

 | Fluid Dynamics & Materials Processing |  |
DOI: 10.32604/fdmp.2022.018537
ARTICLE
Influence of Anthracite-to-Ilmenite-Ratio on Element Distribution in Titanium Slag Smelting in Large DC Furnaces
1Office of Science and Technology, Kunming University, Kunming, 650214, China
2Kunming Metallurgy College, Kunming, 650033, China
3College of Chemistry and Chemical Engineering, Kunming University, Kunming, 650214, China
4Key Laboratory of Yunnan Provincial Higher Education Institutions for Organic Optoelectronic Materials and Devices, Kunming University, Kunming, 650214, China
*Corresponding Author: Zhifeng Nie. Email: niezf123@kmu.edu.cn
Received: 31 July 2021; Accepted: 16 November 2021
Abstract: The distribution of titanium, carbon and associated elements (calcium, magnesium, silicon and aluminum) in a smelting process is studied by means of a chemical equilibrium calculation method for multiphase and multicomponent systems, and verified through comparison with production results. In particular, using the coexistence theory for titanium slag structures, the influence of the AIR (anthracite to ilmenite ratio) on the distribution of such elements is analyzed. The results show that the AIR can be adjusted to achieve a selective reduction of oxides in the melt.
Keywords: Titanium slag; smelting; element distribution
Titanium and titanium dioxide are the main products of titanium metallurgy. Titanium and titanium alloys are widely used in aerospace, electric power, sporting goods, ships, marine engineering, and medicine. The titanium metal can be produced in a pure state, and has limited use in metallurgy. Titanium alloys with iron, copper, aluminum, nickel, and manganese are available as sources of supply, and have a variety of applications [1,2]. The production of titanium-rich materials is the basic industry in titanium metallurgy. Therefore, the preparation of titanium-rich materials suitable for the fluidized chlorination process is an important part of titanium metallurgy. With the depletion of natural rutile, ilmenite has become the main raw material for the production of titanium-rich materials [3]. Titanium-rich materials are normally prepared by the electric furnace melting method. The electric furnace smelting process is relatively simple, with a high production efficiency and a small plant area. The by-product iron and electric furnace gas can be directly utilized [4].
Titanium slag is produced via carbothermal reduction smelting. Titanium dioxide and iron are separated by adding a reducing agent, known as anthracite. The reducing agent increases the furnace bottom temperature and facilitates uniform distribution, thus improving the thermodynamic and kinetic conditions of the reduction reaction. Furthermore, titanium slag has the role of molten salt electrolysis, thus it can save power consumption better than AC electric furnaces. The electrode center temperature of the enclosed DC furnace is high, and the heat is concentrated, which is good for smelting high-melting-point products. The equipment structure is simple and easy to maintain; however, the life of the lining material is relatively short. Graphite is used as the electrode material in an enclosed DC electric furnace; the impurity ash is less than the electrode paste, which is beneficial for improving the quality of high-titanium slag products.
In this study, ilmenite was smelted in a DC furnace, and anthracite was used as a reducing agent. The feeding rates of ilmenite and anthracite were controlled by the feeding system, and the two materials were mixed together before entering the furnace. The materials were fed through a hollow electrode connected to three feeding ports. The direct current flowed between the anode and cathode of the furnace, which is a consumable graphite electrode, and the anode was located in the furnace.
A plasma arc was generated between the electrode and the top of the slag pool. The furnace was lined with refractory materials for the integrity of the refractory lining and shell related to process safety. The principle of “freezing lining” is often employed to ensure that the slag will not corrode refractories. In the slag zone, the cooling and refractory selection ensured the slag “freeze” on the refractory, in order to form a protection between the molten slag and refractory.
The electrode positioning device covered the furnace electrode. This device allowed the electrode column to move precisely in the vertical direction to control the arc length. The device also allowed the addition of new electrodes and sliding of the electrode column. The device ensured electrical connection between the power supply device and the electrode, and the waste gas from the reduction process was discharged from the furnace through a separate outlet on the top of the furnace. The high-temperature gas was quenched and purified by the gas washing equipment near the furnace. The gas discharged from the furnace was controlled by a gas purification system to maintain a slight negative pressure in the furnace.
Ilmenite is a complex solid solution with multiple components based on a ferrotitanium metatitanate lattice. Moreover, it is a solid solution formed by FeO · TiO2 and other impurity oxides. Iron and titanium occur as a mixture of magnetite (Fe3O4) and ilmenite (FeTiO3) with vanadium oxide, usually present within the solid solution of the titanium-bearing magnetite phase [5].
The general formula
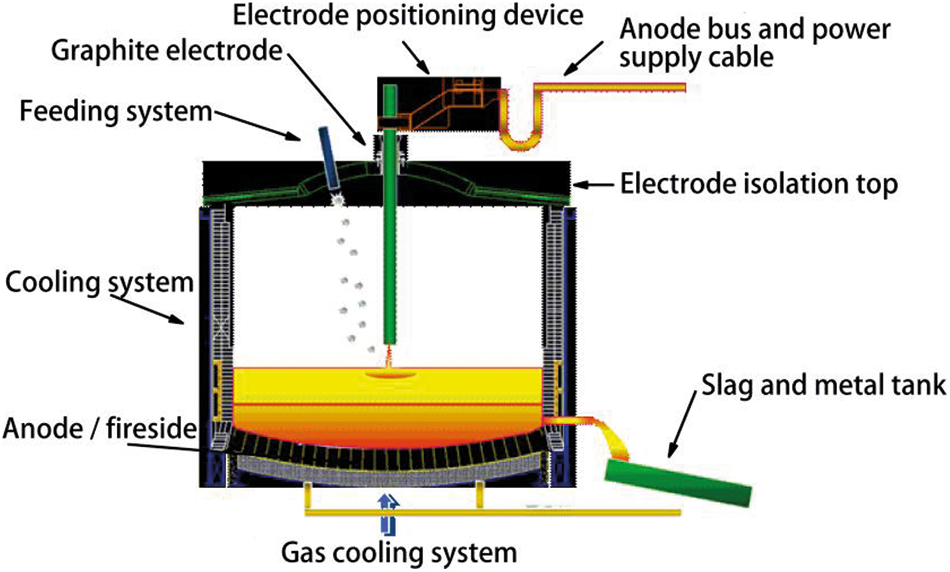
Figure 1: Schematic of DC arc furnace
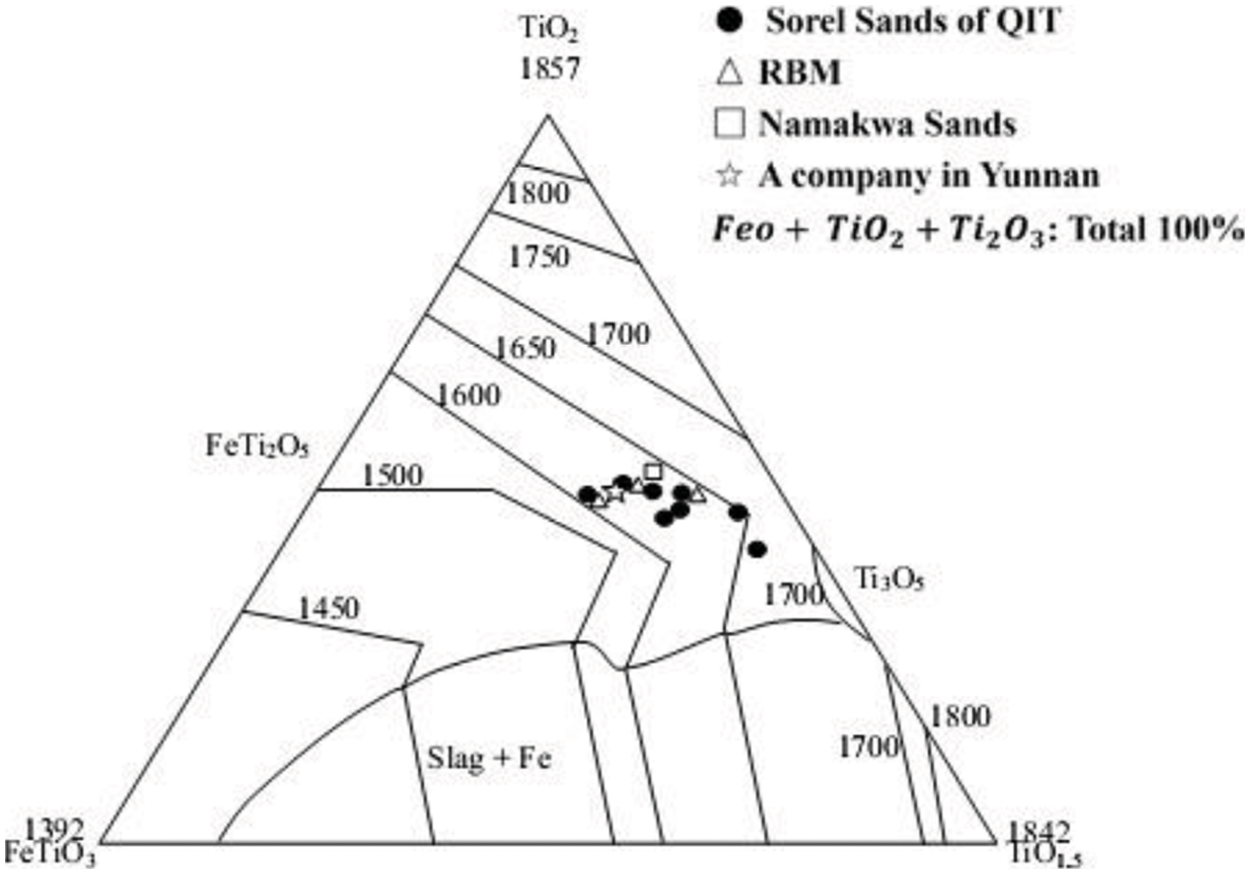
Figure 2: Liquidus diagram of titanium slag from titanium concentrate smelting (°C)
The possible reactions of carbon reduction of ferrometatitanate are as follows [8,9]:
Ilmenite often contains a certain amount of hematite, and its carbon reduction reaction is as follows:
According to the relationship between the change in the standard free energy and temperature of each reaction equation provided above, the change in the standard free energy (Δ
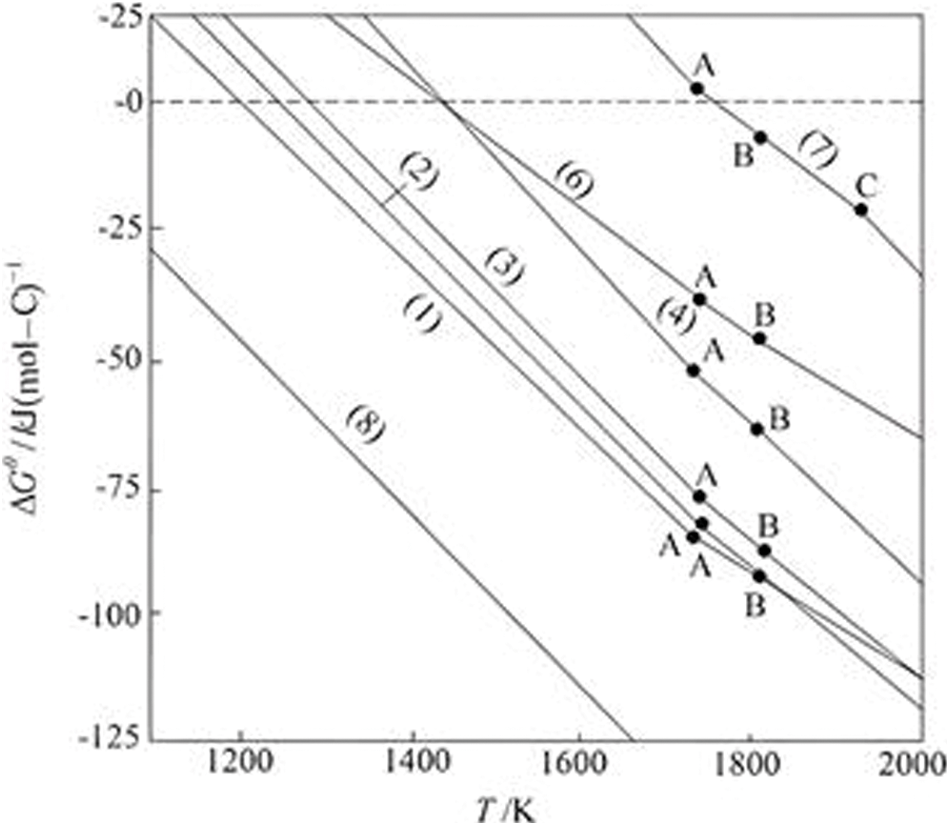
Figure 3: ΔGθ—The relation schema of ilmenite reduction smelting (A: FeTiO3, B: Fe, C: Ti)
The maximum temperature of the reduction smelting ilmenite in an electric furnace is approximately 2000 K. It can be observed from Fig. 3 that at such a high temperature, the values of ΔGT in the reactions of Eqs. (1)–(8) are all negative, which indicates that these reactions can be conducted thermodynamically. With the increase of temperature, the tendency of reaction increases. However, the starting reaction temperature is not the same (i.e., Δ
In the melting process, titanium compounds with different valences coexist, and their proportions vary with the melting temperature and degree of reduction. Solid-phase reduction of iron enables the selective extraction of iron from the ilmenite crystal lattice without diluting the oxide phase with the reducing agent ash [10].
In the process of reduction smelting, in addition to the reduction of carbon, CO produced by the gasification reaction of carbon is also involved in the reaction.
The possibility of the above reactions can be estimated using the equilibrium constants of the reactions and the equilibrium partial pressures of the components in the gas phase. The results show that at a low temperature (1027 K–1273 K), CO energy can reduce Fe2O3 in ore to FeO and Fe according to formulas (13) and (14), respectively.
When the temperature is higher than 1273 K, the reaction formulas (10) and (12) can be obtained; however, because their reaction equilibrium constants are small, only the solid material near the bottom layer can be reduced according to formulas (10) and (12). In a closed electric furnace, the reduction in CO is strengthened.
The possibility of reduction or partial reduction of other impurities in ilmenite, such as MgO, CaO, SiO2, Al2O3, MnO, and V2O5, was studied. To simplify the study, only the reduction reaction of each single oxide with carbon was considered.
Other impurities can also carry out the gas phase reduction reaction of CO, which is only a secondary reaction and will not be described in detail here. The reduction reactions of MgO, CaO, and Al2O3 are conducted according to formulas (15)–(19), respectively. The starting reaction temperatures are 2153 K, 2463 K and 2322 K. It can be observed that they cannot be reduced at a temperature of 2000 K, but the reduction reaction may still occur in the local high-temperature region under the action of the arc. Other impurities, such as SiO2, MnO, and V2O5, are reduced to varying degrees at the reduction smelting temperature of ilmenite, and the reduction products of silicon, manganese, and vanadium are dissolved in the metallic iron phase. However, these impurities are more difficult to reduce than FeO and TiO2, thus it can be predicted that most of the impurities in the ore are enriched in the slag phase, except for some of the SiO2.
These reactions were considered separately. In particular, the reduction smelting process was conducted in a multi-component system at the same time, thus the actual reactions are complex. For example, products, such as FeTi2O5, Ti3O5, Ti2O3, and TiO are dissolved in the unreduced FeTiO3, and non-reducing components, such as MgO, Al2O3, and MnO are also enriched in the slag phase. The activity of FeO in the slag phase decreases under the above two conditions. To accelerate the reduction of ilmenite, the reduction driving force during smelting is increased. The activity of FeO is the major factor controlling the reduction in the driving force. The other factor is the delay in the formation of the pseudobrookite phase, which is a high-melting point precipitation phase. In this system, MgO in ilmenite can be used to form pseudobrookite [11].
Therefore, with the deepening of the reduction smelting process, the activity of FeO in slag becomes increasingly smaller; hence, it becomes increasingly difficult to promote the reduction of FeO, thus promoting the reduction of TiO2. In the later stage of reduction smelting, the FeO concentration in the slag phase is low, thus its reduction is more difficult. Therefore, the energy consumption of reducing the unit weight of FeO increases gradually with the deepening of the reduction smelting process. The results show that the energy consumption for reducing the unit weight of FeO from slag containing 10% FeO is more than 10 times that of reducing the same weight of FeO from slag containing 20% FeO. In actual production, a certain amount of FeO is always present in the slag.
This study investigated the operation process of a DC furnace. According to the coexistence theory of slag structure, the distribution behavior of Ti, Ca, Mg, Si, and Al in the slag was analyzed under the condition of stable input energy and stable composition of titanium concentrate. The distribution behavior of the elements was controlled by controlling the AIR.
Nurgali et al. [12] studied the phase equilibrium in titanium-containing slag from the metallurgical processing of ilmenite concentrates of Shokash deposit on the basis of a five-component system, TiO2-Al2O3-SiO2-MgO-CaO, by thermodynamic analysis (TDA). The phase equilibria in two four-component systems CaO-MgO-Al2O3-SiO2 and TiO2-MgO-Al2O3-SiO2, which are part of the five-component system TiO2-Al2O3-SiO2-MgO-CaO, were clarified and corrected. Thus, data on the phase compositions of congruently (stably) melting compounds of titanium slag and slag of aluminum- and aluminosilicothermic ferrotitanium as well as slag melts from the melting of titanomagnetite ores have been obtained.
Zhao Zhijun and Ma Enquan studied methods to improve the enrichment of V in metal Fe [13]. The research shows that the reduction of ilmenite can be divided into two stages: the first stage is Fe+3 to Fe+2 in the ore, which is easy to conduct, and can be completed in a short time at a low temperature of 1170 K. The second stage is Fe+2 to FeO. The reduction in this stage is complicated. In theory, after Fe+2 to FeO is completed, the reduction products of titanium slag should be Ti3O5; however, because of the existence of other solid solutions and the difficulty of complete reduction of FeO, the content of Ti3O5 in titanium slag is generally 15%–30% after the smelting of ilmenite [14].
Kucukkaragoz et al. [15] concluded that the carbothermal reduction process of titanium ore can be divided into two stages: the first stage is Fe3+ to Fe2+ to Fe, Ti4+ to Ti3+, which is a solid-state reduction process, and the reduction level reaches 50%; the second stage is the reduction of the remaining 50%, which is Ti3+ to Ti2+, and finally, TiO1–x, which is the process of melting and slagging.
Sariev et al. [16] pointed out that in determining the electrical resistivity of ore-coal briquettes with different reducing agents, the most specifically suitable reducing agents for melting (BTSS) are special coke and coal, as briquettes, because they have the maximum electrical resistivity in the temperature range of solid-phase reduction.
Martin-Treceno et al. [17] investigated the reduction process during the electrochemical production of titanium via the electrolysis of molten TiO2-SiO2-Al2O3-MgO-CaO slags. The results showed that the extraction of pure metallic Ti directly from ironmaking slag is difficult without prior removal of SiO2 or other chemical modifications to the system.
2 Experimental Materials and Calculation Methods
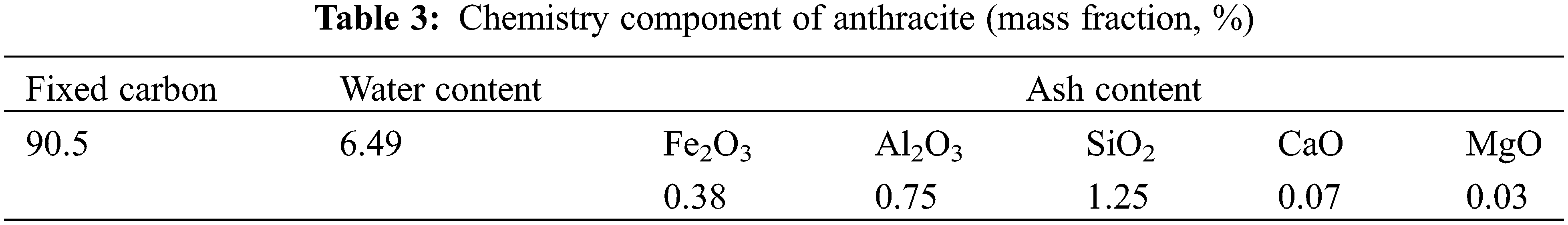
The feed materials used were titanium concentrate and anthracite. The chemical composition of titanium concentrate affects the smelting process and technical and economic indicators, as well as directly determines the grade of TiO2 and the composition and content of impurities in the titanium slag. Considering the actual process and economic rationality, anthracite was selected as the reductant. Anthracite has a wide range of sources and low price; however, the ash content and sulfur content of anthracite are required to be low, to reduce the influence of the enrichment of impurities in the slag phase, resulting in the reduction of titanium slag grade.
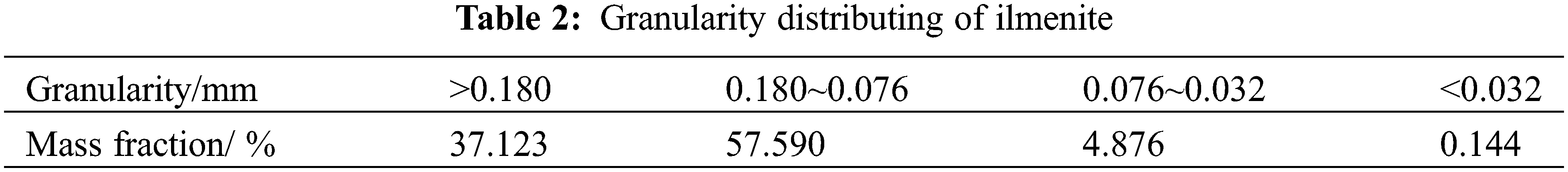
The chemical composition and particle size distribution of the ilmenite concentrate used for smelting titanium slag in a DC furnace are shown in Tables 1 and 2, and the chemical composition of anthracite is shown in Table 3.
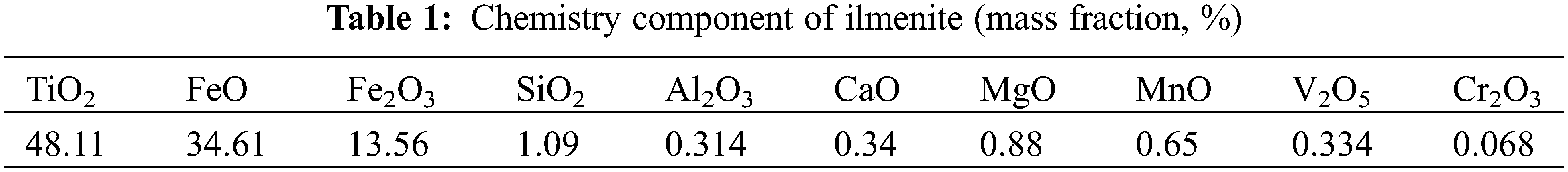
It can be observed from Table 1 that the TiO2 grade is high, which is conducive to the production of titanium slag by DC furnace smelting. However, although the grades of CaO, MgO, and other impurities in the titanium concentrate are low, it is not conducive for obtaining high-grade titanium slag. As shown in Fig. 4, the distribution of these impurities in the titanium concentrate is highly dispersed and uniform, thus it is difficult to separate them by ore dressing.
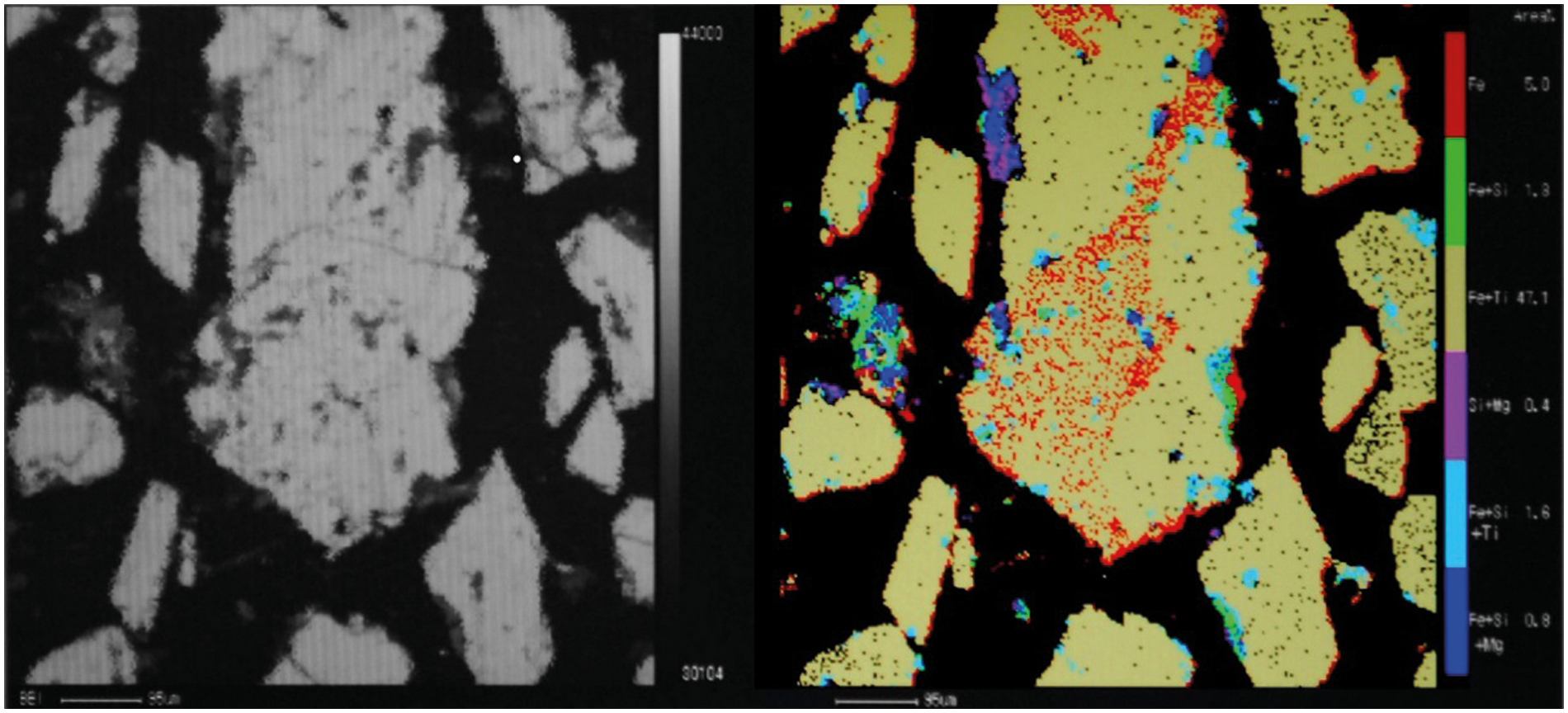
Figure 4: Distribution of impurities in ilmenite
Approximately 75% of the initial anthracite raw materials had particle sizes larger than 8 mm. Anthracite must be crushed and screened after drying in an electric heater. Fig. 5 shows anthracite without crushing, after crushing, and screening.

Figure 5: Anthracite used in the experiments
3 Basic Computing Model of Element Allocation Process
According to the phase diagram of FeO–TiO2 slag system,
Let
According to the mass balance,
Eqs. (23)–(32) are the calculation model of the action concentration of
4 Analysis of Experimental Results
4.1 Effect of AIR on Distribution of Main Elements in Titanium Slag
The impurities in the titanium concentrate are reduced to different degrees under the temperature of reduction smelting. Fig. 6 shows the influence of the AIR on the distribution of elements in the titanium slag. With the increase in AIR, the mass fraction of TiO2 in the titanium slag increased, and the mass fraction of FeO decreased. This is because the lower the carbon content, the greater the carbon consumption of the reducible oxides. When the carbon content is high, the distribution of carbon in the melt oxide reverses, and more carbon is used for the reduction of refractory oxides.
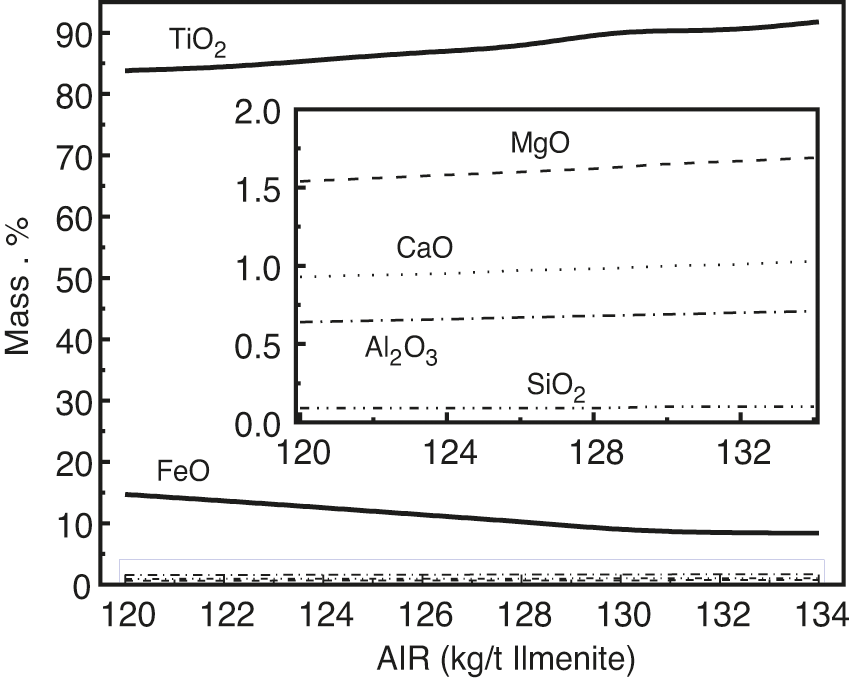
Figure 6: Effect of AIR on distribution of main elements in titanium slag
When the temperature is low and the amount of carbon is small, the distribution of carbon inclines to the easily reducible oxides, which is shown by the decrease in FeO and the enrichment of TiO2 in slag, that is, the mass fraction of TiO2 increases. This is because most of the carbon is used in the reduction of TiO2, resulting in numerous TiO2 enrichment and a sharp decrease in the growth of TiO2. Under this condition, the activity of FeO reached a significantly low value, which further increased the negative deviation from the ideal solution, thus reducing its activity. In addition, the amount of carbon allocated to FeO was small, which led to an almost flat change curve of TiO2. However, the change curve of TiO2 shows a slight upward trend.
The impurity elements MgO, CaO, and Al2O3 in the titanium concentrate are difficult to reduce during reduction smelting. Other impurity elements, such as SiO2, MnO, and V2O5, are reduced to varying degrees at the reduction smelting temperature of ilmenite. When the AIR increased from 120 kg/t ilmenite to 134 kg/t, MgO, CaO, Al2O3, and SiO2 also showed an increasing trend. The contents of MgO, CaO, Al2O3, SiO2, and other oxides further increased. The contents of these impurities are also related to the difficulty of oxidation and reduction. SiO2, MnO, and V2O5 are more difficult to reduce than FeO and TiO2. Therefore, these impurities in the titanium ore are also enriched in titanium slag.
4.2 Effect of AIR on Distribution of Elements in Molten Iron
The amount of carbon had a selective effect on the melt oxide. Fig. 7 shows the effect of AIR on the distribution of elements in the metal. It can be observed that the AIR has an important influence on the distribution behavior of elements in the hot metal.
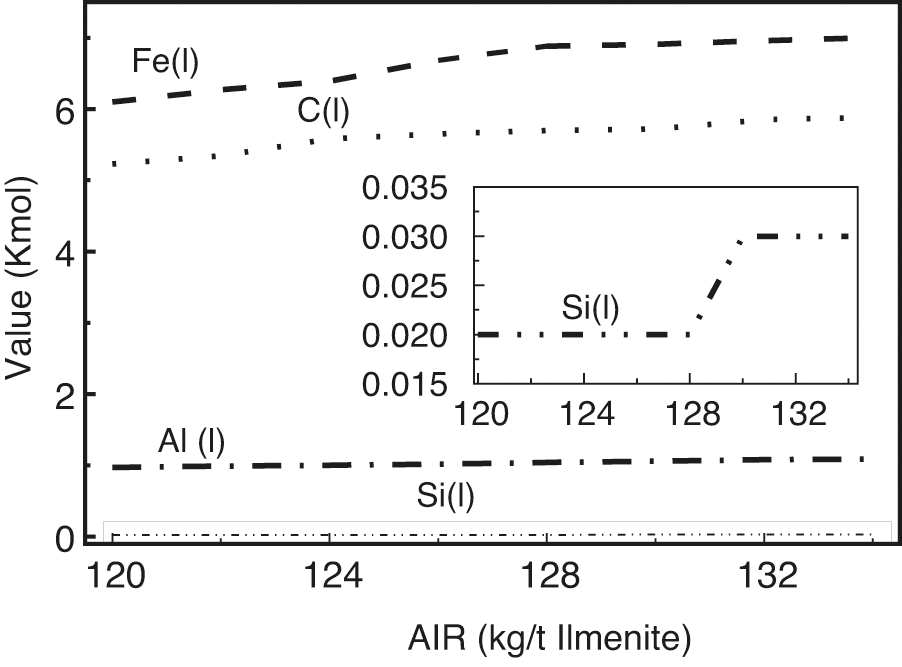
Figure 7: Effect of AIR on the distribution of element in molten iron
With the increase in AIR, the contents of Fe, C, and Si increased. When the AIR reached 130, the amount of reduction increased. Finally, Si and C were dissolved in the Fe phase.
In the process of smelting titanium slag, the reduction potential of FeO in the melt increases, and it is easy to dissociate oxygen and combine with carbon. When the amount of carbon is insufficient, other oxides that are difficult to reduce cannot dissociate oxygen, or the dissociation process with oxygen is inhibited. Therefore, it is difficult to compete with FeO for carbon, thus most of the carbon is used by FeO.
In the case of sufficient or excess carbon, the reduction potential of each reducing substance increases, and the distribution of carbon on each oxide is different. Consequently, the content of each element in the metal increases with the increase in AIR, which shows that the mole fraction of each reduced metal element increases gradually.
4.3 Effect of AIR on the Distribution of C
The effect of the AIR on the distribution of C is shown in Fig. 8. It can be observed that most of the reducing agents are used to reduce TiO2 and FeO, followed by Fe2O3, and part of C also acts as a trace element. When AIR increased from 120 kg/T to 134 kg/T, the amount of C involved in the reduction of TiO2 and FeO increased from 6.3 mol to 7.1 mol, that is, an increase of approximately 13%. The amount of C that became a trace element in the solid solution increased gradually because it was sufficient. AIR had slight effect on the amount of C involved in the reduction of Fe2O3.
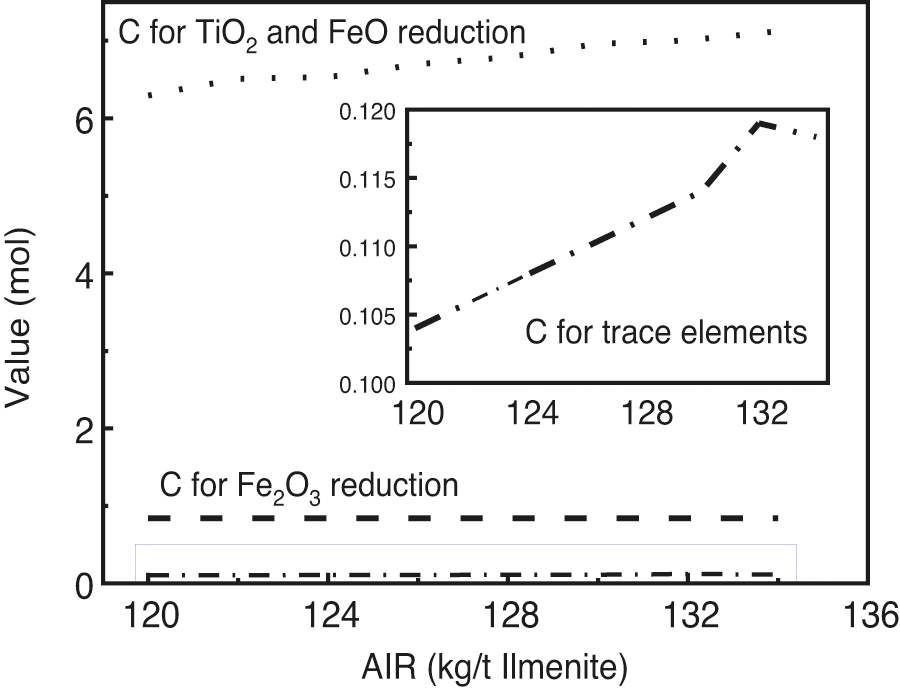
Figure 8: Effect of AIR on the distribution of C
4.4 Effect of AIR on the Distribution of Elements in Exhaust Gas
Fig. 9 shows the effect of the AIR on the distribution of the elements in the gas. It can be observed that with the increase in AIR, the reduction potential of FeO in the melt is large. Moreover, it is easy to dissociate oxygen and combine with carbon, thus the content of CO increases. When AIR is small and carbon is deficient, other oxides cannot dissociate oxygen, or the dissociation process with oxygen is inhibited; thus, most of the carbon is used by FeO, and the amount of CO in the exhaust gas reduces. As the quantity of anthracite increases, the contents of CO2, H2O, and CH4 in the discharged gas have a slightly increasing trend, which shows that the quality of various gases increases gradually.
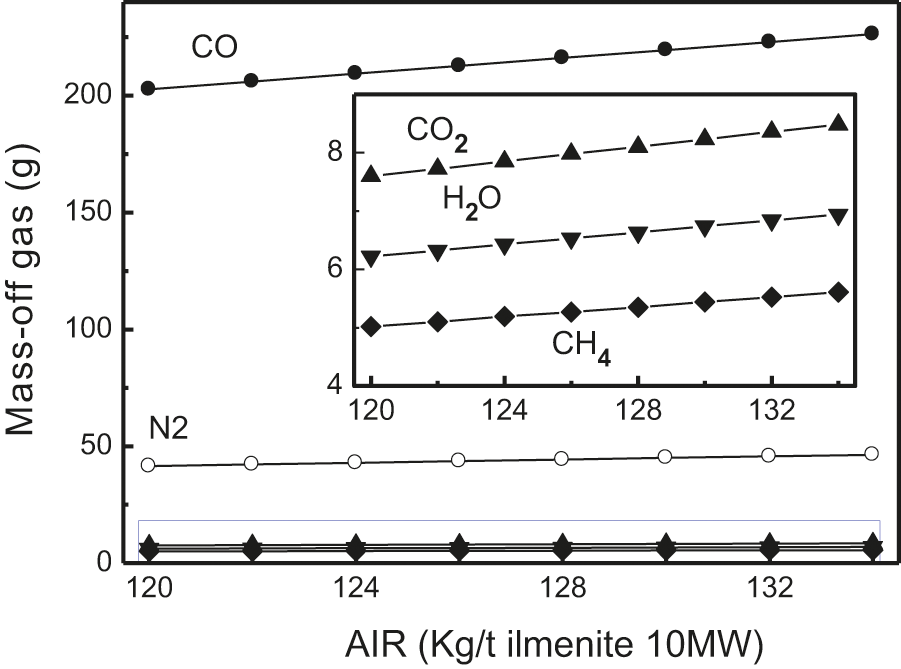
Figure 9: Effect of AIR on the distribution of element in mass-off gas
(1) With the increase in AIR, the mass fraction of TiO2, MgO, CaO, Al2O3, and SiO2 in the titanium slag increased, while the mass fraction of FeO decreased. With an increase in the content of each element in the metal, the mole fraction of the metal element increased gradually. The amount of C involved in the reduction of TiO2 and FeO as well as that dissolved into trace elements increased, while the size of AIR had slight effect on the amount of C involved in the reduction of Fe2O3.
(2) AIR has an important influence on the distribution behavior of elements in titanium slag and hot metal. When AIR increased from 120 kg/t to 134 kg/t, the mass fraction of TiO2 increased from 83.8% to 91.77%, and the mass fraction of FeO decreased from 14.7% to 6.25%. The iron content in hot metal increased from 6.25 kmol to 7.08 kmol, and the carbon content increased from 0.97 kmol to 1.09 kmol. By adjusting the AIR, the selective reduction of oxides in the melt can be realized, the process can be optimized, and the grade of titanium slag can be improved. Considering the production cost, the AIR can be adjusted to achieve the selective reduction of oxides in the melt and reduce the impurities in the slag.
Funding Statement: This work was financially supported by “High-Level Youth Talent Special Support Plan” of Kunming City (C202014002), the National Natural Science Foundation of China (51904137), the Applied Basic Research Projects of Yunnan Province (2019FD044), the Open Projects of State Key Laboratory of Complex Nonferrous Metal Resources Clean Utilization (CNMRCUKF1905), and the Talent Training Project of Kunming University (YJL2102). The authors are grateful to NSFC and “High-Level Talent Special Support Plan” of Kunming City (C201905002).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Gao, F., Nie, Z., Yang, D., Sun, B., Liu, Y. et al. (2018). Environmental impacts analysis of titanium sponge production using kroll process in China. Journal of Cleaner Production, 174, 771–779. DOI 10.1016/j.jclepro.2017.09.240. [Google Scholar] [CrossRef]
2. Akinwamide, S. O., Lemika, S. M., Obadele, B. A., Akinribide, O. J., Abe, B. T. et al. (2019). Study on microstructural and mechanical properties of a stir cast Al (SiC-Mg-TiFe) composite. Fluid Dynamics & Materials Processing, 15(1), 15–26. DOI 10.32604/fdmp.2019.04761. [Google Scholar] [CrossRef]
3. Dang, J., Zhang, G., Chou, K. (2015). Kinetics and mechanism of hydrogen reduction of ilmenite powders. Journal of Alloys and Compounds, 619, 443–451. DOI 10.1016/j.jallcom.2014.09.057. [Google Scholar] [CrossRef]
4. Huang, S., Lei, T., Han, F., Zhou, L. (2014). Effect of ilmenite component and AIR on element distribution of titanium slag smelted by DC arc furnace. Rare Metal Materials and Engineering, 43(12), 2921–2926. DOI 10.1016/S1875-5372(15)60034-0. [Google Scholar] [CrossRef]
5. Geldenhuys, I. J., Reynolds, Q. G., Akdogan, G. (2020). Evaluation of titania-rich slag produced from titaniferous magnetite under fluxless smelting conditions. JOM, 72(10), 1–10. DOI 10.1007/s11837-020-04304-3. [Google Scholar] [CrossRef]
6. Song, B., Lv, X., Miao, H. H., Han, K., Zhang, K. et al. (2016). Effect of Na2 B4O7 addition on carbothermic reduction of ilmenite concentrate. ISIJ International, 56(12), 2140–2146. DOI 10.2355/isijinternational.ISIJINT-2016-338. [Google Scholar] [CrossRef]
7. Deng, G., Wang, X. (2002). Methods of preparing high grade titania feedstock from Panzhihua titanium concentrate. Iron Steel Vanadium Titanium, 23(4), 15–17. DOI 10.3969/j.issn.1004-7638.2002.04.004. [Google Scholar] [CrossRef]
8. Li, D., Liu, H., Zhou, D. (2009). Titanium smelting process. Beijing: Chemical Industry Press. [Google Scholar]
9. Yang, B., Hu, H., He, J. (2015). Titanium based material manufacturing. Beijing: Metallurgical Industry Press. [Google Scholar]
10. Smirnov, K. I., Gamov, P. A. (2021). Pyro-metallurgical processing of ilmenite concentrate with production of iron and titanium oxides. Solid State Phenomena, 316, 385–389. DOI 10.4028/www.scientific.net/SSP.316.385. [Google Scholar] [CrossRef]
11. Dong, H. K., Heo, J. H., Park, H. S., Jin, K. K., Park, J. H. (2020). Improving the production efficiency of high-titania slag in Ti extraction process: Fluxing effect on formation of pseudobrookite. Scientific Reports, 10(1), 6530. DOI 10.1038/s41598-020-63532-4. [Google Scholar] [CrossRef]
12. Nurgali, N., Sariev, O., Mukhambetkaliyev, A., Momenov, B., Kuandykova, A. et al. (2021). Phase composition of titanium-containing raw materials depending on its titanium oxide content. Metalurgija, 60(3–4), 374–376. [Google Scholar]
13. Zhao, Z., Ma, E. (2002). Feasibility study on reducing vanadium content in high titanium’s slag by electric furnace smelting. Titanium Industry Progress, 6, 41–43. DOI 10.3969/j.issn.1009-9964.2002.06.015. [Google Scholar] [CrossRef]
14. Mo, W., Deng, G., Luo, F. (1998). Titanium metallurgy (Second edition). Beijing: Metallurgical Industry Press. [Google Scholar]
15. Kucukkaragoz, C. S., Eric, R. H. (2006). Solid state reduction of a natural ilmenite. Minerals Engineering, 19(3), 334–337. DOI 10.1016/j.mineng.2005.09.015. [Google Scholar] [CrossRef]
16. Sariev, O., Nurgali, N., Beketova, G., Zhanabayev, M., Kainenova, T. et al. (2021). Electrical characteristics of charge mixtures for melting rich titanium slag (RTS). Metalurgija, 60(3–4), 371–373. [Google Scholar]
17. Martin-Treceno, S., Hughes, T., Weaver, N., Marshall, A. T., Bishop, C. M. (2021). Electrochemical study on the reduction of Si and Ti from molten TiO-SiO-AlO-MgO-CaO slag. Journal of the Electrochemical Society, 168(6), 062502. DOI 10.1149/MA2020-02151415mtgabs. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |