 Open Access
Open Access
ARTICLE
Numerical Analysis for the Effect of Irresponsible Immigrants on HIV/AIDS Dynamics
1 Department of Mathematics, Barani Institute of Sciences, Burewala, Pakistan
2 Department of Mathematics, Cankaya University, Balgat, Ankara, 06530, Turkey
3 Department of Medical Research, China Medical University, Taichung, 40402, Taiwan
4 Institute of Space Sciences, Magurele-Bucharest, 077125, Romania
5 Department of Mathematics, Faculty of Sciences, University of Central Punjab, Lahore, 54000, Pakistan
6 Department of Automation, Biomechanics and Mechatronics, Lodz University of Technology, 1/15 Stefanowskiego St., 90-924 Lodz, Poland
7 Department of Mathematics and Statistics, University of Lahore, Lahore, 54590, Pakistan
8 Department of Mathematics, Govt. Maulana Zafar Ali Khan Graduate College Wazirabad, Punjab Higher Education Department (PHED), Lahore, 54000, Pakistan
9 H&BS, MCS, National University of Science and Technology (NUST), H-12, Islamabad, Humayun Road, Rawalpindi, 46000, Pakistan
* Corresponding Author: Ali Raza. Email:
Intelligent Automation & Soft Computing 2023, 36(2), 1479-1496. https://doi.org/10.32604/iasc.2023.033157
Received 09 June 2022; Accepted 12 July 2022; Issue published 05 January 2023
Abstract
The human immunodeficiency viruses are two species of Lentivirus that infect humans. Over time, they cause acquired immunodeficiency syndrome, a condition in which progressive immune system failure allows life-threatening opportunistic infections and cancers to thrive. Human immunodeficiency virus infection came from a type of chimpanzee in Central Africa. Studies show that immunodeficiency viruses may have jumped from chimpanzees to humans as far back as the late 1800s. Over decades, human immunodeficiency viruses slowly spread across Africa and later into other parts of the world. The Susceptible-Infected-Recovered (SIR) models are significant in studying disease dynamics. In this paper, we have studied the effect of irresponsible immigrants on HIV/AIDS dynamics by formulating and considering different methods. Euler, Runge Kutta, and a Non-standard finite difference (NSFD) method are developed for the same problem. Numerical experiments are performed at disease-free and endemic equilibria points at different time step sizes ‘ℎ’. The results reveal that, unlike Euler and Runge Kutta, which fail for large time step sizes, the proposed Non-standard finite difference (NSFD) method gives a convergence solution for any time step size. Our proposed numerical method is bounded, dynamically consistent, and preserves the positivity of the continuous solution, which are essential requirements when modeling a prevalent disease.Keywords
Acquired Immunodeficiency Syndrome (AIDS) is caused by the Human Immunodeficiency Virus (HIV). HIV/AIDS is the most destructive disease faced by humanity. There are serious consequences for the community, economy, and public health. People infected with Human Immunodeficiency Virus (HIV) may harbor the virus for many years without clinical signs of disease. Eventually, it destroys the body’s immune system, increases the risk of certain diseases, damages body organs such as the brain, kidneys, and heart, and results in death. Human Immunodeficiency Virus (HIV) deteriorates the blood deformation of some commonly used immune systems, which protect the body from disease. HIV/AIDS currently kills approximately 2 million people worldwide each year. Sexual relations with an infected person and exchanging infected blood normally cause Human Immunodeficiency Virus (HIV) transmission. The infected mother also transfers Human Immunodeficiency Virus (HIV) to her newborn. People with Acquired Immunodeficiency Syndrome (AIDS) are susceptible to many disease infections that do not normally cause disease in healthy people [1]. Mathematical and Numerical models have demonstrated importance in figuring out the dynamics of Human Immunodeficiency Virus (HIV) disease. These models have been widely used in HIV/AIDS epidemiological research to improve our understanding of the major factors contributing to a disease epidemic. Many researchers have worked intensively on various aspects of this dangerous disease. Busenberg et al. presented a simple model for the transmission of HIV/AIDS in India, and he concluded that the main reason for the spread of HIV/AIDS in the community was the sexual interaction between a core group of prostitutes and young unmarried men [2]. Coutsoudis et al. discussed the effects of free formula milk on infants of Human Immunodeficiency Virus (HIV) infected women [3]. Although it eliminates Human Immunodeficiency Virus (HIV) transmission but carries a risk of increased mortality, breastfeeding has several benefits but carries a risk of Human Immunodeficiency Virus (HIV) transmission. Ogundele et al. studied the mother-to-child transmission (MTCT) of HIV through breastfeeding [4]. Dunn et al. developed a Human Immunodeficiency Virus (HIV) transmission [5]. Newell studied the Human Immunodeficiency Virus (HIV) and concluded that its transmission can occurs before, during, or after birth [6]. An important impact on dangerous sexual behavior is the lack of awareness about HIV/AIDS. Zaleta studied heterosexual disease transmission and proposed a model for a heterosexually transmitted disease [7]. Greenhalgh et al. examined the effects of condom use on the sexual transmission of Human Immunodeficiency Virus (HIV) and Acquired Immunodeficiency Syndrome (AIDS) in a homogeneous male homosexual population [8]. Mukandavire et al. studied a model for heterosexual transmission of HIV/AIDS in a community and formulated a model [9]. The model divided the population into a gendered structure of males and females. The threshold and equilibria of the model were determined, and the stability was examined. The model has been expanded to focus on the impact of condom use as a unique strategic approach to HIV prevention without treatment. Male condom use was initially modeled, and the model was later expanded to include male and female condom use. Basic reproduction numbers were calculated for these models, and the models were analyzed numerically. Din et al. presented a modified model of Human Immunodeficiency Virus (HIV) CD4+T-cells [10]. Abueldahab et al. studied an epidemic model to observe the spread of Human Immunodeficiency Virus (HIV) in Khartoum [11]. Sanusi et al. developed a epidemic model to study and predict the spread of HIV/AIDS [12]. Shaikh et al. proposed the model of the dynamics of HIV/AIDS transmission in the existence of a conscious community using a fractional differential operator [13]. The conditions of existence and uniqueness of the model were obtained. The conditions necessary for disease control were examined to determine unconscious infectious agents’ role in spreading HIV/AIDS. Mickens introduced the concept of Nonstandard finite difference (NSFD) theory [14]. The theory has been widely used to solve mathematical models. Patidar studied developments and applications of Non-standard finite difference (NSFD) methods [15]. Jodar et al. developed two finite difference schemes based on the Nonstandard finite difference (NSFD) theory for an influenza mathematical model [16]. Raza et al. examined a stochastic model of HIV/AIDS based on Nonstandard finite difference (NSFD) theory in a bisexual population considering counseling and antiretroviral therapy. They concluded that the stochastic model of the HIV/AIDS epidemic is relatively more pragmatic than the deterministic model of HIV/AIDS. HIV/AIDS epidemic [17]. The Nonstandard finite difference (NSFD) stochastic scheme retained all the important properties of the disease dynamical model. Jawaz et al. studied a delay epidemic model with diffusion and developed Nonstandard finite difference (NSFD) based scheme to study HIV/AIDS, a delayed reaction-diffusion epidemic model [18]. Iqbal et al. studied a fractional-order HIV/AIDS transmission based on Nonstandard finite difference (NSFD) theory [19]. Ahmed et al. introduced a new Human Immunodeficiency Virus (HIV) CD4+T cells reaction-diffusion model [20]. Arifeen et al. studied the numerical techniques for the higher-order boundary value problems [21]. Shah et al. studied the fractional dynamics of HIV with source term for the supply of new CD4+ T-Cells depending on the viral load via Caputo Fabrizio derivative [22,23]. The well-known methods and infectious diseases models are studied in [24–35]. The rest of the paper is designed: An HIV/AIDS model is considered in Section 2. The existence analysis of the model is also studied in this section. Section 3 is devoted to numerical modeling. Sections 4 and 5 contain the simulation results and conclusions, respectively.
In this work, we consider the HIV/AIDS model presented by [36] for the existence of a solution and reliable numerical study.
With initial conditions
The system (1)–(4) is developed assuming that HIV may be transferred in the population through sexual interaction and through infected blood or needle. Also, it is considered that the rate of irresponsible infective infecting people with the disease is higher than that of responsible infective. In the system (1)–(4),
and non-negative initial conditions are given as,
The model modified into total population
For the sake of simplicity, the above system can be re-written as
where
The classical solution of the deterministic model (5)–(8) together with the initial conditions
The goal of the current section is to show the unique existence of the solution by the well-known Banach fixed theorem stated as.
Theorem (Contraction Mapping Principle)1. Let
and we choose the space of continuous functions
The application of the contraction mapping principle seeks the following two conditions,
•
•
for the operators (15). We see the two conditions in the next two subsections.
In this subsection, we show the explicit estimates for
Here,
The sufficiently small initial conditions can be considered. For arbitrary initial needs, one may choose a larger ball radius. Still, the permissible restriction on the radius can be obtained after the second condition on the contractility of the operator.
Remark 1. The existence of the Schauder fixed point theorem can be guaranteed with the same conditions (17) and (18) with another state of relative compactness using the concepts of equicontinuity and the subsequent Arzela-Ascoli-Theorem.
For the contraction condition, we consider the following two elements from Eq. (15),
The Lipschitz constants
Condition (21) provides the second restriction on the choice of the length of the interval of continuity together with inequality (17). If both conditions are full-filled, then the following result has been established.
Theorem 2. Let the state functions
2.4 Analysis of Two Conditions of Contraction Mapping Principle
To understand the optimal behavior of the solution vector
Let us have a comparison of the two functions appearing on the RHS of the above inequalities
The functions have a specific intersection point
The above important consideration leads to the important result of an optimal solution.
Theorem 3. Suppose the RHS of (5)–(8) are Lipschitz continuous (14), then the unique solution is continuous in the interval
This section is dedicated to discussing the steady states of the system (5)–(8) and their stability analysis. For the steady states put
For the disease-free steady states, there are no infective and full-blown Acquired Immunodeficiency Syndrome (AIDS) patients. Hence
From this, we obtain the reproduction number
The disease persists at the endemic equilibrium state, and E*= gives the equilibrium point
We note here that
The Euler method for the studied model can be obtained as follows
3.2 Fourth Order Runge-Kutta Method
In this section, we make RK- 4 scheme for the studied model. Considering the system of Eqs. (5)–(8), we have,
Step-1
Step-1I
Step-1II
Step-1V
Final Step
3.3 Non-Standard Finite Difference Scheme
Non-Standard finite-difference (NSFD) scheme for the studied model can be constructed as
Use Eq. (1)
Similarly, we obtain
3.4 Stability of the NSFD Scheme
Jacobian matrix for the system (26)–(29) is given by
Clearly two Eigen value i.e.,
Since finding the Eigenvalue of the above matrix is quite tricky, the spectral radius (most considerable Eigen value) was calculated using MATLAB, which is depicted in Fig. 1. The most significant Eigen value is less than 1, which shows that the system (26)–(29) is stable.
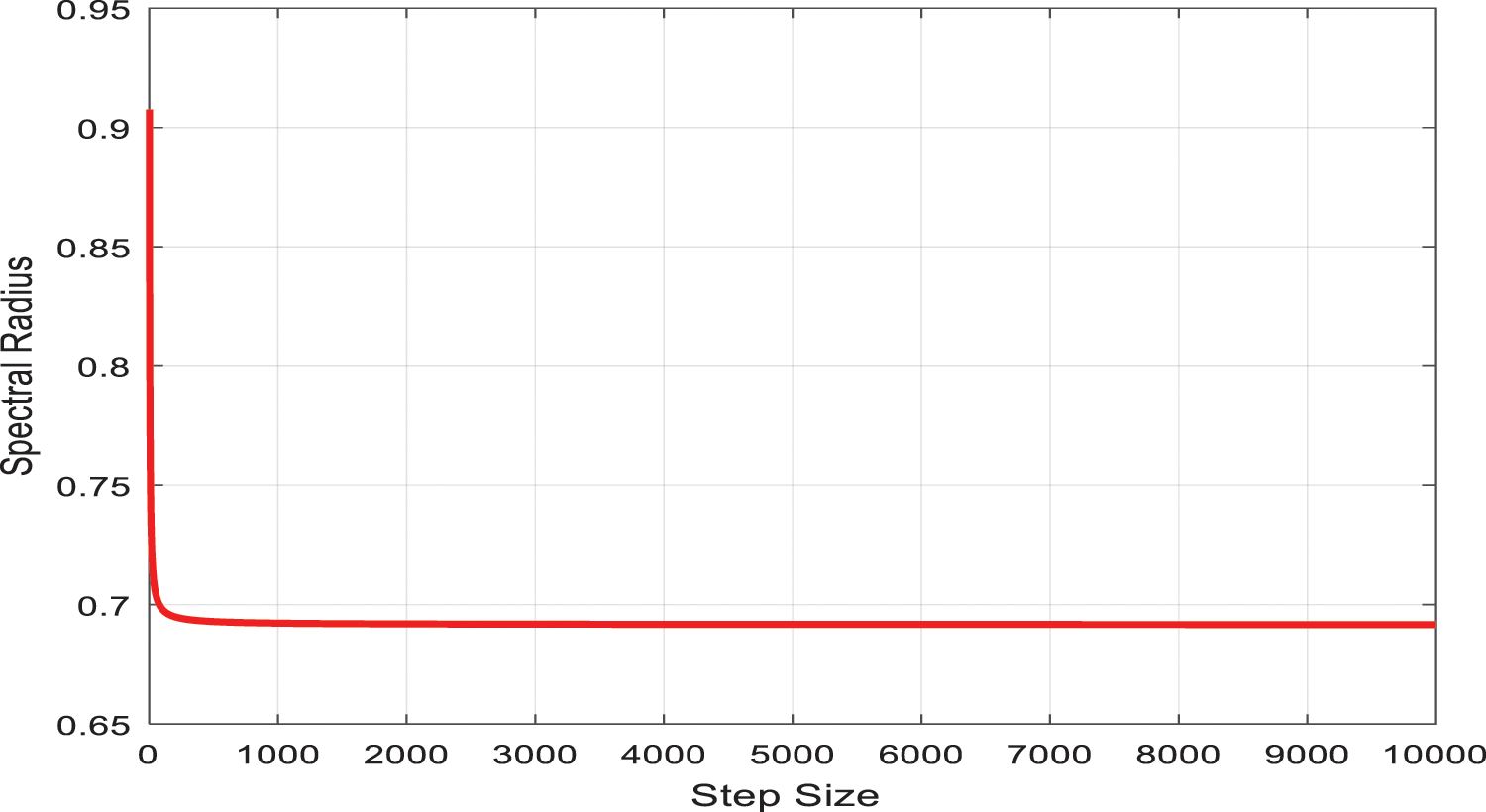
Figure 1: The spectral radius of the largest Eigenvalue
In this portion, we discuss our findings. The following are values of the parameters used in our model.
In Figs. 2a–2d and 3a–3d, all compartments of the proposed model by using the proposed numerical scheme with the time step size
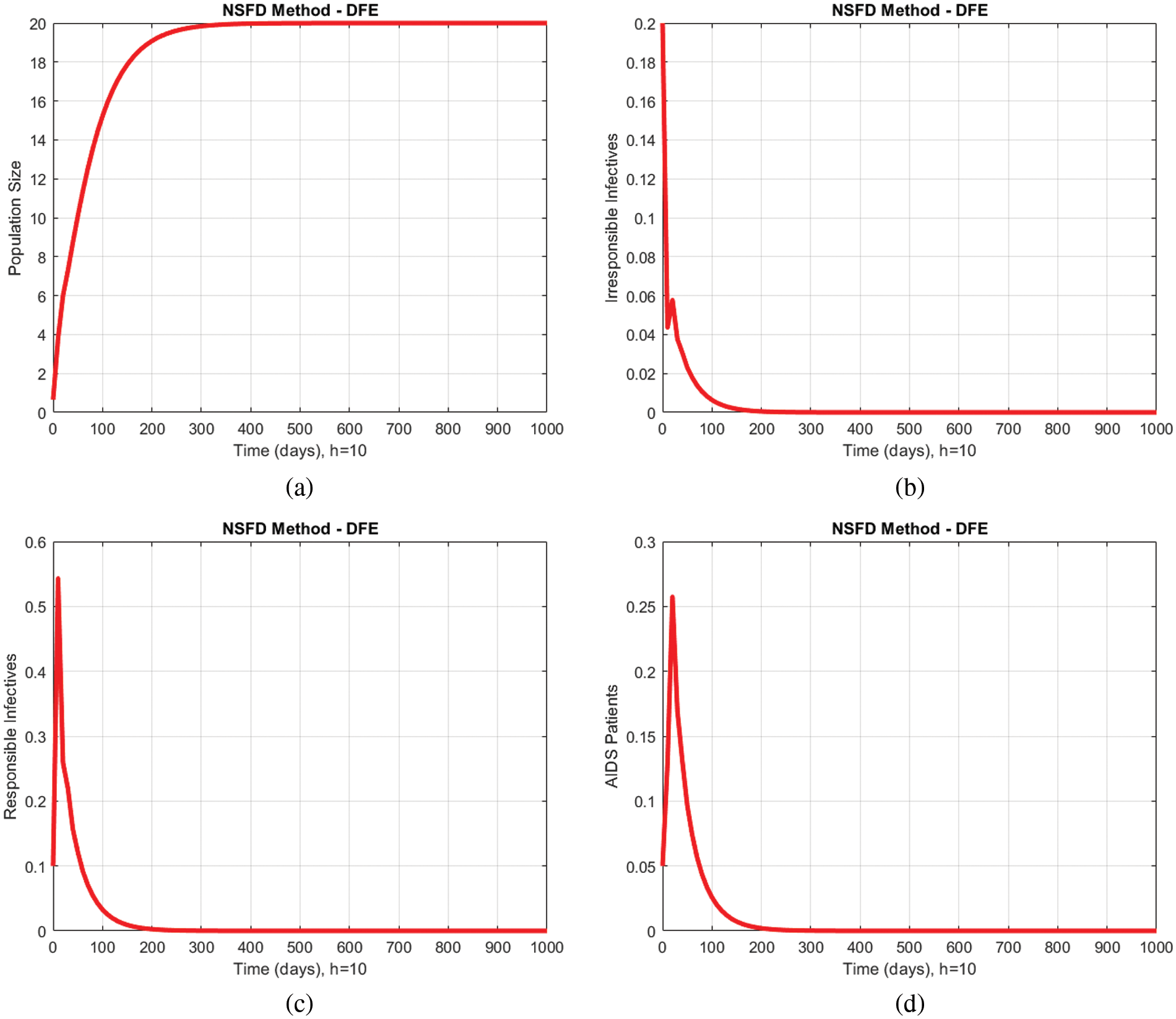
Figure 2: Numerical solutions of all compartments of the proposed model by using the proposed numerical scheme with time step size
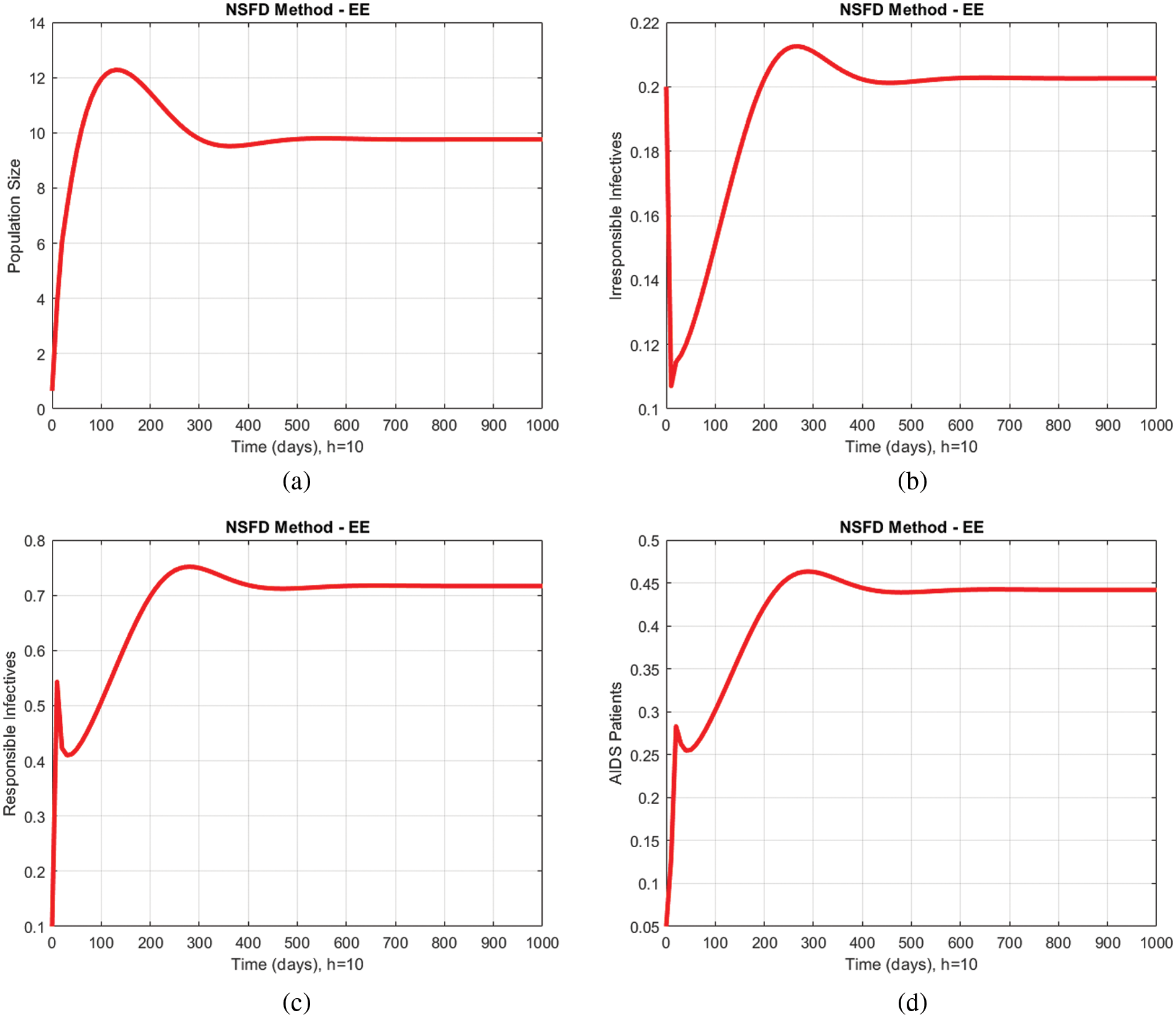
Figure 3: Numerical solutions of all compartments of the proposed model by using the proposed numerical scheme with time step size
4.1 Comparison of NSFD with Euler Method
Figs. 4a–4d shows a graphical comparison of the proposed method with the Euler technique describing the compartment of immunodeficiency patients for different values of
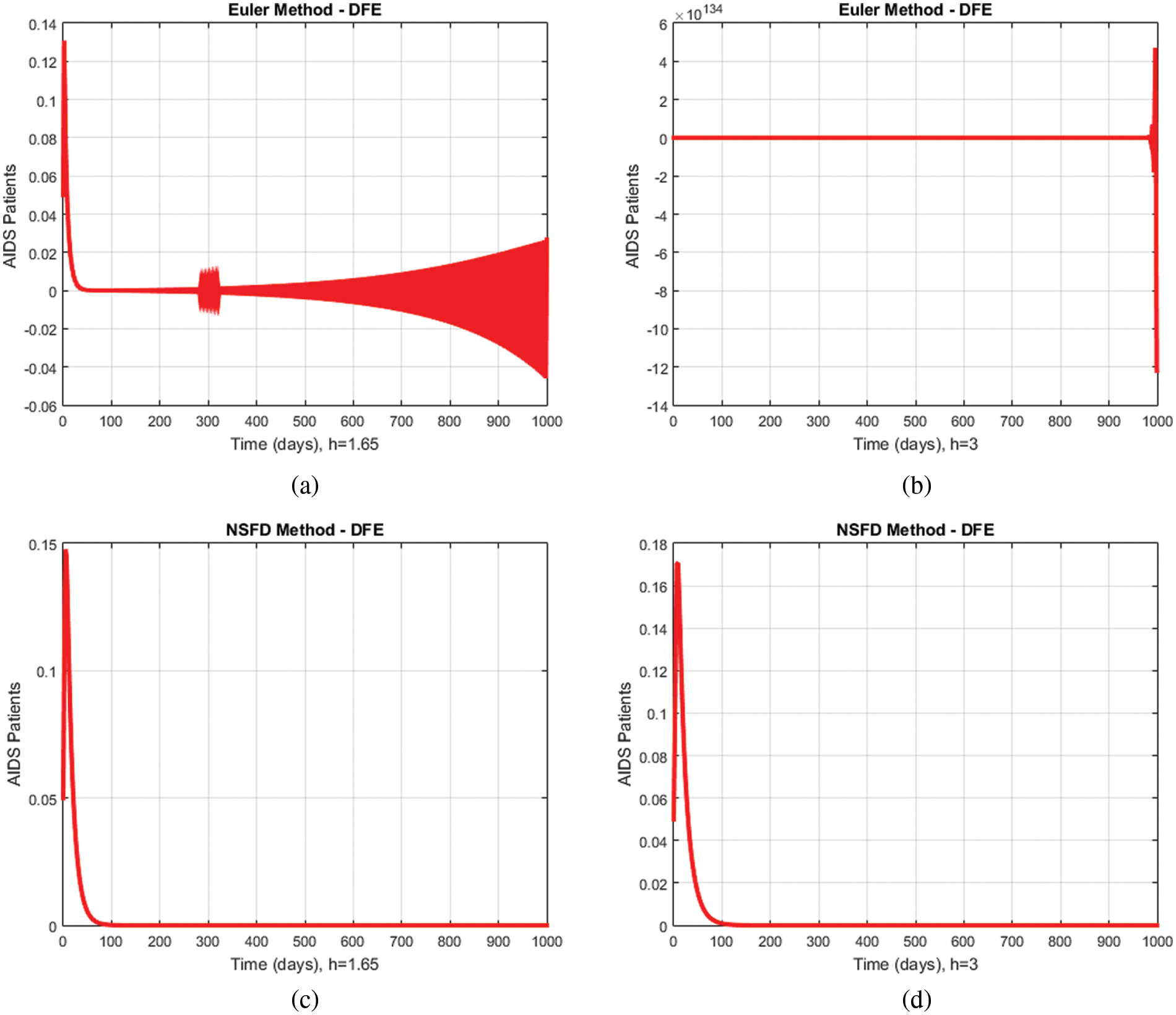
Figure 4: Comparison graphs of the proposed method with the Euler model technique for different values of
Fig. 5 presents another comparison between the proposed method and the Euler technique showing the portion of immunodeficiency patients for different values of
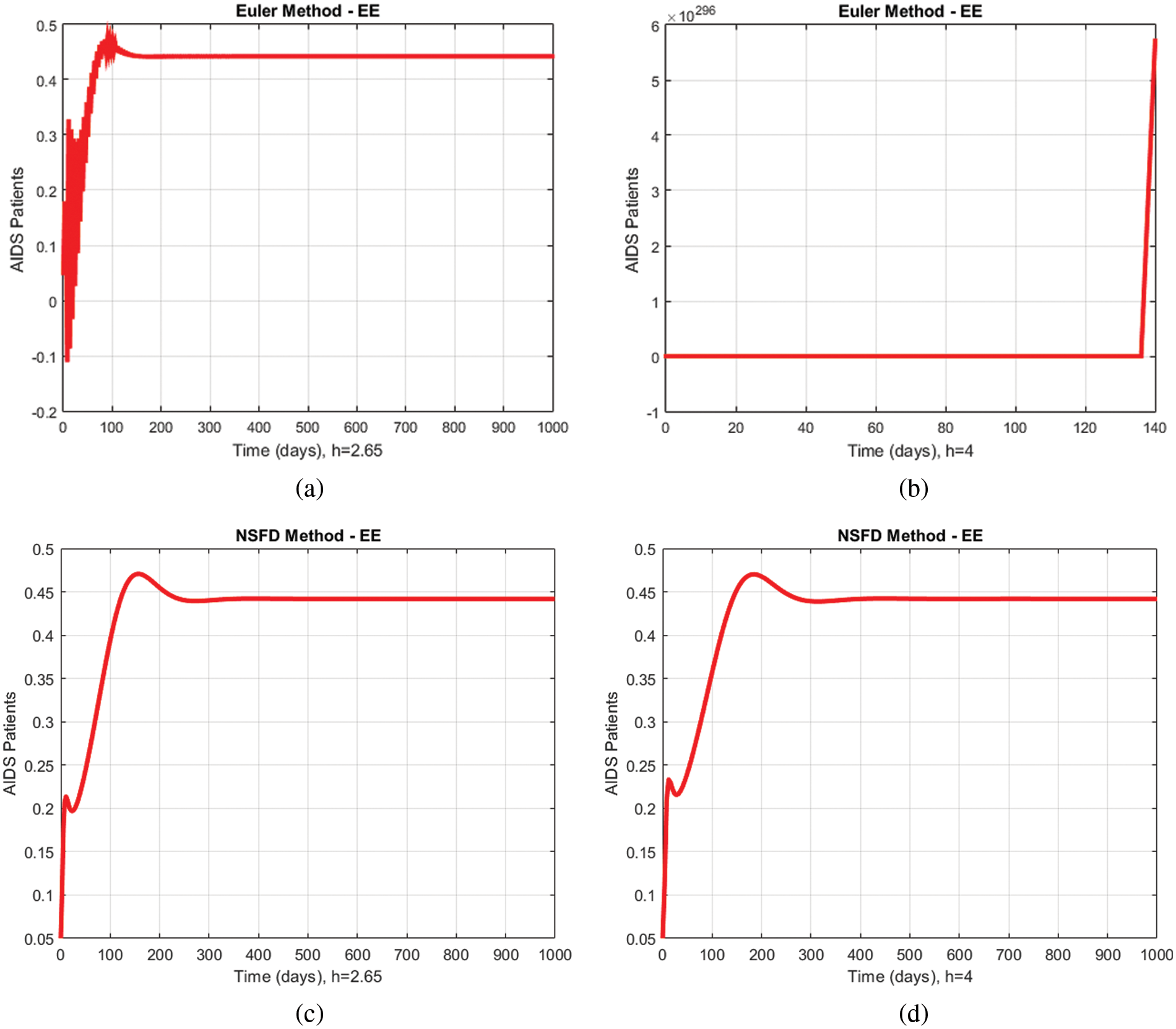
Figure 5: Comparison graphs of the proposed method with the Euler technique of immunodeficiency patients for different values of
4.2 Comparison of NSFD with Runge Kutta Method
A comparison of the proposed method and the Runge Kutta technique describing the proportions of immunodeficiency for different values of
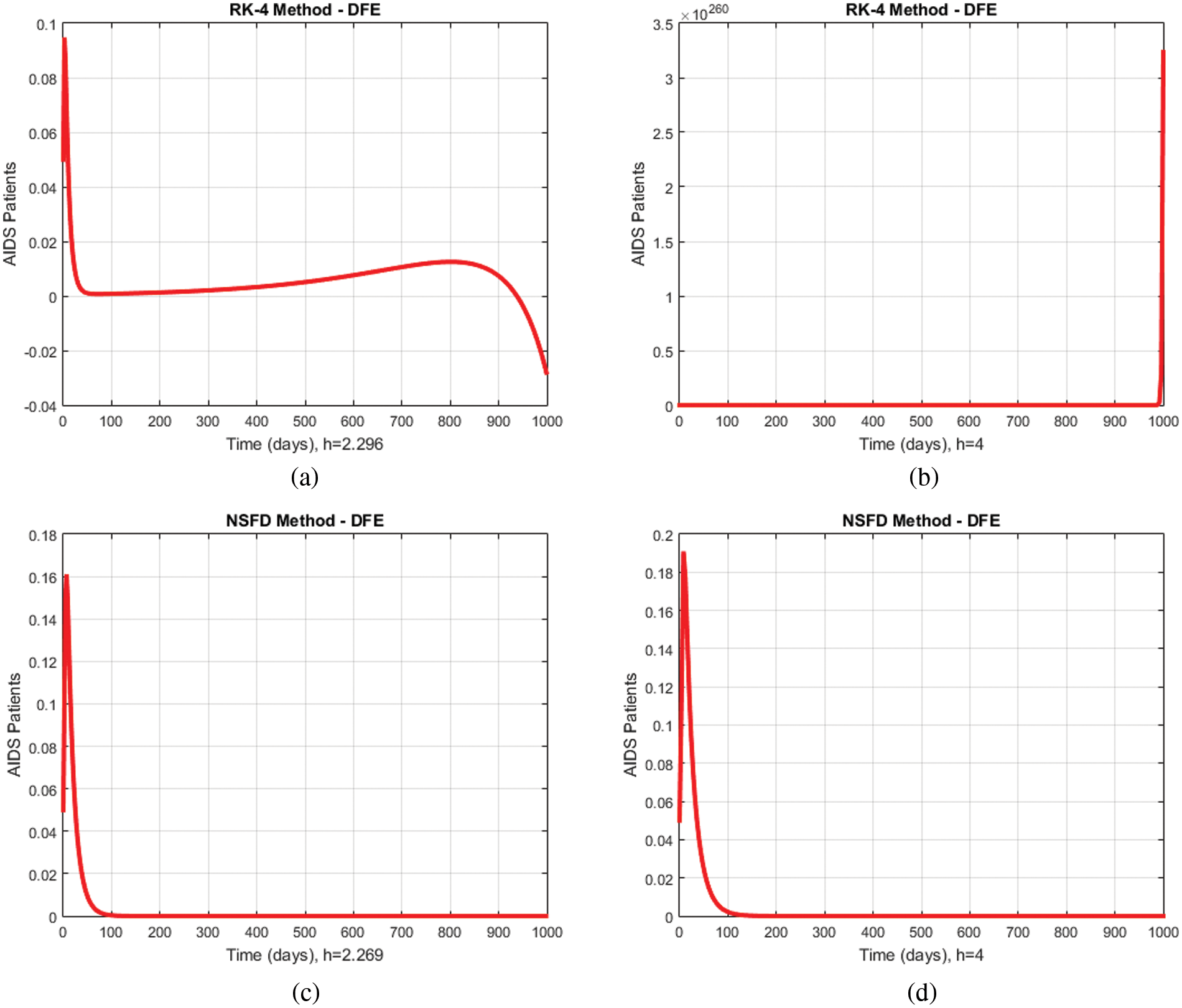
Figure 6: Comparison graphs of the proposed method with Runge Kutta technique of immunodeficiency patients for different values of
In Figs. 7a–7d, a comparison of the proposed method and the Runge Kutta technique describing the compartment of the immunodeficiency patients for different values of
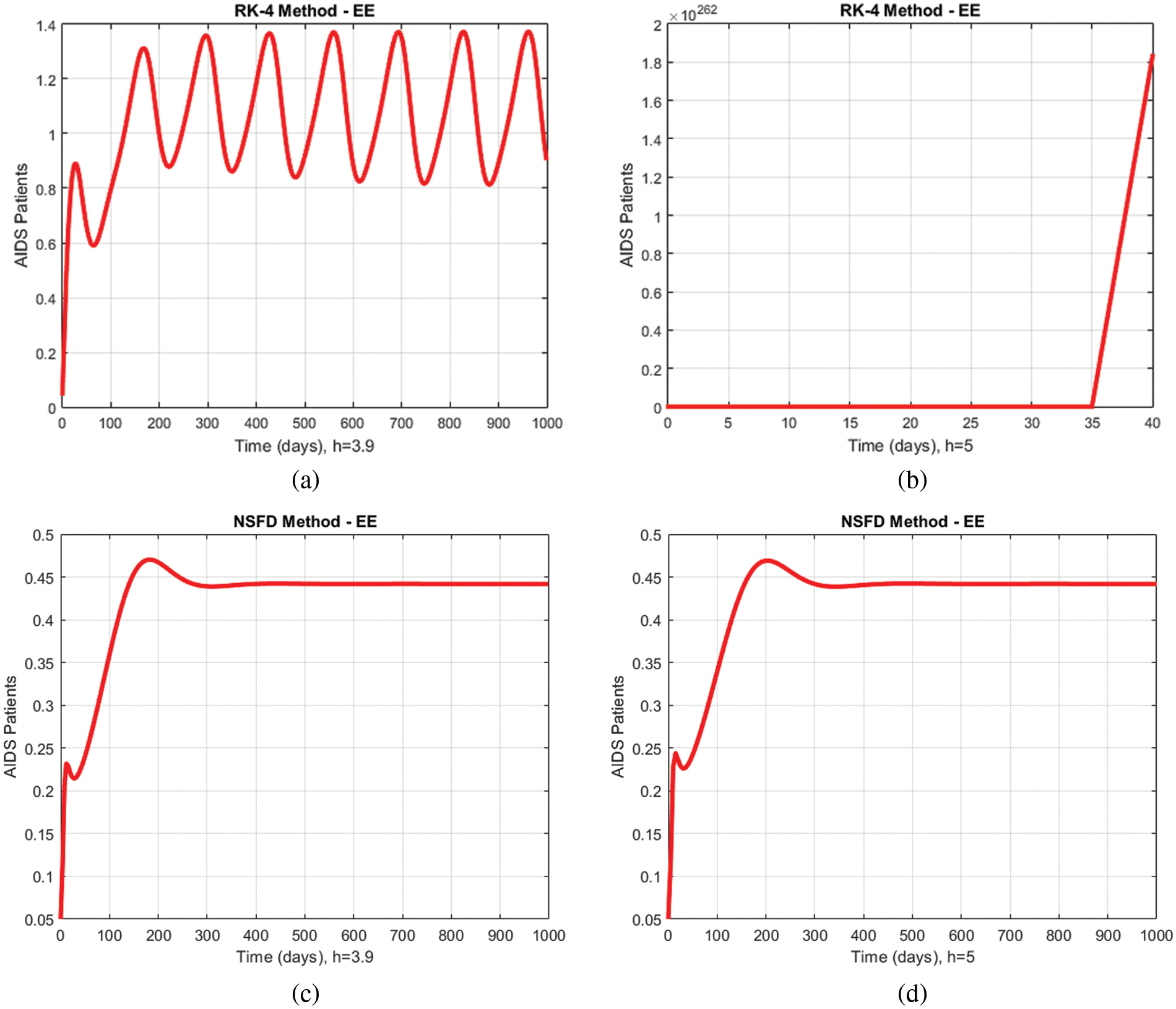
Figure 7: Comparison graphs of the proposed method with Runge Kutta technique of immunodeficiency patients for different values of
The effect of irresponsible immigrants on human immunodeficiency has been studied in this article. The existence of the solutions incorporating the contraction mapping principle and self-mapping is discussed. Euler, Runge Kutta, and Non-standard Finite Difference (NSFD) methods are formulated to solve the studied model. Numerical experiments are performed at disease-free equilibrium (DFE), and endemic equilibrium (EE) points at different time-step sizes. The obtained results are analyzed and compared. We concluded that the Euler and Runge Kutta methods fail to converge at large time step sizes, while the proposed method gives results that combine to actual steady states for any time step size. Moreover, the Non-standard Finite Difference (NSFD) method is bounded, dynamically consistent, and preserves the positivity of the solution, which are essential requirements when modeling a prevalent disease.
Acknowledgement: Thanks to our families and colleagues who supported us morally.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare they have no conflicts of interest to report regarding the present study.
References
1. J. Chin and S. K. Lwanga, “Estimation and projection of adult aids cases: A simple epidemiological model,” Bulletin of the World Health Organization, vol. 69, no. 4, pp. 399–406, 1991. [Google Scholar]
2. S. Busenberg, K. Cooke and H. Y. Heng, “A model for HIV in Asia,” Mathematical Biosciences, vol. 128, no. 1, pp. 185–201, 1995. [Google Scholar]
3. A. Coutsoudis, A. E. Goga, N. Rollins and H. M. Coovadia, “Free formula milk for infants of HIV infected women: Blessing or curse,” Health Policy and Planning, vol. 17, no. 2, pp. 154–160, 2002. [Google Scholar]
4. M. O. Ogundele and J. B. S. Coulter, “HIV transmission through breastfeeding: Problems and prevention,” Annals of Tropical Pediatrics, vol. 23, no. 2, pp. 91–106, 2003. [Google Scholar]
5. D. T. Dunn, B. H. Tess, L. C. Rodrigues and A. E. Ades, “Mother-to-child transmission of HIV: Implication of variation in maternal infectivity,” AIDS, vol. 12, no. 16, pp. 162211–162216, 1998. [Google Scholar]
6. M. L. Newell, “Mechanism and timing of mother-to-child transmission of HIV-1,” AIDS, vol. 12, no. 8, pp. 832–837, 1998. [Google Scholar]
7. C. M. K. Zaleta, “Structured models for heterosexual disease transmission,” Mathematical Biosciences, vol. 160, no. 1, pp. 83–108, 1999. [Google Scholar]
8. D. Greenhalgh, M. Doyle and F. Lewis, “A mathematical treatment of aids and condom use,” Mathematical Medicine and Biology: A Journal of the IMA, vol. 18, no. 3, pp. 225–262, 2001. [Google Scholar]
9. Z. Mukandavire and W. Garira, “Sex-structured HIV/AIDS model to analyse the effects of condom use with application to Zimbabwe,” Journal of Mathematical Biology, vol. 54, no. 5, pp. 669–699, 2007. [Google Scholar]
10. S. T. M. Din, A. Nazir, B. Almohsin, N. Ahmed, U. Khan et al., “On mathematical model of HIV CD4+ T-cells,” Alexandria Engineering Journal, vol. 60, no. 1, pp. 995–1000, 2021. [Google Scholar]
11. S. M. Abueldahab and F. K. Mutombo, “SIR model and HIV/AIDS in khartoum,” Open Access Library Journal, vol. 8, no. 4, pp. 01–10, 2021. [Google Scholar]
12. W. Sanusi, S. Annas, M. I. Pratama and M. Rifandi, “Analysis and simulation of SIPA model for HIV-AIDS transmission,” Journal of Physics: Conference Series, vol. 21, no. 1, pp. 01–09, 2021. [Google Scholar]
13. A. Shaikh, K. S. Nisar, V. Jadhav, S. K. Elagan and M. Zakarya, “Dynamical behaviour of HIV/AIDS model using fractional derivative with Mittag-Leffler kernel,” Alexandria Engineering Journal, vol. 61, no. 4, pp. 2601–2610, 2022. [Google Scholar]
14. R. E. Mickens, “A fundamental principle for constructing nonstandard finite difference schemes for differential equations,” Journal of Difference Equations and Applications, vol. 11, no. 2, pp. 645–653, 2005. [Google Scholar]
15. K. C. Patidar, “Nonstandard finite difference methods: Recent trends and further developments,” Journal of Difference Equations and Applications, vol. 22, no. 6, pp. 817–849, 2016. [Google Scholar]
16. L. Jodar, R. J. Villanueva, A. J. Arenas and G. C. Gonzalez, “Nonstandard numerical methods for a mathematical model for influenza disease,” Mathematics and Computers in Simulation, vol. 79, no. 3, pp. 622–633, 2008. [Google Scholar]
17. A. Raza, M. Rafiq, D. Baleanu, M. S. Arif, M. Naveed et al., “Competitive numerical analysis for stochastic HIV/AIDS epidemic model in a two-sex population,” IET Systems Biology, vol. 13, no. 6, pp. 305–315, 2019. [Google Scholar]
18. M. Jawaz, N. Ahmed, D. Baleanu, M. Rafiq and M. A. Rehman, “Positivity preserving technique for the solution of HIV/AIDS reaction-diffusion model with time delay,” Frontiers in Physics, vol. 7, no. 1, pp. 1–10, 2020. [Google Scholar]
19. Z. Iqbal, N. Ahmed, D. Baleanu, W. Adel, M. Rafiq et al., “Positivity and boundedness preserving numerical algorithm for the solution of fractional nonlinear epidemic model of HIV/AIDS transmission,” Chaos Solitons & Fractals, vol. 134, no. 1, pp. 1–19, 2020. [Google Scholar]
20. N. Ahmed, M. Rafiq, W. Adel, H. Rezazadeh, I. Khan et al., “Structure preserving numerical analysis of HIV and CD4+ T-cells reaction-diffusion model in two space dimensions,” Chaos Solitons & Fractals, vol. 139, no. 1, pp. 1–18, 2020. [Google Scholar]
21. S. U. Arifeen, S. Haq, A. Ghafoor, A. Ullah, P. Kumam et al., “Numerical solutions of higher-order boundary value problems via wavelet approach,” Advances in Difference Equations, vol. 347, no. 1, pp. 1–19, 2021. [Google Scholar]
22. Z. Shah, R. Jan, P. Kumam, W. Deebani and M. Shutaywi, “Fractional dynamics of HIV with source term for the supply of new CD4+ T-cells depending on the viral load via Caputo Fabrizio derivative,” Molecules, vol. 26, no. 6, pp. 1806–1820, 2021. [Google Scholar]
23. T. Q. Tang, Z. Shah, R. Jan, W. Deebani and M. Shutaywi, “A robust study to conceptualize the interactions of CD4+ T-cells and human immunodeficiency virus via fractional-calculus,” Physica Scripta, vol. 96, no. 12, pp. 1–20, 2021. [Google Scholar]
24. A. Raza, A. Ahmadian, M. Rafiq, S. Salashour, M. Naveed et al., “Modeling the effect of delay strategy on transmission dynamics of HIV/AIDS disease,” Advances in Difference Equations, vol. 663, no. 1, pp. 1–19, 2020. [Google Scholar]
25. A. Raza, A. Ahmadian, M. Rafiq, S. Salahshour and I. R. Laganà, “An analysis of a nonlinear susceptible-exposed-infected-quarantine-recovered pandemic model of a novel coronavirus with delay effect,” Results in Physics, vol. 21, no. 1, pp. 1–7, 2021. [Google Scholar]
26. A. Raza, M. S. Arif, M. Rafiq, M. Bibi, M. Naveed et al., “Numerical treatment for stochastic computer virus model,” Computer Modeling in Engineering and Sciences, vol. 120, no. 2, pp. 445–465, 2019. [Google Scholar]
27. M. S. Arif, A. Raza, K. Abodayeh, M. Rafiq and A. Nazeer, “A numerical efficient technique for the solution of susceptible infected recovered epidemic model,” Computer Modeling in Engineering and Sciences, vol. 124, no. 2, pp. 477–491, 2020. [Google Scholar]
28. W. Shatanawi, A. Raza, M. S. Arif, M. Rafiq, M. Bibi et al., “Essential features preserving dynamics of stochastic dengue model,” Computer Modeling in Engineering and Sciences, vol. 126, no. 1, pp. 201–215, 2021. [Google Scholar]
29. M. A. Noor, A. Raza, M. S. Arif, M. Rafiq, K. S. Nisar et al., “Non-standard computational analysis of the stochastic COVID-19 pandemic model: An application of computational biology,” Alexandria Engineering Journal, vol. 61, no. 1, pp. 619–630, 2021. [Google Scholar]
30. K. Abodayeh, A. Raza, M. S. Arif, M. Rafiq, M. Bibi et al., “Numerical analysis of stochastic vector-borne plant disease model,” Computers, Materials and Continua, vol. 63, no. 1, pp. 65–83, 2020. [Google Scholar]
31. K. Abodayeh, A. Raza, M. S. Arif, M. Rafiq, M. Bibi et al., “Stochastic numerical analysis for impact of heavy alcohol consumption on transmission dynamics of gonorrhoea epidemic,” Computers, Materials and Continua, vol. 62, no. 3, pp. 1125–1142, 2020. [Google Scholar]
32. A. Raza, J. Awrejcewicz, M. Rafiq, N. Ahmed, M. S. Ahsan et al., “Dynamical analysis and design of computational methods for nonlinear stochastic leprosy epidemic model,” Alexandria Engineering Journal, vol. 61, no. 10, pp. 8097–8111, 2022. [Google Scholar]
33. A. Raza, M. Rafiq, J. Awrejcewicz, N. Ahmed and M. Mohsin, “Stochastic analysis of nonlinear cancer disease model through virotherapy and computational methods,” Mathematics, vol. 10, no. 3, pp. 1–18, 2022. [Google Scholar]
34. A. Raza, M. Rafiq, J. Awrejcewicz, N. Ahmed and M. Mohsin, “Dynamical analysis of coronavirus disease with crowding effect, and vaccination: A study of third strain,” Nonlinear Dynamics, vol. 107, no. 4, pp. 3963–3982, 2022. [Google Scholar]
35. A. Raza, J. Awrejcewicz, M. Rafiq and M. Mohsin, “Breakdown of a nonlinear stochastic Nipah virus epidemic model through efficient numerical methods,” Entropy, vol. 23, no. 12, pp. 1–20, 2021. [Google Scholar]
36. M. I. Daabo and B. Seidu, “Modelling the effect of irresponsible infective immigrants on the transmission dynamics of HIV/AIDS,” Advances in Applied Mathematical Biosciences, vol. 3, no. 1, pp. 31–40, 2012. [Google Scholar]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools