 Open Access
Open Access
ARTICLE
Production of Light Fraction-Based Pyrolytic Fuel from Spirulina platensis Microalgae Using Various Low-Cost Natural Catalysts and Insertion
1 Energy Conversion and Conservation Laboratory, Department of Chemical Engineering, Faculty of Technology and Vocational Education, Universitas Pendidikan Indonesia, Bandung, 40154, Indonesia
2 Center of Energy Research and Application (CERAP), Faculty of Technology and Vocational Education, Universitas Pendidikan Indonesia, Bandung, 40154, Indonesia
3 Center for Renewable Fuels Research (CRFR), Department of Mechanical and Industrial Engineering, Universitas Negeri Malang, Malang, 65145, Indonesia
4 Center of Advanced Materials for Renewable Energy (CAMRY), Universitas Negeri Malang, Malang, 65145, Indonesias
5 Department of Mechanical Engineering, Universitas Brawijaya, Malang, 65145, Indonesia
6 School of Chemical Engineering and Energy, Universiti Teknologi Malaysia, Johor Bahru, 81310, Malaysia
7 Department of Mechanical Engineering Education, Faculty of Teacher Training and Education, Universitas Sebelas Maret, Surakarta, 57126, Indonesia
* Corresponding Authors: Indra Mamad Gandidi. Email: ; Nugroho Agung Pambudi. Email:
Energy Engineering 2024, 121(12), 3635-3648. https://doi.org/10.32604/ee.2024.054943
Received 12 June 2024; Accepted 09 August 2024; Issue published 22 November 2024
Abstract
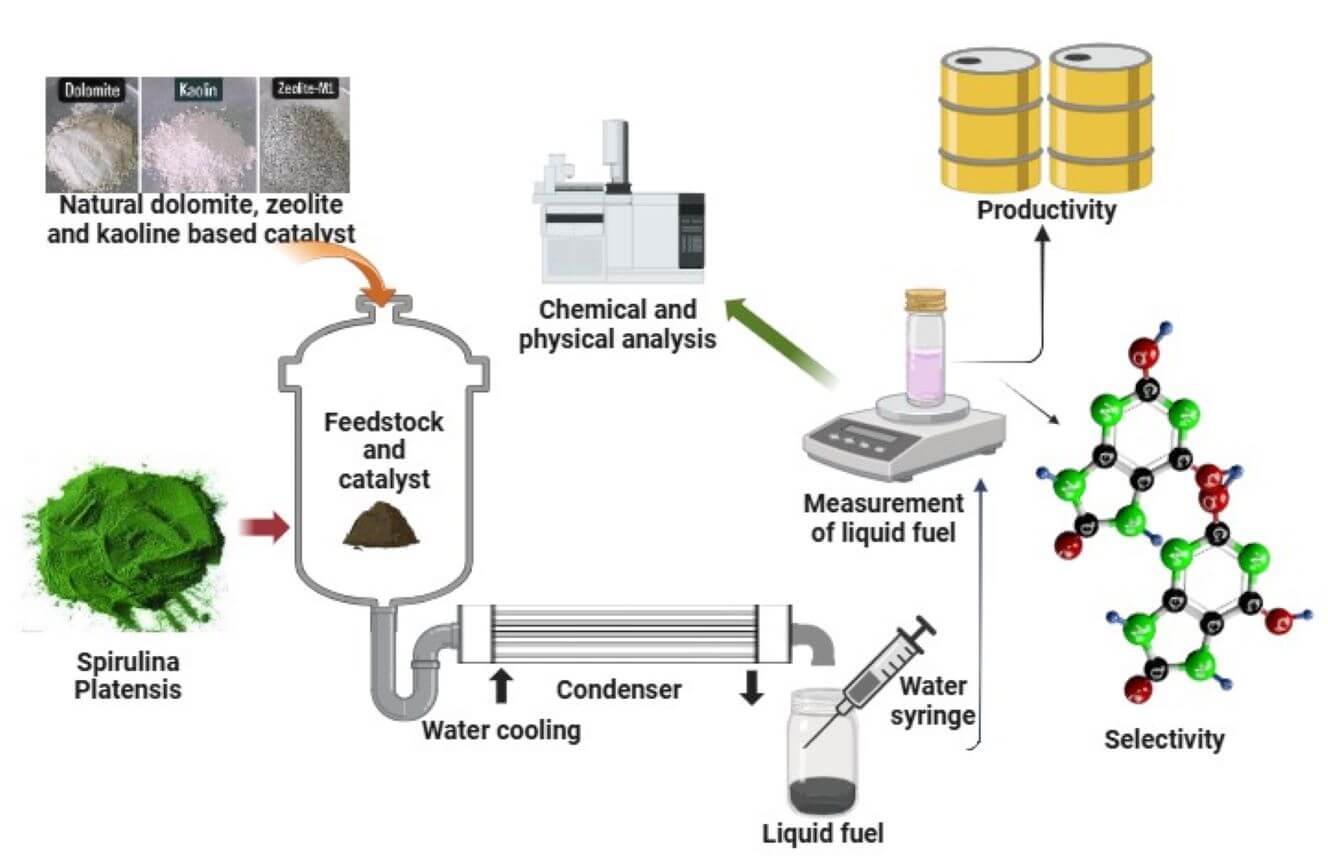
The use of catalysts has significantly enhanced the yield and quality of in-situ pyrolysis products. However, there is a lack of understanding regarding pyrolysis approaches that utilize several low-cost natural catalysts (LCC) and their placement within the reactor. Therefore, this study aims to examine the effects of various LCC on the in-situ pyrolysis of spirulina platensis microalgae (SPM) and investigate the impact of different types of catalysts. We employed LCC such as zeolite, dolomite, kaolin, and activated carbon, with both layered and uniformly mixed LCC-SPM placements. Each experiment was conducted at a constant temperature of 500°C for 60 min. The resulting pyrolytic liquids (bio-oil) and syngas were analyzed using a Gas Chromatography Mass Spectrometry (GC-MS) analyzer to determine the distribution of hydrocarbon compounds. The experimental results indicated that the presence of catalysts significantly influenced the mass yield productivity of liquid fuels and syngas. Activated carbon and zeolite were preferred among the four catalysts for producing liquid fuels (22.4 and 18.6 wt%) when layered and uniformly mixed, respectively. Kaolin with a layered mixture with SPM was more suitable for the production of light fractions (C5–C12), achieving approximately 95.7% peak area, while zeolite with a uniform mixture produced the highest light fraction at about 86.3% peak area. All catalysts except kaolin significantly increased the aromatic compounds in the liquid fuels. Although the amount of oxygenated hydrocarbons in the bio-oil remained relatively high, the final hydrocarbon composition was highly comparable to conventional fuels such as gasoline-88, which has a C5–C12 hydrocarbon distribution of approximately 88.1% peak area. Regarding the syngas products, all catalysts except activated carbon successfully converted nitromethane compounds into tetranitromethane hydrocarbons, with activated carbon predominantly yielding nitromethane compounds.Graphic Abstract
Keywords
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools