

 | Energy Engineering |  |
DOI: 10.32604/EE.2021.014810
ARTICLE
An Experimental Study on Oxidized Mercury Adsorption by Bromide Blended Coal Combustion Fly Ash
1State Key Laboratory of Coal Combustion, School of Energy and Power Engineering, Huazhong University of Science and Technology, Wuhan, 430074, China
2Shenzhen Huazhong University of Science and Technology Research Institute, Shenzhen, 518000, China
3Huaneng Clean Energy Technology Research Institute, Beijing, 102209, China
*Corresponding Author: Guangqian Luo. Email: guangqian.luo@hust.edu.cn
Received: 01 November 2020; Accepted: 11 March 2021
Abstract: The application of forced mercury oxidation technology would lead to an increase of Hg2+ concentration in the flue gas. Although Hg2+ can be easily removed in the WFGD, the mercury re-emission in the WFGD can decrease the total removal of mercury from coal-fired power plants. Hence, it is necessary to control Hg2+ concentration in the devices before the WFGD. Fly ash adsorbent is considered as a potential alternative for commercial activated carbon adsorbent. However, the adsorption efficiency of the original fly ash is low. Modification procedure is needed to enhance the adsorption performance. In this study, the adsorption of Hg2+ by brominated fly ash was studied. The fly ash was collected from the full-scale power plant utilizing bromide-blended coal combustion technology. The brominated fly ash exhibited excellent performance for Hg2+ removal. The flue gas component HBr and SO2 could improve adsorbent’s performance, while HCl would hinder its adsorption process. Also, it was demonstrated by Hg-TPD experiments that the adsorbed Hg2+ mainly existed on the fly ash surface in the form of HgBr2. In summary, the brominated fly ash has a broad application prospect for mercury control.
Keywords: Mercury; fly ash; bromide; adsorbents; flue gas
Due to high volatility and biological toxicity, mercury (Hg) has been received increasing attention in recent years [1]. Coal-fired power plants emitted millions of tons of mercury each year. There are main three forms of mercury in the flue gas, elemental mercury (Hg0), oxidized mercury (Hg2+), and particulate mercury (HgP) [2,3]. By existing air pollutant control devices (APCDs), partial mercury could be captured. Wherein, Hg0 is the trickiest form to be removed by existing APCDs due to its stable physical and chemical properties. Improving the oxidation rate of Hg0 is crucial to mercury control.
Bromide-blended coal combustion technology has proven to improve the oxidation rate of Hg0 significantly [4,5]. Vosteen et al. [6] research found that the oxidation rate could reach 90% when bromide was added into coal. Although Hg2+ is easily water-soluble, the mercury re-emission is not negligible contributed to the reducing substances in the desulfurization slurry. It was reported that the mercury re-emission rate could reach 88% in some power plants [7]. Our previous studies [8,9] also demonstrated that the transformation from Hg2+ to Hg0 occurred in the aqueous phase. Therefore, it is necessary to remove Hg2+ as much as possible before the wet flue gas desulfurization systems (WFGD). Activated carbon (AC) injection technology is already commercially available in the United States, which was considered as one of the most mature mercury removal technologies [10,11]. It exhibited a good performance on Hg0 removal. However, the cost of commercial activated carbon is high, which hinders its large-scale utilization. As a by-product of coal-fired power plants, fly ash adsorbent is considered as a potential alternative to AC [12,13]. However, the Hg removal efficiency of the original fly ash is poor due to its undeveloped pore structure and low content of unburned carbon. So it has to be modified before its application. It was reported that the mercury removal efficiency could be improved by attaching active materials (such as halogens and sulfur) on fly ash surface [14–16]. Many researchers [17–19] have investigated the catalytic oxidation removal of elemental mercury in flue gas by brominated fly ash. But little attention was paid to the Hg2+ adsorption performance of fly ash.
Even though, the adsorption efficiency of Hg2+ by fly ash still needs more investigation. The Hg2+ adsorption performance of brominated fly ash produced from bromide-blended coal combustion technology is worth being studied. Hence, the Hg2+ adsorption performance of brominated fly ash adsorbents was studied in this paper. We collected different fly ash samples from the coal-fired power plant utilizing bromide-blended coal combustion technology. Meanwhile, Hg2+ adsorption experiments were carried out in the laboratory. Hg2+ adsorption efficiencies were compared to select the optimal fly ash modification strategy. Also, Hg-TPD experiments were also conducted in order to characterize the bonding nature of Hg2+ on fly ash surface. Finally, the possibility of utilizing the brominated fly ash to remove Hg2+ was verified in this research.
All the fly ash samples were collected from coal-fired power plant which utilized bromide-blended coal combustion technology. The bromide mass proportion in coal corresponding to the fly ash was 0, 25, 75, 130 ppm, respectively. The collected fly ash was dried and then screened, and the 80~200 mesh particles were selected as samples. The samples were named as BCFA0, BCFA25, BCFA75, and BCFA130. Here, BC represents calcium bromide which was used to be blended with coal. FA denotes represents for fly ash. The numbers (0, 25, 75 and 130) refer to the bromide proportion blended with coal.
2.2 Hg2+ Adsorption Experiments
The Hg2+ adsorption experimental apparatus (see Fig. 1) consists of four parts: a gas generator, a reaction unit, a test unit, and exhaust gas treatment system. The gaseous HgCl2 was generated by the 3315 module of the Hg-CEMS system (Tekran, America). The concentration of HgCl2 at the export of the 3315 module was 10.88 μg/m3. The HBr, HCl, and SO2 gases were controlled by the mass flowmeters. The HgCl2 and other gases were mixed with a gas mixer. The quartz reactor was placed inside a tubular furnace, and the adsorbents were fixed in the middle of the reactor with quartz cotton. The heating temperature was 110°C. The concentration of Hg2+ was detected by the 3320 and 2537 module of Hg-CEMS systems. Exhaust gas was purged by the exhaust gas treatment systems and then emitted into the atmosphere.
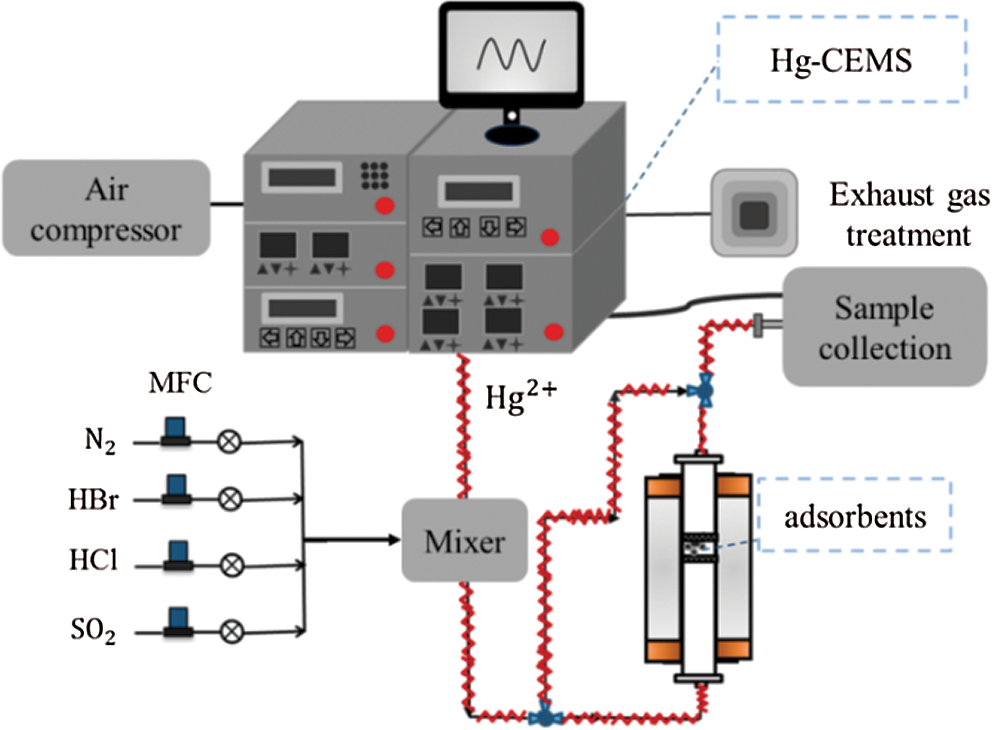
Figure 1: Schematic of Hg2+ adsorption experiments
2.3 Temperature-Programmed Desorption (TPD) Experiment
The temperature-programmed desorption (TPD) experiment (see Fig. 2) was carried out in a fixed bed reactor to identify the binding form of adsorbed Hg2+ by fly ash adsorbent. 1 L/min high purity N2 controlled by a mass flowmeter was used as the carrier gas. The gas produced by TPD was detected by the 3320 and 2537 module of Hg-CEMS systems. The temperature range was controlled between 40°C–60°C, and the heating rate was set as 8 °C/min. Before the experiment, 100 mg adsorbed fly ash adsorbents were weighed and placed in the middle of the reactor. The exhaust gas was purged into the atmosphere swept by high purity N2.
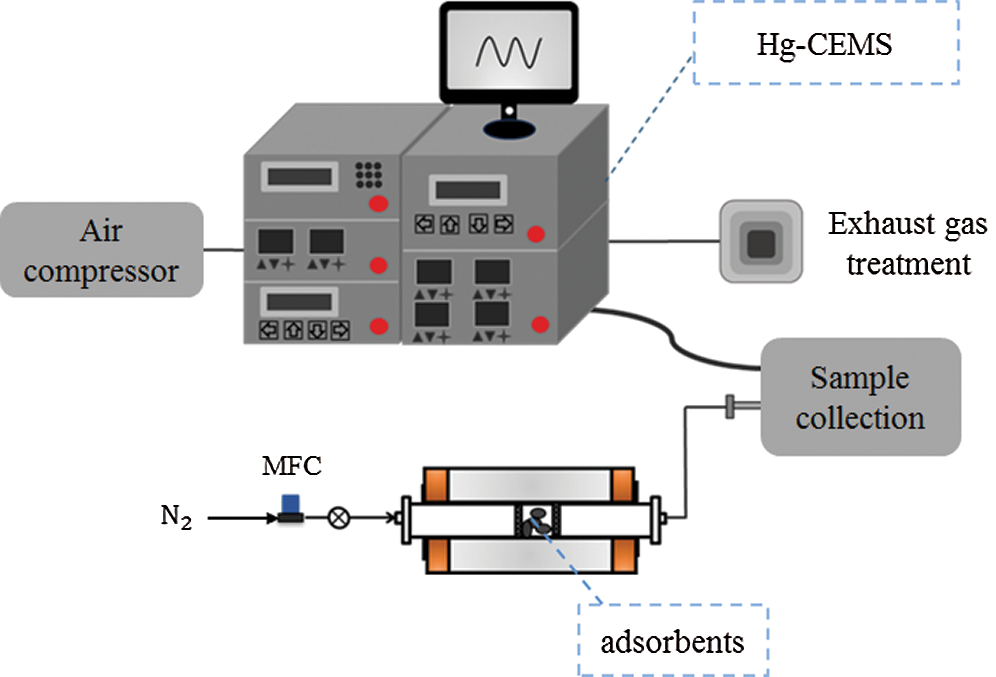
Figure 2: Schematic of mercury TPD experiments
3.1 Effects of Bromide Proportion in Coal on Hg2+ Adsorption by Fly Ash
In order to investigate the effects of bromide proportion on Hg2+ adsorption efficiency, fly ash adsorbents produced by bromide-blended coal combustion were utilized to adsorb oxidized mercury. The Hg2+ adsorption experiment was conducted at 110°C. The concentration of Hg2+ at the reactor inlet was 10.88 µg/m3. The space velocity is 4123.8 h-1. It can be seen from Fig. 3a that the initial Hg2+ removal efficiency of BCFA0 is around 17.79%, which is the lowest among all the fly ash absorbents. With the increase of bromide concentration in coal, the adsorption efficiency of fly ash is enhanced gradually. Moreover, the breakthrough time of fly ash adsorbents became longer. It was implied that bromine could promote the Hg2+ adsorption capacity of fly ash. However, the difference in the initial Hg2+ removal efficiency between BCFA75 and BCFA130 is not obvious, which means the Hg2+ adsorption capacity could not be improved significantly with high bromide concentration. Previous study [4] has shown that bromide in coal was transformed into HBr largely in the flue gas. A large amount of HBr could be adsorbed by the active sites on the fly ash surface. The bromine sites have an ability to capture Hg2+, which is the reason why brominated fly ash could adsorb Hg2+. When the bromide proportion in coal increased, the bromine adsorption sites in fly ash also increased. Hence, Hg2+ adsorption efficiency was improved. However, the adsorption efficiency of BCFA75 and BCFA130 were very close to each other. In other words, the increase of Br content did not continue to improve the adsorption efficiency of fly ash. According to the study of Niksa [20], the bromine could bind to the adsorption site on unburned carbon which could adsorb Hg2+. It might be that the number of bromine adsorption sites on the two adsorbents is similar, so the adsorption efficiency of BCFA75 and BCFA130 were similar.
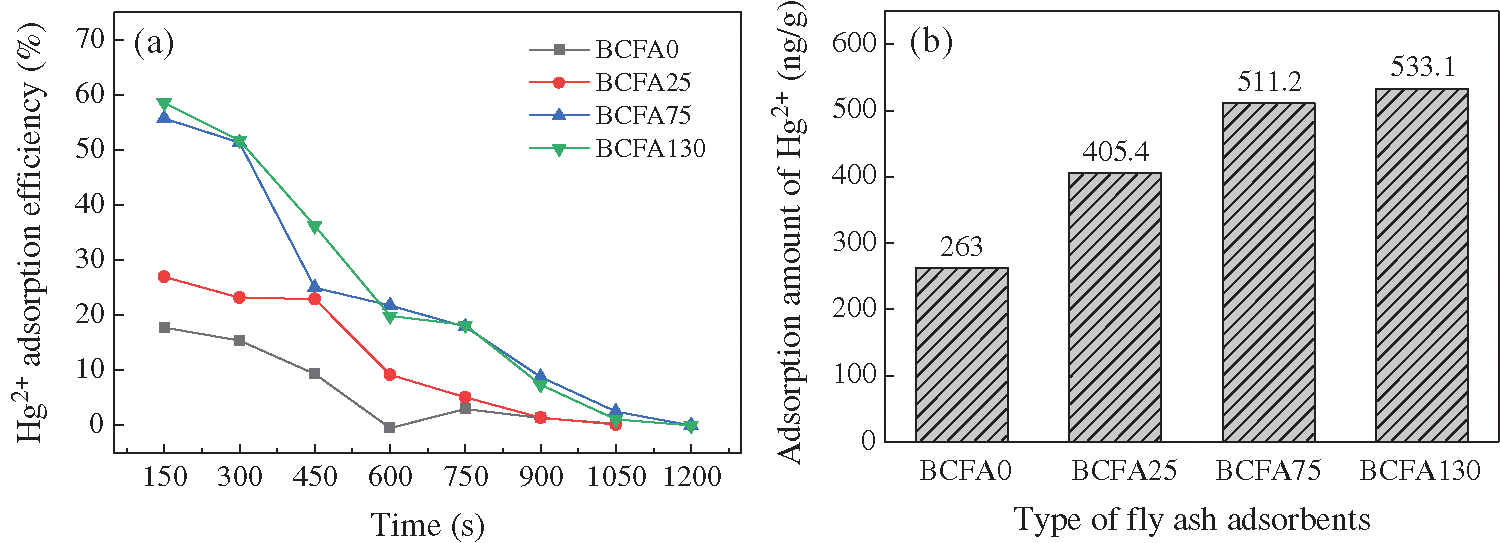
Figure 3: (a) Effects of bromide proportion in coal on Hg2+ adsorption efficiency. (b) Hg2+ adsorption amount of different fly ash adsorbents
3.2 Effects of Temperature on Hg 2+ Adsorption by Fly Ash
Temperature is a significant factor that could affect the adsorption efficiency of absorbents. The BCFA75 was chosen to investigate the effects of temperature. The adsorption temperature range was selected from 110°C to 180°C. It can be seen from Fig. 4a that the initial Hg2+ removal efficiency declined with the increase of temperature. Obviously, the adsorption capacity was also greatly reduced (Fig. 4b). Both physical adsorption and chemical adsorption occurred in the Hg2+ adsorption process. The Hg2+ compounds were firstly adsorbed on the fly ash surface by physical adsorption, and then they were bound to functional groups. On the one hand, the physical adsorption process is due to the intermolecular Van der Waals force. Because of the increased thermal motion of molecules, Van der Waals force was weakened under higher temperature. On the other hand, chemical bonds formed by chemical adsorption could be broken with the increase of the temperature. Therefore, the Hg2+ adsorption efficiency of BCFA75 decreased. In addition, the breakthrough time also shortened from the 1200 s to 900 s, which indicated that high temperature would hamper the Hg2+ adsorption.
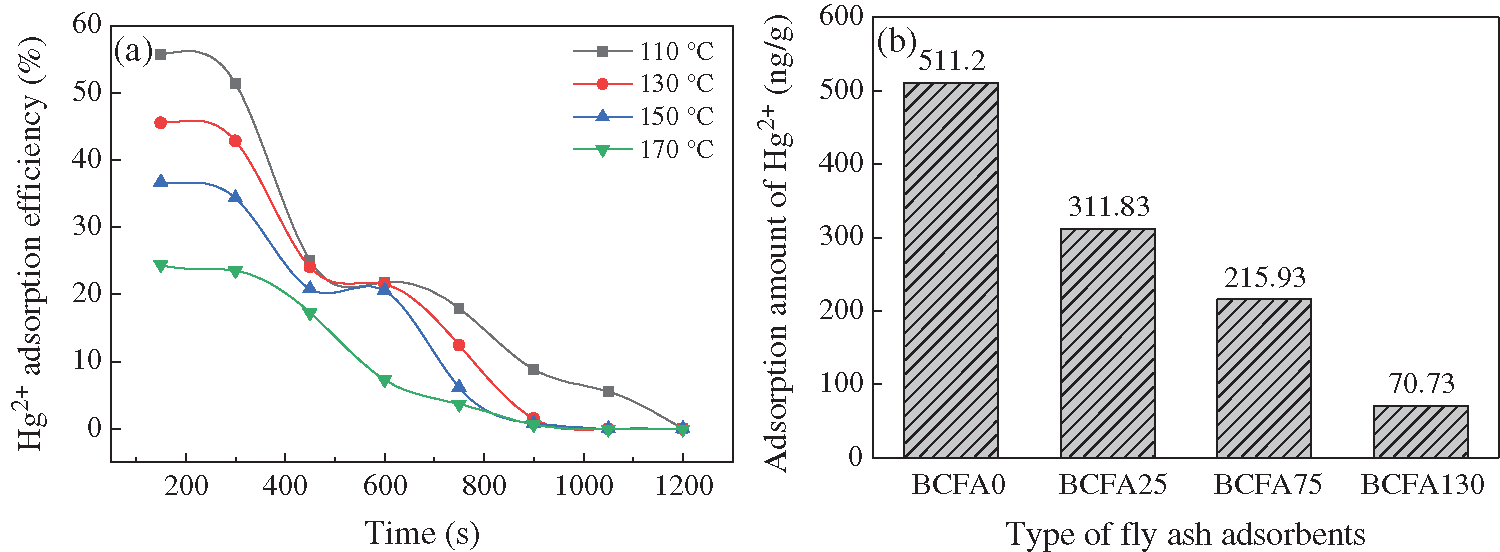
Figure 4: (a) Effects of temperature on the Hg2+ adsorption efficiency. (b) Hg2+ adsorption amount of adsorbents
3.3 Effects of Space Velocity on Hg2+ Adsorption by Fly Ash
BCFA130 was chosen to determine the effects of space velocity in this section. The experiment was conducted at 110°C. The space velocities were set as 16497.0 h−1, 8247.6 h−1, and 4123.8 h−1, respectively. It can be observed from Fig. 5 that the initial adsorption efficiency and the breakthrough time decreased with the increase of space velocity, which indicated that low space velocity could improve the removal of Hg2+. It is due to lower space velocity corresponds to more amounts of adsorbents. Higher doses of adsorbents could certainly absorb more Hg2+.
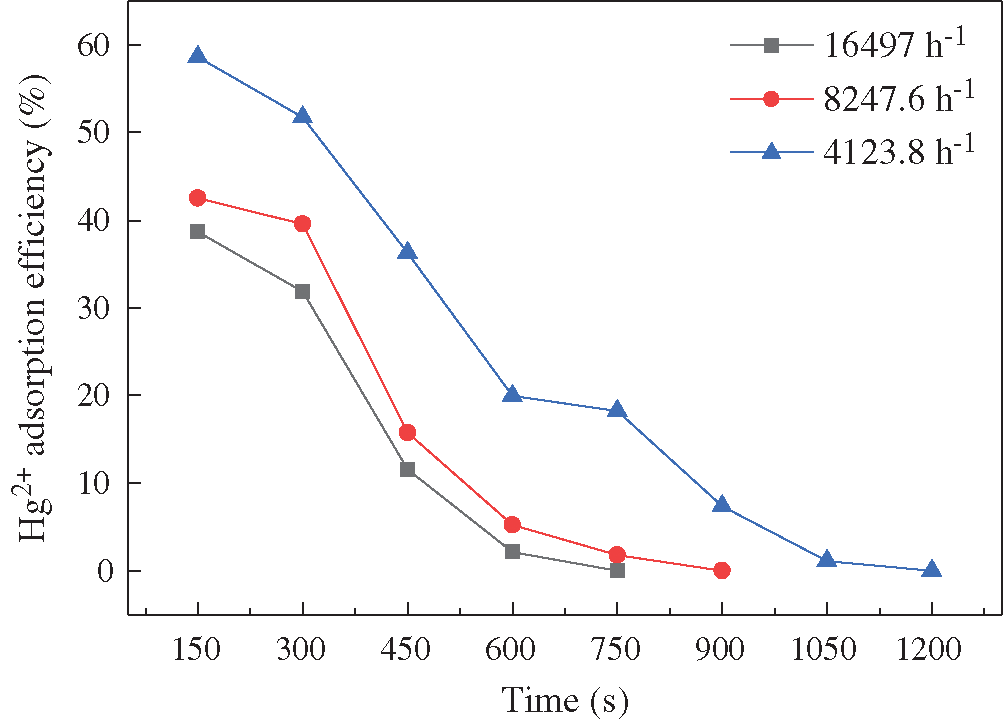
Figure 5: Effects of space velocity on the Hg2+ adsorption
3.4 Effects of Flue Gas Compositions on Hg2+ Adsorption by Fly Ash
The HBr, HCl and other components in realistic flue gas could also affect the adsorption performance of fly ash adsorbents. The simulated gas was used to conduct adsorption experiments. The experimental conditions were summarized in Tab. 1.
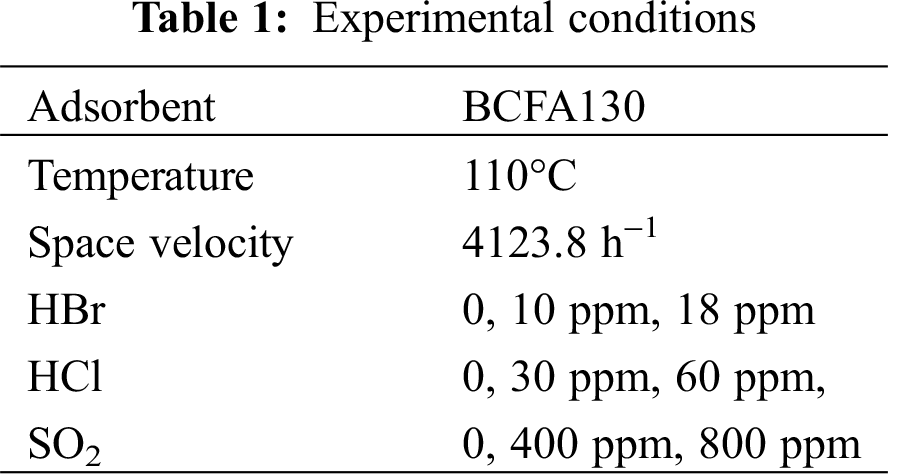
As described in the previous section, HBr has unignorable influences on Hg2+ adsorption. Yang et al. [4] found that the concentration of HBr in flue gas could not exceed 18 ppm no matter how much bromide was added in the coal combustion process. Accordingly, the HBr concentration of 0, 10, 18 ppm was chosen in our adsorption experiments. The results were depicted in Fig. 6. It can be seen that the adsorption efficiency decreased by 20% when HBr was added. As previously mentioned, HBr could bind to the adsorption sites of fly ash, which affected the adsorption process of Hg2+. The competitive adsorption of HBr with Hg2+ reduced Hg2+ removal efficiency. We can also see that the BCFA130 adsorbents were broken through after 1200 s in the absence of HBr. However, the BCFA130 adsorbents showed stable adsorption capacity in the presence of HBr. This was because HBr could supplement the brominated active sites on the fly ash surface, which enabled the BCFA130 adsorbents to adsorb Hg2+ continuously. It was indicated that we could make full use of the adsorption sites of fly ash in the flue gas contained HBr.
Figure 6: Effects of HBr concentration on Hg2+ adsorption efficiency
There was a large amount of HCl in the actual flue gas. The concentration of 0, 30, 60 ppm HCl was selected to investigate the HCl effects. From Fig. 7, we can see that the Hg2+ removal efficiency declined from 58.6% to 47.0% when HCl was added. Moreover, the breakthrough time also decreased. It was inferred that HCl preempted the adsorption sites on the fly ash, which led to the decrease of Hg2+ adsorption process. In contrast to HBr, the addition of HCl did not improve the adsorptive performance. It was because most of Hg2+ existed in the flue gas in the form of HgCl2. The chlorinated sites on the fly ash surface could not adsorb the HgCl2. Therefore, the critical problem is the solution to the adverse effects of HgCl2 on fly ash adsorbents.
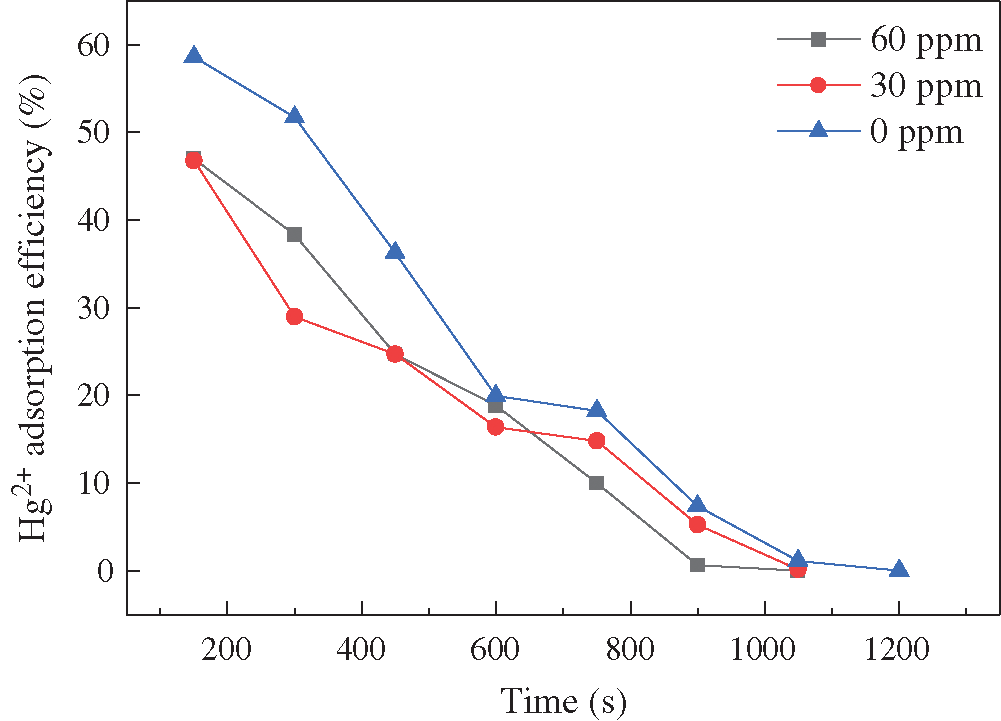
Figure 7: Effects of HCl concentration on Hg2+ adsorption efficiency
A large amount of SO2 was added in the experiments to observe its effects on the fly ash adsorbent. It can be seen from Fig. 8 that the adsorbents were not broken through during the experiment in the presence of SO2. But the initial Hg2+ adsorption efficiency was dropped. Previous research [21] has shown that the SO2 molecules are easily adsorbed inside the microporous structure on the surface of the adsorbent because of their strong polarity. The adsorbed SO2 could react with O2 and H2O to form sulfur-containing groups, which have a strong adsorption capacity to Hg2+ [22, 23]. Therefore, SO2 can improve the adsorption performance of fly ash adsorbents. Due to the competitive adsorption process, the adsorption efficiency of Hg2+ was reduced in the concentration of 800 ppm SO2 compared with 400 ppm. It was shown that the fly ash adsorbents are a promising candidate for being used in the equipment the upstream of the WFGD system.
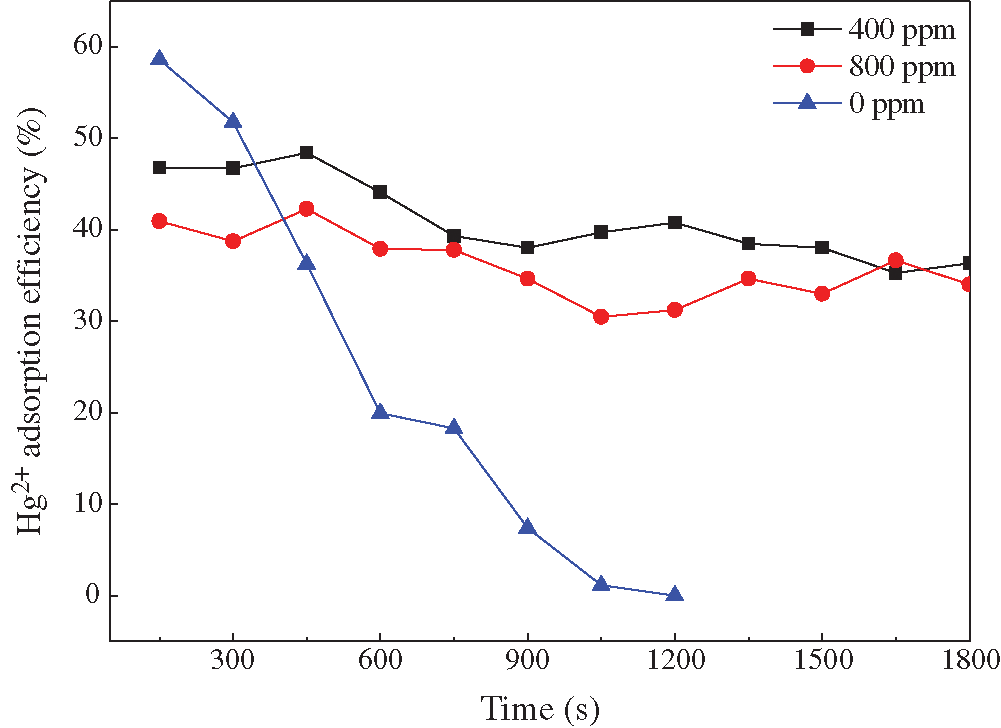
Figure 8: Effects of SO2 concentration on the Hg2+ adsorption efficiency
In order to investigate the binding form of Hg2+ with fly ash adsorbents, the Hg-TPD experiments were conducted on the BCFA130 adsorbents, which were broken through. It can be seen from Fig. 9 that the maximum precipitation peak was located at 260°C. It was proved that the precipitation peak at 260°C corresponds to HgBr2 [22]. The mechanisms of the Hg2+ adsorption process are as follows [20]:
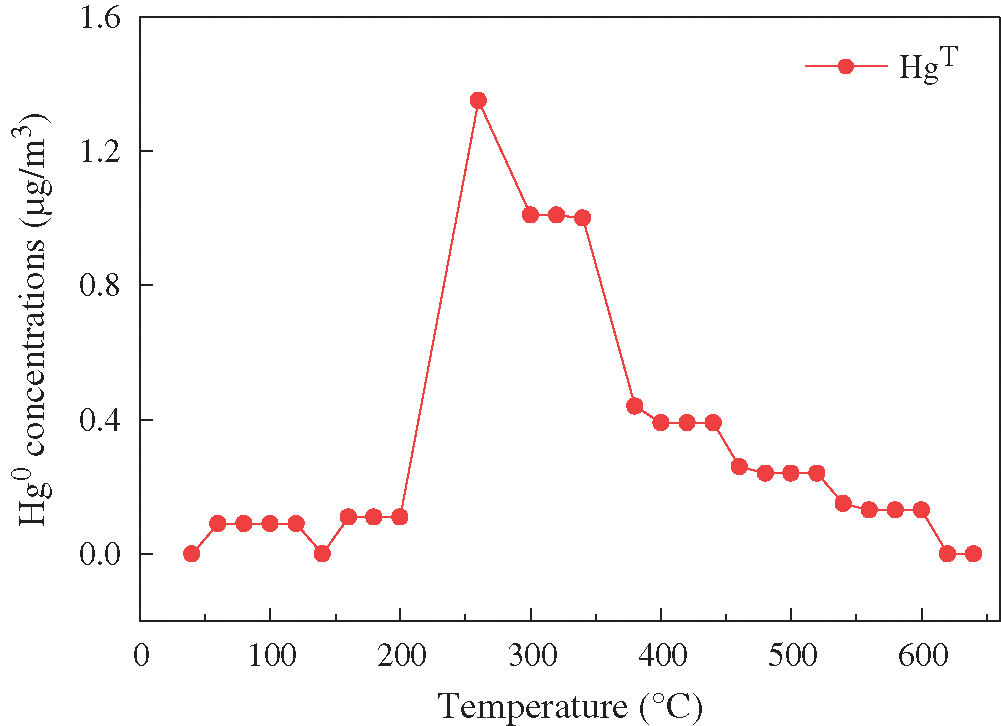
Figure 9: Mercury TPD results of spent fly ash adsorbents
The Hg2+ adsorption performance of brominated fly ash adsorbents was investigated in this paper. The effects of various parameters on Hg2+ adsorption efficiency were explored systematically by Hg2+ adsorption experiments. The main conclusions of this research are as follows. (1) Moderate bromide proportion in coal, lower temperature, and smaller space velocity could enhance the Hg2+ adsorption efficiency. In the realistic coal-fired power plants, the performance of brominated fly ash adsorbents can be greatly improved under suitable working conditions. (2) The HBr and SO2 can extend the breakthrough time of brominated fly ash adsorbents. The HCl would hinder adsorbents’ adsorption efficiency. The HBr, SO2, and HCl will compete with Hg2+ for adsorption sites on brominated fly ash surface. (3) The Hg2+ adsorption process mainly takes place by chemical adsorption. The adsorbed mercury mainly exists on the fly ash surface in the form of HgBr2.
As a by-product of the bromide-blended coal combustion technology, the brominated fly ash adsorbent is easily available, economical and efficient.
Acknowledgement: The Huaneng Clean Energy Technology Research Institute was appreciated for providing the experimental bench and technical support. The Analytical and Testing Center of Huazhong University of Science and Technology was gratefully acknowledged for the assistance of experimental measurements.
Funding Statement: This research was financially supported by the National Key Research and Development Program of China (Grant No. 2016YFB0600603), National Natural Science Foundation of China (Grant No. 51776084) and Shenzhen Science and Technology Innovation Committee (Grant No. JCYJ20190809095003718).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Wang, S., Zhang, L., Zhao, B., Meng, Y., Hao, J. (2012). Mitigation potential of mercury emissions from coal-fired power plants in China. Energy & Fuels, 26(8), 4635–4642. DOI 10.1021/ef201990x. [Google Scholar] [CrossRef]
2. Gao, Y., Zhang, Z., Wu, J., Duan, L., Umar, A. et al. (2013). A critical review on the heterogeneous catalytic oxidation of elemental mercury in flue gases. Environmental Science & Technology, 47(19), 10813–10823. DOI 10.1021/es402495h. [Google Scholar] [CrossRef]
3. Zhao, S., Pudasainee, D., Duan, Y., Gupta, R., Liu, M. et al. (2019). A review on mercury in coal combustion process: Content and occurrence forms in coal, transformation, sampling methods, emission and control technologies. Progress in Energy and Combustion Science, 73(123), 26–64. DOI 10.1016/j.pecs.2019.02.001. [Google Scholar] [CrossRef]
4. Yang, Y., Xu, W., Wu, Y., Xiong, J., Zhu, T. et al. (2016). Effect of HBr formation on mercury oxidation via CaBr 2 addition to coal during combustion. RSC Advances, 6(64), 59009–59015. DOI 10.1039/C6RA11468G. [Google Scholar] [CrossRef]
5. Feeley III, T. J., O’Palko, B. A., Jones, A. P. (2008). Developing mercury control technology for coal-fired power plants-from concept to commercial reality. Main Group Chemistry, 7(3), 169–179. DOI 10.1080/10241220802302556. [Google Scholar] [CrossRef]
6. Vosteen, B. W., Kanefke, R., Koser, H. (2006). Bromine-enhanced mercury abatement from combustion flue gases-recent industrial applications and laboratory research. VGB Powertech, 86(3), 70–75. [Google Scholar]
7. Lee, S. J., Seo, Y., Jang, H., Park, K., Baek, J. et al. (2006). Speciation and mass distribution of mercury in a bituminous coal-fired power plant. Atmospheric Environment, 40(12), 2215–2224. DOI 10.1016/j.atmosenv.2005.12.013. [Google Scholar] [CrossRef]
8. Yu, M., Luo, G., Sun, R., Zou, R., Li, X. et al. (2021). A mechanism study on effects of bromide ion on mercury re-emission in WFGD slurry. Chemical Engineering Journal, 406(8), 127010. DOI 10.1016/j.cej.2020.127010. [Google Scholar] [CrossRef]
9. Qiu, Y., Wu, H., Luo, G. Q., Yao, H. (2014). Effects of pH on Hg0 re-emission in WFGD. Advanced Materials Research, 960, 462–468. DOI 10.4028/www.scientific.net/AMR.960-961.462. [Google Scholar] [CrossRef]
10. Sjostrom, S., Durham, M., Bustard, C. J., Martin, C. (2010). Activated carbon injection for mercury control: Overview. Fuel, 89(6), 1320–1322. DOI 10.1016/j.fuel.2009.11.016. [Google Scholar] [CrossRef]
11. Zhao, W., Geng, X., Lu, J., Duan, Y., Liu, S. et al. (2021). Mercury removal performance of brominated biomass activated carbon injection in simulated and coal-fired flue gas. Fuel, 285(5), 119131. DOI 10.1016/j.fuel.2020.119131. [Google Scholar] [CrossRef]
12. Zhang, Y., Duan, W., Liu, Z., Cao, Y. (2014). Effects of modified fly ash on mercury adsorption ability in an entrained-flow reactor. Fuel, 128, 274–280. DOI 10.1016/j.fuel.2014.03.009. [Google Scholar] [CrossRef]
13. Maroto-Valer, M. M., Zhang, Y., Granite, E. J., Tang, Z., Pennline, H. W. (2005). Effect of porous structure and surface functionality on the mercury capacity of a fly ash carbon and its activated sample. Fuel, 84(1), 105–108. DOI 10.1016/j.fuel.2004.07.005. [Google Scholar] [CrossRef]
14. Gu, Y., Zhang, Y., Lin, L., Xu, H., Orndorff, W. et al. (2015). Evaluation of elemental mercury adsorption by fly ash modified with ammonium bromide. Journal of Thermal Analysis and Calorimetry, 119(3), 1663–1672. DOI 10.1007/s10973-014-4376-0. [Google Scholar] [CrossRef]
15. Zhu, T. Y., Kuang, J. Y., Xu, W. Q., Ye, M., Guo, Y. Y. et al. (2012). Study on mercury adsorption performance of modified fly ash. Advanced Materials Research., 343, 246–249. [Google Scholar]
16. Deng, S., Shu, Y., Li, S., Tian, G., Huang, J. et al. (2016). Chemical forms of the fluorine, chlorine, oxygen and carbon in coal fly ash and their correlations with mercury retention. Journal of Hazardous Materials, 301, 400–406. DOI 10.1016/j.jhazmat.2015.09.032. [Google Scholar] [CrossRef]
17. Geng, X., Duan, Y., Zhao, S., Xu, Y., Huang, T. et al. (2019). Study of mercury-removal performance of mechanical2013; chemical-brominated coal-fired fly ash. Energy & Fuels, 33(7), 6670–6677. DOI 10.1021/acs.energyfuels.9b01034. [Google Scholar] [CrossRef]
18. Zhang, Y., Zhao, L., Guo, R., Wang, J., Cao, Y. et al. (2017). Influences of NO on mercury adsorption characteristics for HBr modified fly ash. International Journal of Coal Geology, 170, 77–83. DOI 10.1016/j.coal.2016.10.002. [Google Scholar] [CrossRef]
19. Sasmaz, E., Kirchofer, A., Jew, A. D., Saha, A., Abram, D. et al. (2012). Mercury chemistry on brominated activated carbon. Fuel, 99(2–3), 188–196. DOI 10.1016/j.fuel.2012.04.036. [Google Scholar] [CrossRef]
20. Niksa, S., Naik, C. V., Berry, M. S., Monroe, L. (2009). Interpreting enhanced Hg oxidation with Br addition at Plant Miller. Fuel Processing Technology, 90(11), 1372–1377. DOI 10.1016/j.fuproc.2009.05.022. [Google Scholar] [CrossRef]
21. Mathieu, Y., Tzanis, L., Soulard, M., Patarin, J., Vierling, M. et al. (2013). Adsorption of SOx by oxide materials: A review. Fuel Processing Technology, 114, 81–100. DOI 10.1016/j.fuproc.2013.03.019. [Google Scholar] [CrossRef]
22. Ie, I., Chen, W., Yuan, C., Hung, C., Lin, Y. et al. (2012). Enhancing the adsorption of vapor-phase mercury chloride with an innovative composite sulfur-impregnated activated carbon. Journal of Hazardous Materials, 217(5), 43–50. DOI 10.1016/j.jhazmat.2012.02.035. [Google Scholar] [CrossRef]
23. Hsu, C., Chen, Y., Hsi, H. (2020). Adsorption of aqueous Hg2+ and inhibition of Hg0 re-emission from actual seawater flue gas desulfurization wastewater by using sulfurized activated carbon and NaClO. Science of the Total Environment, 711(2), 135172. DOI 10.1016/j.scitotenv.2019.135172. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |