DOI:10.32604/iasc.2021.017453

| Intelligent Automation & Soft Computing DOI:10.32604/iasc.2021.017453 |  |
| Article |
Strategies for Reducing the Spread of COVID-19 Based on an Ant-Inspired Framework
Information Systems Department, College of Computer Sciences, Najran University, 1988, Saudi Arabia
*Corresponding Author: Ghassan Ahmed Ali. Email: alhabeb@gmail.com
Received: 30 January 2021; Accepted: 01 May 2021
Abstract: Many living organisms respond to pandemics using strategies such as isolation. This is true, for example, of social insects, for whom the spread of disease can pose a high risk to colony survival. In light of such behaviors, the present study investigated a different way of developing strategies to mitigate the effects of the coronavirus pandemic. Specifically, we considered the strategies ants use to handle epidemics and limit disease spread within colonies. To enhance our understanding of these strategies, we explored ants’ social systems and how they specifically respond to infectious diseases. The early warning threshold system reflects the importance of building mechanisms and making early decisions to enable appropriate actions to be taken to control the spread of disease and develop alternative plans. Determining thresholds is a complex process with several factors related to decision-making. For example, reliable warnings about pathogens among ants require the defense system to allocate more members to more valuable resources. The effectiveness of ants’ disease-control strategies is rooted in behaviors such as social coordination between members, organizational immunity, disease-outbreak reduction, behavioral plasticity, and risk-threshold determination. Understanding such behaviors among ants could inspire the development of new strategies for limiting the spread of COVID-19 as well as future pandemics.
Keywords: COVID-19; ant framework; artificial intelligence; prediction model
In March 2020, the World Health Organization declared the COVID-19 outbreak a pandemic [1]. While some countries implemented various disease-control strategies (ranging from stringent lockdowns to more permissive approaches), others focused more on containment. Nevertheless, a failure to determine the correct mechanisms caused COVID-19 to worsen in many places. Thus, selecting the right disease-control strategies plays an important role in stemming outbreaks and estimating their prevalence [2]. It is clear that confusion persists regarding the best strategies for combating COVID-19, and proper management strategies are still urgently needed [3]. Such strategies mainly aim to reduce disease spread rather than target the disease itself [3].
Among the many COVID-19 response strategies, artificial intelligence (AI) has been widely used as an early warning system to detect outbreaks, shape the healthcare structure, model public health strategies, provide faster decision-making for healthcare authorities, and trace the contacts of infected people [4]. Deep learning, computer vision, and machine learning are useful for monitoring social distancing, which can greatly reduce infection rates.
Conventional approaches such as active surveillance and case isolation can be useful containment strategies for COVID-19. However, some strategies have proven ineffective in certain places or have been ignored in others. There is no conclusive evidence regarding which approaches are the best and how to effectively implement them. Factors such as pandemic severity and healthcare-system capacity often determine response strategies. In this regard, examining insect behavior could provide insight into developing more effective strategies. There are, indeed, similarities between social insects and human societies, and both groups are subject to disease outbreaks [5].
Social insects have a suite of defense mechanisms and diverse strategies they use to prevent or reduce infectious disease. These include disease detection and estimating the effect of disease on all colony members. Such strategies could provide guidance for combating disease in human societies [6–9]. Relatedly, Pull et al. [10] noted that exploring insect defenses against pathogens could help researchers to better understand complicated immune systems. It has been suggested, moreover, that human models can be improved by exploring insect organisms through tuning or mimicking insect models [11]. In particular, an epidemiological model of human society could be developed by simulating how insect societies reduce disease [7,12]. In short, exploring insect societies and their behavioral mechanisms and responses related to containing disease could provide new approaches for human societies.
The present study focused specifically on ant colonies to understand behaviors they use to combat disease. These behaviors have been shown to be unique, highly adaptive, successful, and capable of preventing future disease [10,13]. Such effectiveness is based on features such as social coordination between members, organizational immunity, disease-outbreak reduction, behavioral plasticity, and risk-threshold determination [13–19]. Therefore, ant research could prove useful for formulating strategies to combat COVID-19. To this end, this study analyzed natural phenomena related to disease-prevention strategy. Fig. 1 illustrates the way an ant-inspired framework could be used to control COVID-19.
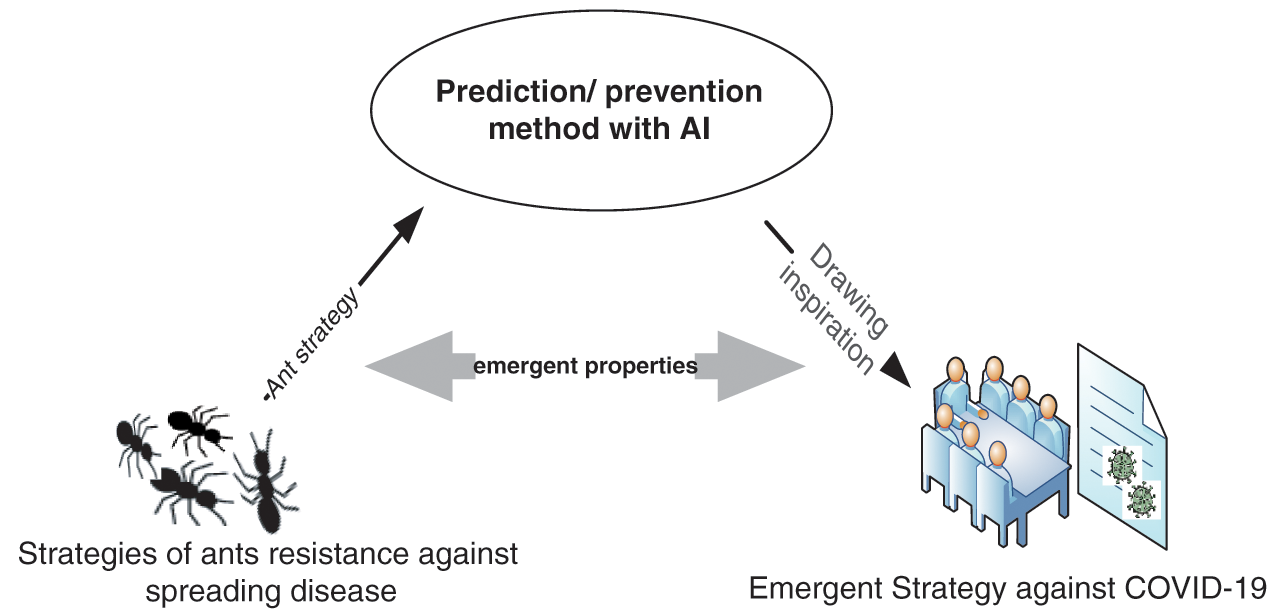
Figure 1: Ant-inspired framework for controlling COVID-19
The rest of this paper is organized as follows. Section 2 describes ant strategies used to combat disease, including proactive strategies and thresholds after event detection. Section 3 presents the principles of strategies used to combat disease—namely, avoidance strategy, organizational immunity strategy, and fault tolerance. Section 4 concludes the paper and discusses the limitations and future work.
2 Strategies Ants Use to Combat Disease
The proactive strategy for disease detection helps ant colonies control disease before it spreads. Colony members prioritize colony preservation and prophylactic activities against pathogens [20]. Early, reliable warnings about pathogens among ants are essential for detecting disease and reducing new infections [21]. The characteristics of proactive-stage, prepandemic preparation include the following:
• Division of labor, in which detection tasks are managed by different functions.
• Cleaning up colonies, which can reduce pathogens and prevent diseases from reaching places where they could spread. Undertaking environmental hygiene at different times can positively affect survival and reduce disease [22].
• Aside from monitoring mechanisms, specific plans and actions are important for disease prevention in ant colonies.
2.2 After Detection of Event R0
Initially, an ant cannot determine the severity of a detected disease, whether it is an accidental disease, a nonmeasurable habitual condition, or a pandemic. Therefore, the ant deals with the event according to its characteristics, as well as the criteria established to determine the severity of the disease. Ant behavior indicates that ants prefer to live with known risks rather than unknown risks [22]. They are not ignorant of dangers, but they have difficulty dealing with pathogens they lack previous experience with.
Ants have developed an early warning system to detect unknown pathogens based on the features of the detected disease [23–25]. Early detection of pathogens is critical for colony survival, despite its costs in terms of time and energy [14]. The early warning threshold is characterized by adjustable reactions to a disease according to severity, morbidity, and colony fitness [26]. Ants react to a disease according to infection records, including age (susceptibility to infection), infection history, and risk adjustment [26]. Determining thresholds is a complex process with several factors related to decision-making [14]. Multiple parties—not just one—are empowered to determine the risk and decide when and where to take specific actions. Likewise, when it is difficult to avoid risks, decisions are made based on priorities, and sorting is undertaken based on short-term effects. In this context, it is important to consider external and internal factors in terms of the nature of the disease and how many are infected or at risk of infection.
The estimated risk threshold of the early warning system may differ based on the frequency of events, the number of infected members, and the effect of events on the environment, as follows:
• High risk: characterized by common events or unusual behavior among the majority of individuals that will cause serious damage or destroy the environment.
• Medium risk: characterized by common events or unusual behavior among the majority of individuals that may cause damage to the environment.
• Low risk: characterized by very rare events or unusual behavior among a few individuals that will not cause damage to the environment.
The aspects of ant thresholds that can be used to determine a COVID-19 threshold are as follows:
- The importance of early identification and disclosing whether new viruses have been detected anywhere in the world. An early warning system will enable appropriate actions to be taken to control the spread of disease and develop alternative plans [27].
- Early warning failure may cause the rapid spread of a disease, as with COVID-19 [28,29]. Safety violations should be disclosed to the public [30]. Rapid recognition and prognosis are crucial to combating the spread of a disease. Early warning and quick reaction, as seen in ant colonies, can limit disease spread.
- The collaboration and communication seen in ant colonies can integrate management structures and facilitate collaboration between several parties. In the human context, parties such as health and research centers, organizations, and governments can collaborate to more effectively respond to epidemics without wasting resources. Policies related to risk determination can be derived from such integration to move from short-term priorities to long-term planning.
Fig. 2 shows the thresholds of an early warning system with regard to decision-making for strategy implementation.
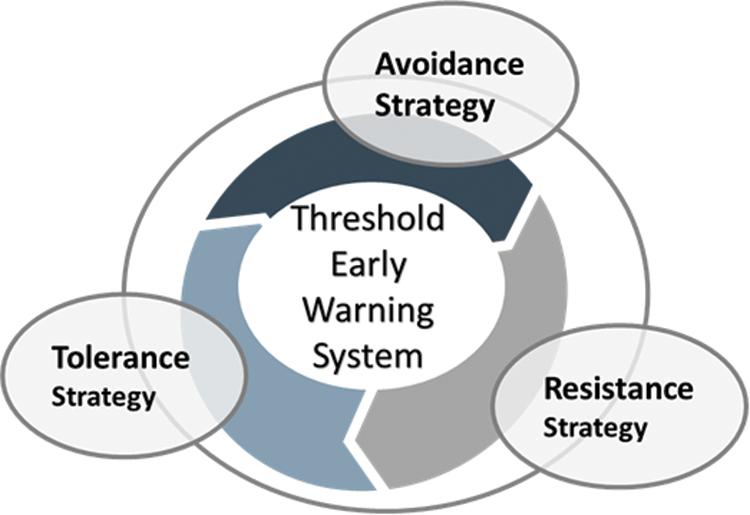
Figure 2: Thresholds of early warning system strategies
3 Principles of Strategies for Combating Disease
Ants try to reduce risk by implementing an avoidance strategy as a first line of defense. This strategy includes the following:
• Avoiding (with strict compliance) visiting areas with high pathogen density or hotspots of parasites [8].
• Isolating infected members in a special zone and encouraging others to stay away [31].
• Limiting and adjusting contact between infected and noninfected members [32,33]; noninfected members should not touch infected members but shield them and warn others not to come close [17,32].
• Infected members typically become aware of risk, and their changed behavior increases their chances of survival [17,34].
Fig. 3 shows the characteristics of the avoidance strategy.
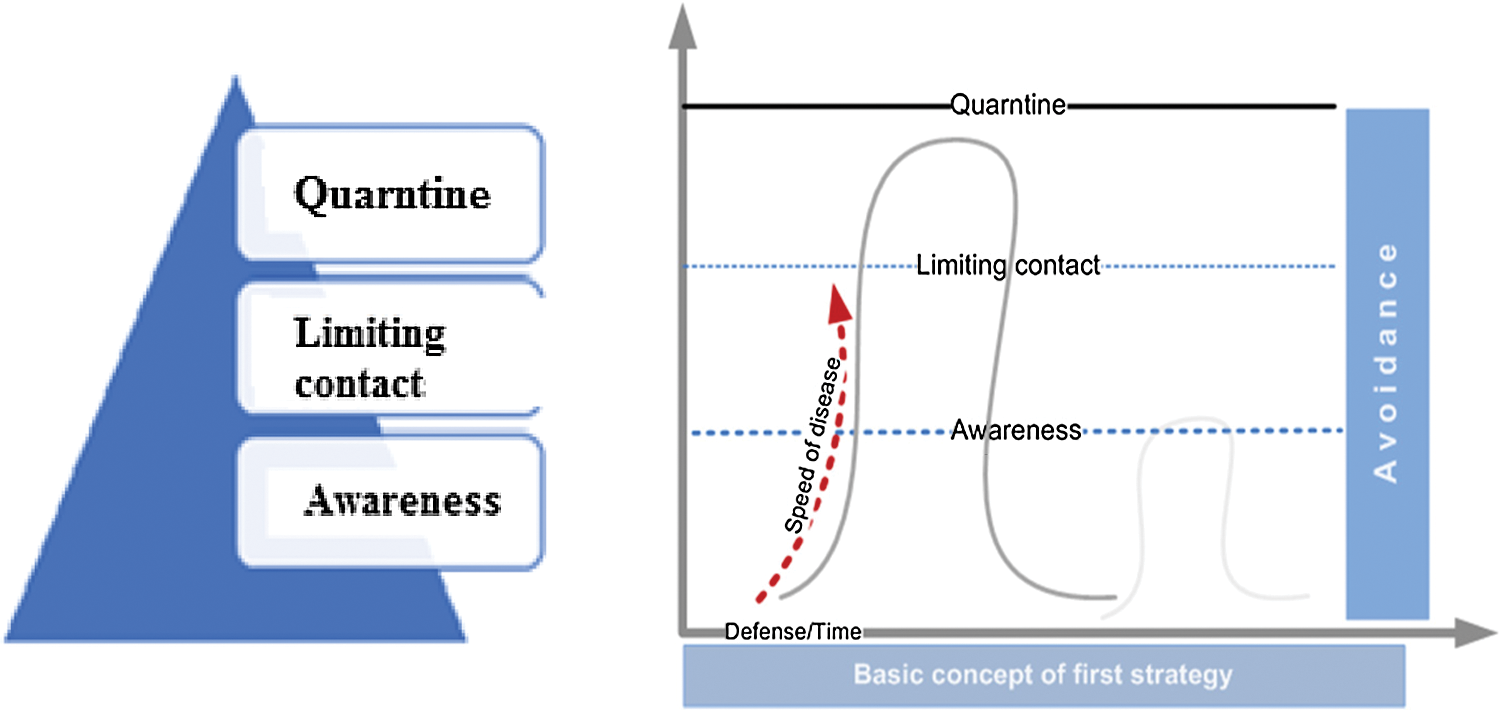
Figure 3: The avoidance strategy
Yet, the spread of disease (among other factors) makes implementing the avoidance strategy difficult and not universally effective, especially when a disease has successfully passed the first line of defense. Therefore, using another strategy is preferred [10].
3.2 Organizational Immunity Strategy
The second strategy ants use to reduce disease transmission is changing the contact structure and the interaction network structure. Ants suppress epidemic risk by changing social connections within the colony [15]. Altering these “rules” of the communication network is an important factor that helps reduce disease. In this stage, ants try to break the pathogen life cycle by keeping it from infecting new members [10,35]. The aspects of the organizational immunity strategy that mitigate disease outbreaks are as follows:
- Intensive care and surveillance of members at high risk [35].
- Reducing the duration of contact [33]. Duration is a critical factor compared to contact alone. In this strategy, duration is minimized as much as possible.
- Spatial compartmentalization and physical separation [19]. This trait can limit movement and restrict mobility, thus reducing the overall infection rate.
- Behavioral structuring [36]. All members should adjust their behavior according to the risk that may threaten the whole colony [10].
Fig. 4 shows the characteristics of the organizational immunity strategy.
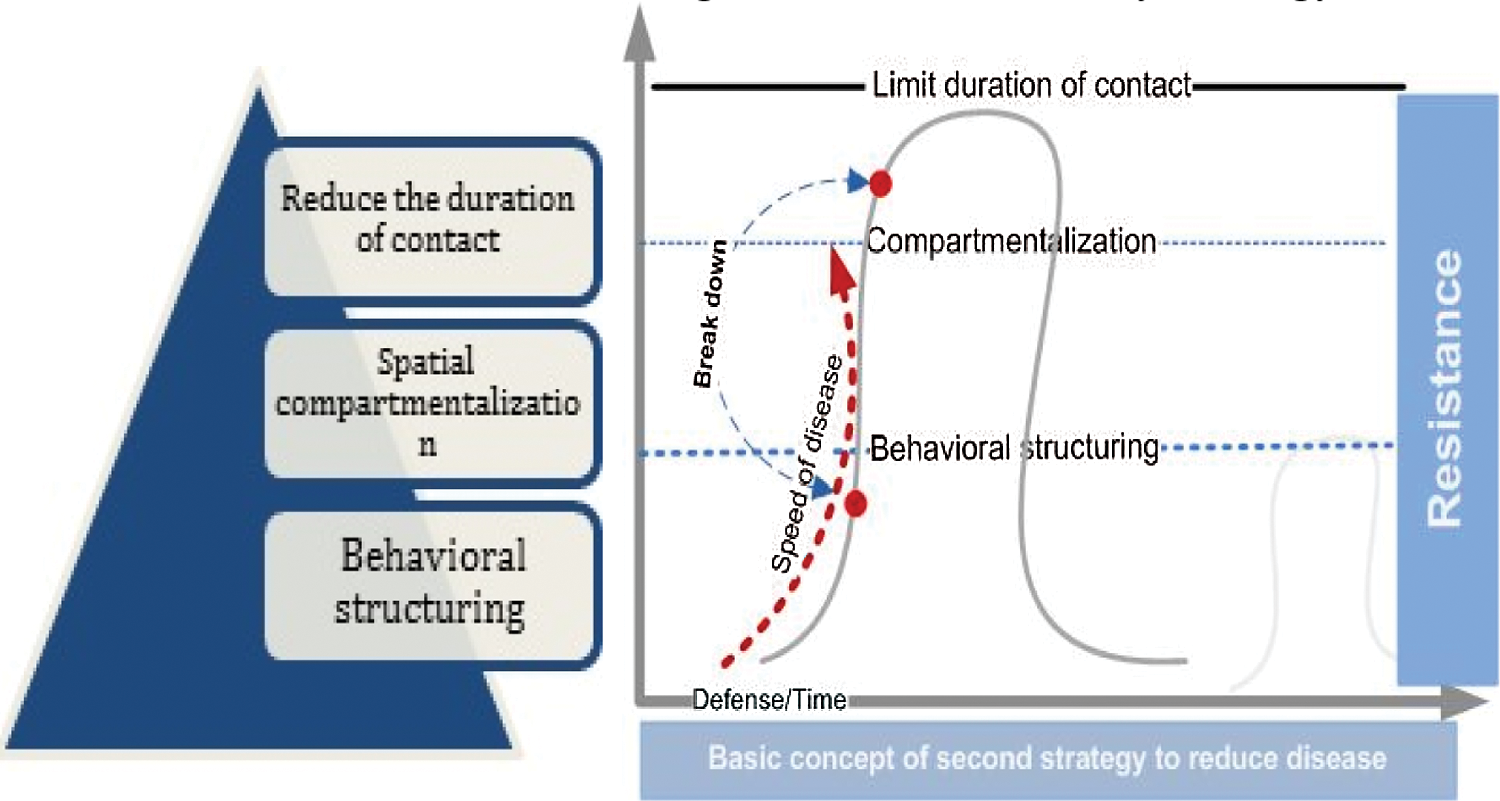
Figure 4: The organizational immunity strategy
Fault tolerance has to do with keeping a system working and continuing processes, even after the failure of certain components [37]. This is the third strategy ants use to flatten the disease curve [35]. If the disease cannot be completely removed, the aim is to at least limit its effects. This strategy may alleviate pathologies, depending on understanding how the disease works and how to cope with its negative effects [38]. Fault tolerance strategies can limit a disease’s effects without causing the whole system to fail. Here, strategies are adopted that focus on reducing risks rather than directly resisting the disease [39]. Fig. 5 shows the characteristics of the fault tolerance strategy.
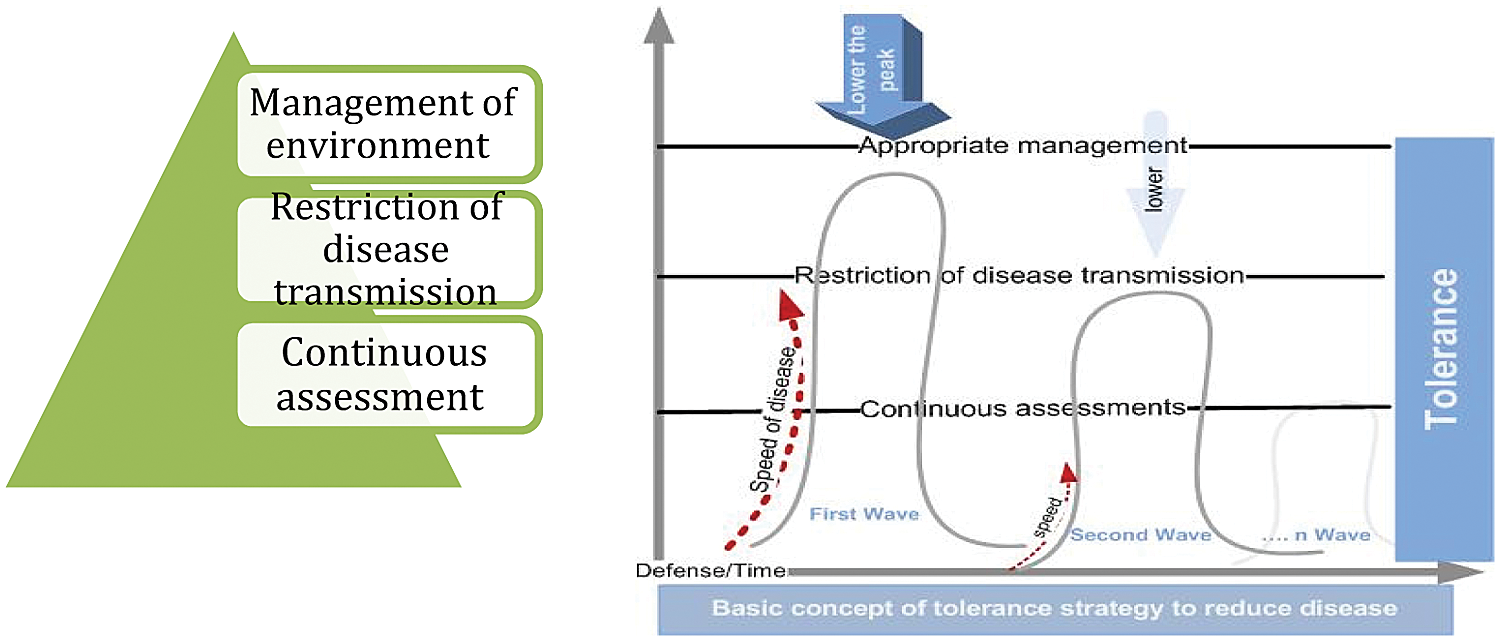
Figure 5: The fault tolerance strategy
In this stage, the success of limiting morbidity and mortality depends on preventing transmission in terms of the following:
• Appropriate management of the environment [40], especially where disease hotspots are detected (i.e., “source reduction”).
• Limiting disease expansion to new areas.
• Tight restriction of disease transmission between members.
• Continuous assessment of the effect of each phase.
The fault tolerance strategy aims to reduce risks, recover overall tasks in the environment, and effectively respond to changes in the environment. Studies have focused on fault tolerance as an important approach to reducing the spread of COVID-19.
3.4 Ant Strategies and Security Policy
COVID-19 has given rise to privacy and security issues in areas such as information collection and data protection. With regard to computer security, security policy is at least as important as security countermeasures. Security policy provides a framework for making decisions, such as which defense mechanisms to use. Security policy establishes the rules by which a system must operate—what is allowed and not allowed—and without it, security development can be ambiguous and unfocused. At the most basic level, security policies partition the states of a system into a set of authorized or secure states and unauthorized or insecure states [41].
In ant colonies, the “security policy” architecture begins by identifying rules and guidance regarding security issues. The “security policy” pertains to protecting either the colony and its contents or the rights of individual members. Security behavior involves distributing tasks based on necessity. Workers are assigned to a group as a member based on certain criteria (e.g., age, strength, priority of assignment). Each group has a specific task. Additional tasks may be assigned based on the requirement that some ants may engage in defense-related tasks, but they also regulate other activities in the colony as additional tasks. Though the main task of guard ants is to identify and detect intruders, they may also recruit other ants to defend against more aggressive intruders [13].
Ants’ security tasks include guarding, monitoring, detection, early warning, and assessment [9]. Although ant members congregate around the queen and focus on her reproductive activities, the colony appears to be controlled by the consensus its workers. An intracolonial hierarchy seems to be responsible for distributing security tasks, and there are primary prerequisites for each task. For example, workers become guards only after performing certain jobs in the colony. There are consequences for security breaches, and if a guard ant is removed, another ant assumes its role [13].
Supervision and surveillance arise in ant colonies after “rules” are designated and tasks are assigned. The main purpose of surveillance is to ensure that the colony system functions properly. Fig. 6a shows the “security policy” architecture in an ant colony and how it works. Fig. 6b shows how an ant colony can guide humans to improve their security policies.
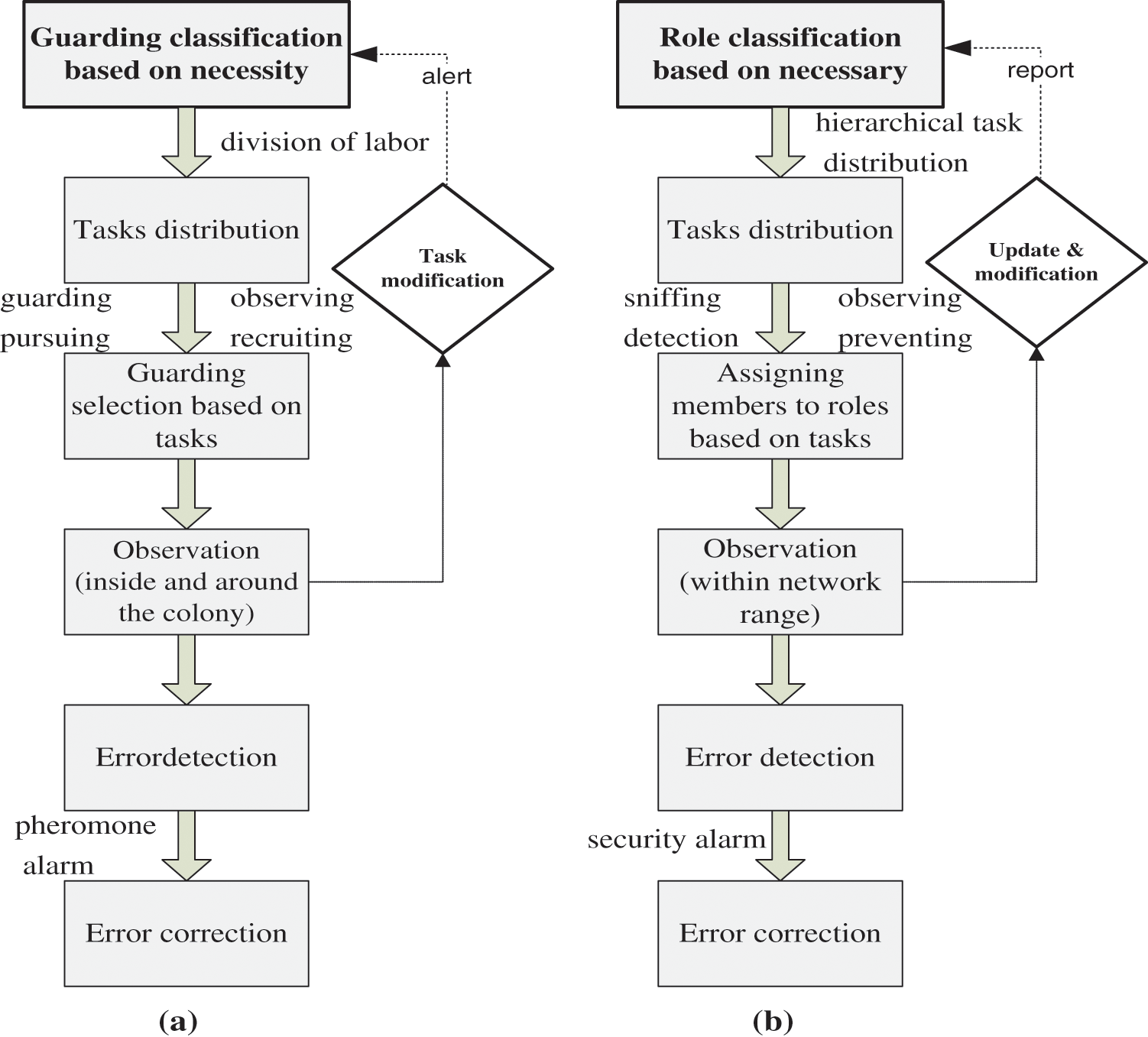
Figure 6: (a) Security policy in ant colonies. (b) Approach to modeling security policy using AI
Many strategies have been proposed or adopted to limit the transmission of COVID-19. The outcomes of these strategies have been widely varied. However, it is generally advantageous to have a strategy as opposed to fighting a pandemic with no strategy at all.
The early warning threshold system found in ant colonies highlights the importance of establishing mechanisms and making early decisions with regard to detecting a disease outbreak. This study investigated three noteworthy disease-defense strategies used by ants. Most of the strategies focus on using physical isolation to reduce disease transmission. Physical distancing can reduce transmission in many cases, including unknown or suspected cases. This study provides new insights, on the basis of ant-colony systems, for defining rules and guidelines that could help improve COVID-19 security policy. More broadly, this study highlights the relevance of studying social insect behaviors for application to human situations. In this regard, the present study may help to explain how ant strategies can be adapted to reduce disease transmission among humans. This could inspire humans to “think outside the box” when searching for effective strategies to deal with current and future epidemics or pandemics.
This study is limited in that it evaluated ant behavior regardless of ant species or classification. Moreover, in some cases, the manner in which ants react to disease is not evident, and some strategies overlap. In other cases, strategies are implemented simultaneously. Future studies can more thoroughly investigate such phenomena.
Funding Statement: The author would like to express his gratitude to the ministry of education and the deanship of scientific research – Najran University – Kingdom of Saudi Arabia for their financial and technical support under code number (NU/ESCI/18/001).
Conflicts of Interest: The author declares that he has no conflicts of interest to report regarding the present study.
1. World Health Organization (WHONovel Coronavirus (2019-nCoVSituation report 1. 21. (Accessed 15 October 20202020. [Online]. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf. [Google Scholar]
2. P. Daszak, K. J. Olival and H. Li, “A strategy to prevent future pandemics similar to the 2019-nCoV outbreak,” Biosafety and Health, vol. 2, no. 1, pp. 6–8, 2020. [Google Scholar]
3. M. A. Shereen, S. Khan, A. Kazmi, B. Nadia and R. Siddique, “COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses,” Journal of Advanced Research, vol. 24, no. 4, pp. 6–8, 2020. [Google Scholar]
4. A. Alamri and S. Alamri, “Live data analytics with IoT intelligence-sensing system in public transportation for COVID-19 pandemic,” Intelligent Automation & Soft Computing, vol. 27, no. 2, pp. 441–452, 2021. [Google Scholar]
5. T. Fountain and W. O. H. Hughes, “Weaving resistance: Silk and disease resistance in the weaver ant Polyrhachis dives,” Insectes Sociaux, vol. 58, no. 2, pp. 453–458, 2011. [Google Scholar]
6. B. L. Hart, “Behavioural defences in animals against pathogens and parasites: Parallels with the pillars of medicine in humans,” Philosophical transactions of the Royal Society of London. Series B, Biological sciences, vol. 366, no. 1583, pp. 3406–3417, 2011. [Google Scholar]
7. S. C. Stearns and J. C. Koella, Evolution in health and disease, New York, NY, USA: Oxford University Press, pp. 195–197, 2008. [Google Scholar]
8. S. Cremer and M. Sixt, “Analogies in the evolution of individual and social immunity,” Philosophical Transactions of the Royal Society B: Biological Sciences, vol. 364, no. 1513, pp. 129–142, 2009. [Google Scholar]
9. J. R. Shorter and O. Rueppell, “A review on self-destructive defense behaviors in social insects,” Insectes Sociaux, vol. 59, no. 4, pp. 1–10, 2012. [Google Scholar]
10. C. D. Pull, L. V. Ugelvig, F. Wiesenhofer, A. V. Grasse, S. Tragust et al., “Destructive disinfection of infected brood prevents systemic disease spread in ant colonies,” Elife, vol. 7, no. e32073, pp. 1–29, 2018. [Google Scholar]
11. S. A. Babayan and D. S. Schneider, “Immunity in society: Diverse solutions to common problems,” PLOS Biology, vol. 10, no. e1001297, pp. 10–12, 2012. [Google Scholar]
12. R. G. Loreto, S. L. Elliot, M. L. R. Freitas, T. M. Pereira and D. P. Hughes, “Long-term disease dynamics for a specialized parasite of ant societies: A field study,” PLoS One, vol. 9, no. e103516, pp. 1–7, 2014. [Google Scholar]
13. C. De Bekker, I. Will, B. Das and R. M. M. Adams, “The ants (Hymenoptera: Formicidae) and their parasites: Effects of parasitic manipulations and host responses on ant behavioral ecology,” Myrmecol. News, vol. 28, no. 1, pp. 1–24, 2018. [Google Scholar]
14. S. Cremer, S. A. O. Armitage and P. Schmid-Hempel, “Social immunity,” Current Biology, vol. 17, no. 16, pp. R693–R702, 2007. [Google Scholar]
15. N. Stroeymeyt, A. V. Grasse, A. Crespi, D. P. Mersch, S. Cremer et al., “Social network plasticity decreases disease transmission in a eusocial insect,” Science, vol. 362, no. 6417, pp. 941–945, 2018. [Google Scholar]
16. C. Kurze, N. E. Jenkins and D. P. Hughes, “Evaluation of direct and indirect transmission of fungal spores in ants,” Journal of Invertebrate Pathology, vol. 172, no. 107351, pp. 1–8, 2020. [Google Scholar]
17. H. Pereira and C. Detrain, “Pathogen avoidance and prey discrimination in ants,” Royal Society open science, vol. 7, no. 191705, pp. 1–16, 2020. [Google Scholar]
18. J. B. Leclerc and C. Detrain, “Impact of colony size on survival and sanitary strategies in fungus-infected ant colonies,” Behavioral ecology and sociobiology, vol. 72, no. 3, pp. 1–10, 2018. [Google Scholar]
19. T. Chouvenc and N. Y. Su, “When subterranean termites challenge the rules of fungal epizootics,” PLoS One, vol. 7, no. e34484, pp. 1–7, 2012. [Google Scholar]
20. L. Diez, P. Lejeune and C. Detrain, “Keep the nest clean: Survival advantages of corpse removal in ants,” Biology letters, vol. 10, no. 20140306, pp. 1–4, 2014. [Google Scholar]
21. L. Pontieri, S. Vojvodic, R. Graham, J. S. Pedersen and T. A. Linksvayer, “Ant colonies prefer infected over uninfected nest sites,” PLoS One, vol. 9, no. e111961, pp. 1–6, 2014. [Google Scholar]
22. N. H. Feffermann, J. F. A. Traniello, R. B. Rosengaus and D. V. Calleri, “Disease prevention and resistance in social insects: Modeling the survival consequences of immunity, hygienic behavior, and colony organization,” Behavioral Ecology and Sociobiology, vol. 61, no. 4, pp. 565–577, 2007. [Google Scholar]
23. R. B. Rosengaus, C. Jordan, M. L. Lefebvre and J. F. A. Traniello, “Pathogen alarm behavior in a termite: A new form of communication in social insects,” Naturwissenschaften, vol. 86, no. 11, pp. 544–548, 1999. [Google Scholar]
24. N. Wilson-Rich, R. J. Stuart and R. B. Rosengaus, “Susceptibility and behavioral responses of the dampwood termite Zootermopsis angusticollis to the entomopathogenic nematode Steinernema carpocapsae. J. Invertebr,” Journal of Invertebrate Pathology, vol. 95, no. 11, pp. 17–25, 2007. [Google Scholar]
25. T. Brütsch, A. Felden, A. Reber and M. Chapuisat, “Ant queens (Hymenoptera: Formicidae) are attracted to fungal pathogens during the initial stage of colony founding,” Myrmecological News, vol. 20, no. 9, pp. 71–76, 2014. [Google Scholar]
26. M. Konrad, C. D. Pull, S. Metzler, K. Seif, E. Naderlinger et al., “Ants avoid superinfections by performing risk-adjusted sanitary care,” Proceedings of the National Academy of Sciences of the United States of America, vol. 115, no. 11, pp. 2782–2787, 2018. [Google Scholar]
27. L. Y. Victor and L. C. Madoff, “ProMED-mail: An early warning system for emerging diseases,” Clinical Infectious Diseases, vol. 39, no. 2, pp. 227–232, 2004. [Google Scholar]
28. E. Gu and L. Li, “Crippled community governance and suppressed scientific/professional communities: A critical assessment of failed early warning for the COVID-19 outbreak in China,” Journal of Chinese governance, vol. 5, no. 2, pp. 160–170, 2020. [Google Scholar]
29. N. C. Peeri, N. Shrestha, M. S. Rahman, R. Zaki, Z. Tan et al., “The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: What lessons have we learned?,” International Journal of Epidemiology, vol. 49, no. 3, pp. 717–726, 2020. [Google Scholar]
30. G. J. Kost, W. Ferguson, A. T. Truong, J. Hoe, D. Prom et al., “Molecular detection and point-of-care testing in Ebola virus disease and other threats: A new global public health framework to stop outbreaks,” Expert review of molecular diagnostics, vol. 15, no. 10, pp. 1245–1259, 2015. [Google Scholar]
31. J. Heinze and B. Walter, “Moribund ants leave their nests to die in social isolation,” Current Biology, vol. 20, no. 3, pp. 249–252, 2010. [Google Scholar]
32. P. I. Marikovsky, “On some features of behaviour of the ants Formica rufa L. infected with fungous disease,” Insectes Sociaux, vol. 9, no. 2, pp. 173–179, 1962. [Google Scholar]
33. L. V. Ugelvig and S. Cremer, “Social prophylaxis: Group interaction promotes collective immunity in ant colonies,” Current Biology, vol. 17, no. 22, pp. 1967–1971, 2007. [Google Scholar]
34. W. O. Hughes, J. Eilenberg and J. J. Boomsma, “Trade-offs in group living: Transmission and disease resistance in leaf-cutting ants,” Proceedings of the Royal Society of London. Series B: Biological Sciences, vol. 269, no. 1502, pp. 1811–1819, 2002. [Google Scholar]
35. S. Cremer, C. D. Pull and M. A. Fürst, “Social immunity: Emergence and evolution of colony-level disease protection,” Annual Review of Entomology, vol. 63, no. 8, pp. 105–123, 2018. [Google Scholar]
36. P. Schmid-Hempel, “Parasites in social insects,”Parasites in Social Insects,Princeton, NJ: Princeton Univ. Press., pp. 409,1998. [Google Scholar]
37. L. L. Li, H. Luo, S. X. Ding, Y. Yang and K. X. Peng, “Performance-based fault detection and fault-tolerant control for automatic,” Automatica, vol. 99, no. 39, pp. 308–316, 2019. [Google Scholar]
38. J. S. Ayres, “Surviving COVID-19: A disease tolerance perspective,” Science Advances, vol. 6, no. 18, pp. eabc1518, 2020. [Google Scholar]
39. J. S. Ayres, N. Freitag and D. S. Schneider, “Identification of Drosophila mutants altering defense of and endurance to Listeria monocytogenes infection,” Genetics, vol. 178, no. 3, pp. 1807–1815, 2008. [Google Scholar]
40. S. J. Waddington and W. O. H. Hughes, “Waste management in the leaf-cutting ant Acromyrmex echinatior: The role of worker size, age and plasticity,” Behavioral ecology and sociobiology, vol. 64, no. 8, pp. 1219–1228, 2010. [Google Scholar]
41. C. Taylor, B. Endicott-Popovsky and D. A. Frincke, “Specifying digital forensics: A forensics policy approach,” Digital investigation, vol. 4, no. S1, pp. 101–104, 2007. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |