 Open Access
Open Access
ARTICLE
Gamma Radiation Induced Mutual Grafting of Quaternary Ammonium-Based Monomer on to Rayon Fibers to Improve its Water Absorbency and Antibacterial Efficacy
1
Shriram Institute for Industrial Research, 19, University Road, Delhi-110007, India.
2
Amity Institute of Applied Sciences, AIAS, Amity University, Noida-201303, Uttar Pradesh,
India.
3
Bhabha Atomic Research Centre, Trombay, Mumbai-400085, India.
* Corresponding Author: e-mail:
Journal of Polymer Materials 2022, 39(1-2), 55-70. https://doi.org/10.32381/JPM.2022.39.1-2.4
Abstract
The present work is focused on the study of antimicrobial and water absorption properties of the surface of grafted rayon fiber with quaternary ammonium-based salts containing 3- Acrylamidopropyl trimethyl ammonium chloride (APTAC) monomer induced by gamma radiation. Grafting was qualitatively confirmed by FTIR-ATR,TGA, SEM and by calculating grafting yield and grafting efficiency. The performance was examined for water absorbency and antibacterial efficacy (R) against Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus). Study showed that water absorption, grafting yield and grafting efficiency increased steadily with the increase in monomer concentration and absorbed gamma irradiation dose up to a certain level followed by either slowdown or leveling off the increasing trend. The water absorption and antibacterial efficacy (R) of the APTAC-grafted rayon fiber was found to be substantially enhanced as compared to pristine rayon fiber showing a remarkable 435% increase in water and a four log cycle decrease in the number of living bacteria after 24 hours of incubation.Keywords
The absorbent cores of most of the commercially available hygiene products such as sanitary napkins, tampons, diapers, incontinence products are conventionally made of wood pulp fluff[1] with synthetic super absorbent polymers (SAP) or synthetic super absorbent fibers (SSF) evenly distributed in the wood pulp fluff matrix. The wood pulp having 70% water absorbency and SAPs/SSFs having water absorbency of 10-1000 times of its weight enabling the absorbent core to imbibe and hold 300-400 % body fluid even under pressure meeting the functional requirement of these products. Presently, SAPs and SSFs are the materials of immediate choice for the manufacturers as superabsorbent materials. Although natural SAPs and cellulosic wood pulps are biodegradable but they have lower water absorption capacity, higher cost and poses functional challenges such as gel blocking and proliferation of bacteria and fungi resulting in poor hygienic conditions and decreases the life of the end products[2]. On the other hand, high gel strength of synthetic SAPs upon swelling, although eliminates the problem of gel blocking by absorbing and holding a large amount of fluid under pressure due to a large number of void spaces (interstitial spaces) between the particles but rewet value or wet feeling of the absorbent core is sometimes compromised when interstitial liquid is surplus in the saturated state. Synthetic SAPs also have several disadvantages such as non- biodegradability, restrictions of their use beyond a certain limit as they tend to physically dislodge from the cellulosic wood pulp fluff during manufacturing and transportation. This results in poor fluid absorption and distribution of acquired liquid throughout the storage layer due to significant absence of liquid wicking. Moreover, such absorbent core might also lack enough strength to retain its dry structure, shape, and integrity. Modified cellulosic fibers grafted with suitably selected monomers with the enhanced water absorbency and antibacterial efficacy has the potential either to replace SAPs/SSFs from the absorbent core or SAPs/SSFs-wood pulp fluff as a whole without compromising the performance and functionality of these hygiene products.
Physical entrapment and coating of antibacterial compounds such as quaternary ammonium- based monomers or silver nanoparticles (AgNPs) at the manufacturing stage[3] were found to be ineffective in imparting long-lasting antibacterial activity due to the leaching of the physically trapped or coated antibacterial compounds leading to induced toxicity with the decrease in antibacterial efficacy. Grafting of cellulosic fibers to covalently bond the antibacterial compounds has been emerging as a popular route adopted by researchers worldwide since past few decades to impart antibacterial activity in various technical and smart textile products intended for hygiene application, sports, personal & health care sectors such as clothing for hospital staff and patients, hospital beddings, sports clothing, armbands, underwear, ladies tights, shoe linings, sleeping bags, toys for children[4–6]. Researchers all across the globe reported the incorporation and modification of various physico-chemical functionalities in cellulosic fibers and fabrics by grafting with suitable monomers either by conventional processes such as chemicals, enzymes, and photochemical means[7] or by radiation grafting[8]. Radiation-induced grafting was found to be advantageous over the conventional chemical grafting[9] and proved to be a simple and effective means of grafting of various vinyl monomers onto polymeric materials or textile fabrics for modification of various physico- chemical properties[10,11] such as flame retardance[12], water absorption[2], water impermeability, abrasion resistance and anti- crease properties[13], rot resistance[14], thermo- responsive character[15], biomedical applications[10, 16]. The crease recovery of viscose rayon was found to be improved by grafting with the acrylic acid monomer[12].The effect of process parameters such as substrate backbone, monomer concentration, solvent type and composition, ambiance (air or argon), polyvalent cations (Fe+2, Co+2, Ni+2), storage time and thermal treatment after irradiation on grafting yield in radiation-induced grafting of viscose rayon fabrics with acrylic acid monomer and in turn its influence on physico-chemical properties such as tensile strength, elongation, swelling, moisture absorption, crease recovery angle and dyeing properties of the fabrics were thoroughly studied[12, 17]. Water uptake, water retention, and antibacterial properties of the cotton fabric were found to be improved by gamma radiation-induced grafting with cationic quaternary ammonium and phosphonium compounds containing monomers such as vinylbenzyltrimethylammonium chloride (VBT)[18], 2-(Methacryloyloxy)ethyl trimethylammonium chloride (MAETC)[19] and 2-(Acryloyloxyethyl) trimethylammonium chloride (AETC)[2]. Enhancement of antimicrobial properties was also reported by grafting target substrate with a polycationic antimicrobial agent such as quaternary ammonium and phosphonium salts[3, 20–22].
The primary objective of the present work is to replace the SAPs/SSFs from the absorbent core or SAPs/SSFs-wood pulp fluff as a whole of hygiene products by modified super absorbent and antibacterial rayon fibers. An effort was made to address the low water absorbency and microbial susceptibility of rayon fiber restricting their potential use in the absorbent core of hygiene products by immobilizing the antibacterial chemical APTAC onto rayon fiber. This was done by covalently grafting APTAC onto the rayon fiber by high energy gamma radiation at varying absorbed radiation doses (1 kGy, 3 kGy, and 5 kGy) using different APTAC monomer concentrations (10%, 20%, 30%, and 40% v/v) in aqueous medium under an atmospheric ambiance. Rayon being generated from naturally occurring cellulose such as wood pulp could easily be blended with wood pulp fluff than SSF during commercial processing. Process waste of the rayon fiber industry was used in this ‘waste-to-wealth' initiative of producing biodegradable, eco- friendly, cost-effective and sustainable hygiene products. Grafted rayon fiber was characterized for functional groups using FTIR-ATR technique, surface morphology using SEM, thermal stability by thermo-gravimetric analysis (TGA) as well as by calculating grafting yield and grafting efficiency. The performance of the grafted rayon fiber was examined for water absorption and antibacterial efficacy (R) against Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus).
Rayon fiber was procured from Grasim Industries, Birlagram, Nagda, Madhya Pradesh, India. Laboratory reagent grade 3-Acrylamidopropyl trimethyl ammonium chloride (APTAC) obtained from S-D Fine Chem Limited, Mumbai was used. Distilled water produced for captive consumption in Shriram Institute, Delhi was used as the process media.
2.2.1 Gamma radiation induced mutual grafting of APTAC on to rayon fiber
A fully automatic, PLC-based computerized gamma radiation facility (800 Kci design facility & 485 Kci operation facility) of Shriram Applied Radiation Centre (SARC), located at Shriram Institute, Delhi, India was used for the present study. The plant was designed asper the prescribed norms of the Board of Radiation and Isotope Technology (BRIT) and Atomic Energy Regulatory Board (AERB) in collaboration with the Bhabha Atomic Research Centre (BARC), Mumbai, India. The facility houses radioactive Cobalt-60 (60Co y-ray) pencils as the radiation source. The irradiator is a panoramic, pool type in which a sealed source is contained under a storage pool of water and is fully shielded with high density concrete walls when not in use. The cell volume of the plant is 100m3 with product overlap source geometry.
Rayon fibers were thoroughly washed with distilled water followed by soaking in a 2% aqueous solution of Sodium hydroxide (NaOH) to bring down the hemicellulose content to the desired level required for the intended application. The rayon fibers were then completely immersed in respective aqueous APTAC solutions in a stoppered glass bottle in such a way so as to keep the ratio of the substrate to grafting solution constant in all the experiments and kept for one hour. APTAC monomer molar concentrations in aqueous solutions were so maintained as to graft the rayon fibers with 10%, 20%, 30%, and 40% (v/v) monomers solutions respectively. The stoppered glass bottles containing the rayon samples and respective APTAC monomer solution were then packed in standard 7 ply corrugated cardboard cartons of outer dimension 59 cm (L) x 43 cm (W) x 33 cm (H). The temperature inside the gamma chamber during the grafting reaction was 30±2°C. The dose rate (2.5 kGy.h-1) was ascertained by the Fricke dosimeter prior to irradiation. Once loaded onto the conveyor at the automatic box transfer station they get transferred into carriers having 5 shelves. These carriers travel at the predetermined speed on the overhead monorail that enters the irradiation cell through a labyrinth and returns to the box transfer station. After each cycle, the boxes were progressively transferred to the next shelf and on completion of the fifth cycle, all the boxes were uniformly exposed to the desired levels of radiation dose i.e. 1 kGy, 3 kGy, and 5 kGy respectively, and were then get unloaded. The grafted samples were taken out of the glass bottles, washed thoroughly, and then dried initially at room temperature and finally at 40±1°C under vacuum till constant weight was achieved.
The grafted fibers were removed from the reaction tube after the desired irradiation dose. An excess amount of distilled water was added and stirred for 10 minutes to extract homopolymers. The residual homopolymers were then removed from the grafted fibers by washing with boiling water. Both the washing was collected for subsequent determination of grafting efficiency. The fiber samples were then dried in a hot air circulating oven at 50°C to constant weight. The grafting yield (degree of grafting, G) was then determined as the percentage increase in weight using the following relationship.
Where G is the degree of grafting, Wi and Wf are the weights of initial and final grafted samples respectively.
The remnant grafting solution in the reaction tube and washings obtained from the process of grafting yield determination described in section 2.2.2 were collected and homopolymer was precipitated by adding methanol. This process was continued until no further precipitation of homopolymer was observed. The precipitate was dried to constant weight (W4). The grafting efficiency (GE) was calculated from the equation:
Where GE is the grafting efficiency, W1 & W2 are the initial & final weights of the rayon fibers; W3 & W4 are the weights of monomer initially used and homopolymer.
The grafted rayon fiber sample was placed in a weighing bottle and dried in a vacuum oven at 100°C - 110°C for two hours to ensure the complete removal of moisture, then kept in a desiccator to cool down to room temperature. The initial weight of the dry sample (Wi) was recorded. The dry sample was then kept completely immersed in distilled water for 24 hours at room temperature. Excess water was then wiped off by blotting paper and reweighed the wet grafted sample (Wf). The percent moisture absorption was then calculated from the following relationship:
Where Wi and Wf are the weights of initial and final grafted samples respectively.
Breaking strength were measured using a Universal Tensile Testing Machine (Instron Japan Co., Ltd.). The rayon filaments sample from the yarn of length 30 cm was taken. The speed of movement of jaw was kept 3 cm/min during the test.
2.2.6 Antibacterial activity and efficacy
Antibacterial activity is the property of a product to inhibit the growth of bacteria. Both the pristine rayon and APTAC-grafted rayon were evaluated for antibacterial activity and efficacy as per JIS L1902: 2008 guidelines which is a Japanese method specification to test antibacterial activity and efficacy on textile products. Two bacteria cultures viz. E. coli JM109 and S. aureus ATCC 6538 were prepared in nutrient agar slants and maintained at 4°C used as the test organism. 40 g of nutrient agar was dissolved in 1000 mL of distilled water in a conical flask and autoclaved at 121°C for 15 minutes to prepare a medium for bacteria growth. Each individual bacterium was then streaked onto the nutrient agar slant and incubated for 24 hours at 37°C followed by the adjustment in order to have an active bacteria count 1x108 cfu/mL equivalent to 0.5 Mcfarland at densitometer. Individual bacterial culture for inoculation was then prepared with 0.85% Sodium chloride (NaCl) solution by serial dilution (400 μL/0.4 mL) method and subjected to validation by plate count method so as to have active bacteria count of 105 cfu/mL as any textile substrate conventionally to be inoculated by 1x105 cfu/mL for bacterial study.
A set of 3 pieces APTAC-grafted rayon samples and that of pristine rayon fiber samples for each bacteria S. aureus & E. coli having dimension 50 mmx50 mm were kept in sterilized Petri plates and inoculated with 200 μL of each inoculum of 105 cfu/mL spreading onto the samples. Serial dilutions were immediately done after inoculation to have an active bacterial count at ‘0' hours of the untreated rayon (U0) by plate count method.
After ‘0’ hour count estimation, the other 3 pieces of pristine rayon samples and 3 pieces of APTAC-grafted rayon samples were inoculated with 200 μL of each inoculum of 105 cfu/mL spread onto the samples and incubated at 35°C for 24 hours. Serial dilutions were done immediately after ‘24’ hours to have an active bacterial count of the pristine rayon samples (Ut) and APTAC-grafted rayon samples (At) by plate count method.
Antibacterial efficacy (R) of APTAC-grafted rayon fiber was evaluated by bacteriostatic activity value which was calculated by subtracting the difference between the logarithm value of the number of living bacteria on the APTAC-grafted samples after incubation and immediately after inoculation from the difference between the logarithm value of the living bacteria on the untreated samples after incubation and immediately after inoculation after the inoculation of bacteria on the grafted samples and the untreated samples.
Where R is the antibacterial efficacy, U0 and Ut are the count of bacteria (cfu/piece) inoculated at 0 and after 24 hours of the untreated sample respectively and At is the count of bacteria (cfu/piece) inoculated after 24 hours of the grafted sample.
2.2.7 Fourier-transform infrared spectroscopy (FTIR)
FTIR analysis of the pristine and APTAC-grafted rayon fiber was performed by attenuated total reflection (ATR) mode. ATR technique gives information about the changes on the surface due to the introduction of the additional functionality by the grafting with monomer. FTIR-ATR was recorded on Shimadzu IR, AFFINITY-1S spectrophotometer, in the region of 4000-400 cm-1. The running conditions were 4 cm-1 spectral resolution and 25 scans per sample.
2.2.8 Thermogravimetric analysis (TGA)
Thermal stability studies of the samples were done to record the changes in weight as a function of temperature over a certain period on thermogravimetric analyzer NETZSCH STA model 449F3 Jupiter under inert nitrogen (N2) ambiance from room temperature to 600°C at a rate of 10°C/min and TGA curves were recorded simultaneously.
2.2.9 Scanning electron micrography (SEM)
SEM of each variety of fiber was taken on SEM Instrument, Make SEC, Korea, Model No. SNE-4500 M plus with a secondary electron detector at a voltage of 10 kV at × 400 magnifications to analyze microstructural changes that occurred after gamma radiation grafting. Before analysis by SEM, the samples were dried using a vacuum drying oven at 45°C and fixed on stubs with sputter coated with gold.
3.1 Gamma radiation induced mutual grafting of APTAC on to rayon fiber
In mutual grafting, rayon fiber substrate and aqueous solution of APTAC monomer of different concentrations were irradiated together by high energy gamma radiation. Monomer solutions were used in the mutual grafting technique[23]. Since there was usually more solvent, the irradiation generated free radicals in the solvent, the monomer, and on the substrate. It was assumed that the reaction goes via the solvent radicals, which could react with both the monomer and the polymer substrate. The clear disadvantage of mutual grafting technique by radiation was the formation of a homopolymer[24], which could be suppressed by using homopolymer suppressors viz. Mohr-salt (Fe(NH4)2 (SO4)2(6H2O)[24] or the addition of styrene[25] if ethylenically unsaturated reactive monomers such as acrylic acid, methacrylic acid or acryl amide were present. On the other hand, the advantages of mutual grafting were the lower degradation of the cellulose substrate and the higher yields since each radical created on the cellulose can immediately initiate a graft copolymerization reaction.
The irradiation caused homolytic cleavage by hydrogen atom abstraction at the C2-C3 glycol group as well as at the C6 hydroxyl group of methylol (-CH2OH) unit of the an hydroglucose unit of the rayon fiber and forms cellulose free- radicals. Grafting was mainly initiated at these hydrogen-abstracted sites on the cellulose backbone. It was established that the grafting occurs mainly at the C2-C3 glycolic hydroxyl unit and to a lesser extent at the C6 hydroxyl unit. As during the mutual irradiation, monomer radicals and active (grafting) sites on the fiber backbone were generated simultaneously, hence, grafting could be achieved either by the reaction between the growing polymeric radicals and the active sites on the cellulosic backbone (‘grafting onto’ method) or by the direct initiation of the monomer by the active sites on the cellulose backbone (‘grafting from’ method)[26]. Termination of the grafting process occurred by the formation of a covalent bond between two growing radicals either by combination or by disproportionation. Based on the above mutual grafting mechanism, a schematic representation of the APTAC-grafted rayon fiber was proposed in Figure 1.

Figure 1: Schematic representation of the APTAC-grafted rayon fiber.

Figure 2: Effect of increasing APTAC monomer concentration and absorbed gamma radiation dose on (a) grafting (b) grafting efficiency.
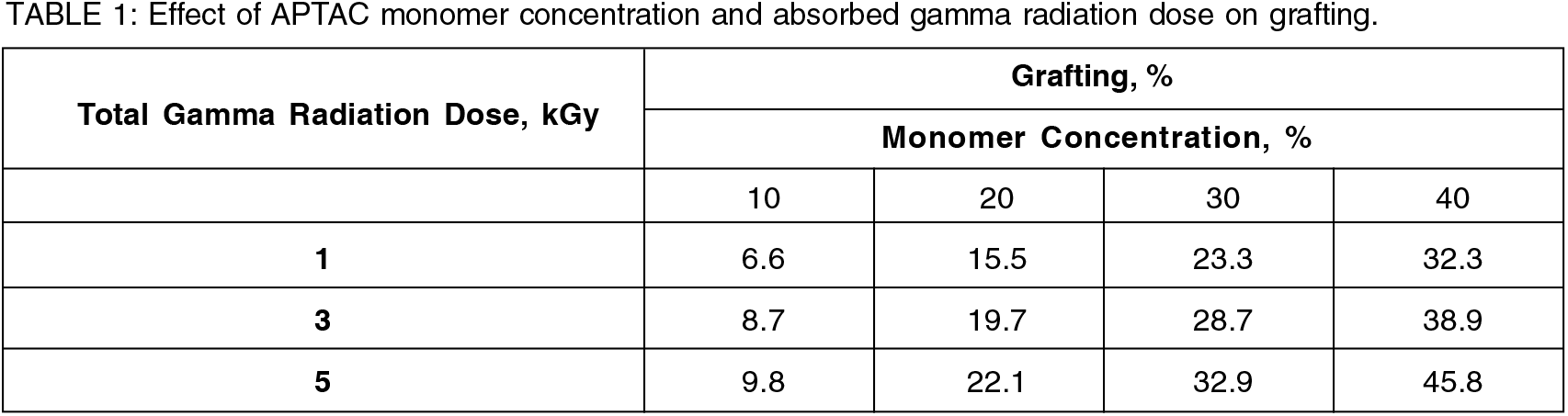
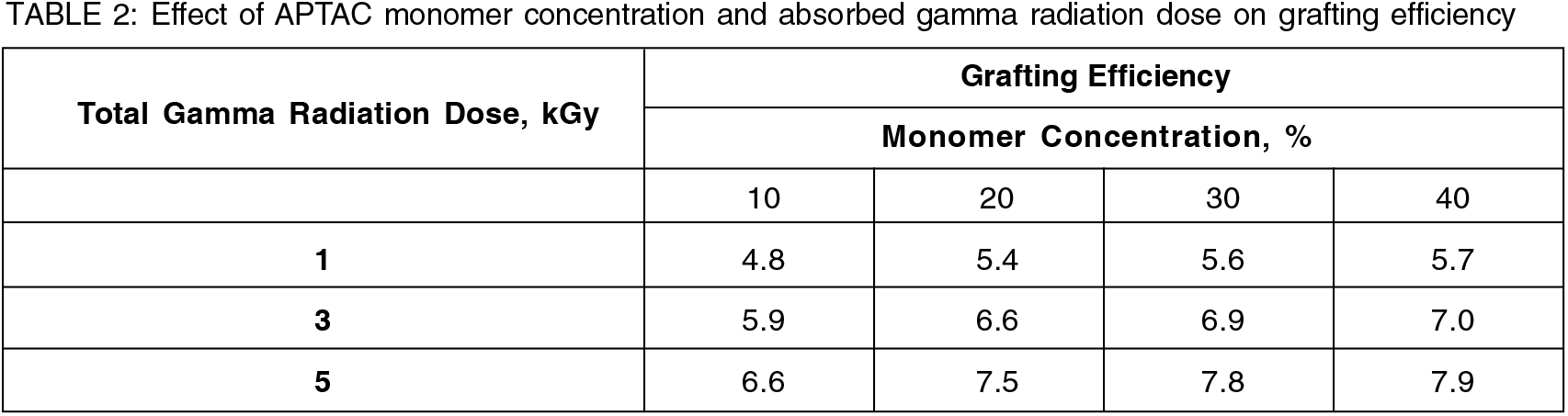
3.2 Effect of APTAC monomer concentration on percent grafting, grafting efficiency
Grafting yield and grafting efficiency was found to be steadily increasing with the increase in APTAC monomer concentrationup to 20 % (v/v) at a fixed gamma radiation dose followed by the leveling off or increase at a slower rate at higher monomer concentration beyond 20% (Table 1, 2 & Fig. 1). The initial increasing trend could be attributed to the increased interactions between the radical grafting sites on substrate rayon fiber with more APTAC monomer molecules[2 18].
3.3 Effect of absorbed gamma radiation dose on Percent grafting, grafting efficiency
The dependence of grafting yield and grafting efficiency on the absorbed gamma radiation dose at a fixed APTAC monomer concentration, on the other hand, showed a similar trend as that of dependence on APTAC monomer concentration at fixed total gamma radiation dose and could be observed from the Table.1, 2 of section 3.2.
Initial steady increase up to 3 kGy absorbed gamma radiation dose was due to the increased number of radical grafting sites on rayon fiber substrate could interact more with the monomer molecules. The slower rate or leveling off the trend at higher absorbed gamma radiation doses beyond 3 kGy was due to the energy deposition taking place predominantly in the bulk of the solution resulting in higher radical density leading to monomer exhaustion through homo-polymerization by recombination of radicals and its subsequent gelation. This restricted the monomer diffusion to the reactive site of substrate rayon fiber and propagating chains due to the high viscosity of the bulk of the grafting mixture resulting in the slowdown or leveling off the rate of increase of grafting efficiency and in turn grafting yield[2, 18].
3.4 Effect on water absorption
Incorporation of ionic monomer such as APTAC into a less hydrophilic substrate like rayon always increased water uptake as radiation induced poly(APTAC) was highly hygroscopic[2]. Therefore, APTAC-grafted rayon resulted in the present study, was expected to have the highest water absorbency (hydrophilic) than pristine rayon. In line with the expectation, the water absorption values (Table 3) of APTAC-grafted rayon were found to be manifold increased and comparable to vinylbenzyltrimethylammonium chloride (VBT)-grafted cotton[18], 2-(Methacryloyloxy)ethyl trimethylammonium chloride (MAETC)-grafted cotton[19] & 2-(Acryloyloxyethyl) trimethylammonium chloride (AETC)-grafted cotton[2]. The improved water absorption capacity of the APTAC-grafted rayon fiber was due to the presence of carboxyl groups in APTAC molecules which facilitates the formation of hydrogen bonding with water molecules.
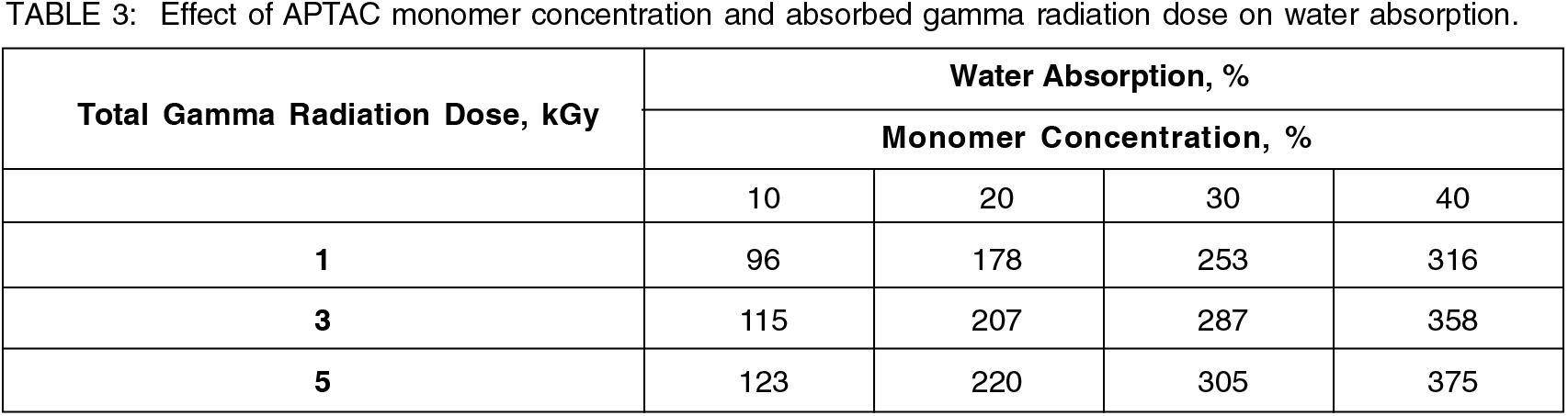
Monomer concentration and absorbed gamma radiation dose were also found to be affecting the water absorption similarly as it affected grafting yield and grafting efficiency as water absorption was linearly correlated with the grafting yield as reported by earlier researchers[18]. Water absorption was found to be steadily increasing with the increase in APTAC monomer concentration up to 20% and absorbed gamma radiation dose up to 3 kGy followed by the leveling off or increasing at a slower rate at higher monomer concentration beyond 20% and higher absorbed gamma radiation dose beyond 3 kGy (Table 3).
Desired water absorption level of 300-400% was achieved with APTAC-grafted rayon fiber using combination of 30% APTAC monomer concentration and 5 kGy gamma radiation dose (305%) and (40%) APTAC monomer concentration and 1 kGy (316%), 3 kGy (358%), 5 kGy (375%) gamma radiation doses respectively (Table 3 and Figure 3).

Figure 3: Effect of increasing quaternary ammonium-based monomer concentration and absorbed gamma radiation dose on Water absorption percentage.
3.5 Characterization of grafted rayon fiber
Rayon fiber grafted with 30% (v/v) APTAC concentration at a total gamma radiation dose of 3 kGy was taken for all the characterizations as the grafted fiber obtained at higher monomer concentration and gamma radiation dose became a bit stiffer as evaluated by sensory perception[27] and not suitable for further processing.
The breaking strength of rayon fiber samples grafted with APTAC were determined and the results obtained are shown in table shows that breaking strength of the grafted samples has decreased. This decrease in the breaking strength of rayon fiber shows that the tenacity of the viscose rayon fabrics increased after grafting[20]

3.5.2 Fourier-transform infrared spectroscopy (FTIR)
It had been observed that researchers all across the globe confirmed grafting qualitatively mostly by FTIR-ATR technique. Generally, the ATR technique gave information about the changes due to grafting in the surface of the graft copolymer[28], whereas KBr technique indicated the changes occurred both inside and on the surface of the grafted product[23]. Grafting of VBT on to cotton cellulose matrix was confirmed by the exhibition of additional characteristic peaks at 1428 cm-1 (C-H bending of methylgroups), 1488 cm-1 (scissoring of methylene groups) and 890 cm-1 (out of plane bending of aromatic ring C-Hbonds) evaluated by FTIR spectroscopy in ATR mode[29]. Glycidyl methacrylate (GMA) grafting of cotton cellulose was also qualitatively confirmed by FTIR spectroscopy with the additional absorption peak at 1728 cm-1 attributable to the stretching vibrations of the carbonyl group[27].
The FTIR-ATR spectrum of pristine rayon fiber showed broadband at 3325 cm-1 (vO-H str), 2897 cm-1 (vC-H str), 1020 cm-1 (vC-O-C str), and 895 cm-1 (vC-C str) vibrations[26]. Fingerprint region of the spectrograph of APTAC grafted rayon fiber showed the appearance of the additional peaks at 1645 cm-1 corresponding to C-O stretch of the ester group, 1479 cm-1 to C-H symmetric bending[30, 31] and 1413 cm-1 to C-N stretching vibration of quaternary ammonium salt[32, 33] substantiated the grafting of APTAC on to rayon fiber. FTIR spectra of pristine rayon and radiation-induced APTAC-grafted rayon fiber are presented in Figure 4.

Figure 4: FTIR spectrum of pristine (untreated) rayon and gamma radiation-induced grafted rayon fiber with 30% APTAC at 3 kGy absorbed gamma radiation dose.
3.5.3 Thermogravimetric analysis (TGA)
Thermal degradation or stability of cellulose could also change with grafting. It had been reported that the thermal stability of cellulose- g-PAN or cellulose-g-PAA was higher than that of ungrafted cellulose[34]. A similar improvement in the thermal stability was observed for polyacrylamide-g rafted carboxy methyl cellulose[35] and N-isopropylacrylamide- and methyl acrylate-grafted cellulose[36], poly(acrylic acid)-grafted cellulose microfiber[37], cellulose- graft-poly(N,N- dimethylacrylamide)[38], NIPAM-grafted-cellulose derivatives[39], and polyacrylamide-grafted cellulose[40].
It was observed that the APTAC-grafted rayon resulted in lower weight loss in comparison to pristine fiber up to temperature 325°C in line with the findings of the earlier researchers[2, 12, 16, 34, 36–38]. APTAC-grafted rayon encountered in higher weight loss in comparison to pristine fiber beyond 325°C may be due to the decomposition of quaternary groups and removal of pendant groups[2].
3.5.4 Scanning Electron Microscopy (SEM)
Figure 5 represents the SEM micrograph of pristine fiber and rayon fiber grafted with 30% (v/v) APTAC monomer concentration at a total absorbed gamma radiation dose of 3 kGy. The comparison of the SEM of the untreated rayon and the APTAC-grafted rayon fiber showed a clear indication of the change in the topology of the grafted samples. Grafting of APTAC on the rayon backbone opened up its matrix and showed considerable deposition of poly (APTAC) on the surface of the backbone polymers. Micrographs also clearly showed that the surface morphology of the rayon fibers visibly changed from a rough to smooth surface with an increase in the diameter of the fiber due to grafting[2].

Figure 5: TGA thermogram of pristine (Untreated) rayon and gamma radiation-induced grafted rayon fiber with 30% APTAC at 3 kGy absorbed gamma radiation dose.
4 Antibacterial Activity and Efficacy
Findings of antibacterial activity and efficacy study as per JIS L 1902:2008 of pristine rayon fiber and that of radiation-induced APTAC-grafted rayon fiber with 30% APTAC concentration irradiated at a total gamma radiation dose of 3 kGy have been summarized in Table 4 and pictorially depicted in Figure 7.
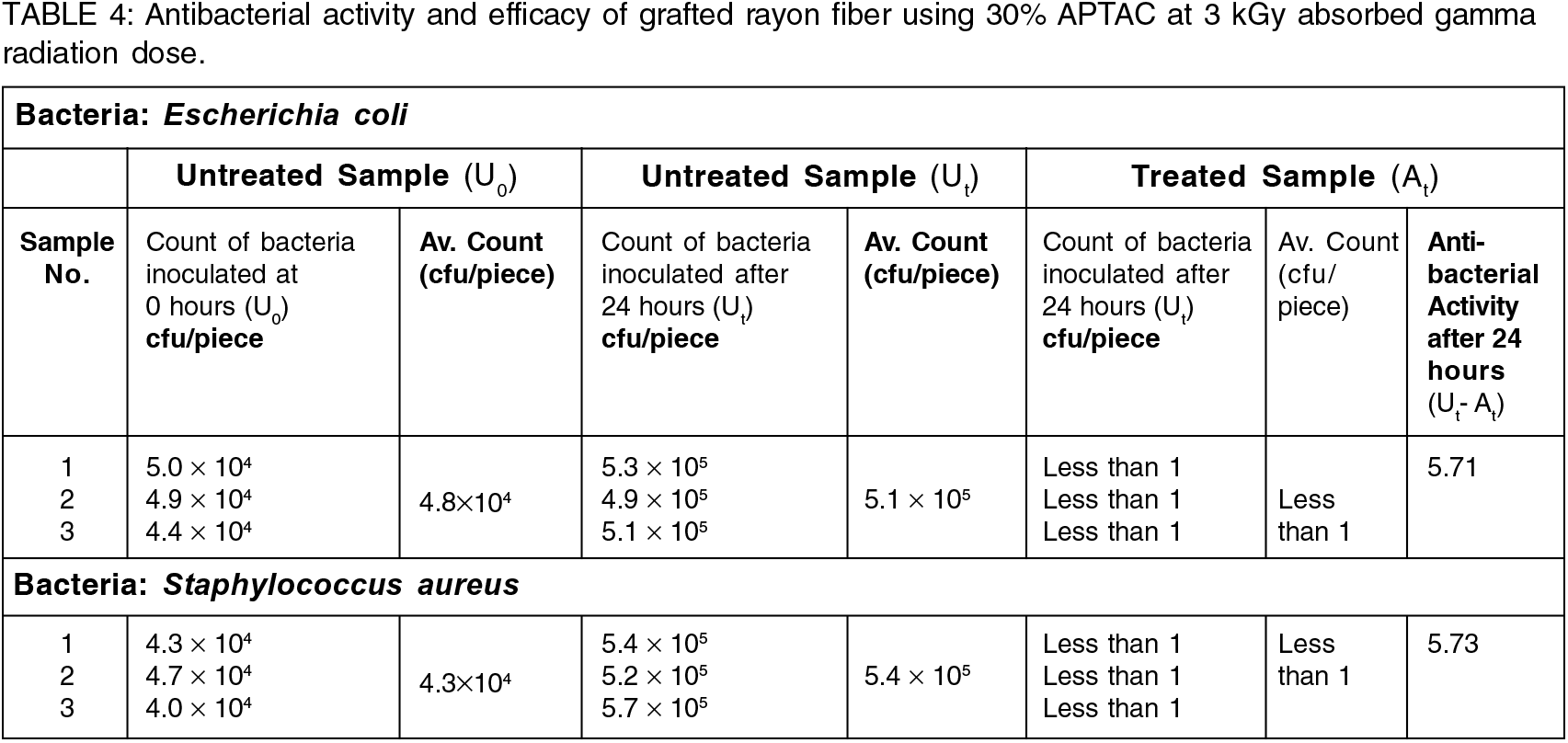

Figure 6: SEM micrograph of (a) untreated rayon and (b) gamma radiation-induced grafted rayon fiber with 30% APTAC & 3 kGy total gamma radiation dose.

Figure 7: Antibacterial activity & efficacy of grafted rayon fiber using 30% APTAC at 3 kGy gamma radiation dose. (a) & (d) Count of bacteria inoculated at 0 hours (U0) with untreated rayon against E. coli & S. aureus (b) & (e) Count of bacteria inoculated after 24 hours (Ut) with untreated rayon against E. coli & S. aureus (c) & (f) Count of bacteria inoculated after 24 hours (At) with treated rayon against E. coli & S. aureus
Rayon fiber grafted with 30% APTAC concentration at a total irradiation dose 3 KGy showed four log cycle decrease in the active bacterial count after 24 hours resulted in antibacterial activity and efficacy (R) of 5.71 against Escherichia coli and 5.73 against Staphylococcus aureus which was much higher than the value 2.0 as prescribed in the guidelines JIS L 1902:2008 for a substrate to be called or declared as antimicrobial. Hence, the grafted rayon sample showed improved antibacterial activity compared to pristine rayon fiber.
Enhanced antibacterial activity of the grafted rayon fibers could be attributed to the adsorption of the cations of the APTAC molecules of the APTAC-grafted rayon fiber on the bacteria cell surface due to negatively charged phosphate groups on microbial cell wall followed by the penetration of the a polar alkyl chains disrupting the cell wall resulting leakage of Adenosine triphosphate (Nucleic acid). This led to cell lysis and ultimately to cell death[18] destroying bacteria inhibiting the growth of a wide variety of bacteria such as E. coli and S. aureus.
It can be concluded based on work presented above that water absorbency and antibacterial efficacy of the pristine rayon fiber have been enhanced manifold by radiation-induced grafting with APTAC. Antibacterial activities of the grafted rayon fiber against E. coli and S. aureus were found to be substantially enhanced as compared to pristine rayon fiber showing four log cycle decrease in the number of living bacteria after 24 hours of incubation. Water absorption was also remarkably increased by 435 % compared to its pristine counterpart and found to be linearly correlated with the grafting yield showing a steady increasing trend up to a certain level of monomer concentration and absorbed gamma radiation dose followed by either slowing down or leveling off the increasing trend. The APTAC-grafted rayon fiber thus developed could find applications in various bio-medical and hygiene applications such as sanitary napkins, tampons, diapers, incontinence products, and wound dressings where antibacterial activity and super absorbency are prerequisites.
Acknowledgement: The authors would like to thank the Board of Research of Nuclear Sciences (BRNS), Mumbai, India (Sanction No- 2009/35/8/BRNS) for their financial support and also to the management of Shriram Institute for Industrial Research, Delhi, India for their constant guidance & support while carrying the research work.
REFERENCES
1. H. Liu, Y. Zhang, J. Yao, Fibers. Polym. 2014, 15: 145-152. https://doi.org/10.1007/s12221-014-0145-8 [Google Scholar] [CrossRef]
2. N.K. Goel, V. Kumar, M.S. Rao, Y.K. Bhardwaj, S. Sabharwal, Radiat Phys Chem. 2011, 80: 12331241. https://doi.org/10.1016/j.radphyschem.2011.04.12 [Google Scholar] [CrossRef]
3. B. Gottenbos, H.C. Van Der Mei, F. Klatter, P. Nieqwenhuis, H.J. Busscher, Biomaterials. 2002, 23: 1417-1423. https://doi.org/10.1016/S0142-9612(01)00263-0 [Google Scholar] [CrossRef]
4. R. Kumar, S. Kumari S, B. Rai, R. Kumar, S. Sirohi, and G. Kumar, J Polym Environ. 2020, 28: 2761–2770. https://doi.org/10.1007/s10924-020-01808-6 [Google Scholar] [CrossRef]
5. R. Poonguzhali, S.K. Basha, V.S. Kumari, Int J Biol Macromol., 2017,105, 111-120. https://doi.org/10.1007/s10924-020-01808-6 [Google Scholar] [CrossRef]
6. A. Hasan, G. Waibhaw, S. Tiwari, K. Dharmalingam, I. Shukla, L.M. Pandey, J Biomed Mater Res., Part A. 2017, 105: 2391-2404. https://doi.org/10.1002/jbm.a.36097 [Google Scholar] [CrossRef]
7. R. Khullar, V.K. Varshney, S, Naithani, P.L. Soni, Express Polym Lett 2. 2008, 12-18. https://doi.org/10.3144/expresspolymlett.2008.3 [Google Scholar] [CrossRef]
8. E. Princi, S. Vicini, N. Proietti, D, Capitani, Eur Polym J. 2005, 41: 1196-1203. https://doi.org/10.1016/j.eurpolymj.2005.01.009 [Google Scholar] [CrossRef]
9. A. Bhattacharya, B.N. Misra, Prog Polym Sci. 2004, 29: 767-814. https://doi.org/10.1016/j.progpolymsci.2004.05.002 [Google Scholar] [CrossRef]
10. J. Chen, Y.C. Nho, A.S. Hoffman, J Biomater Sci Polym. 2004, Ed 15: 841-849. https://doi.org/10.1163/1568562041271138 [Google Scholar] [CrossRef]
11. H.A. Abd-El-Rehim, E.A. Hegazy, A.M. Ali, J Appl Polym Sci. 1999, 74: 806-815. https://doi.org/10.1002/(SICI)1097-4628(19991024)74:4<806::AID-APP6>3.0.C0;2-B [Google Scholar] [CrossRef]
12. F.M. Barakat, K.M. El-Salmawy, A.H. Zahran, Open J Polym Chem. 2016, 06: 27-42. https://doi.org/10.4236/ojpchem.2016.63004 [Google Scholar] [CrossRef]
13. H. Lönnberg, L. Fogelström, L. Berglund, M.A.S.A. Samir, L. Berglund, E. Malmström, A. Hult, Eur Polym J. 2008, 44: 2991-2997. https://doi.org/10.1016/j.eurpolymj.2008.06.023 [Google Scholar] [CrossRef]
14. M.L. Sagu, K.K. Bhattacharyya, J. Macromol Sci, Part A - Chem. 1986, 23: 1099-1105. https://doi.org/10.1080/00222338608081115 [Google Scholar] [CrossRef]
15. H. I. Melendez-Ortiz, E. Bucio, G, Burillo, Radiat Phys Chem. 2009, 78: 1-7. https://doi.org/10.1016/j.radphyschem.2008.08.003 [Google Scholar] [CrossRef]
16. B. Gupta, R. Jain, N. Anjum, H. Singh, Radiat Phys Chem. 2006, 75: 161-167. https://doi.org/10.1016/j.radphyschem.2005.04.003 [Google Scholar] [CrossRef]
17. I. Ishigaki, T, Sugo, K. Senoo, T. Okada, J. Okamoto, S. Machi, J Appl Polym Sci. 1982, 27: 1033-1041. https://doi.org/10.1002/app.1982.070270322 [Google Scholar] [CrossRef]
18. V. Kumar, Y.K. Bhardwaj, K.P. Rawat, S. Sabharwal, Radiat Phys Chem. 2005, 73: 175-182. https://doi.org/10.1016/j.radphyschem.2004.08.011 [Google Scholar] [CrossRef]
19. N.K. Goel, M.S. Rao, V. Kumar, Y.K. Bhardwaj, C.V. Chaudhari, K.A. Dubey, S. Sabharwal, Radiat Phys Chem. 2009, 78: 399-406. https://doi.org/10.1016/j.radphyschem.2009.03.011 [Google Scholar] [CrossRef]
20. Barakat, M.F., El-Salmawy, K.M. and Zahran, A.H., Open Journal of Poolymer Chemistry, 2017, 7: 118. https://doi.org/10.4236/ojpchem.2017.71001 [Google Scholar] [CrossRef]
21. K.Y. Chen, Y.S. Lin, C.H. Yao, M.H. Li, J.C. Lin, J Biomater Sci, Polym., 2012. Ed 21: 429-443. https://doi.org/10.1163/156856209X424378 [Google Scholar] [CrossRef]
22. A. Kanazawa, T. Ikeda, T. Endo, J Polym Sci Part A Polym Chem. 1994. 32: 1997-2001. https://doi.org/10.1002/pola.1994.080321024 [Google Scholar] [CrossRef]
23. H.J. Patel, M.G. Patel, A.K. Patel, K.H. Patel, R.M. Patel, 2008. Express Polym Lett, 2: 727-734. https://doi.org/10.3144/expresspolymlett.2008.86 [Google Scholar] [CrossRef]
24. L. Wojnarovits, C.M. Foldvary, E. Takacs, 2010. Radiat Phys Chem., 79: 848-862. https://doi.org/10.1016/j.radphyschem.2010.02.006 [Google Scholar] [CrossRef]
25. E. Takacs, H. Mirzadeh, L. Wojnarovits, J. Borsa, M. Mirzateheri, N. Benke, 2007. Nucl Instruments Methods Phys Res Sect B Beam Interact with Mater Atoms, 265: 217-220. https://doi.org/10.1016/j.nimb.2007.08.098 [Google Scholar] [CrossRef]
26. S.M. Badawy, A.M. Dessouki, H.M. Nizam El-Din, 2001. Radiat Phys Chem, 61: 143-148. https://doi.org/10.1016/S0969-806X(00)00431-X [Google Scholar] [CrossRef]
27. I. Kaur, N. Sharma, V. Kumari, 2013. J Adv Res, 4: 547-557. https://doi.org/10.1016/jJare.2012.11.003 [Google Scholar] [CrossRef]
28. G. Desmet, E. Takacs, L. Wojnarovits, J. Borsa, 2011. Radiat Phys Chem., 80: 1358-1362. https://doi.org/10.1016/j.radphyschem.2011.07.009 [Google Scholar] [CrossRef]
29. C. Mao, Y. Qiu, H. Sang, H. Mei, A. Zhu, J. Shen, S. Lin, 2004. Adv Colloid Interface Sci, 110: 5-17. https://doi.org/10.1016/j.cis.2004.02.001 [Google Scholar] [CrossRef]
30. V. Kumar, N.K. Goel NK, Y.K. Bhardwaj, S. Sabharwal, L. Vershney, 2012. Sep Sci Technol 47:1937-1947. https://doi.org/10.1080/01496395.2012.664599 [Google Scholar] [CrossRef]
31. Y. Song, Y. Sun, X. Zhang, J. Zhou, L. Zhang, 2008. Biomacromolecules, 9: 2259-2264. https://doi.org/10.1021/bm800429a [Google Scholar] [CrossRef]
32. W. Sajomsang, P. Gonil, S. Tantayanon, 2009. Int J Biol Macromol, 44: 419-427. https://doi.org/10.1016/j.ijbiomac.2009.03.003 [Google Scholar] [CrossRef]
33. S. Pal, D. Mal, R.P. Singh, 2005. Carbohydr Polym. 59: 417-423. https://doi.org/10.1016/j.carbpol.2004.06.047 [Google Scholar] [CrossRef]
34. D. Roy, J.S. Knapp, J.T. Guthrie, S. Perrier, 2008. Biomacromolecules, 9: 91-99. https://doi.org/10.1021/bm700849j [Google Scholar] [CrossRef]
35. W.Dahou, D. Ghemati, A. Oudia, D. Aliouche, 2010. Biochem Eng J, 48: 187-194. https://doi.org/10.1016/j.bej.2009.10.006 [Google Scholar] [CrossRef]
36. L.N. Fu, W. Wang, L.J. Yu, S.M. Zhang, G. Yang, 2009. Mater Sci Forum, 610: 1034-1038. https://doi.org/10.4028/www.scientific.net/MSF.610-613.1034 [Google Scholar] [CrossRef]
37. T. Toledano-Thompson, M.I. Loria-Bastarrachea, M.J. Aguilar-Vega, 2005. Carbohydr Polym, 62: 67-73. https://doi.org/10.1016/j.carbpol.2005.06.024 [Google Scholar] [CrossRef]
38. M.S. Hiltunen, J. Raula, S.L. Maunu, 2011. Polym Int., 60:1370-1379. https://doi.org/10.1002/pi.3090 [Google Scholar] [CrossRef]
39. A.A. Shvedova, V.E. Kagan, V. Fadeel, 2010. Annu Rev Pharmacol Toxicol., 50: 63-88. https://doi.org/10.1146/annurev.pharmtox.010909.105819 [Google Scholar] [CrossRef]
40. K.C. Gupta, K. Khandekar, 2002. J Appl Polym Sci, 86: 2631-2642. https://doi.org/10.1002/app.11448 [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2022 The Author(s). Published by Tech Science Press.
Copyright © 2022 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools