 Open Access
Open Access
ARTICLE
Effect of Kaempferol Enriched Green Polymer-based Encapsulation for Effective Shelf-life Enhancement
Centre for Food Technology, Department of Biotechnology, Anna University, Chennai, India.
* Corresponding Author: e-mail:
Journal of Polymer Materials 2022, 39(1-2), 71-87. https://doi.org/10.32381/JPM.2022.39.1-2.5
Abstract
In this study, a multi-layered green polymer-based packaging composite assembled by active flavonoid Kaempferol grafted with starch-chitosan was prepared, and its characteristics and effect on meat storage, including physicochemical characteristics and antioxidant capacity, were analysed and evaluated at refrigeration conditions given four treatments: CON (control— without coating), SEC (with starch+chitin), P01 (with starch+chitin + 1% of Kaempferol) and P02 (with starch+chitin + 2% of Kaempferol). The addition of Kaempferol increased the antioxidant activity of meat and inhibited the lipid oxidation until day 24 upon migration effect; also improving meat acceptability. The Rancimat induction time confirmed the stability of both P01 and P02 in the presence of polyphenols, where regular storage up to 3 weeks and accelerated storage up to 3rd day (equivalent to 3 months) were achieved, confirming it as a potential packaging module in the postharvest storage and transportation of meat.Keywords
Facilitating meat storage, seems a real challenge for the food industries without the interference of preservative-based packaging and/ or cold storage facility, resulting in increasing environmental concerns. To address this problem, much attention has been paid to developing the biodegradable polymers from renewable resources, that originate from various natural resources, such as starch, cellulose, chitosan and proteins viz. in conjunction with their renewable, low-cost, ecologically friendly, decomposable, and biocompatible features.
Secondly, cold storage conditions, where the meat is liable to thermal shock and fibrillation after thawing. As they are sensitive to oxidative degradation due to physical fluctuations resulting in high degree of unsaturation of the fats, which potentially reduce the nutritional quality and affects the sensory characteristics of the foods (Miyashita, Uemura, & Hosokawa, 2018). For example, because of fat content, rancid odors, discolouration eventually leads to stale meat. Though, different strategies have been developed to minimize the meat oxidation, mainly including the addition of antioxidants either synthetic or natural or semi-synthetic (Wang et al. 2018b), microencapsulation (Encina, Vergara, Giménez, Oyarzún-Ampuero, & Robert, 2016; Vishnu et al. 2017; Yildiz et al. 2018), and incorporation into emulsion formulation (Miyashita et al. 2018), till the most recent technique of microencapsulation with a modified layering structure (Maria Jenita, Sukumar M et al. 2020).
Considering the highly perishable foods, vacuum and other modified packaging techniques is mostly used to extend the shelf life, so when meat is packed under vacuum or a modified atmosphere, it develops a purple-red colour associated with the formation of deoxymyoglobin (Moczkowska et al. 2017). Although film packaging is more economic than vacuum packaging, exposure to oxygen favours oxidation, one of the major causes of deterioration in meat quality, as well as myoglobin oxidation, which results in the accumulation of metmyoglobin in the meat and a dis-coloration from a red to a brown colour, with stench (Mancini and Hunt 2005). Thus, reduction of oxidation reactions in meats attributing to two-three modifications like hurdle technology, is proven to extend the shelf-life of meat (Jeremiah LE, 2001).
Thus, one alternative to avoid oxidation could be the utilisation of edible coating and films (Vital et al. 2016), to prevent meat -dehydration, reducing microbial growth, deterioration from the oxidative process and also to reduce respiratory reactions, with the aim of preserving the meat's texture, colour and flavour, thus extending its shelf-life (Fang et al. 2017).
Previous studies show that during storage of meat at 30°C for 48 h or 4°C for 10 days, ∼0.5–1.0% chitosan inhibited the growth of spoilage bacteria, reduced lipid oxidation, putrefaction, resulting in better sensory attributes. Chitosan also had a good effect on the development of the red colour of meat during storage. Generally edible based coatings are used to improve the technological properties based on the intended application, here we have selected natural rice-based starch and natural chitin as a green edible polymer, which are abundantly available for use (Olaiya et al. 2019). Chitin is hydrophobic, while starch, a polysaccharide with a hydrophilic nature, is biocompatible and an absorbable natural-polymer, that have exhibited biocompatibility and biodegradability in medicinal drug release. Thus, along with antioxidants which retard lipid oxidation by acting as free radical scavengers, oxygen scavengers, metal chelators (Kumar et al. 2015). Synthetic antioxidants (butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), tertiary butylhydroquinone (TBHQ) and others) are used in the food industry to delay meat oxidation (Mansour and Khalil, 2000). However, these antioxidants are gradually falling out of the favor of the consumers due to their synthetic nature and side effects on health on a long run (Salami et al. 2016). When encapsulation can effectively protect the meat from being oxidized, usage of natural starches like gum arabic, modified starches, modified chitosan, pectin have been frequently applied as a material to encapsulate these antioxidants as in multi-layered microencapsulation structure of antioxidants of plant-based polyphenols (AOX) and wall material (WM) (Maria Jenita et al. 2020).
While many previous studies have focused on essential oils such as rosemary, oregano, garlic, basil, thyme, clove, cinnamon, grape seed, garlic, and others (Kesavan Radha Krishnan et al. 2014, Perdones et al. 2016), as natural additives. However, their extraction can be expensive and their flavour can be accentuated limiting their application to foods. Considering the locally grown and easily available plants, such as pandan Pandanus amaryllifolius could be an alternative as a natural antioxidant. Pandanus amaryllifolius is a tropical plant in the Pandanus genus, which is commonly known as pandan and rambai leaf in Tamil nadu, India. It has fragrant leaves which are used widely as a flavouring herb in Southeast Asian cuisine. An annual, aromatic herb, which has been used both as a culinary herb and in traditional medicine, and also has compounds with antioxidant activity, particularly because of the presence of the bioactive compound Kaempferol (Resmi et al. 2009). The high preference to use chemical food preservatives need to be counterbalance with an effort to develop safer natural preservatives. Pandan leaves are often used as additives in a lot of meat based dishes in India as it flavours the meat a lot. But besides those usability pandan also has anti-bacterial properties and curative limits. This study verifies the effects of starch+chitin-based green edible coating with pandan polyphenols on meat characteristics during storage under refrigerated conditions.
The meat used for this study was cut-Longissimus dorsi (LD) muscle (n = 8) from a free range pasture goat, weighing 4± 2.9 kg and slaughtered at 24-months old. Homogenous steaks of 2.5 cm thick were then obtained from each muscle (approximately 20 steaks) and distributed randomly for experimental treatment and analysis.
The pandan leaves was procured from nursery in Chennai (authorized by NBA-National Biodiversity of India). The leaves were sanitized (200 ppm with sodium chloride), washed, dried in a fluidized bed reactor (with air circulation 25°C), packed and stored under vacuum until the preparation of the extract. Soxhlet extraction gave around 32.20% Pandan oil extract-Kaempferol from 500g of dry sample, confirmed by GC-MS analysis.
Green polymer-based edible-coating solutions and treatments
Chitin was isolated from the dried shells of Fenneropenaeus indicus shrimp wastes, following Salaberria et al. 2015 method with minor modification. Briefly, proteins were first removed using 2 M NaOH solution at 30°C for 24 h under vigorous stirring; then, minerals (CaCO3) were removed by 2 M HCl (30% w/w) solution at 30°C for ∼3 h. The sample were dried at 65°C for 14 h and then dispersed in a solution of 2 M HCl (35% w/w) at 100°C for 3 h under reflux for acid hydrolysis, and finally; pigments were extracted using 2% KMnO4 solution at 550°C for 3 h under vigorous stirring. The chitin sample were dried at 35°C overnight in an oven.
Preparation of polymeric starch-chitin edible coating
Around 2.0 g of rice starch was dispersed in 100 mL of deionized water and aliquots of 0, 1.0, 3.0, 5.0, 7.0, and 9.0 wt %, based on rice starch were added to the suspension. The mixture was stirred for ∼30 min at 70°C, the samples were cooled to room temperature and were ready for coatings. For coating with pandan extract, the pandan was mixed in sterile distilled water (1:100 and 1:50 w/v) for 30 min at 70°C, then filtered and the extract used to dissolve the conc rice starch+chitin under magnetic stirring. The meat was submerged in the starch+chitin solution (1 min), drained, submerged in calcium chloride solution (1:50 w/v) (30 s) and drained for a further 30s and more. The samples were packaged individually (polystyrene tray overwrapped with a retractile film) and displayed in a refrigerated and illuminated display with light (fluorescent lamp-1200 lx; 12 h/day) at 2-5°C.
Samples were divided and distributed into four treatments: CON (control—without coating), SEC (with starch+chitin), P01 (with starch+chitin + 1% of Kaempferol) and P02 (with starch+chitin + 2% of Kaempferol). The analyses were carried on days 1, 7, 14 and 21 of display.
Total phenolic content (TPC) and antioxidant activity (DPPH and ABTS radical scavenging) of kaempferol and meat
The phenolic content and antioxidant activity were analyzed for SEC, P01, P02, CON at regular day's interval. Meat extracts were obtained by homogenization, centrifugation (4000 rpm, 25°C, 15 min) and filtration (filter paper), the extracts were used directly for further analyses.
An aliquot of each extract (100 mL) was mixed with 100 mL of Folin–Ciocalteu reagent (1:1 deionized water) and 2250 mL sodium carbonate (28 g/L). The solutions were then incubated in dark chamber (25°C, 30 min) and the absorbance was read at 750 nm (UV–visible spectrophotometer, Thermo Scientific). Results were expressed in equivalent of gallic acid (GAE) (mg GAE/g); a standard curve with gallic acid (0–500 mg/ L) was prepared.
Samples (150 μL) were mixed with a DPPH (50 μM) in a methanolic solution (2550 μL)°C. After 30 min, the absorbance was read (515 nm). The activity was recorded as follows:
where At: absorbance read at 30 min of reaction. At = 0: absorbance read at the beginning.
The same assay was followed from Re R et al. 1999, with the following modifications, 7 mM ABTS (5 mL) with 140 mM potassium persulfate (88 μL) was
mixed and incubated in the dark at 28 °C for 16 h. The ABTS activated radical was diluted with ethanol to an absorbance of 0.75 ± 0.02. The radical scavenging activity (%) was measured at 735 nm. Samples (50 μL) were mixed with ABTS solution (1950 μL) and absorbance was recorded at 6 min. The radical scavenging activity (%) was calculated using the formulae:
where At: absorbance read at 6 min of reaction; At=0: absorbance read at the beginning.
The oxidation (malonaldehyde/ MDA content) was evaluated using TBA (Souza et al. 2011). The meat (15 g), of each sample, was mixed with TCA (trichloroacetic acid) solution (10 mL; 7.5% TCA, 0.1% gallic acid and 0.1% EDTA), homogenized, centrifuged (15 min, 5000 rpm and 4°C), filtered and mixed with TBARS reagent (1:1 v/v; 1% thiobarbituric acid, 15% TCA and 570.5 μM, HCl), heated (10 min; 100°C), cooled, and the absorbance read at 532 nm. A standard curve (using 1,3,3-tetramethoxypropane – 0 to 75 μM) was used and the results expressed as mg MDA/kg of meat.
Scanning electron microscopy (SEM)
Particle morphologies of the microcapsules produced with CON, PO1 and PO2 were evaluated by employing a scanning electron microscope. A small amount of each sample was attached to a double side adhesive tape fixed to stubs, coated with 3-5 mA palladium under vacuum. They were examined with a QUANTA 400F Field Emission scanning electron microscope (IIT Madras). This was operated at 20 kV with a magnification of ∼10000x.
The meat samples were weighed each day of analyses and results were expressed in relation to the percentage of weight loss compared to the day 0th.
The values of pH were verified using a pH meter equipped with a penetration pH-electrode (HI99163).
The shear force (kgf) was measured with a texturometer (TAXT Plus), with a Warner-Bratzler blade, 5 kg of load cell, 1 mm/s of speed. Cooked and raw samples with 1 cm X 1 cm X 2 cm were used for the experiment.
The color was measured during display, using the CIE Lab system (Minolta CR-400 Chroma meter), with a ∼10° view angle, D65 illuminant, 8 mm of aperture with a close cone; the light (L*), red (a*) and yellow (b*) were obtained.
Chroma and hue values were calculated as follows:
Evaluation of the shelf life of green edible coating
1. Real-Time Shelf Life Testing
The real-time shelf life testing of natural oils was typically undertaken with assessment by GC, by determining the marker compounds of lipid oxidation. Sensory determination also attributes the analytical data.
2. Accelerated Shelf Life Testing (ASLT)
ASLT is an advanced approach to determine the shelf life of food matrices under accelerated conditions; by predicting the shelf life under real-time storage. Temperature being the important acceleration factor/variable to determine the oxidative rancidity of samples, while oxygen pressure, catalyst addition, and reactant contact are other factors of consideration (Ragnarsson and Labuza, 1977). Where, the temperature dependence of lipid oxidation is attributed to the overall energy of activation. Ragnarsson et al. suggests that activation energy may be higher in the presence of antioxidants and have to offer considerations for ASLT testing data obtained at 60-65°C. Oxidation induction time (Rancimat test), expressed as the oxidation induction time (hr) was measured with the Rancimat equipment (Metrohm, Herisau Switzerland installed at IIT, Madras). Oil sample (5g) was warmed to 120°C with an air flow of 10 L/hr; the conductivity cells with 60 mL of deionized water (2 μS/cm); the time taken for the appearance of sudden water conductivity rise, caused by the adsorption of volatiles derived from oxidation of oil, was recorded as the induction time (hrs). The antioxidant efficiency was expressed as protection factor (PF) as
Where IPa is the induction period in the presence of an antioxidant and IPc is the induction period in the absence of antioxidant, respectively.
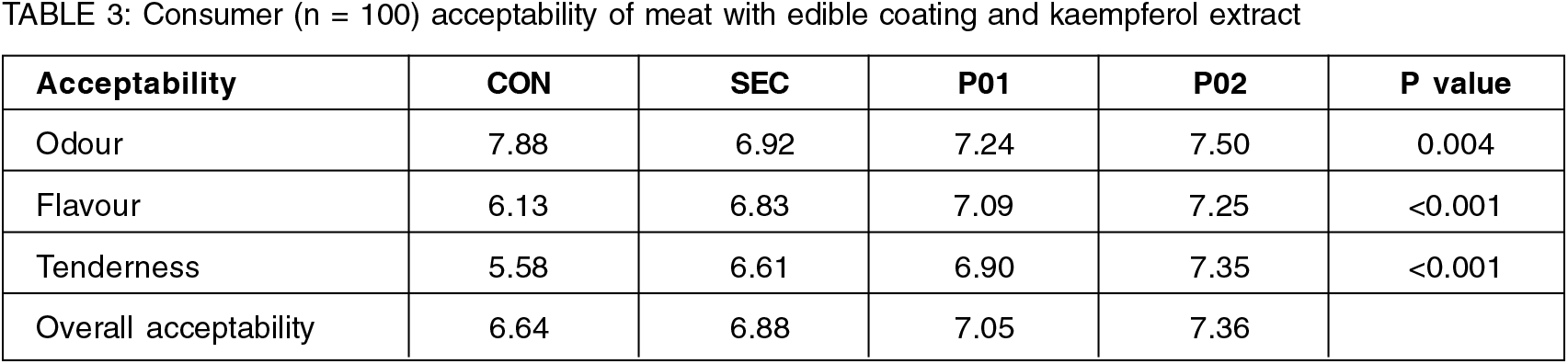
The consumer test was performed with the Sensory panel. The samples were assessed on acceptability basis displayed from day 1 till day 30, evaluating all the four samples, following a randomized design, Macfie et al. (1989). Each meat portion was cooked (∼70°C) in a grill and kept warm (∼50°C) until the evaluation (less than 10 min). The attributes of odour, flavour, tenderness and overall acceptability was assessed by the panel using a 10 hedonic scale (1 = dislike extremely to 9 = like extremely, without the medium level).
The data obtained were analysed by analysis of variance with the general linear model (GLM)–SPSS s/w (v.23.0). All data are presented as mean±standard deviation for 3 average replications for each experiment. The results were significant at P<0.05 level. Principal components analysis (PCA) was used to identify the relationship between treatments and meat attributes using XLSTATs/w (v.7.5.3).
Lipid oxidation or oxidative stability of natural oils in perishable foods could be evaluated using different approaches, namely chemical, physical, and sensory evaluation. Chemical methods include analysis of primary oxidation products (peroxide value [PV] and conjugated dienes) and secondary oxidation products (para-anisidine values [pAV], thiobarbituric acid reactive substances [TBARS], and gas chromatography–mass spectrometry [GC–MS]). While physical methods measure the outcome of oxidative reactions of lipids/fats through changes in conductivity (Rancimat).
Sensory analysis could be used to complement the analytical parameters, and typically descriptive analysis is undertaken to profile the flavor and aroma changes in fats/fat containing foods. Gas-sensor arrays (electron nose) (installed at Centre for Food Technology, Anna University Chennai) have been used in association with GC–MS for rapid screening of volatiles (Olsen et al. 2005).
These two concentrations of pandan extract (1 and 2%) were used in order to not damage the gel formation and still add a good amount of active compounds to improve the shelf life, as found in preliminary tests, also as concentrations more than 3% made the gel fragile and difficult to form, also imparting a dominating flavour to the food sample. P01 and P02 showed a DPPH radical scavenging of 78.5% and 90.0%. In relation to ABTS radical scavenging, pandan showed a radical scavenging of 65.5 (1:50 w/v) and 37.9% (1:100 w/v). The TPC found was 17.9 mg GAE/g pandan.
In relation to meat, the starch+chitin coating (SEC) did not alter (P > 0.05) the antioxidant activity in comparison with the CON treatment (Table 1). On the other hand, both levels (1 or 2%) of kaempferol addition to the starch+chitin edible coating increased (P > 0.01) antioxidant activity and also the TPC, compared with CON and SEC treatments (Table 1). Differences in meat with edible coating and both levels of kaempferol were observed only by the DPPH assay (P > 0.001). Antioxidant capacity of pandan by hydrogen transfer measured by DPPH and ABTS radical scavenging showed greater sequestration for ABTS radical. Thus, the addition of pandan extract increases the antioxidant activity of coated meat and can positively influence the maintenance of meat quality. The role of chitin was also major to hold the protein together from getting denatured.
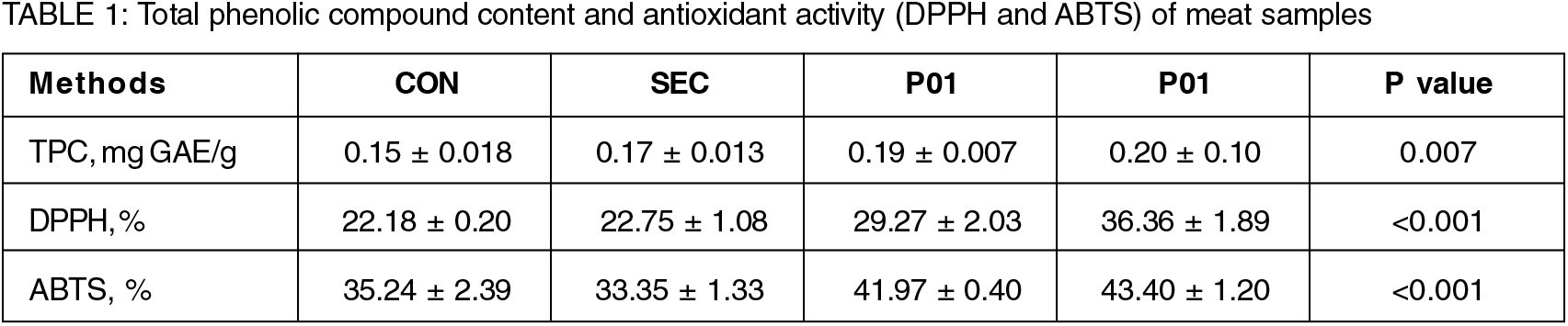
Results are expressed as mean and standard deviation
CON—Meat without edible coating, SEC—Meat with starch+chitin edible coating without kaempferol extract, P01-Meat with starch+chitin edible coating and 1% of kaempferol extract and P02—Meat with starch+chitin edible coating and 2% of kaempferol extract. TPC- Total phenolic compound, GAE- Gallic acid equivalent, DPPH- Radical scavenging of 1,1-Diphenyl-2-picry- hydrazil and ABTS- Radical scavenging of 2, 20-Azino-bis(3-ethyl-benzothiazoline-6-sulfonic acid) diammonium salt.
Before ageing (day 1), the TBARS values were similar (P>0.05) in the meats from CON and SEC treatments and lowest from treatments with 1 or 2% of the kaempferol (Fig. 3). At the 14th and 21st ageing day, the values of TBARS were lower (P < 0.05) in the treatments with kaempferol, intermediate in the SEC treatment and higher (P<0.05) in the CON treatment.
Thus, in all ageing days, the coated meat with addition of pandan extract into starch+chitin edible coating presented better results. Besides that, SEC also reduced the lipid oxidation at day ∼14 and 21, as the coating can minimize the contact with atmosphere and increase water barrier properties, as the starch forms a thin gel like and works as a perfect arresting barrier.
CON—Meat without edible coating, SEC—Meat with starch+chitin edible coating without kaempferol extract, P01- Meat with starch+chitin edible coating and 1% of kaempferol extract and P02—Meat with starch+chitin edible coating and 2% of kaempferol extract. TPC- Total phenolic compound, GAE- Gallic acid equivalent, DPPH- Radical scavenging of 1,1-Diphenyl-2-picry-hydrazil and ABTS- Radical scavenging of 2,20-Azino-bis(3-ethyl-benzothiazoline-6-sulfonic acid) diammonium salt.
Lipid oxidation increased, as expected, during display as shown in Fig. 1, mainly in the CON treatment, with the highest values. The kaempferol addition into starch+chitin edible coating (1 or 2%) decreased (P < 0.05) lipid oxidation. At the 21st day, the TBARS values were approximately 1.44, 1.20, 0.75 and 0.81 mg MDA/kg of meat from CON, SEC, P01 and P02, respectively, corresponding to a decrease in lipid oxidation of approximately 52.5 and 42.4% for meat with coating with pandan extract- kaempferol. Oxidation, growth of microorganisms, leads to the deterioration of quality in foods, loss of quality during display (Johnson and Decker 2015; Vieira et al. 2017). To ensure oxidative stability in the product during its shelf-life period, the balance between anti- and pro-oxidant needs to be maintained (Johnson and Decker 2015). In this way, this study demonstrated that the use of kaempferol (a natural plant polyphenol) was effective in retarding the oxidation of meat during >21 days of display. Even with the reduction in TBARS values of meat with coating, the MDA levels of all treatments after 21 days of display were below (1.5 mg MDA/kg meat) the limit for acceptability.

Figure 1.: Effect of active edible coating on lipid oxidation (TBARS) expressed as mg malonaldehyde kg-1 raw meat during display
The secondary oxidation reactions are measured using the Rancimat test, expressed as induction period (hr) which represents the time needed for decomposition of hydroperoxides produced by sample oxidation (Läubli & Bruttel, 1986). The Rancimat test was carried out for a selected concentration (P1000) that gave highest stability in primary oxidation. Table 2 shows the induction period (hr) and protection factors for the experimental samples. Increasing induction period in the presence of an antioxidant is associated with the efficiency of the antioxidant, referred to as protection factor (PF). A better PF (1.86) in P01 and (2.05) in P02 during AS (accelerated storage) than in PF of control and SEC was seen. Similar observations have been made in different samples in the presence of various other natural antioxidants. (Chu and Hsu, 1999) inferred that catechin (1,000 ppm) with phospholipids (1,500 ppm) and rosemary (1,000 ppm) extract enhanced oxidative stability of oil in terms of higher induction period of 16.89 hr compared with the shorter induction period 4.77 hr in the control.
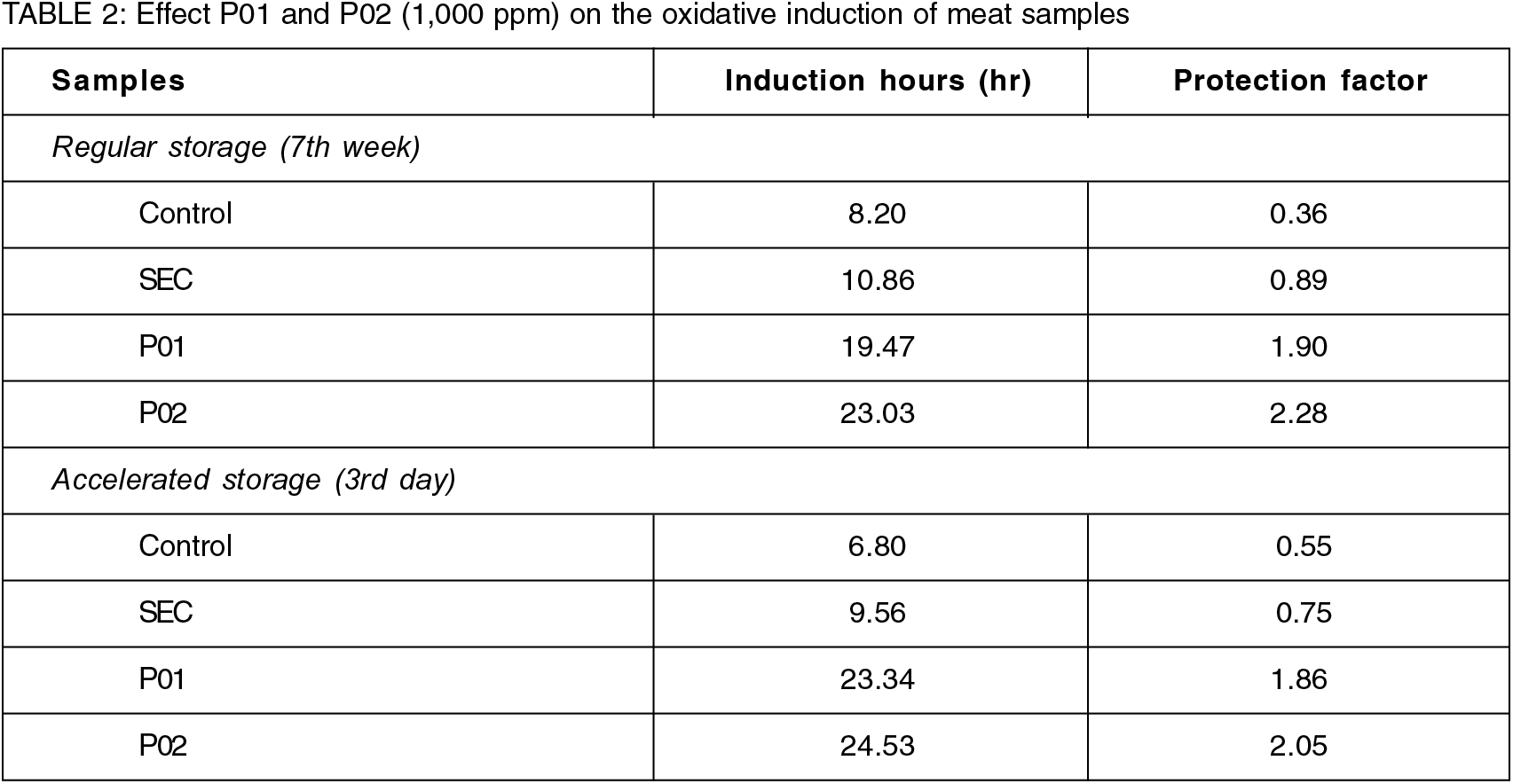
CON—Meat without starch+chitin edible coating, SEC—Meat starch+chitin coating without kaempferol extract, P01—Meat with starch+chitin coating and 1% kaempferol extract and P02—Meat with starch+chitin edible coating and 2% kaempferol extract
The secondary oxidation reactions in the samples were measured using the Rancimat test, expressed as induction period (hr) which represents the time needed for decomposition of hydroperoxides produced by edible layer oxidation (Läubli & Bruttel, 1986). The Rancimat test was carried out for a selected concentration of P1000 that gave highest stability in primary oxidation. Table 2 shows the induction period (hr) and protection factors for the experimented samples. Increasing induction period in the presence of an antioxidant is associated with the efficiency of the antioxidant, referred to as protection factor (PF). A mild variation showed in the PF of P01 and P02 during regular storage than its counter accelerated storage was seen in comparison. However, the slow- release of the second layer of polyphenols in P02, acted upon the meat during the accelerated storage, thus retarding the metabolite oxidation, as the Kaempferol decreases the oxidative damage directly via reacting with free radicals or indirectly by inhibiting the activity or expression of free radical generating enzymes.
There were no significant differences (P> 0.05) in pH values among treatments and display. Thus, edible coating with pandan extract in meat did not alter pH. Vital et al. (2016) observed that with essential oils increased the pH of meat, and was associated with the coating pH (∼ 6). As starch+chitin have similar gelling property, were we pronounced the use of natural rice starch+chitin. As observed in the treatments carried out, the display (1, 7, 14 and 21 days) did not alter the pH of meat (Fig. 2). The pH values ranged from 5.60 to 5.90.

Figure 2: pH, weight losses and texture (shear force) of meat with edible coating during display
CON—Meat without edible coating, SEC—Meat with starch+chitin edible coating without kaempferol extract, P01- Meat with starch+chitin edible coating and 1% of kaempferol extract and P02—Meat with starch+chitin edible coating and 2% of kaempferol extract.
The starch+chitin edible coating decreased (P >0.01) the weight loss (WL) in the meat samples at 1, 7, 14 and 21 days of display (Fig. 2). However, the addition of kaempferol did not alter (P >0.05) the WL in all evaluated days, when compared to Vital et al. (2016) with essential oils being used as edible coating. Thus, edible coating (starch+chitin-based) protects the meat against water losses. The WHC progressively increased (P <0.01) for all treatments during display, ranging from 3.2-10.65, 1.4-9.20, 1.48.52 and 1.4-8.04% for CON, SEC, P01 and P02, respectively (Fig. 2). During ageing of the meat, some water loss is expected as a consequence of changes in muscular fibers caused by rigor mortis and modifications of myofibrillar structure (Pearce et al. 2011).
Texture (Warner–Bratzler shear force- WBSF)
All treatments with coating, SEC, P01 and P02, were more tender than CON (P < 0.05; FIG 2). The WBSF decreased (P < 0.05) with display time for all experimental samples, although less intense in CON group, which had the highest WBSF throughout display time (Fig 2). This difference in meat tenderness may be associated to the water loss reduction by coating, which maintained the water in the samples resulting in a more tender meat (Pearce et al. 2011). Other factor, might be associated with atmospheric contact (the coating reduces this contact), oxidation of lipids and proteins of meat protected by the chitin (Lund et al. 2011).
The L* (lightness) values were higher (P <0.001) in CON- without addition of starch+chitin edible coating studied at 1, 7, 14 and 21days, when compared to the value of L* from meat coated (Fig. 3). However, for the SEC, P01 and P02 treatments the L* values were similarly (P> 0.05) observed on the same days. The L* values decreased (P <0.01) during display for all the experimented treatments. CON showed a higher L* value, probably associated to changes in the structure of meat related to the oxidative condition, which may increase light dispersion (MacDougall, 1982).


Figure 3: L*, a*, b*, chroma and hue values of meat without and with an edible coating during display
CON—Meat without edible coating, SEC—Meat with starch+chitin edible coating without kaempferol extract, P01- Meat with starch+chitin edible coating and 1% of kaempferol extract and P02—Meat with starch+chitin edible coating and 2% of kaempferol extract.
Contrary to the L* values observed in meat samples, a* values (red) were higher (P <0.01) in meat samples with starch+chitin edible coating, with or without the addition of pandan extract, also probably related to the exudates in the coated meat, which enhances the red coloration. Fresh meat usually becomes lighter and less red after a few days. However, the meat with coating and pandan extracts maintained the lightness and redness during the 14 days of ageing and further. While the conversion of oxy- to metmyoglobin results in discoloration of meat and interactions between lipid oxidation and discoloration was observed in control samples (Faustman et al. 2010; Soladoye et al. 2015). Thus, the coating, as well as reducing the lipid oxidation, also acts in the maintenance of the meat colour. In the meat samples from SEC, P01 and P02 the yellowness (b* values) was higher (PÂ0.05) than the CON (the starch+chitin edible coating had yellowish and brownish colour). At day 21, SEC, P01 and P02 expressed values consistent with the meat's natural degradation process (Moczkowska et al. 2017). The yellowish colour of the active biofilm is advantageous if it is also used to protect light- catalyzed deterioration reactions (Cardoso et al. 2016). Chroma of the samples with starch+chitin coating was significantly higher than CON (P<0.05, FIG 3), which means that the samples with starch+chitin had more vivid colour and maintained same smell which was more accepted by consumers at the time of purchase (Cardoso et al. 2016). The hue value showed a difference (P <0.001) among the treated samples (FIG 3); while during all display time, CON had the lowest hue values. The hue values were similar (P >0.05) among SEC, P01 and P02 treatments at days 1, 7, 14 and 21. In this way, the starch+chitin edible coating protected the meat from a greater discoloration as that observed in the CON treatment, as reported by (Cardoso et al. 2016). During display, hue values increased (P <0.01) in the CON treatment, while the hue values of meat with starch+chitin edible coating decreased 14 to 21st day. On the other hand, the hue value of meats treated with pandan extract (especially P02) did not alter during the display period, indicating minimal colour deterioration, while the industries and research sectors are searching for new ways to improve/extend products' shelf-life. The green polymer based- edible coating with plant extracts such as pandan can reduce or inhibit degradation processes during the meat display.
Morphology of the experimented samples
The microencapsulated particles obtained under different experimented conditions obtained by spray drying were characterized using scanning electron microscopy (SEM) technique. As shown in Fig. 4a, the surface structure of spray dried SEC microcapsules was affected, with slightly wrinkled surfaces. Coating material composition and core: coating ratio might be responsible for dented characteristics of the spray dried microcapsules, that may occur due to sudden moisture loss. The preferred structure in the microencapsulation process is a uniform and smooth surface, slightly spherical shape with minimum cracks and collapses on its walls (Barros and Srtingheta, 2016). In this case, the coating material mixture PO2 gave the best results (Fig. 4c). The microencapsulated PO1 had more distorted layers (Fig. 4b). The particles with asymmetrical surfaces are more sensitive to oxidation reaction compared to those of smooth surfaces because of their irregular surface areas. It was observed that core: coating ratio had a significant effect on the uniformity of the experimented microspheres as stated by Maria Jenita et al., 2020. Likely, when the ratio was changed from 1:1 to 1:2, the number of small particles sticking on the targeted microcapsules were increased considerably as shown in Fig. 4c. These contain slow release of phenolic content and act as desirable microcapsules since they decrease radical activity overtime.

Figure 4: SEM of multi-layered microencapsulation structure of antioxidants (AOX) and wall material (WM)
SEC—meat with starch+chitin coating without kaempferol extract, P01—meat with starch+chitin coating and 1% kaempferol extract, P02—Meat with starch+chitin edible coating and 2% kaempferol extract.
The effect of different temperature conditions on microcapsules were also investigated on the samples since it demonstrated the highest TPC and antioxidant activity in chemical methods. Both core: coating material ratios (1:1 and 1:2) led to an increase on the numbers of particle size, and also the quantity of clustered units with rising temperature; similar results were reported in the literature (Tonon et al. 2008), whereas a uniform thickness of the microcapsules was clearly observed in PO2 sample (Fig. 4c).
In relation to odour and flavour, meat with edible coating (SEC) did not present differences compared to CON (P>0.05). On the other hand, when pandan extract was added at the concentration of ∼2%, meat had higher notes (PÂ0.01), also with regard to tenderness, meat with pandan (P02) was also the preferred meat (P>0.001); and all treatments with coating received higher notes than the CON, perhaps associated with the lower water loss in these treatments, resulting in a tender meat, as observed in WBSF analysis (Fig. 2). As observed in the other sensorial attributes assessed, the meat with coating and pandan (P02) received the best notes (P<0.001), demonstrating a greater acceptance and preference of this treatment by consumers. The addition of natural bioactive compounds as antioxidants prevents the formation of secondary metabolites as a result of oxidation and, consequently, which decreases the deterioration in flavour, increasing acceptability scores—another factor that may be associated with greater acceptance of samples with coating and pandan extract, especially the P02. In addition, the flavour conferred on the meat by the pandan may also have influenced the acceptance of the product (Table 3).
Principal components analysis (PCA)
Information about meat preferences of the sensory panel is graphically summarized in Fig. 1 which represents the PCA - scores for odour, flavour, tenderness and overall acceptability of meat with and without edible coatings. Principal component analysis of the scores for odour, flavour, tenderness and overall acceptability of meat with and without edible coatings. CON—meat without starch+chitin edible coating, SEC—meat with starch+chitin coating without kaempferol, P01—meat with starch+chitin coating and 1% kaempferol, P02—Meat with starch+chitin edible coating and 2% kaempferol has been explained in Figure 5. Both axes explained 98.2% of the variance.

Figure 5: Principal component analysis of the scores for odour, flavour, tenderness and overall acceptability of meat with and without polymeric edible coatings.
CON—meat without starch+chitin edible coating, SEC—meat with starch+chitin coating without kaempferol extract, P01—meat with starch+chitin coating and 1% kaempferol extract, P02—Meat with starch+chitin edible coating and 2% kaempferol extract.
Attributes of odour, flavour, tenderness and overall acceptability are situated on the right side of the Figure, closely located to the P01 and P02 treatments. CON and SEC (without kaempferol) are located on the left side of the graph, inversely related to acceptability attributes. As demonstrated in other consumer studies (Eiras et al. 2017), there are different groups of consumers with different preferences/ perceptions in relation to meat acceptability, with different market niches that demand products with different characteristics (Font-i-Furnols and Guerrero, 2014), especially products that improve the quality of the product and reduces the use of synthetic compounds, being coatings with natural additives of greater interest for this market in practical availability and usability.
The kaempferol presented a large amount of phenolic compounds and greater antioxidant activity. The inclusion of kaempferol in the starch+chitin based edible coating increased the antioxidant activity and reduced the meat lipid oxidation more effectively than the coating without kaempferol. The green polymer coating also decreased weight loss during display and also increased tenderness of meat. Coated meat was darker, redder, mild yellow and had a more intense colour. Nevertheless, the inclusion of pandan extract in the starch+chitin based edible coating improved meat acceptability. It is evident that pandan polyphenols- kaempferol suppress the formation of free radicals in meat (both P01 and P02) thereby inhibiting 800 ppm) were effective in retarding primary and secondary oxidation based on their concentration. The same concentration showed the stabilizing activity up to 3rd day (equivalent to almost 3 months) in accelerated conditions. No significant change in the color and refractive index of meat was observed. The results provide scientific substantiation for the prospects of P02 as natural polyphenols in meat and meat- based products.
Besides, complex wall materials of encapsulation that exhibit a great reduction in oxidation. When pea protein isolate- modified starch complexes are used to encapsulate docosa-hexaenoic acid (DHA)-rich canola oil, the peroxide value (PV) of the microcapsules decreased by over 60% compared to bulk oil (Yildiz et al. 2018). Thus, incorporation of highly sensitive fatty carboxylic acid into oil-in-water (O/W) emulsions is additionally a wide used approach to limit the oxidisation with advantage of the benefit of preparation and low value (McClements et al. 2017; McClements & Decker, 2000, 2017; Miyashita et al. 1993) also claiming the slow disintegration with respect to time, temperature, acids (Maria Jenita, Sukumar M et al. 2020).
The carrier structure like the unsaturation degree of oil is merited by the consistency of the wall material, yet because the thickness and also the charge of oil/water interface, considerably influence the oxidisation rate of edible film (Figure 2); based on the modified emulsion structure of the matrix and wall material patented by Maria Jenita, Sukumar M et al. 2020, relates to method of preparing multi- layered microencapsulation. Thus, edible coatings containing natural compounds with antioxidant activity may be applied in meat products with the aim of improving their characteristics during shelf-life.
References
1. American Oil Chemists' Society (1994). Official methods and recommended practices of the AOCS, (5 ed.) Washington, DC. [Google Scholar]
2. Chu, Y. H., & Hsu, H. F. (1999). Effects of antioxidants on peanut oil stability. Food Chemistry, 66: 29-34. [Google Scholar]
3. Fang Z, Zhao Y, Warner RD, Johnson SK (2017). Active and intelligent packaging in meat industry. Trends Food Sci Technol, 61:60-71. [Google Scholar]
4. Faustman C, Sun Q, Mancini R, Suman SP (2010). Myoglobin and lipid oxidation interactions: mechanistic bases and control. Meat Sci, 86:86–94. [Google Scholar]
5. Font-i-Furnols M, Guerrero L (2014). Consumer preference, behaviour and perception about meat and meat products: an overview. Meat Sci, 98:361-371. [Google Scholar]
6. Jeremiah LE (2001). Packaging alternatives to deliver fresh meats using short-or long-term distribution. Food Res Int, 34:749-772. [Google Scholar]
7. Johnson DR, Decker EA (2015). The role of oxygen in lipid oxidation reactions: a review. Ann Rev Food Sci Technol, 6:171-190. [Google Scholar]
8. Kesavan Radha krishnan, Srinivasan Babuskin, Packirisamy Azhagu Saravana Babu, Mohammed Abbas Fayidh, Muthusamy Sukumar, (2014). Bio protection and preservation of raw beef meat using pungent aromatic plant substances. Journal of the Science of Food and Agriculture. Vol 94: 12, https://doi.org/10.1002/jsfa.6580. [Google Scholar] [CrossRef]
9. Kumar Y, Yadav DN, Ahmad T, Narsaiah K (2015). Recent trends in the use of natural antioxidants for meat and meat products. Comprehen Rev Food Sci Food Saf, 14:796-812. [Google Scholar]
10. Lund MN, Heinonen M, Baron CP, Estevez M (2011). Protein oxidation in muscle foods: a review. Mol Nutr Food Res, 55:83-95. MacDougall DB (1982). Changes in the colour and opacity of meat. Food Chem, 9:75-88. [Google Scholar]
11. Macfie HJ, Bratchell N, Greehoff K, Vallis LV (1989). Designs to balance the effect of order of presentation and first order carry over effect in hall tests. J Sens Stud, 4:129-148. [Google Scholar]
12. Mancini RA, Hunt MC (2005). Current research in meat color. Meat Science, 71:100-121. [Google Scholar]
13. Mansour EH, Khalil AH (2000). Evaluation of antioxidant activity of some plant extracts and their application to ground beef patties. Food Chem, 69:135-141. [Google Scholar]
14. Maria Jenita, M. Sukumar, U. Lalithapriya, V. Renuka (2020). Formulation of stable edible oil by incorporating microencapsulated natural polyphenols. Indian Patent Journal, (201941043744 A45/ 2020. [Google Scholar]
15. Moczkowska M, Półtorak A, Montowska M, Pospiech E, Wierzbicka A (2017). The effect of the packaging system and storage time on myofibrillar protein degradation and oxidation process in relation to beef tenderness. Meat Sci, 130:7-15. https://doi.org/10.1016/j.meatsci.2017.03.008 [Google Scholar] [CrossRef]
16. Olaiya N., Surya I., Oke P., Rizal S., Sadiku E., Ray S., Farayibi P., Hossain M.S., Abdul Khalil H. (2019). Properties and Characterization of a PLA-Chitin-Starch Biodegradable Polymer Composite. Polymers, 11:1656. doi: https://doi.org/10.3390/polym11101656 [Google Scholar] [CrossRef]
17. Pearce KL, Rosenvold K, Andersen HJ, Hopkins DL (2011). Water distribution and mobility in meat during the conversion of muscle to meat and ageing and the impacts on fresh meat quality attributes—A review. Meat Sci, 89:111-124. https://doi.org/10.1016/j.meatsci.2011.04.007 [Google Scholar] [CrossRef]
18. Perdones A, Chiralt A, Vargas M (2016). Properties of film-forming dispersions and films based on chitosan containing basil or thyme essential oil. Food Hydrocoll, 57:271-2. [Google Scholar]
19. Ragnarsson, J.O., Labuza, T.P., (1977). Accelerated shelf life testing for oxidative rancidity in foods—a review. Food Chem, 2 (4291-308. [Google Scholar]
20. Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med, 26:1231-1237. https://doi.org/10.1016/S0891-5849(98)00315-3. [Google Scholar] [CrossRef]
21. Resmi Aini (2016). Pandan leaves extract Pandanus amaryllifolius Roxb as a food preservative, Indonesian journal of medicine and health, 7(4166-173. [Google Scholar]
22. Salaberria, A.M, Diaz, R.H, Labidi, J, Fernandes, S.C.M. Role of chitin nanocrystals and nanofibers on physical, mechanical and functional properties in thermoplastic starch films. Food Hydrocoll. 2015, 46: 93-102. [Google Scholar]
23. Salami SA, Guinguina A, Agboola JO, Omede AA, Agbonlahor EM, Tayyab U (2016) Review: in vivo and postmortem effects of feed antioxidants in livestock: a review of the implications on authorization of antioxidant feed additives. Animal, 1:1375-1390. [Google Scholar]
24. Soladoye OP, Juárez ML, Aalhus JL, Shand P, Estévez M (2015). Protein oxidation in processed meat: mechanisms and potential implications on human health. Compreh Rev Food Sci Food Saf, 14:106-122. [Google Scholar]
25. Souza FN et al. (2011). Antioxidant status and biomarkers of oxidative stress in bovine leukemia virus-infected dairy cows. Vet Immunol Immunopathol, 143:162-166. [Google Scholar]
26. Vieira SA, Zhang G, Decker EA (2017). Biological implications of lipid oxidation products. J Am Oil Chem Soc, 94:339-351. [Google Scholar]
27. Vital ACP et al. (2016). Effect of edible and active coating (with rosemary and oregano essential oils) on beef characteristics and consumer acceptability. Plos One, 1:1-15. https://doi.org/10.1016/0168-1591(86)90115-2 [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2022 The Author(s). Published by Tech Science Press.
Copyright © 2022 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools