 | Fluid Dynamics & Materials Processing |  |
DOI: 10.32604/fdmp.2023.021499
ARTICLE
Experimental Study on the Treatment of Tertiary Oil Recovery Wastewater via a Novel Electro-Coagulation Method
1Petroleum Engineering Technology Research Institute of Sinopec Northwest Oilfield Company, Urumchi, 830011, China
2Key Laboratory of Enhanced Oil Recovery in Carbonate Fractured-Vuggy Reservoirs, Urumchi, 830011, China
*Corresponding Author: Wei Cui. Email: tgzy0403@163.com
Received: 18 January 2022; Accepted: 20 February 2022
Abstract: At present, methods for treating tertiary oil recovery wastewater via electro-coagulation are still in their early stage of development. In this study, a device for electro-coagulation wastewater treatment was built and tested in an oil field. The effects that the initial pH value, electrode type, and connection mode have on the coagulation and separation effect were assessed by measuring the mass fraction and turbidity of oil. The results have shown that when the electro-coagulation method is used, the effectiveness of the treatment can be significantly increased in neutral pH conditions (pH = 7), in acidic conditions, and in alkaline conditions. Compared to an Al electrode, the floc that is produced by an Fe electrode is smaller; thus, it does not easily coagulate and settle in a short time. Using the oil removal rate, turbidity removal rate and energy consumption as a basis to assess the performances, the results have demonstrated that the combined aluminum alloy iron composite electrode should be used as electrolytic electrode.
Keywords: Electrocoagulation; oily sewage; polymer; experiment study
In recent years, development of electrocoagulation technology has attracted significant research attention because of its high efficiency, and it has been increasingly and widely used in many industrial applications such as electroplating, pulp, food, printing and dyeing, wastewater treatment, landfill leachate, and other fields [1–3]. Numerous studies have proven that electrocoagulation can quickly reduce turbidity, chemical oxygen demand (COD), and biochemical oxygen demand (BOD); moreover, it can also aid in effective removal of heavy metal ions, suspended solids, and other pollutants. Electrocoagulation offers unique advantages compared to the traditional flocculation method. Specifically, in electrocoagulation, the functions of flocculation, air flotation, and redox reaction occur simultaneously; moreover, addition of chemicals is not required, and secondary pollution is avoided. The electrocoagulation device and its operation are relatively simple, the floor area is small, and the process can easily achieve automation [4,5].
The application prospects of electrocoagulation technology have attracted tremendous research attention at the global scale. For instance, Bensadok et al. [6] used the batch method and studied the electrocoagulation process. They measured COD, turbidity, and pH in circulating liquid. Karhu et al. [7] conducted experimental research on water circulation treatment in the intermittent mode using a horizontal flow electrode configuration. They evaluated the effect of an electrocoagulation demulsifier by measuring COD, total organic carbon, total surface charge, and turbidity. Mohora et al. [8] proposed a new continuous treatment electrocoagulation method using a bipolar aluminum electrode to remove arsenic from groundwater. Al-Shannag et al. [9] used a carbon steel electrode to remove Cu2+, Ni2+, Zn2+, and Cr3+ from electroplating wastewater via electrocoagulation. Guvenc et al. [10] used electrocoagulation to reduce the COD content in papermaking wastewater and to remove phenol and Ca2+. Bazrafshan et al. [11] found that electrocoagulation could be used to reduce COD in actual textile wastewater by more than 93.1%, and dye removal of more than 98.6% was achieved. Zheng et al. [12] developed a method to determine Faradaic power consumption as a function of current density and estimated the energy consumption per unit coagulant dose for electrocoagulation reactors that were operated over a wide range of conditions. Liu et al. [13] developed a multiple-stage treatment process by employing electrocoagulation with biogas pumping to simultaneously reclaim anaerobic digestion effluent and to clean biogas. The experimental results showed that the removal efficiencies of total solids, COD, total nitrogen, and total phosphorus were 30%, 81%, 37%, and 99.9%, respectively. Qi et al. [14] reported an integrated electrocoagulation process that was based on horizontal bipolar electrodes and investigated the pollutant removal efficiency and energy consumption in different configurations of a two-dimensional electrocoagulation system.
The above-mentioned literature studies indicate that many scholars have systematically studied the electrocoagulation mechanism, potential applications, and technical improvements. However, the research and application of electrocoagulation in polymer-containing wastewater treatment have not been carried out extensively. Moreover, the pH value of wastewater, and electrode material of the electrocoagulation device affect its performance in wastewater treatment. The study and optimization of these factors are expected to improve the treatment efficiency. However, to date, systematic experimental study on the treatment of polymer-containing wastewater in oil fields has never been reported. Therefore, treatment of polymer-containing wastewater via electrocoagulation is studied to provide a theoretical basis for optimizing the process. In this study, an electrocoagulation device that is close to field application was established and used in the research of the system. The effects of the initial pH value, electrode type, and connection mode on the performance of oilfield wastewater treatment via electrocoagulation were studied.
2 Experimental Principle and Method
2.1 Principle and Experimental Scheme
Electrocoagulation can remove pollutants through many different specific ways. Schematic illustration of electrocoagulation process is shown in Fig. 1. Metal ions that are produced by sacrificial anode dissolution are hydrolyzed and polymerized in water to form a series of polynuclear hydrolysates. These new hydrolysates are flocculants with high activity and strong adsorption capacity [15–17]. At the same time, tiny bubbles are generated on the cathode via electrolysis. When these bubbles float to the surface of water, they adhere to pollutants, such as colloidal particles and emulsified oil. Thus, pollutants float to the surface of water and can effectively be removed. The bubbles that are generated in electrolysis are much smaller than those generated via ordinary pressurized air flotation, and they float particularly well in acidic medium (the cathode produces new ecological hydrogen with strong reducibility, and this can reduce the content of heavy metal ions and organic matter). Moreover, under the action of current, some organic compounds in raw water are oxidized into low-molecular weight organic compounds and can even be directly oxidized into carbon dioxide and water. The new hydrogen that is produced at the cathode has strong reduction ability and can degrade pollutants via reduction processes.
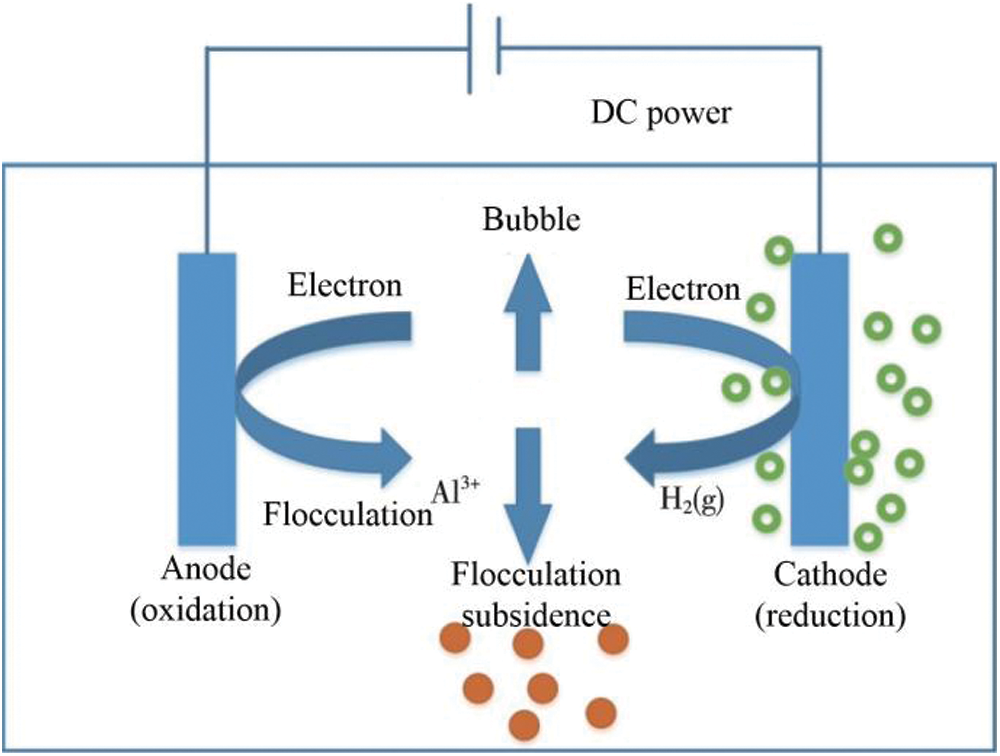
Figure 1: Schematic illustration of electrocoagulation process
In this study, a wastewater treatment system that contains polymers was established. The experimental system is mainly composed of a reaction tank, an electrode plate, a direct current (DC) power supply, a magnetic stirrer, and the polymer-containing sewage. The reaction tank with an effective volume of 2.4 L and dimensions of 320 mm × 120 mm × 150 mm, is made of plexiglass. The effective volume of treated wastewater is 2000 mL, and the wall thickness is 15 mm. Parallel vertical grooves are arranged on the inner wall of the reaction tank to facilitate the placement of electrodes. The electrodes are made of pure aluminum with thickness of 3 mm. According to the experimental requirements, the spacing of plates can range from 5 to 100 mm. A DC power supply is used to control the voltage and current. The output voltage is controlled to be in the range of 0–40 V, and the output current is controlled to be in the range of 0–10 A. A magnetic mixer is used to achieve the basic purpose of mixing the liquid in the container. The principle of magnetic field is stirring the liquid in the container with the help of the magnetic field, accompanied by the heating temperature control system, it can heat and control the sample temperature according to the specific experimental requirements, maintain the temperature conditions required by the experimental conditions, and ensure that the liquid mixing meets the experimental requirements.
2.2 Sewage Configuration and Test
Simulated polymer-containing wastewater was prepared according to the characteristics of oilfield tertiary oil recovery wastewater. Sodium dodecylbenzene sulfonate, oil, and anionic polyacrylamide were added to water to prepare the simulated sewage. After shearing and stirring with a high-speed shear device, the liquid was poured into a separating funnel and left to stand for 24 h, in order to separate the liquid into a surface oil film and an oily water layer. The lower layer of the prepared solution was used as the simulated sewage. To improve the conductivity, sodium chloride was added, and sulfuric acid and sodium hydroxide were used to adjust the pH of the simulated sewage to a specific level. To ensure the consistency with the on-site sewage, the initial oil content and polymer concentration were controlled to be 600 and 300 mg L−1, respectively.
In this study, two parameters, namely, the oil removal rate and turbidity reduction rate were used to evaluate the effectiveness of electrocoagulation process. The measurement and analysis methods are as follows:
(1) Oil content measurement
The oil content in the polymer-containing wastewater was determined by ultraviolet (UV) spectrophotometry. Notably, the oil contains aromatic compounds with conjugated bonds and benzene rings and have characteristic absorption in the UV region [18,19]. Therefore, the content of oil can be determined by measuring the concentration of conjugated double bonds. The standard curve of oil content is shown in Fig. 2.
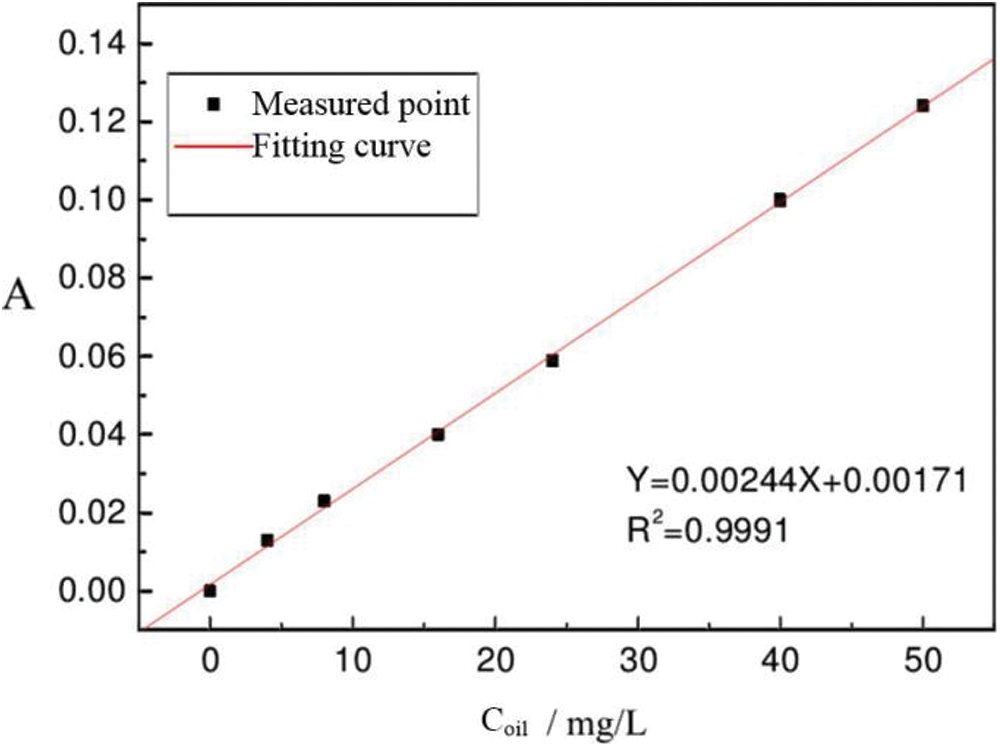
Figure 2: Standard curve of oil content
(2) Turbidity measurement
Turbidity refers to the degree of obstruction of suspended solids in a liquid with respect to the propagation of light. It can be measured using a turbidimeter in NTU (which is the turbidity measurement unit) [20–22]. The main instruments and specific models that were used in the experiment are listed in Table 1.
In the experiment, each working condition was repeated three times. Every time when a sewage sample was taken, the oil content and turbidity were tested. The results are presented by using the method of averaging the results.
3 Analysis of Factors That Influence Wastewater Treatment via Electrocoagulation
The initial pH of the polymer-containing wastewater significantly impacted the electrocoagulation process. Different values of initial pH led to different hydrolysates at the electrode. Moreover, the corresponding stability of different types of flocculants was different, and this affected the final quality of the treated water. To study the effects of the initial pH of polymer-containing wastewater on oil and turbidity removal, the pH of simulated polymer-containing wastewater was adjusted to acidic, neutral, and alkaline conditions, respectively. Other experimental conditions were consistent with the experimental conditions: distance between electrodes was 1.8 cm, magnetic stirring speed was 550 rpm, current density was 4.5 mA m2, experimental electrode was aluminum electrode, concentration of polymers in sewage is 800 mg/L. The change curves for the oil removal rate and turbidity data that were obtained under the experimental conditions of different values of initial pH (pH = 5, 6, 7, 8, 9) are shown in Figs. 3 and 4, respectively.
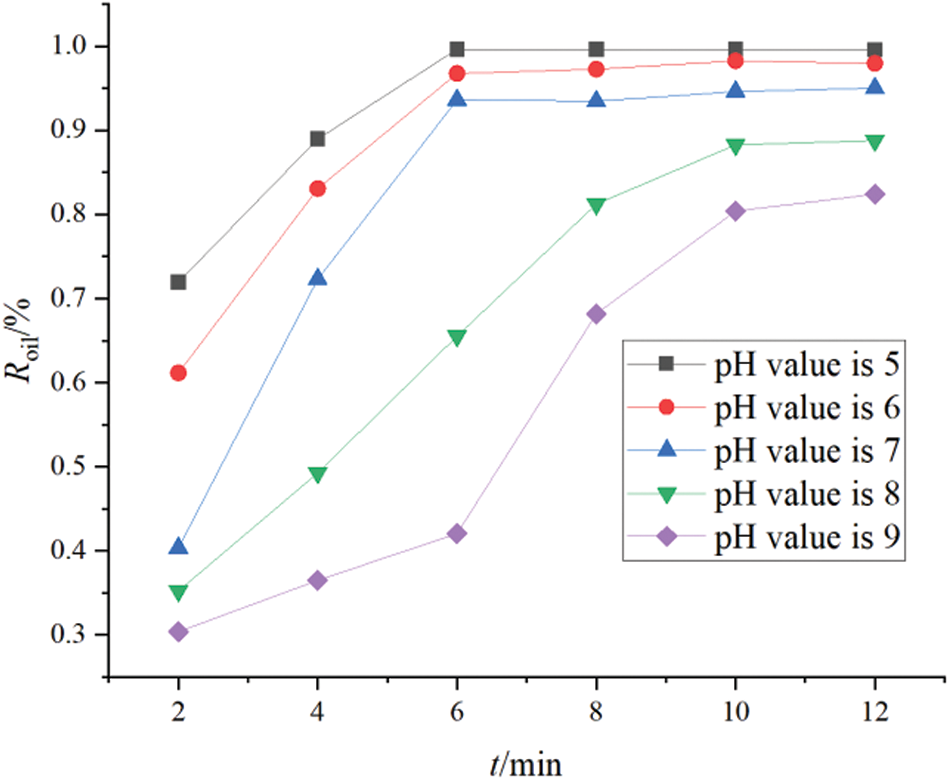
Figure 3: Oil removal rates at different pH values
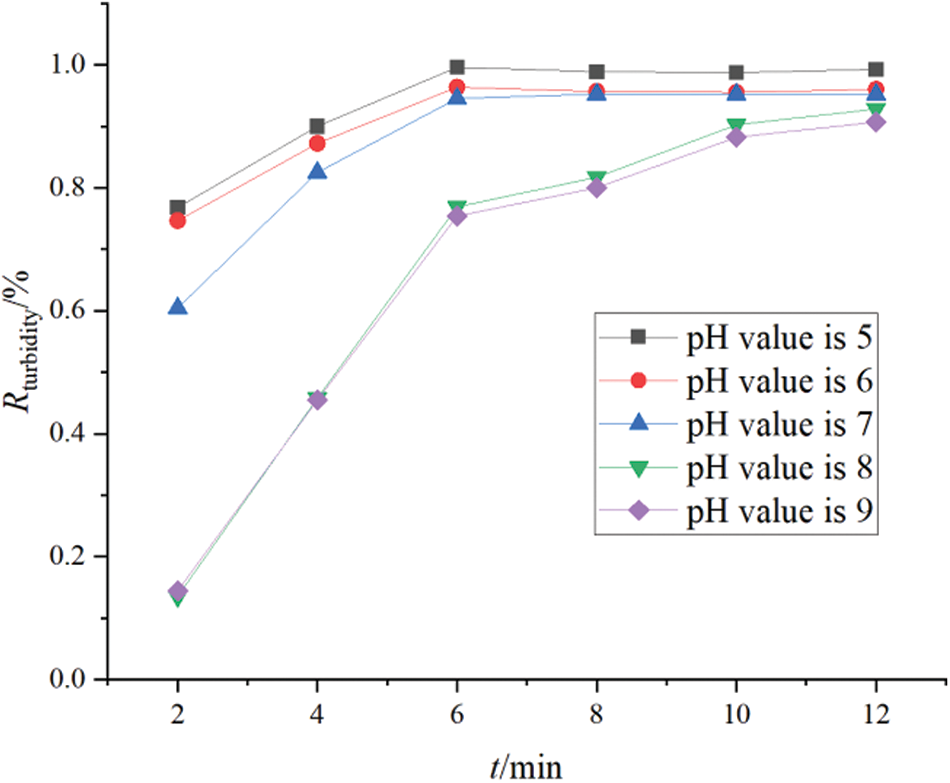
Figure 4: Turbidity removal rates at different pH values
The figure illustrates that the treatment effects in 6–8 min in acidic and neutral conditions is better, the oil removal rate and turbidity removal rate increase sharply, and each of them then settle to a stable state. With longer treatment time, a change in the oil removal rate is no longer obvious. Compared to acidic and neutral conditions, an obvious oil removal effect in the early stage of the treatment process in alkaline conditions is observed. However, the oil removal rate is slightly lower than that in acidic and neutral conditions. Under alkaline conditions, the purification process did not reach a 100% stable state in the entire experiment. In general, the effect of electrocoagulation is the best under neutral conditions followed by acidic conditions, and it was the worst in alkaline conditions.
Aluminum (Al) and iron (Fe), which are common soluble metal elements, were also studied as anode and cathode materials, respectively. Comparative analysis of the electrocoagulation effects observed when these materials were used as electrodes is shown in Figs. 5 and 6. The trends of oil removal effect and turbidity removal effect are similar.
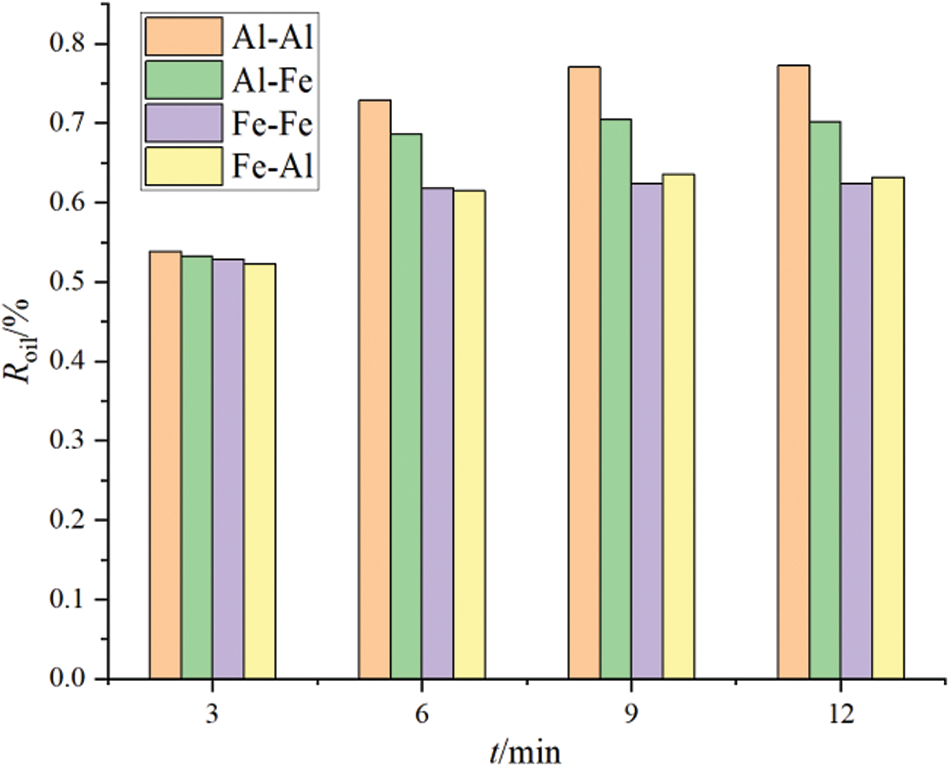
Figure 5: Oil removal rate with different electrode types
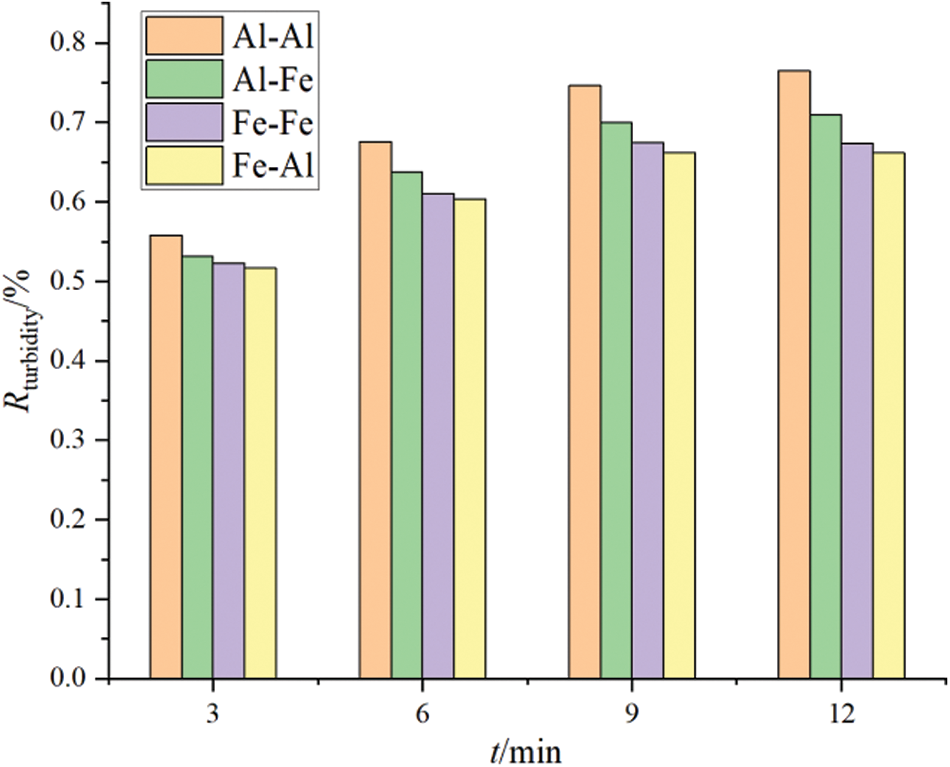
Figure 6: Turbidity removal rate with different electrode types
The water samples were treated for 3, 6, 9, and 12 min, respectively. The color of the treated water samples changed from green to light green after 3 min of electrolysis, and became dark green after 10 min of electrolysis. After standing for 1 h, the electrolyte turned yellow. This result is mainly attributed to the oxidation of Fe2+ ions in the electrolyte to Fe3+ by oxygen in the air. After standing for 12 h, the upper part of the electrolyte was clear, and the flocs settled to the bottom of the container.
Compared with the water sample treated with an aluminum electrode, the floc that was produced by the iron electrode was smaller, and thus, it did not easily coagulate and precipitate in a short time. This is not conducive to the rapid treatment of oily wastewater. In a similar study, Kaliniichuk et al. [23] used aluminum and stainless-steel plates to conduct a matching test of electrocoagulation treatment of organic substances in ship bottom wastewater. They showed that when an aluminum plate was used as the anode and cathode in an electrocoagulation treatment system, higher sewage treatment efficiency could be achieved via electrolysis. Moreover, when an iron electrode was used as the anode and cathode in an electrocoagulation treatment system, chromaticity was easily produced in the electrolysis process, and this was not found to be conducive to sewage treatment in accordance with standards.
3.3 Influences of Electrode Connection Mode
Theoretically, conductive substances (copper, iron, aluminum, zinc, etc.) can be used as electrodes to treat wastewater. However, wastewaters with different quality need to meet different treatment standards, and the compositions of waters with different quality can be very different. Therefore, to achieve the desired treatment objective, it is very important to select an appropriate electrode and its connection mode. In this study, iron, aluminum, and iron-combined aluminum alloy electrodes were used in electrolytic cells. The turbidity removal rate, and energy consumption were considered to select the best electrode connection mode.
There are three electrode connection modes: single-stage type, double-stage (multi-stage) type, and combined type. The single-stage type refers to liquid in an electrolytic cell. All the electrodes are connected to a power supply in the multi-stage connection, which is relatively simple. In an electrolytic cell, only the electrode plates on both sides are, respectively, connected to the positive and negative poles of the power supply, and other electrode plates do not need to be connected with the power supply. The combined type is a connection mode that is between the single-stage type and multi-stage type. A schematic illustration of the electrode connection modes is shown in Fig. 7. The test conditions are as follows: pH of wastewater samples was 6, plate spacing was 2 cm, current intensity was 4 mA m2, and electrolysis time was 60 min. The experimental results are shown in Fig. 8.
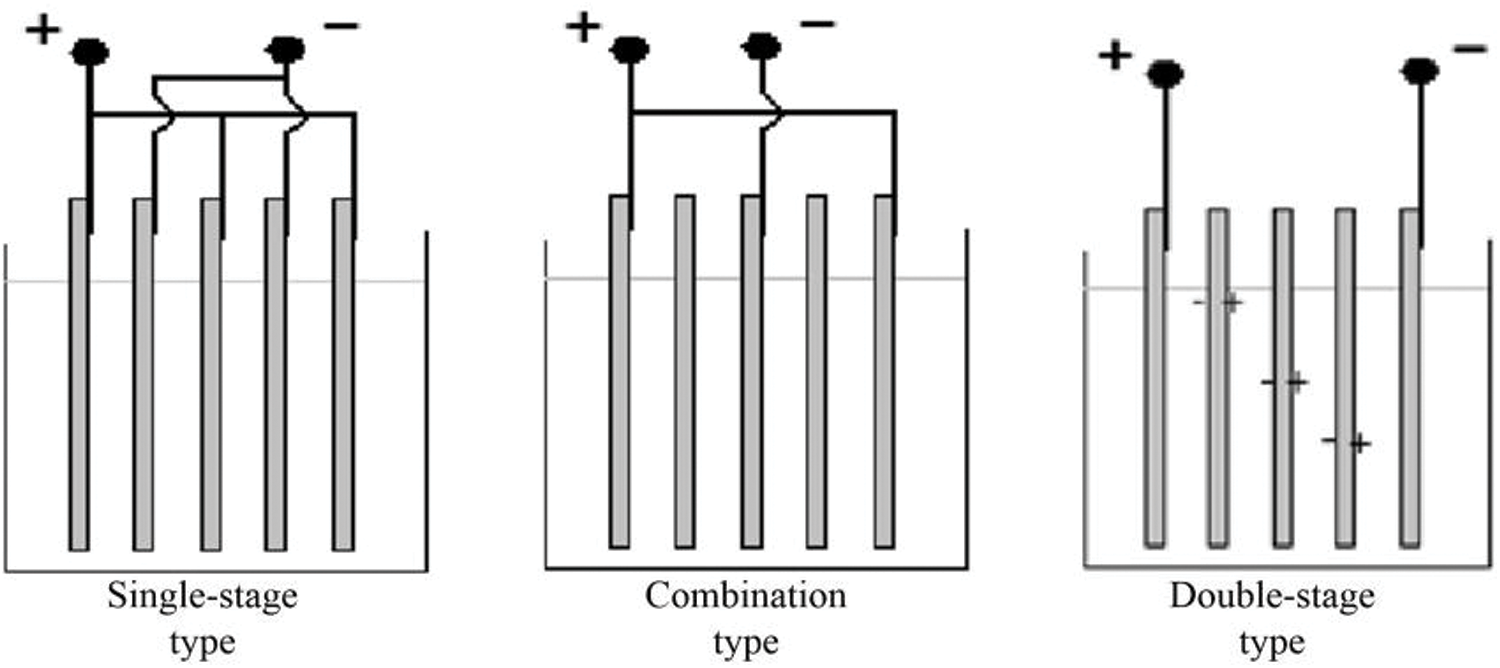
Figure 7: Schematic illustration of different electrode-connection methods
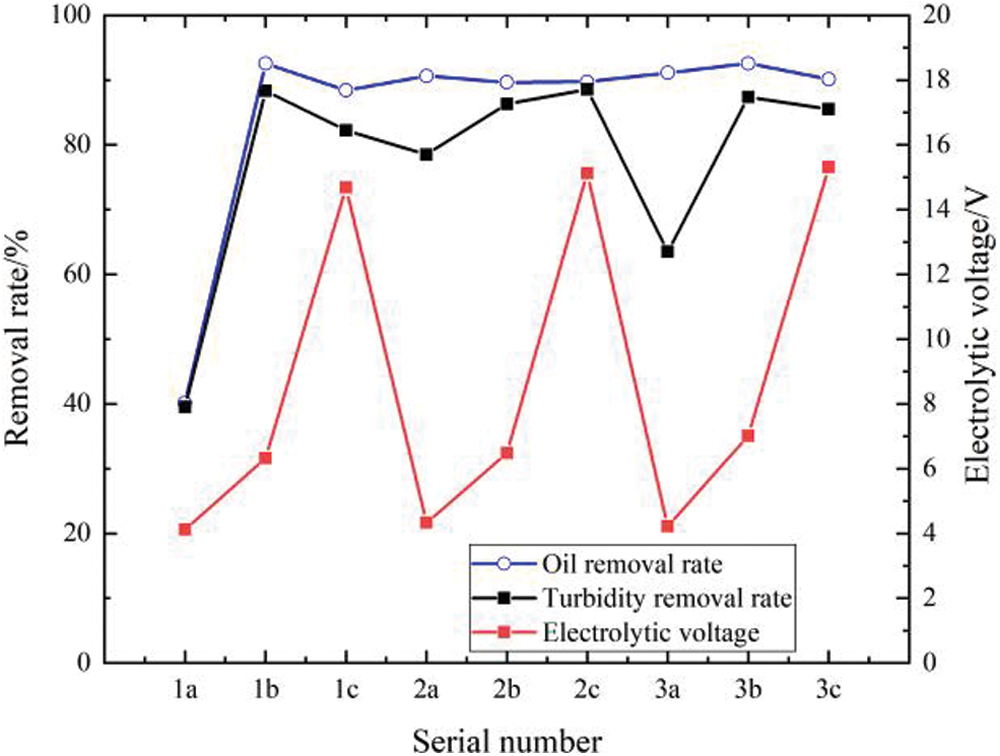
Figure 8: Relationships between the connection mode and the electrode, removal rate, and voltage
In Fig. 8, the numbers 1, 2, and 3 represent the iron electrode, aluminum electrode, and aluminum alloy iron composite electrode, respectively; the letters a, b, and c represent the single-stage type, combined type, and double-stage (multi-stage) type, respectively. Based on the comparative analysis, the following conclusions can be drawn:
(1) The energy consumption of the single-stage type is significantly lower than that of the double-stage type and combined type. Among all the connection modes of the single-stage type, the energy consumption was the smallest when iron was used as the electrode plate. However, the oil removal rate and turbidity removal rate were very low.
(2) The turbidity removal rates of the combined type and double-stage type were significantly higher than that of the single-stage type. However, the energy consumption of the double-stage type was significantly higher than that of the combined type. Therefore, from a comprehensive consideration of these two aspects, it is determined that the connection mode of the combined type is better than those of the other two types.
(3) The oil removal rates of the combined type and double-stage type were significantly higher than that of the single-stage type. However, considering the turbidity removal rate and energy consumption, the combined type was the best.
(4) In terms of the color of the treated water sample, the water sample treated with an aluminum electrode was colorless; however, the color of the treated water sample was slightly yellow as a result of the dissolution of iron ions in the wastewater treatment process with the iron electrode and aluminum alloy iron composite electrode. This is also the reason why the turbidity removal rate of the water sample that was treated with an aluminum electrode was the highest.
(5) For the combined connection mode, the aluminum alloy iron composite electrode exhibited the highest oil removal rate. The aluminum electrode showed the highest turbidity removal, but it was only a little higher than that of the aluminum alloy with iron glue as the electrode plate. In terms of energy consumption, there was not much difference in the energy consumption values of the three electrodes. Therefore, from these three aspects, the treatment effect of the aluminum alloy iron composite electrode as a soluble electrode was found to be the best.
Based on the above-mentioned points, the combined aluminum alloy iron composite electrode was selected as the electrolytic electrode for the electrocoagulation treatment of oilfield-produced water.
In this study, a set of electrocoagulation treatment devices that are similar to the field application was built to study the treatment of tertiary oil recovery wastewater via electrocoagulation. The effects of initial pH and electrode type (aluminum and iron) on the wastewater treatment performance of the electrocoagulation device were studied by single factor analysis.
The pH value significantly impacts the treatment effect. The best treatment environment is neutral conditions, followed by acidic conditions, and then alkaline conditions.
Aluminum and iron were used as anode and cathode materials, respectively, and different combination schemes were used to treat tertiary oil recovery wastewater. Compared with the aluminum electrode, the floc that was produced by the iron electrode electrocoagulation treatment was smaller, which did not easily coagulate and precipitate in a short time. Thus, the iron electrode is not conducive to the rapid treatment of wastewater.
According to the comparative analysis of the oil removal rate, turbidity removal rate, and energy consumption, the combined aluminum alloy iron composite electrode is selected as the electrolytic electrode for the treatment of simulated oilfield-produced water via electrocoagulation.
Funding Statement: This work was supported by Key Laboratory of Enhanced Oil Recovery in Carbonate Fractured-Vuggy Reservoirs.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Nasrullah, M., Zularisam, A. W., Krishnan, S., Sakinah, M., Singh, L. et al. (2019). High performance electrocoagulation process in treating palm oil mill effluent using high current intensity application. Chinese Journal of Chemical Engineering, 27(1), 216–225. DOI 10.1016/j.cjche.2018.07.021. [Google Scholar] [CrossRef]
2. Lee, A. K., Lewis, D. M., Ashman, P. J. (2013). Harvesting of marine microalgae by electroflocculation: The energetics, plant design, and economics. Applied Energy, 108(8), 45–53. DOI 10.1016/j.apenergy.2013.03.003. [Google Scholar] [CrossRef]
3. Raeisossadati, M., Moheimani, N. R., Bahri, P. A. (2021). Evaluation of electrocoagulation, flocculation, and sedimentation harvesting methods on microalgae consortium grown in anaerobically digested abattoir effluent. Journal of Applied Phycology, 18, 1–12. DOI 10.1007/s10811-021-02403-5. [Google Scholar] [CrossRef]
4. Ivanov, O. M., Anisimova, T. A., Beznosova, V. V., Kassir, I. A. (2020). Theoretical analysis of the effect of nap charge in electroflocculation technology. Fibre Chemistry, 52(2), 83–87. DOI 10.1007/s10692-020-10156-z. [Google Scholar] [CrossRef]
5. Sasson, M. B., Calmano, W., Adin, A. (2009). Iron-oxidation processes in an electroflocculation (electrocoagulation) cell. Journal of Hazardous Materials, 171(1), 704–709. DOI 10.1016/j.jhazmat.2009.06.057. [Google Scholar] [CrossRef]
6. Bensadok, K., Benammar, S., Lapicque, F., Nezzal, G. (2008). Electrocoagulation of cutting oil emulsions using aluminium plate electrodes. Journal of Hazardous Materials, 152(1), 423–430. DOI 10.1016/j.jhazmat.2007.06.121. [Google Scholar] [CrossRef]
7. Karhu, M., Kuokkanen, V., Kuokkanen, T., Rämö, J. (2012). Bench scale electrocoagulation studies of bio oil-in-water and synthetic oil-in-water emulsions. Separation & Purification Technology, 96, 296–305. DOI 10.1016/j.seppur.2012.06.003. [Google Scholar] [CrossRef]
8. Mohora, E., Ron, S., Agbaba, J., Tubić, A., Mitić, M. et al. (2014). Removal of arsenic from groundwater rich in natural organic matter (NOM) by continuous electrocoagulation/flocculation (ECF). Separation & Purification Technology, 136, 150–156. DOI 10.1016/j.seppur.2014.09.006. [Google Scholar] [CrossRef]
9. Al-Shannag, M., Al-Qodah, Z., Bani-Melhem, K., Qtaishat, M. R., Alkasrawi, M. (2015). Heavy metal ions removal from metal plating wastewater using electrocoagulation: Kinetic study and process performance. Chemical Engineering Journal, 260, 749–756. DOI 10.1016/j.cej.2014.09.035. [Google Scholar] [CrossRef]
10. Guvenc, S. Y., Erkan, H. S., Varank, G., Bilgili, M. S., Engin, G. O. (2017). Optimization of paper mill industry wastewater treatment by electrocoagulation and electro-fenton processes using response surface methodology. Water Science & Technology, 76, 2015–2031. DOI 10.2166/wst.2017.327. [Google Scholar] [CrossRef]
11. Bazrafshan, E., Alipour, M. R., Mahvi, A. H. (2016). Textile wastewater treatment by application of combined chemical coagulation, electrocoagulation, and adsorption processes. Desalination and Water Treatment, 57, 9203–9215. DOI 10.1080/19443994.2015.1027960. [Google Scholar] [CrossRef]
12. Zheng, G., Liao, Z., Schulz, M., Davis, J. R., Baygents, J. C. et al. (2015). Estimating dosing rates and energy consumption for electrocoagulation using iron and aluminum electrodes. Industrial & Engineering Chemistry Research, 48(6), 3112–3117. DOI 10.1021/ie801086c. [Google Scholar] [CrossRef]
13. Liu, Z., Stromberg, D., Liu, X., Wei, L., Yan, L. (2015). A new multiple-stage electrocoagulation process on anaerobic digestion effluent to simultaneously reclaim water and clean up biogas. Journal of Hazardous Materials, 285(21), 483–490. DOI 10.1016/j.jhazmat.2014.10.009. [Google Scholar] [CrossRef]
14. Qi, Z., You, S., Liu, R., Chuah, C. J. (2020). Performance and mechanistic study on electrocoagulation process for municipal wastewater treatment based on horizontal bipolar electrodes. Frontiers of Environmental Science & Engineering, 14(3), 40. DOI 10.1007/s11783-020-1215-3. [Google Scholar] [CrossRef]
15. Lobo, F. L., Wang, H., Huggins, T., Rosenblum, J., Linden, K. G. et al. (2016). Low-energy hydraulic fracturing wastewater treatment via AC powered electrocoagulation with biochar. Journal of Hazardous Materials, 309, 180–184. DOI 10.1016/j.jhazmat.2016.02.020. [Google Scholar] [CrossRef]
16. Tang, S. F., Liu, F. (2006). Treatment of oily waste water in polymer flooding by using air-flotation technique. Journal of Oil and Gas Technology, 28(4), 131–133 (in Chinese). DOI 10.3969/j.issn.1000-9752.2006.04.037. [Google Scholar] [CrossRef]
17. Jiang, W., Chen, M., Yang, J., Deng, Z., Liu, Y. et al. (2017). Dynamic experimental study of a new electrocoagulation apparatus with settlement scheme for the removal process in oilfield. Journal of Electroanalytical Chemistry, 801, 14–21. DOI 10.1016/j.jelechem.2017.07.017. [Google Scholar] [CrossRef]
18. Maher, E. K., O’Malley, K. N., Dollhopf, M. E., Mayer, B. K., Mcnamara, P. J. (2020). Removal of estrogenic compounds from water via energy efficient sequential electrocoagulation-electrooxidation. Environmental Engineering Science, 37(2), 99–108. DOI 10.1089/ees.2019.0335. [Google Scholar] [CrossRef]
19. Elabbas, S., Ouazzani, N., Mandi, L., Berrekhis, F., Perdicakis, M. et al. (2016). Treatment of highly concentrated tannery wastewater using electrocoagulation: Influence of the quality of aluminium used for the electrode. Journal of Hazardous Materials, 319, 69–77. DOI 10.1016/j.jhazmat.2015.12.067. [Google Scholar] [CrossRef]
20. Gatsios, E., Hahladakis, J. N., Gidarakos, E. (2015). Optimization of electrocoagulation (EC) process for the purification of a real industrial wastewater from toxic metals. Journal of Environmental Management, 154, 117–127. DOI 10.1016/j.jenvman.2015.02.018. [Google Scholar] [CrossRef]
21. Liu, J., Xu, S., Sun, Z. (2007). Toward an understanding of the turbidity measurement of heterocoagulation rate constants of dispersions containing particles of different sizes. Langmuir, 23, 11451–11457. DOI 10.1021/la701426u. [Google Scholar] [CrossRef]
22. Naismith, J. (2005). Membrane integrity-direct turbidity measurement of filtrate from mf membrane modules at an operating potable water treatment plant. Desalination, 179(1), 25–30. DOI 10.1016/j.desal.2004.11.052. [Google Scholar] [CrossRef]
23. Kaliniichuk, E. M., Vasilenko, I. I., Shchepanyuk, V. Y., Sukhoverkhova, N. A., Makarov, I. S. (1976). Treating refinery wastewaters to remove emulsified oils by electrocoagulation and electroflotation. Angewandte Chemie International Edition, 16(3), 434–435. DOI 10.1021/i360060a018. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |