DOI:10.32604/iasc.2022.020151

| Intelligent Automation & Soft Computing DOI:10.32604/iasc.2022.020151 |  |
| Article |
Design of Virtual Reality System for Organic Chemistry
1Department of Game Engineering, Pai Chai University, Daejeon, 35345, Korea
2College of Pharmacy, Jeju National University, Jeju, Korea
3Interdisciplinary Graduate Program in Advanced Convergence Technology & Science, Jeju National University, Jeju, Korea
4Department of Computer Engineering, Jeju National University, Jeju, Korea
*Corresponding Author: Soo Kyun Kim. Email: kimsk@jejunu.ac.kr
Received: 11 May 2021; Accepted: 03 July 2021
Abstract: Virtual reality (VR) is an advanced technology widely used in many fields. Education is essential for human resources development, and the use of technology in education can enhance teaching and learning methods. This study aims to present new methods and tools for visual and interactive education in organic chemistry. The experimental design and chemical equipment used in this research are based on the basic theory of organic chemistry, and the related materials are simulated as three-dimensional (3D) models to perform the experiments in a VR system. Chemical reactions are simulated by mixing the chemicals, and the students can observe the chemical reactions that occur. In addition, we measured the impact of using virtual organic chemistry experiments. The system was evaluated by students of the organic chemistry department, and the effectiveness of the experimental apparatus was evaluated in discussions and surveys. The results indicate that VR technologies may be considered suitable for adoption as an accessible educational tool, and that simulating laboratory circumstances through a VR system can improve students’ learning outcomes in experimental chemistry.
Keywords: Virtual reality; Head-mounted displays; Organic chemistry; Chemical reaction; Simulation; Virtual laboratory; Reaction equation; Platform
Science is important in daily life because humans use tools or technological products based on knowledge, science, and creativity to facilitate life and work. Science helps people to develop critical thinking, reasoning, research skills, creativity, ability to solve problems systematically, make informed decisions, and analyze evidence. Science is essential in a knowledge society. Therefore, everyone must learn science to understand nature and man-made technology and to apply knowledge rationally, creatively, and morally.
Chemistry, a branch of science, is a precise language that describes the reactions and changes observed in natural biological phenomena. The molecules—corresponding to the words—enable more elaborated explanations in the mechanism of action and operative methods of nature and surrounding environments. For this reason, chemistry is necessary to solve the problems of daily life, and many applied sciences use chemistry [1].
Both theory and practice are fundamental in learning chemistry. Conducting experiments can enhance learning motivation and practical capabilities in dealing with the molecules. In particular, for synthetic chemists who prepare the molecules artificially and supply them where needed, experimental proficiency is fundamental for the success in research or industry, such as biotechnology and the pharmaceutical field. The execution of an experiment is meant not to improve productivity but to provide practical training in proposing and verifying various hypotheses. Therefore, the curriculums of the university laboratory practice are intended to cultivate logical and systemic thinking skills.
Traditional education methods can meet the needs of students in certain subjects, but they cannot provide the best learning experience for all students in all subjects [2]. Current VR technology involves both virtual and real objects. Virtual simulation experiments can provide a visual 3D response. Thus, students can observe the chemical mechanism anytime and anywhere, which improves learning outcomes and the interest in learning, which helps students to learn and develop their skills [3]. To ensure safety, students can practice or be tested in virtual environments before the actual operations in the laboratory.
This study aims to create a virtual environment for organic chemistry experiments to support students in learning chemistry using 3D model simulations. The system helps students to learn in a safe environment before conducting real experiments. We used Unity 3D with C# programming to design and develop a chemical reaction simulation system in which users experimentally interact with 3D objects using Oculus head-mounted display (HMD) and a hand controller. In addition, we explain the evaluation results: the system was tested in scenarios in which users or students performed organic chemistry experiments.
Educational virtual environments solve many educational problems. In particular, the interconnection between devices is essential for creating a virtual educational environment. The application demonstrates a virtual educational environment that can be seen through a VR headset and controlled by a motion controller [4]. In addition to VR, other technologies have emerged to simulate VR experiences, including augmented reality (AR) and mixed reality (MR). Other established and emerging technologies are beyond the scope of this study, and they have become complicated with the rapid development of technology. Different forms of digital interactions can be referred to as “XR” or “AR.” In experiments with various technologies, HTC Vive was found to be effective for simulation purposes. In addition, HTC Vive enables us to design the experience for a group of people in the same space. However, technology is constantly evolving, and many ideas in this study are not limited to VR and can be easily extended to all types of XR technology if the cost allows. The users can “touch” the simulated reality [5] and perform spatial manipulation with enough precision so that workers can carry it. Posting the details of adjusting and rearranging atoms in this research, we can refer to people who use VR as “participants” instead of “users,” realizing that VR is different from other forms of human–machine interactions because humans can participate in the virtual world.
An Android program used a VR method for 3D visualization of reaction rate materials. The visualization method provided cyberspace in three renderings. The image display projected an active stereo reproduction of an image onto the screen, which required shutter glasses. During the verification, materials experts focused on learning and materials, and media experts focused on audiovisual and software engineering [6].
In the current prototype [7], users controlled the virtual reactor manually or using a simulated computer screen inside the VR environment. The purpose was to copy the original program and learn the automatic and manual control of today’s chemical plants. Knowledge acquisition was gradually provided in the training mode (“Training Layout in VR”) instead of immediately feeding all VR and chemical operation knowledge to provide opportunities for exploration, guiding practice, and continuous feedback. Subsequently, performance was evaluated for trainees and trainers. The prototype was evaluated by industrial partners and tested against their limitations in classroom teaching and pilot plants. The goal was to design a VR training system that compensates for the shortcomings of traditional training and supplements the entire training system.
In LeMo’s evaluation [8], most students liked to use this new application to learn. They appreciated LeMo because real-time pop-up messages pointed out their mistakes; moreover, they found it interesting and challenging, and it aroused positive emotions.
Organic chemistry educators have new-generation educational technologies that affect classroom education. Thus, students become more isolated and may not develop the social skills needed in today’s workplace. So far, new computing technologies have had less impact on university classrooms than expected [9]. In the context of higher education, emerging technologies enable teaching complex molecular topics. New methods may help or even replace traditional methods, such as molecular models, textbook images, and old screen computing environments. The teaching of organic chemistry relies on models to demonstrate the molecular procedures, structures, properties, interactions, behaviors, and physics that drive chemical transformation [10].
In Jinkun et al. [11], a VR organic chemistry system was constructed using a wireless HMD and Leap Motion. The system used a wireless network to integrate servers and clients without additional wearable. The VR experience in organic chemistry Leap Motion training was used to detect position, and hand and finger motion data were sent to the VR server and the client. The virtual environment was shown in the user’s view through the HMD. Using a smartphone with specific processing capabilities, users controlled their avatars in the virtual environment of an organic chemistry experiment by interacting with the virtual objects in real-time.
VR Multi-Sense Classroom (VRMC) [12] was developed as an immersive learning environment. In VR headsets, learners used hand movements to reconstruct hydrocarbon molecules and experience tactile feedback through gloves. Learners from different backgrounds conducted the first prototype evaluation are reported on VRMC’s ability to support high motivation, interest, and a wide range of learning methods for mastering organic chemistry. This new type of VR classroom supports learning of molecular organic chemistry using a touch system, and it supports multi-sensory learning.
A 3D organic chemistry lab (VCL) for organic chemistry experiments demonstrated and explained the potential of VCL to improve student learning using advanced 3D interfaces. The VCL aimed to create a virtual 3D environment to interact with chemicals and conduct high-performance chemical experiments. High-school students evaluated VCL and found it to be beneficial for effective learning of organic chemistry [13]. Furthermore, a virtual organic chemistry lab can be used as an alternative of real chemistry labs, especially when it is impossible to establish and maintain a standard organic chemistry laboratory because of financial constraints (for example, in some schools). The nature of the virtual chemical laboratory—to try different ideas—makes the chemical practice effective and important, and it can be used to supplement and support existing schools and chemical laboratories. Therefore, science high schools are recommended to combine virtual organic chemistry laboratory with practical chemistry. For example, higher education science schools in Nigeria [14] do not have real chemical laboratories, and virtual laboratories can be used to meet their needs.
In STEM education, laboratory subjects such as chemistry are important for the professional development of students. However, it was observed that the students were anxious and unconfident when conducting laboratory experiments. Students’ belief in their experimental skills was called “experimental self-efficacy” (ESE) [15], and researchers investigated four main factors that affected ESE in chemical labs. With the development of tools, their study characterized ESE and pre-lab interventions. It was measured how the virtual laboratory (VL) influenced ESE and students’ conceptual knowledge. An analysis using statistical methods, such as t-tests and difference matrices, confirmed that VL improved the students’ ESE.
A learning environment based on AR [16,17] and VR [18,19] may help to overcome this difficulty because it provides new visualization methods. The learners can directly interact with chemical objects and openly experiment with them. Students can understand modern chemistry better by combining VR technology with studying real chemistry research topics. Thus, they would be prepared to study future courses that provide a realistic view of modern chemistry. Combining practical learning with digital methods facilitates learning, and lectures can focus on both VR and contemporary chemistry. In Xiaoyun et al. [20], MR chemistry laboratory demonstrated and provided new educational experiences. Students simulated chemistry experiments in a VL and interacted with objects using Oculus helmet–mounted displays (HMD) and handheld controls.
2.3 Background Knowledge for Organic Chemistry Lab
Benzoic acid, known since the 16th century, has been used as a preservative when manufacturing foods and pharmaceuticals and to treat bacterial infections [21]. Apart from its direct use, benzoic acid significantly contributed as a reaction reagent and/or product that can be converted into different compounds or functional groups [22]. Synthetic organic chemistry is a branch of organic chemistry that synthesizes organic substances, which can be used as pharmaceutical materials. In particular, we study the oxidation reaction to synthesize benzoic acid from toluene, which has important applications. The chemical reaction equations are as follows [23]. Eq. (1) shows the main reaction formula, Eq. (2) is a chemical process for the deactivation of the remained oxidizing agent, and Eq. (3) is the critical step to separate the product from the crowded reaction condition. The role of oxidation is played by KMnO4, and water is used as a solvent for dissolving the solutes in the reaction system. The reaction can be confirmed visually: the purple reaction mixture turns black, indicating that the reaction is complete. The work-up of the reaction commences with quenching the one equivalent of KMnO4 theoretically and removing the MnO2, a by-product generated in the process (Fig. 1. Eq. (2)). The product produced during the actual reaction exists as sodium benzoate because of NaOH (Fig. 1. Eq. (1)), and additional acidification is performed to convert it to benzoic acid, which is a free acid (Fig. 1. Eq. (3)). The produced benzoic acid is no longer soluble in water and naturally leaves the reaction system. Therefore, the product can be easily purified by filtering the precipitated solid.
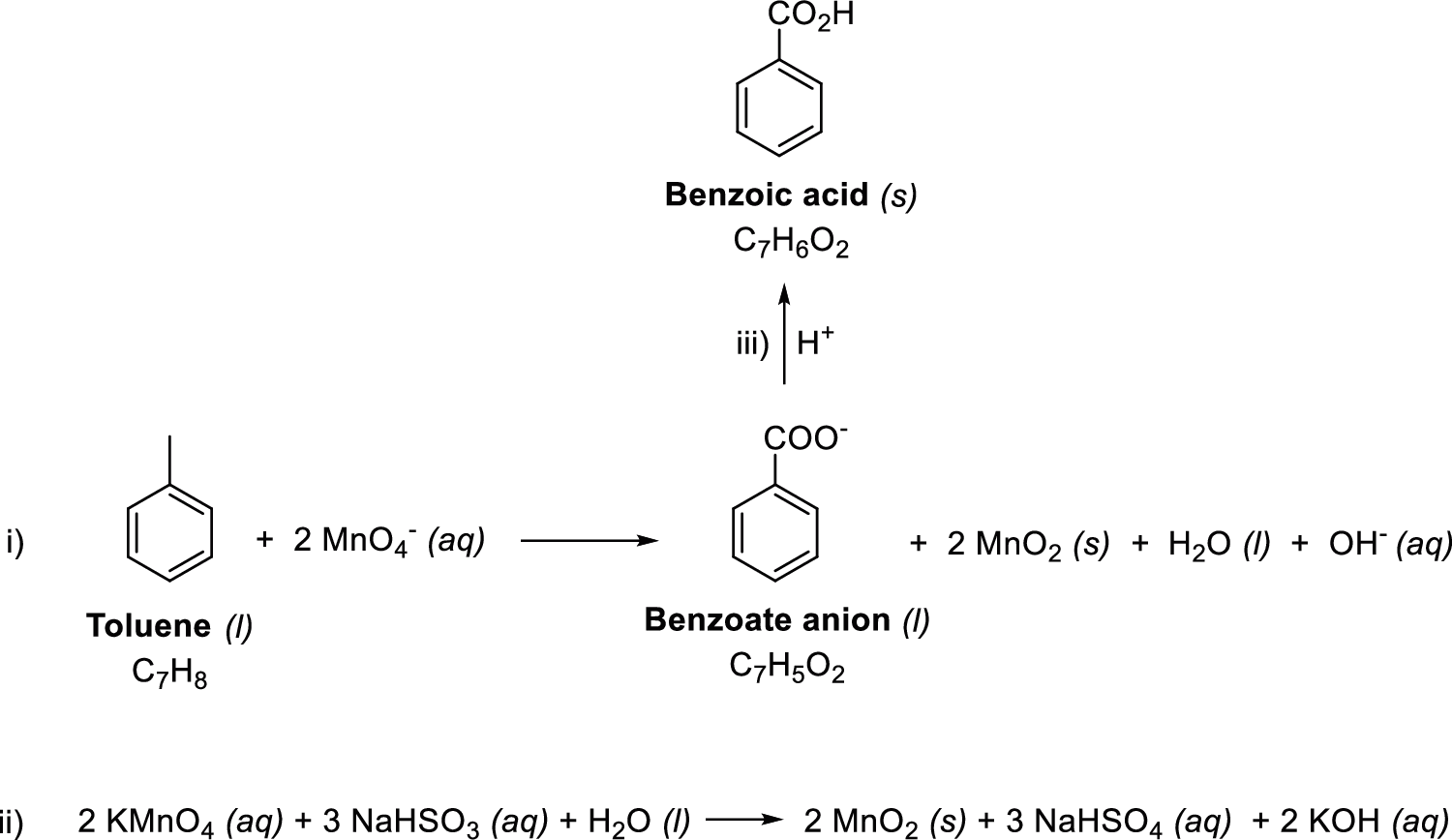
Figure 1: Chemical reaction equations: Oxidation of toluene to benzoic acid, and work-up equations
3 Design of VR Organic Chemistry Experiments
The proposed system provides experimental procedures that learners can use in their experiments by taking a close look at the reaction scheme and checking the reagents to be used. Such a reaction scheme allows users to interact with 3D objects and choose the reagent with an apparatus preparation to set a reaction. The users can run, monitor, and check the reaction. Finally, they can stop the reaction, purify and isolate the molecule, identify its structure, and analyze the results, as shown in Fig. 2.
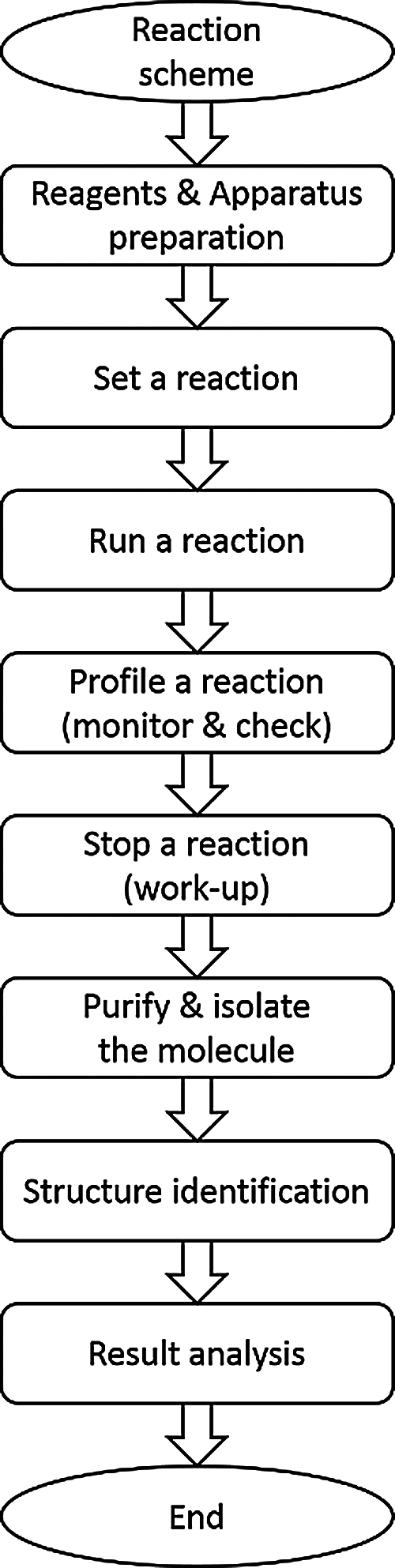
Figure 2: System overview
Fig. 2. shows an overview of the workflow of the proposed chemical reaction simulation scheme, and Fig. 3. illustrates the architecture of the experimental system, showing the main software and hardware platforms. The prototype implementation was based primarily on the Unity 3D platform, and used the functionality of the Unity Asset Store to create models and interactions. Once an application is published through the Oculus platform, users can run the application directly on an Oculus rift device. Then, users interact with the VR equipment according to its operating procedures. In this prototype, users were able to perform a series of basic interactions using the Oculus rift controller. The users were able to enter a simulated 360-degree 3D scene by wearing a VR headset. The Oculus Rift device is capable of tracking the user’s head in 3D. Therefore, the users can intuitively perceive a 3D space by moving their head, and the VR user’s device display can be synchronized with a computer.
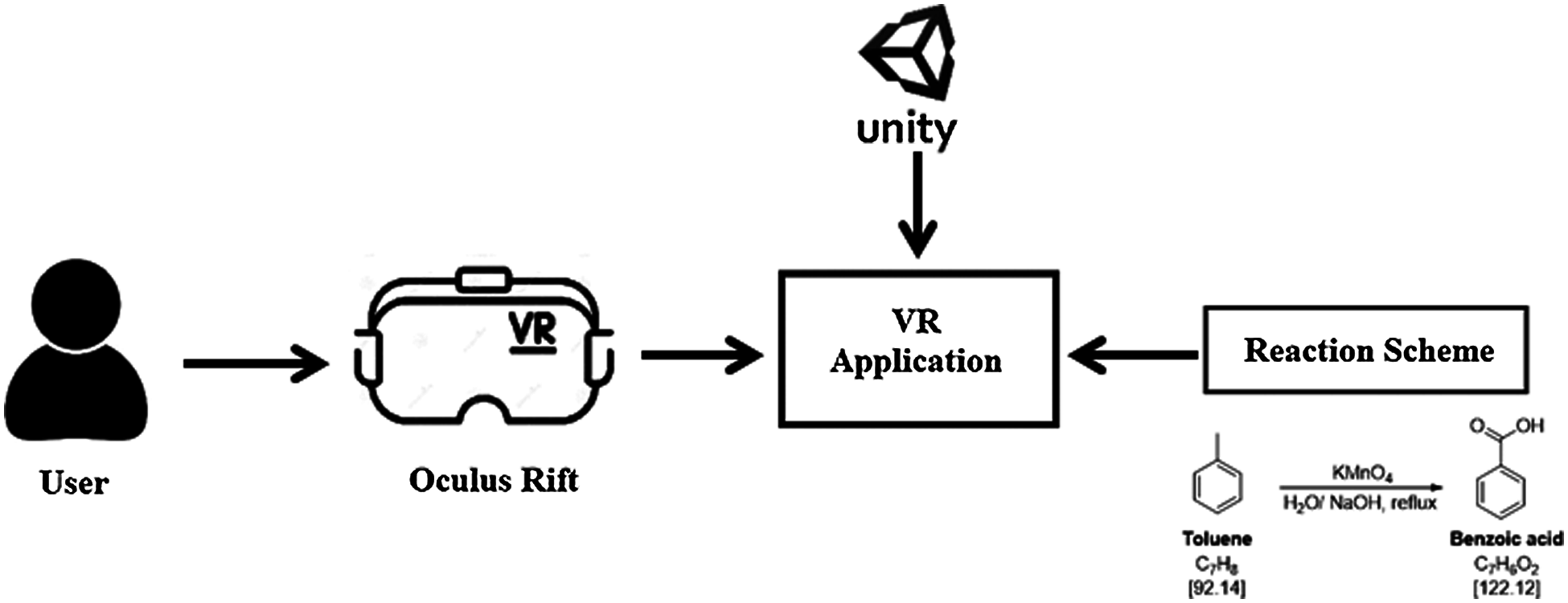
Figure 3: VR system architecture
3.2 VR Organic Chemistry Lab Scene
Fig. 4 below shows an example of the interior design features displayed in the virtual chemical laboratory; (a) shows an exterior perspective of the virtual laboratory and (b) provides a first-person view of the interior setting. The model was mainly imported from the Unity Asset Store, so as to simulate the real world as closely as possible. The developed system adopted a first-person camera perspective setting to deepen the users’ perceptions of immersion in the virtual environment.

Figure 4: VR environment (a) virtual laboratory (b) interior setting
After developing some VR 3D scenes, we have created the VR organic chemistry lab environment in which users can choose an experiment. The scene looks like a simple, real laboratory with an organic chemistry laboratory equipment set on the table, as shown in Fig. 5. where (a) shows the chemical apparatus or 3D models for interaction with a monitor, and users can watch an organic chemical scenarios video before the experiment; (b) shows the user guidelines or chemical reaction schemes that users can read before testing; (c) shows how to mix chemistry into the flask that contains the stirring bar, and (d) shows how to start the reaction by turning on the stirring switch and heating switch.
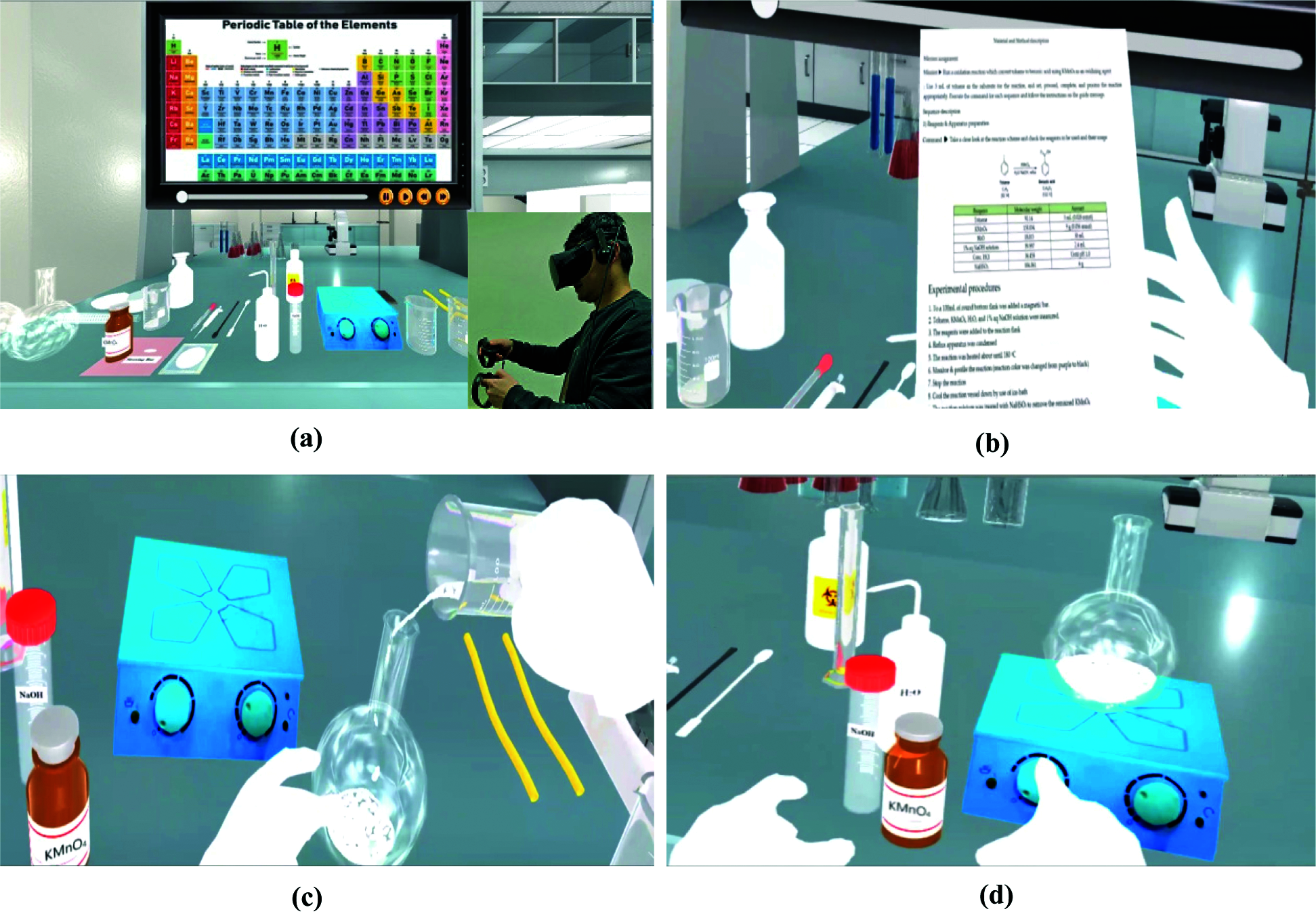
Figure 5: Laboratory table
The experimental environment for this study is an Intel(R) Core(TM) i5-8400T CPU @ 1.70 GHz, 16 GB RAM, and Windows 10 (version 2019) Personal (64 bit) of the Unity 3D development engine. The experiment is to run an oxidation reaction that converts toluene to benzoic acid using KMnO4 as an oxidizing agent. The learners should use 3 mL of toluene as the substrate for the reaction and set, proceed, complete, and process the reaction appropriately. Fig. 6. shows the experimental procedures to which users can refer during the experiment: the reaction scheme, reagents to be used, and their usage.
This experiment was performed in a physically touchable space in the laboratory of Bio-Health Materials Core-Facility in Jeju National University with the use of the corresponding spectroscopic analytic apparatus. Running the reaction requires high temperatures, handling a flammable liquid, and the highest concentration of the hydrochloric acid solution.
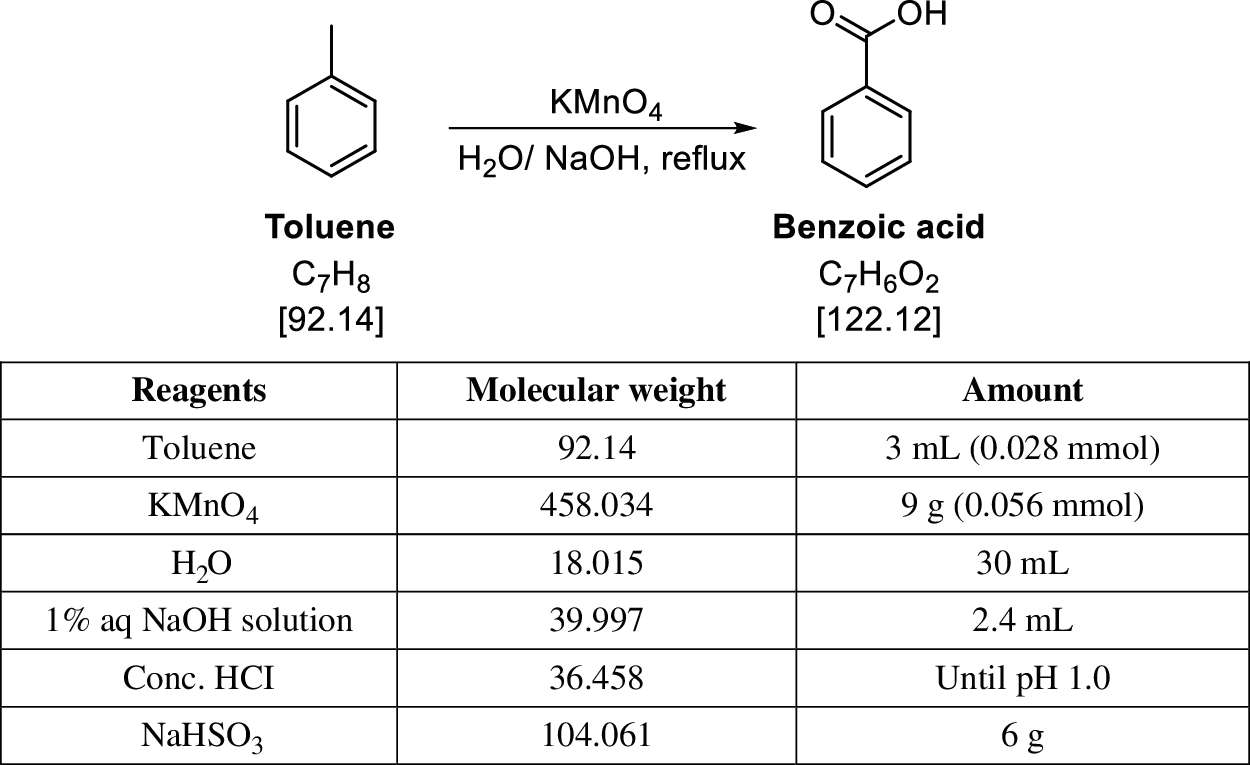
Figure 6: Reagents & apparatus preparation
Experimental procedures:
1. Add a magnetic bar to a 100 mL round bottom flask.
2. Measure toluene, KMnO4, H2O, and 1% NaOH(aq) solutions.
3. Add the reagents to the reaction flask.
4. Condense the reflux apparatus.
5. Heat the reaction until approximately 180oC.
6. Monitor & profile the reaction (the reaction color changes from purple to black).
7. Stop the reaction.
8. Cool the reaction vessel down by use of ice-bath.
9. Treat the reaction mixture with NaHSO3 to remove the remaining KMnO4.
10. Remove the precipitated solid (MnO2) using filtration.
11. Collect the filtrate (product candidate) and acidify it with concentrated HCl.
12. Filter the generated solid under reduced pressure. Wash it several times in water to remove impurities.
13. Acquire and isolate the solid. Dry it.
14. Confirm the structure by NMR analysis.
15. Calculate the chemical yield of the reaction.
This section describes the evaluation of the VR organic chemistry laboratory. This experiment was developed for students studying organic chemistry or those involved in chemical experiments. In an educational environment, students must achieve the following goals:
1. To understand chemical experiments, learn to observe and record experimental phenomena, and practice basic chemical research methods.
2. To use the interaction between practical results and the model to observe and understand chemical structures from different angles.
3. To imagine the difference between virtual and real objects.
4. To practice and be tested in VL before real-world operations to ensure safety, especially in the case of hazardous substances and valuable reagents, when the experimental process is replicated in a VL.
5. To motivate students’ interest in chemistry and increase their impression of chemical reactions.
6. To cultivate students’ scientific attitudes toward the facts, objectives of the experiment, and being careful and strict in discipline.
We selected 42 students of the chemistry department of Paichai University as experimental subjects (See Fig. 7). Two types of users or students and teachers were divided into two groups, an experimental group and a reference group, with 21 members in each group (not separated by gender). The experimental group used the Oculus HMD, and the reference group also used the Oculus HMD after observation. Two chemistry teachers participated in the evaluation. Before the evaluation, all subjects were familiarized with the equipment and its operation under the guidance of the teacher.

Figure 7: Student immersive experience in VR chemical reaction simulation
4.2 Experimental Results and Discussion
For the evaluation, we prepared reference questionnaires in discussions with those who used this system to explore students’ conceptual knowledge and immersion. This study involved a survey, interviews, and quantitative data collection to investigate students’ attitudes toward the use of VR chemical reaction simulation. The results show the students’ opinions regarding their performance after using this system, focusing on control scenes through the use of VR applications. In total, 42 students (including 2 teachers) participated in this simulation and answered the questionnaires or surveys. The questionnaire used a 5-point Likert scale: 5 for “strongly agree” (4.50–5.00 during the evaluation), 4 for “agree” (3.50–4.49), 3 for “undecided” (2.50–3.49), 2 for “disagree” (1.50–2.49), and 1 for “strongly disagree” (1.00–1.49). Tab. 1. shows the results of survey responses and quantitative data analysis.

In Tab. 1. the mean score 3.73 (“agree”) indicates that the surveyed users or students found that the VR organic chemistry reaction scene and the interface were easy to understand. The mean scores 3.83 and 3.78 (“agree”) indicate that the students found 3D object manipulation to be easy and intuitive to use, with a high interaction accuracy. The mean scores of 4.04 and 4.45 (“agree”) indicate that the students found the VR application useful in learning chemistry and improving their learning skills. The mean scores of 4.57 and 4.52 (“strongly agree”) conclusively prove that in the VR organic chemistry reaction scene, the 3D models were beautiful, clear, and realistic, and the VR organic chemistry simulation was enjoyable and engaging for the students. In addition, the mean score 3.95 (“agree”) indicates that students found that the organic chemistry experiments in a VL and interactions with 3D objects using Oculus HMD and a hand controller were better than those with keyboard and mouse controls but also caused dizziness. We also expanded the results from five perspectives corresponding to five dimensions including hardware, immersion, education, interaction, and time required for a user sufficiently to familiarize themselves with the use of the devices, as shown in Tab. 2 below.
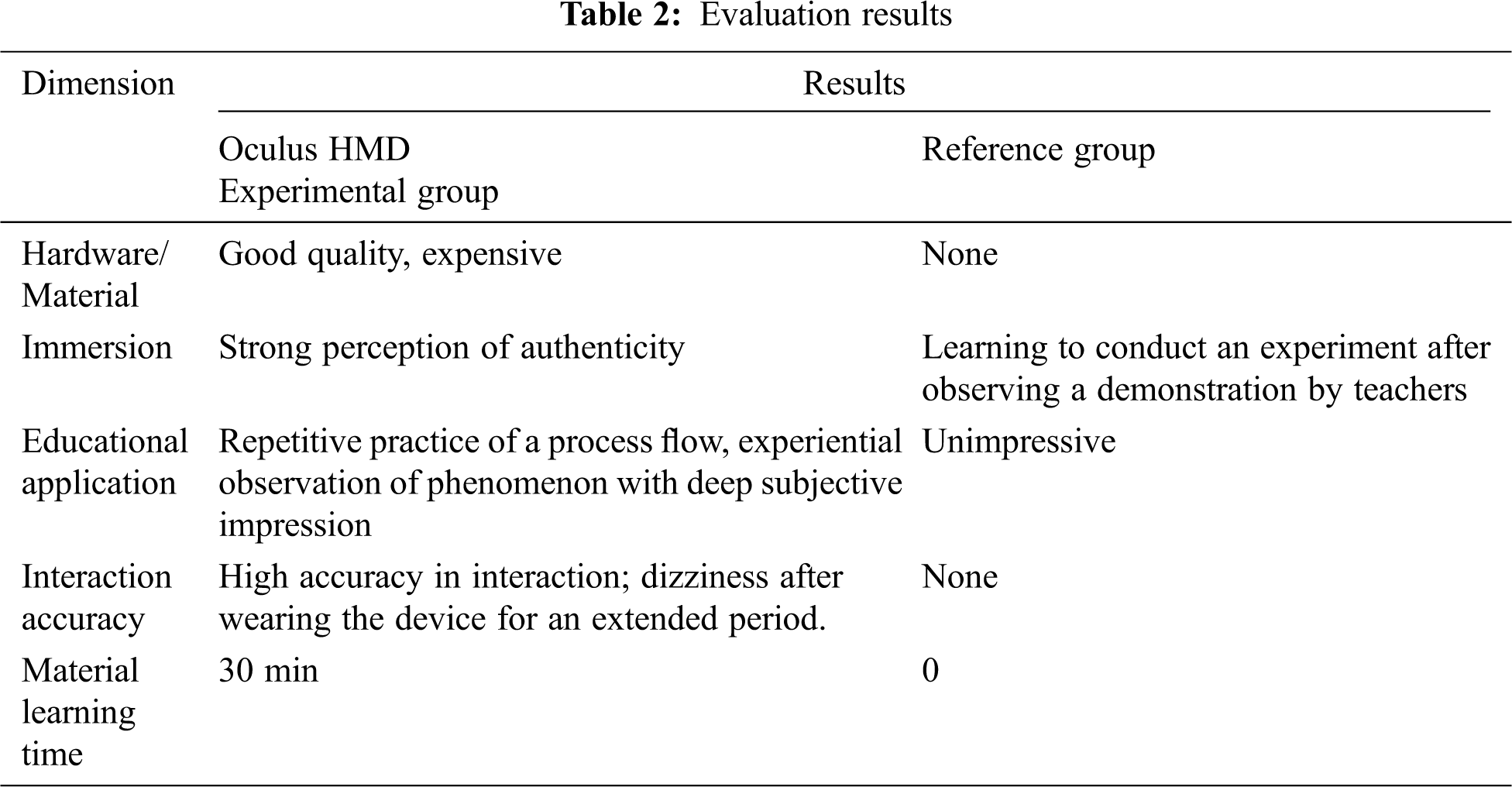
The results shown in Tab. 2 were obtained from scientific questionnaires and interviews conducted with students, and respondents were invited to discuss their experiences and perceptions after participating in the VR chemistry reaction laboratory simulation.
We have proposed new techniques and tools for creating visual and interactive organic chemistry experiments. The experimental design and chemical equipment in this study are based on the basic theory of organic chemistry. Related materials were implemented in 3D models to perform the experiments in a VR system, in which chemical reactions were simulated by the mixing of chemicals. Moreover, we have used surveys, interviews, and quantitative data to investigate students’ attitudes toward the use of VR organic chemical reaction simulation. We believe that simulating laboratory circumstances through a VR system can improve students’ learning outcomes in experimental chemistry. This sort of convergence research is expected to provide significant benefits to students. They are free from the potential dangers of real laboratory environments. Unintended exposure to chemicals caused by inexperience has sometimes involved large and small safety accidents. Students running VR simulations can perform interesting experiments repeatedly without any consumption of real compounds. Therefore, VR can be adopted as an easy-to-use educational tool. Experimental mistakes can be easily corrected, and students can acquire high-level competencies through this opportunity. Although some students have mentioned discomfort, such as dizziness, we plan to address it in future work.
Funding Statement: This work was supported by the Research Grant of Jeju National University in 2021.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. W. Tarng, Y. J. Lin and K. L. Ou, “A virtual experiment for learning the principle of Daniell cell based on augmented reality,” Apply Science, vol. 11, no. 2, pp. 726–786, 2021. [Google Scholar]
2. J. T. Bell and H. C. Fogler, “The application of virtual reality to (chemical engineering) education,” IEEE Virtual Reality, pp. 217–218, 2004. [Google Scholar]
3. W. Zhang, “Research on chemical reaction simulation platform based on animation model,” Chemical Engineering Transactions, vol. 59, pp. 649–654, 2017. [Google Scholar]
4. S. A. Awadhi, N. A. Habib, D. A. Murad, F. A. Deei, M. A. Houti et al., “Interactive virtual reality educational application, Advances in Science,” Technology and Engineering Systems Journal, vol. 3, no. 4, pp. 72–82, 2018. [Google Scholar]
5. M. O’Conner, J. B. Simon, M. D. Helen, J. B. Alexander, J. J. Alex et al., “Interactive molecular dynamics in virtual reality from quantum chemistry to drug binding: An open-source multi-person framework,” Journal of Chemical Physics, vol. 150, no. 22, pp. 220901, 2019. [Google Scholar]
6. M. Suleman, K. H. Sugiyarto and J. Ikhsan, “Development of media three-dimensional (3D) visualization using virtual reality on chemistry education,” ICREMS 6, Journal of Physics: Conference Series, vol. 1397, no. 1, pp. 012034, 2019. [Google Scholar]
7. Y. Tehreem and T. Pfeiffer, “Immersive virtual reality training for the operation of chemical reactors,” in Lecture Note in Informatics (LNI), 2020. [Google Scholar]
8. F. S. Silva, T. Alves, J. Braz and S. Piccara, “Studying chemical molecular structures through gaming,” Proceedings of the 4th Int. Conf. on Computer Supported Education, vol. 1, pp. 506–516, 2012. [Google Scholar]
9. E. P. Harry, “How should chemistry educators respond to the next generation of technology change?,” Journal of Education Science, vol. 10, no. 2, pp. 34, 2020. [Google Scholar]
10. J. B. Simon, E. R. Kara, D. Helen, E. G. Heather, J. M. Adrian et al., “Teaching enzyme catalysis using interactive molecular dynamics in virtual reality,” Journal of Chemical Education, vol. 96, no. 11, pp. 2488–2496, 2019. [Google Scholar]
11. H. Jinkun, T. Yifei, S. Wei and F. Simon, “An implementation of VR chemistry experiment system,” Proc. of Int. Conf. on Big Data and Internet of Thing, pp. 205–208, 2017. [Google Scholar]
12. I. B. Bosede, S. B. Kevin, F. P. Rui and D. C. Adrian, “Haptic virtual reality and immersive learning for enhanced organic chemistry instruction,” Virtual Reality, vol. 23, no. 4, pp. 363–373, 2019. [Google Scholar]
13. A. Numan, U. Sehat, A. Aftab and R. Jumal, “3D interactive virtual chemistry laboratory for simulation of high school experiments,” Int. Conf. on Computer Graphics, Animation and Gaming Technologies, EURASIA Turkey, 2014. [Google Scholar]
14. F. Aliyu and A. T. Corrienna, “Virtual chemistry laboratory: A panacea to problems of conducting chemistry practical at science secondary schools in Nigeria,” International Journal of Engineering and Advanced Technology (IJEAT), vol. 8, no. 5C, pp. 544–549, 2019. [Google Scholar]
15. V. K. Kolil, S. Muthupalani and K. Achuthan, “Virtual experimental platforms in chemistry laboratory education and its impact on experimental self-efficacy,” International Journal of Educational Technology in Higher Education, vol. 17, no. 1, pp. 117, 2020. [Google Scholar]
16. J. A. N. Sabah, M. A. N. Ammar, A. Nawaiseh and M. A. Emad, “The impact of using augmented reality on the developing the tenth graders motivation towards learning: An applied study on the chemistry courses,” European Journal of Business and Management, vol. 12, no. 15, pp. 118–122, 2020. [Google Scholar]
17. K. Kounlaxay, Y. S. Shim, S. J. Kang, H. Y. Kwak and S. K. Kim, “Learning media on mathematical education based on augmented reality,” KIIS Transactions on Internet and Information Systems, vol. 15, no. 3, pp. 1015–1029, 2021. [Google Scholar]
18. M. Frevert and D. S. D. Fuccia, “Possibilities of learning contemporary chemistry via virtual reality,” World Journal of Chemical Education, vol. 9, no. 1, pp. 1–7, 2021. [Google Scholar]
19. E. Neresian, A. Spryszynski and M. J. Lee, “Integration of virtual reality in secondary STEM education,” 2019 IEEE Integrated STEM Education Conf. (ISECpp. 83–90, 2019. [Google Scholar]
20. D. Xiaoyun, S. J. Kang, J. I. Choi and S. K. Kim, “Mixed reality system for virtual chemistry lab,” KIIS Transactions on Internet and Information Systems, vol. 14, no. 4, pp. 1673–1688, 2020. [Google Scholar]
21. A. D. Olmo, J. Calzada and M. Nuñez, “Benzoic acid and its derivatives as naturally occurring compounds in foods and as additives: Uses, exposure, and controversy,” Critical Reviews in Food Science and Nutrition, vol. 57, no. 14, pp. 3084–3103, 2015. [Google Scholar]
22. P. Chand, Y. S. Babu, S. Bantia, N. Chu, L. B. Cole et al., “Design and synthesis of benzoic acid derivatives as influenza neuraminidase inhibitors using structure-based drug design,” Journal of Medicinal Chemistry, vol. 40, no. 25, pp. 4030–4052, 1997. [Google Scholar]
23. A. Shaabani, F. Teimouri and D. G. Lee, “Ion exchange catalysis in oxidation of organic compounds with KMnO4,” Synthetic Communications, vol. 33, no. 6, pp. 1057–1065, 2003. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |