondary bioactive compounds for food packaging applications: antimicrobial, mechanical, and gas barrier properties. Polymers. 2021;14(12):2395. doi:10.3390/polym14122395. [Google Scholar] [PubMed] [CrossRef]
15. Ranjha MMAN, Irfan S, Nadeem M, Mahmood S. A comprehensive review on nutritional value, medicinal uses, and processing of banana. Food Rev Int. 2022;38:199–225. doi:10.1080/87559129.2020.1725890. [Google Scholar] [CrossRef]
16. Tripathi S, Mishra S. Antioxidant, antibacterial analysis of pectin isolated from banana peel and its application in edible coating of freshly made mozzarella cheese. Asian Food Sci J. 2021;20(7):82–92. doi:10.9734/afsj/2021/v20i730324. [Google Scholar] [CrossRef]
17. Florez M, Guerra-Rodríguez E, Cazon P, Vazquez M. Chitosan for food packaging: recent advances in active and intelligent films. Food Hydrocoll. 2022;124:107328. doi:10.1016/j.foodhyd.2021.107328. [Google Scholar] [CrossRef]
18. Priyadarshi R, Rhim J. Chitosan-based biodegradable functional films for food packaging applications. Innov Food Sci Emerg Technol. 2020;62:102346. doi:10.1016/j.ifset.2020.102346. [Google Scholar] [CrossRef]
19. Jiang A, Patel R, Padhan B, Palimkar S, Galgali P, Adhikari A, et al. Chitosan based biodegradable composite for antibacterial food packaging application. Polymers. 2022;15(10):2235. doi:10.3390/polym15102235. [Google Scholar] [PubMed] [CrossRef]
20. Wrońska N, Katir N, El Kadib A, Lisowska K. Biodegradable chitosan-based films as an alternative to plastic packaging. Foods. 2022;12(18):3519. doi:10.3390/foods12183519. [Google Scholar] [PubMed] [CrossRef]
21. Kumar S, Mukherjee A, Dutta J. Chitosan based nanocomposite films and coatings: emerging antimicrobial food packaging alternatives. Trends Food Sci Technol. 2020;97:196–209. doi:10.1016/j.tifs.2020.01.002. [Google Scholar] [CrossRef]
22. Fahrasmane L, Parfait B, Aurore G. Bananas, a source of compounds with health properties. Acta Horticulturae. 2014;1040:75–82. doi:10.17660/ActaHortic.2014.1040.9. [Google Scholar] [CrossRef]
23. Falguera V, Quintero JP, Jiménez A, Muñoz JA, Ibarz A. Edible films and coatings: structures, active functions and trends in their use. Trends Food Sci Technol. 2011;22:292–303. doi:10.1016/j.tifs.2011.02.004. [Google Scholar] [CrossRef]
24. Dhall RK. Application of edible films and coatings on fruits and vegetables. In: Edible films and coatings. Boca Raton, FL, USA: CRC Press; 2016. vol. 19. doi:10.1007/978-0-387-92824-1. [Google Scholar] [CrossRef]
25. Alharaty G, Ramaswamy HS. The effect of sodium alginate-calcium chloride coating on the quality parameters and shelf life of strawberry cut fruits. J Compos Sci. 2020;4:123. doi:10.3390/jcs4030123. [Google Scholar] [CrossRef]
26. Ghasemnezhad M, Shiri MA, Sanavi M. Effect of chitosan coatings on some quality indices of apricot (Prunus armeniaca L.) during cold storage. Caspian J Environ Sci. 2010;9:25–33. [Google Scholar]
27. Greener IK, Fennema O. Lipid-based edible films and coatings. Lipid Technol. 1992;4:34–8. [Google Scholar]
28. Guilbert S, Gontard N, Cuq B. Technology and applications of edible protective films. Packag Technol Sci. 1995;8(6):339–46. doi:10.1002/pts.2770080607. [Google Scholar] [CrossRef]
29. Javanmard MS. Shelf life of apples coted with whey protein concentrate-gellan gum edible coatings. J Food Biosci Technol. 2011;1:55–62. [Google Scholar]
30. Jiwan SS, Tasleem AZ. Bioactive compounds in banana fruits and their health benefits. Food Qual Saf. 2018;2:4. doi:10.1093/fqsafe/fyy019. [Google Scholar] [CrossRef]
31. Ju J, Xie Y, Guo Y, Cheng Y, Qian H, Yao W. Application of edible coating with essential oil in food preservation. Crit Rev Food Sci Nutr. 2019;59(15):2467–80. doi:10.1080/10408398.2018.1456402. [Google Scholar] [PubMed] [CrossRef]
32. Khaliq G, Mohamed MTM, Ali A, Ding P, Ghazali HM. Effect of gum arabic coating combined with calcium chloride on physico-chemical and qualitative properties of mango (Mangifera indica L.) fruit during low temperature storage. Sci Hortic. 2015;190:187–94. doi:10.1016/j.scienta.2015.04.020. [Google Scholar] [CrossRef]
33. Kocira A, Panasiewicz K, Staniak M, Szpunar-Krok E, Hortynska P. Polysaccharides as edible films and coatings: characteristics and influence on fruit and vegetable quality—a review. Agronomy. 2021;11:813. doi:10.3390/agronomy11050813. [Google Scholar] [CrossRef]
34. Kraśniewska K, Galus S, Gniewosz M. Biopolymers-based materials containing silver nanoparticles as active packaging for food applications—a review. Int J Mol Sci. 2020;21:698. doi:10.3390/ijms21030698. [Google Scholar] [PubMed] [CrossRef]
35. Garcia MPM, Carmen MGG, Lopez-Caballero ME, Barbosa-C´novas GV. Edible films and coatings: fundamental and applications. 1st ed. Boca Raton, USA: CRC Press; 2016. doi:10.1201/9781315373713. [Google Scholar] [CrossRef]
36. Embuscado ME, Huber KC. Edible films and coatings for food applications. New York, USA: Springer; 2009. vol. 9, p. 169–208. [Google Scholar]
37. Elsherief MF, Devecioglu D, Saleh MN, Karbancioglu-Guler F, Capanoglu E. Chitosan/alginate/pectin biopolymer-based Nanoemulsions for improving the shelf life of refrigerated chicken breast. Int J Biol Macromol. 2024;264:130213. doi:10.1016/j.ijbiomac.2024.130213. [Google Scholar] [PubMed] [CrossRef]
38. Murmu SB, Mishra HN. The effect of edible coating based on Arabic gum, sodium caseinate and essential oil of cinnamon and lemon grass on guava. Food Chem. 2018;245:820–8. doi:10.1016/j.foodchem.2017.11.104. [Google Scholar] [PubMed] [CrossRef]
39. Natalia S, Emma S, Lanny S, Karsono SP. Improving shelf life of Cavendish Banana using Chitosan edible coating. Procedia Chem. 2014;9:113–20. doi:10.1016/j.proche.2014.05.014. [Google Scholar] [CrossRef]
40. Gol NB, Rao TVR. Influence of zein and gelatin coatings on the postharvest quality and shelf life extension of mango (Mangifera indica L.). Fruits. 2014;69:101–15. doi:10.1051/fruits/2014002. [Google Scholar] [CrossRef]
41. Olivas GI, Mattinson DS, Barbosa-Cánovas GV. Alginate coatings for preservation of minimally processed ‘Gala’ apples. Postharvest Biol Technol. 2007;45(1):89–96. doi:10.1016/j.postharvbio.2006.11.018. [Google Scholar] [CrossRef]
42. Parafati L, Vitale A, Restuccia C, Cirvilleri G. The effect of locust bean gum (LBG)-based edible coatings carrying biocontrol yeasts against Penicillium digitatum and Penicillium italicum causal agents of postharvest decay of mandarin fruit. Food Microbiol. 2016;58:87–94. doi:10.1016/j.fm.2016.03.014. [Google Scholar] [PubMed] [CrossRef]
43. Senturk Parreidt T, Schmid M, Müller K. Effect of dipping and vacuum impregnation coating techniques with alginate-based coating on physical quality parameters of cantaloupe melon. J Food Sci. 2018;83(4):929–36. doi:10.1111/1750-3841.14091. [Google Scholar] [PubMed] [CrossRef]
44. Shahidi F. Antioxidants in food and food antioxidants. Food/Nahrung. 2000;44(3):158–63. doi:10.1002/1521-3803(20000501)44:3<158::AID-FOOD158>3.0.CO;2-L. [Google Scholar] [PubMed] [CrossRef]
45. Suhag R, Kumar N, Trajkovska Petkoska A, Upadhyay A. Film formation and deposition methods of edible coating on food products: a review. Food Res Intern. 2020;136:109582. doi:10.1016/j.foodres.2020.109582. [Google Scholar] [PubMed] [CrossRef]
46. Susmitha Reddy K, Jatinder S. Edible coatings in fruits—a review. Int J Curr Microbiol Appl Sci. 2020;9:11. [Google Scholar]
47. Shian TE, Abdullah A, Musa KH, Mohammad YM, Ghani MA. Antioxidant properties of three banana cultivars (Musa acuminata `Berangan', `Mas' and `Raja') extracts. Sains Malays. 2012;41(3):319–24. [Google Scholar]
48. Tavassoli-Kafrani E, Shekarchizadeh H, Masoudpour-Behabadi M. Development of edible films and coatings from alginates and carrageenans. Carbohydr Polym. 2016;137:360–74. doi:10.1016/j.carbpol.2015.10.074. [Google Scholar] [PubMed] [CrossRef]
49. Vyas PB, Neeta BG, Rao TVR. Postharvest quality maintenance of papaya fruit using polysaccharide-based edible coatings. Int J Fruit Sci. 2013;14(1):81–94. doi:10.1080/15538362.2013.801753. [Google Scholar] [CrossRef]
50. Mahhfuzah WWI, Dalila NND, Hanif MA, Siti AH, Hanis NAY, MohdReza WIWH. Study on efficacy of edible protein based fruit coating to delay the ripening of banana (MUSA SPP). Int J Agr Forest Plant. 2020;10:70–3. [Google Scholar]
51. Saha A, Gupta RK, Tyagi YK. Effect of chitosan based edible coating on quality and shelf life of sapota (Manilkara zapota) fruits during storage. Int Conf. 2015;8:1. [Google Scholar]
52. Rahmadiawan D, Abral H, Shi S-C, Huang T-T, Zainul R, Ambiyar HN. Tribological properties of Polyvinyl Alcohol/Uncaria gambir extract composite as potential green protective film. Tribol Ind. 2023;45:367–74. doi:10.24874/ti.1482.05.23.06. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools