

 | Energy Engineering |  |
DOI: 10.32604/EE.2021.016157
REVIEW
Advancements in the Development of Various Types of Dye-Sensitized Solar Cells: A Comparative Review
1Department of Mechanical Engineering, College of Engineering, University Malaysia Pahang, Pahang, 26300, Malaysia
2Research Centre for Nano-Materials and Energy Technology, School of Engineering and Technology, Sunway University, Selangor, 47500, Malaysia
3Higher Institution Centre of Excellence, UM Power Energy Dedicated Advanced Centre, University of Malaya, Kuala Lumpur, 59990, Malaysia
4Department of Mechanical Engineering, Institute of Engineering and Technology, GLA University, Mathura, 281406, India
5School of Energy Management, Shri Mata Vaishno Devi University, Katra, Jammu and Kashmir, 182320, India
*Corresponding Authors: A. K. Pandey. Email: adarsh.889@gmail.com; adarshp@sunway.edu.my; M. Samykano. Email: mahendran@ump.edu.my
Received: 12 February 2021; Accepted: 21 April 2021
Abstract: The global increase in energy demand has resulted in the depletion of non-renewable resources and caused environmental degradation. Consequently, emerging renewable technologies are a potential solution to fulfil energy demand and mitigate the effect of global warming. Low-cost solar energy harvesting technologies are most feasible technologies. Various solar cells technologies have been developed with improved overall performance and conversion efficiency. However, due to low cost and a wide range of applications, dye-sensitized solar cells (DSSCs) have been immensely focused on one of the most promising third-generation solar cells. The highest conversion efficiency of DSSC achieved after three decades of research is more than 14%, but the commercialization of this technology is still a challenge. In this review paper, an attempt has been made to present the comparison of different articles published, that gives the in-depth study of recent developments in various types of DSSCs based on architectural assembly and physical appearance. An overview of the limitations and challenges with their possible improvement strategies have also been discussed. This review paper concludes that appropriate selection of electrolytes dramatically affects the performance of DSSC, and quasi-solid-state electrolyte proves to be a better option. Besides, it also concludes that tandem structures are widely agreed with the approach to expand light utilization spectrum for an overall increase in its performance. However, still, the research is required, which could efficiently widen the applications of the DSSCs.
Keywords: Dye-sensitized solar cells; solar energy; architectural assembly; quasi-solid electrolyte; tandem structures
Nomenclature
| CIGS | Copperindium gallium selenide |
| ITO | Indium-doped tin oxide |
| DSSC | Dye sensitized solar cells |
| LUMO | Lower unoccupied molecular orbit |
| FTO | Fluorine-doped tin oxide |
| NREL | National Renewable Energy Laboratory |
| HOMO | Highest occupied molecular Orbital |
| PCE | Power conversion efficiency |
The global increase in energy demand from the last few decades is due to rapid growth in industrialization and an upgraded lifestyle. Global energy demand is projected to be doubled by the year 2050 due to this rapid growth [1,2]. Fossil fuel such as oil, natural gas and coal are deemed potential sources of energy from many years to fulfil this global energy demand. This has resulted in the depletion of these non-renewable energy resources and environmental concerns. However, for energy security and mitigation of environmental problems, it is necessary to emphasis on renewable energy sources such as solar, biomass, wind energy, etc. These sustainable energy sources can easily fulfil the increase in energy demand and mitigate environmental pollution [3,4].
Solar energy is the crucial choice between renewable energy resources due to its abundant energy supply. Sunlight is the most available and efficient energy source among other renewable resources. However, the cost and reliability of solar energy production is the greatest obstacle to switching to environmentally friendly and sustainable technologies [5]. Solar cells are the most suitable among all solar energy harvesting technologies for directly converting solar energy into electrical energy, and these are termed as photovoltaic devices. Photovoltaic technology is essential for future energy generation [6]. Various solar cells have been developed and successfully tried in a real environment, but few of them could only be commercialized because of many challenges [1]. Most commercial solar cells are made up of silicon and has high conversion performance, but their large-scale application is still limited due to the high cost. Various solar cells technologies and their power conversion efficiency are illustrated in Fig. 1 [7].
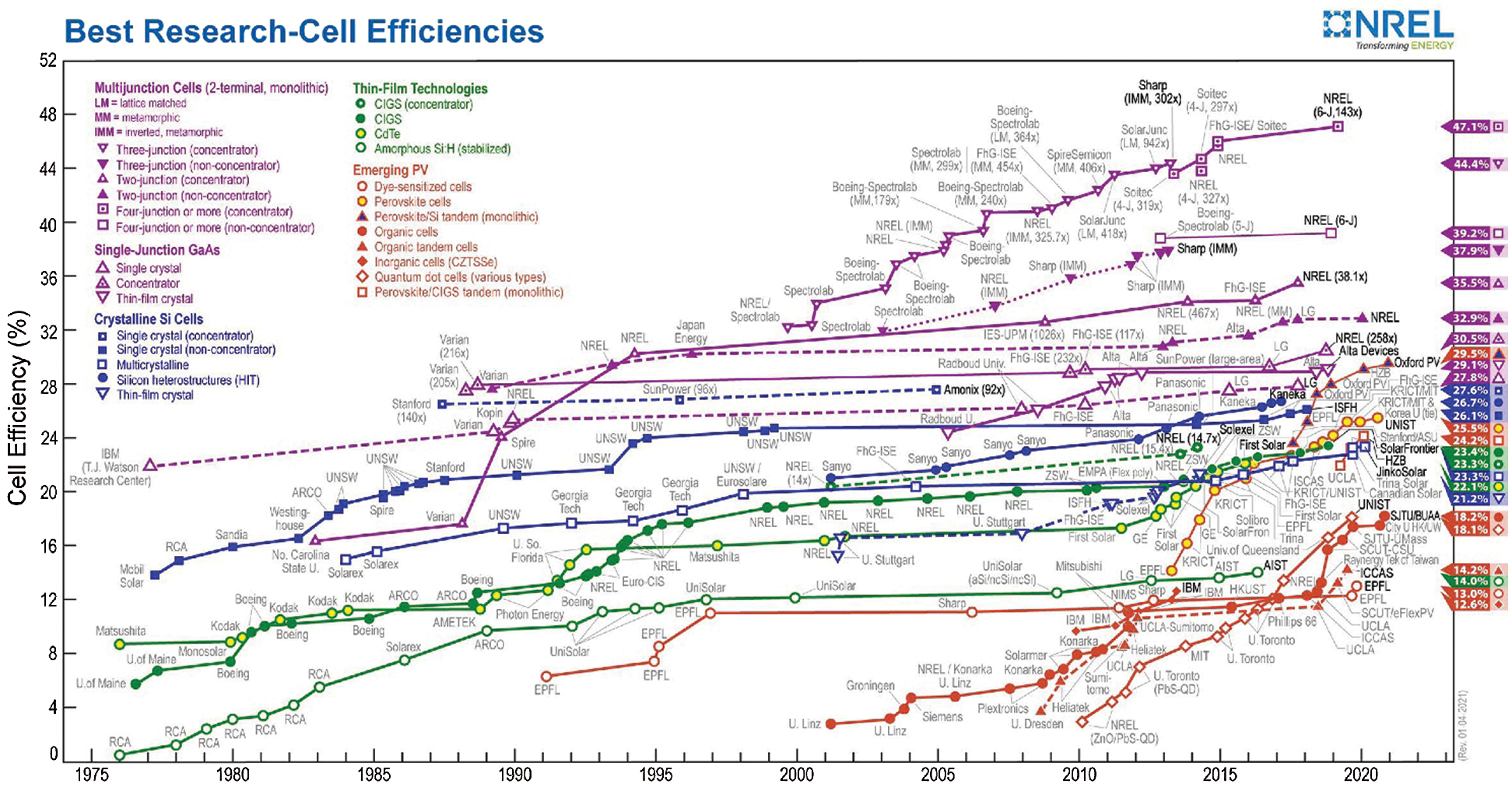
Figure 1: Efficiency graph of various solar cells technologies [7]
There are limited commercial applications of silicon-based solar devices because of the complex and expensive fabrication process, limited integration flexibility and weight of the modules. Recently, however, many organic thin-film solar cells have been discovered that proves more merits over traditional silicon-based solar cells concerning lightweight, durability and low-cost panel. Additionally, the DSSC has proven to be one of the most appropriate replacements to traditional silicon solar cells in cost-effectiveness, optimum performance, and simple manufacturing processes. The highest conversion efficiency of DSSCs achieved after three decades of research is more than 12% which is certified efficiency from National Renewable Energy Laboratory (NREL) [7]. Fig. 2 represents the increasing efficiency trend of DSSC from approximately 7% to 14.2% in between the year 1990 to 2020, respectively [6,8,9]. In recent years, DSSC cell power conversion efficiency under 1-sun illumination has also been reported above 14% [10–12].
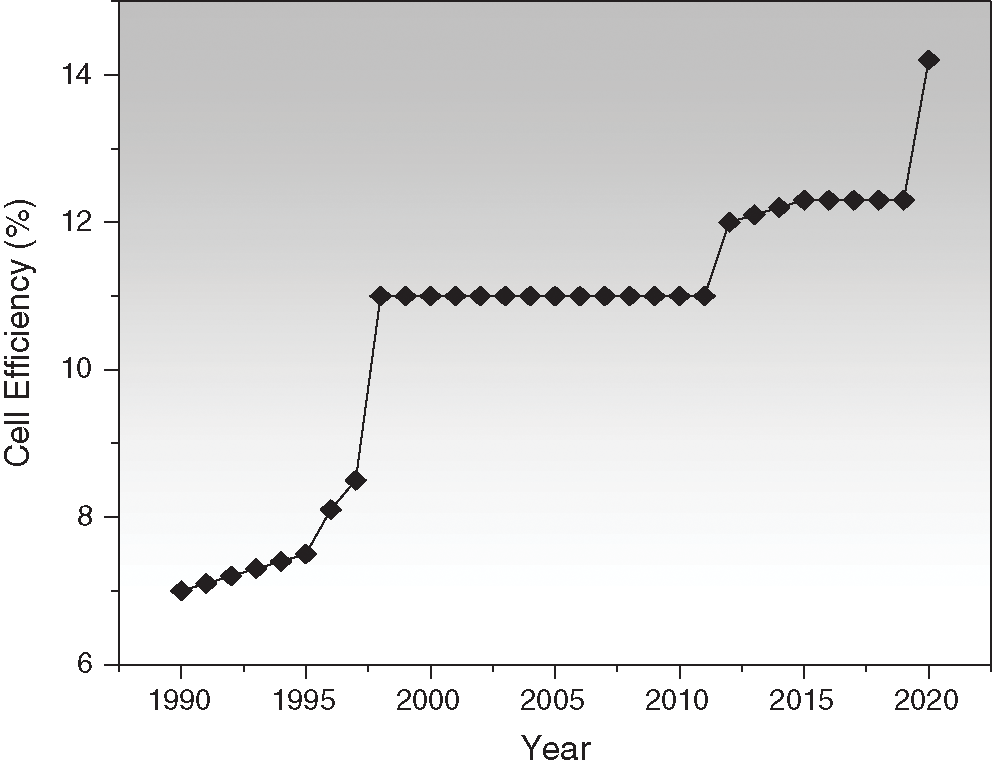
Figure 2: Three-decade efficiency trend of DSSC [6,8,9]
DSSC comprises of various components, i.e., conductive substrate or conductive film-coated substrate, sensitizer (dye), the coated counter electrode, semi-conductor photoanode, electrolyte and catalyst [13]. The schematic diagram of the DSSC has been shown in Fig. 3a. DSSCs are easy to manufacture because they require comparatively very less manufacturing temperature and insensitive to pollutants from the atmosphere. Even in darker conditions or in cloudy weather, these solar cells function better. Such effective use of diffused light makes DSSCs an excellent choice for artificial lighting in indoor applications. A significant rise in the publications over the past three decades has paved the path for intensive research [14].
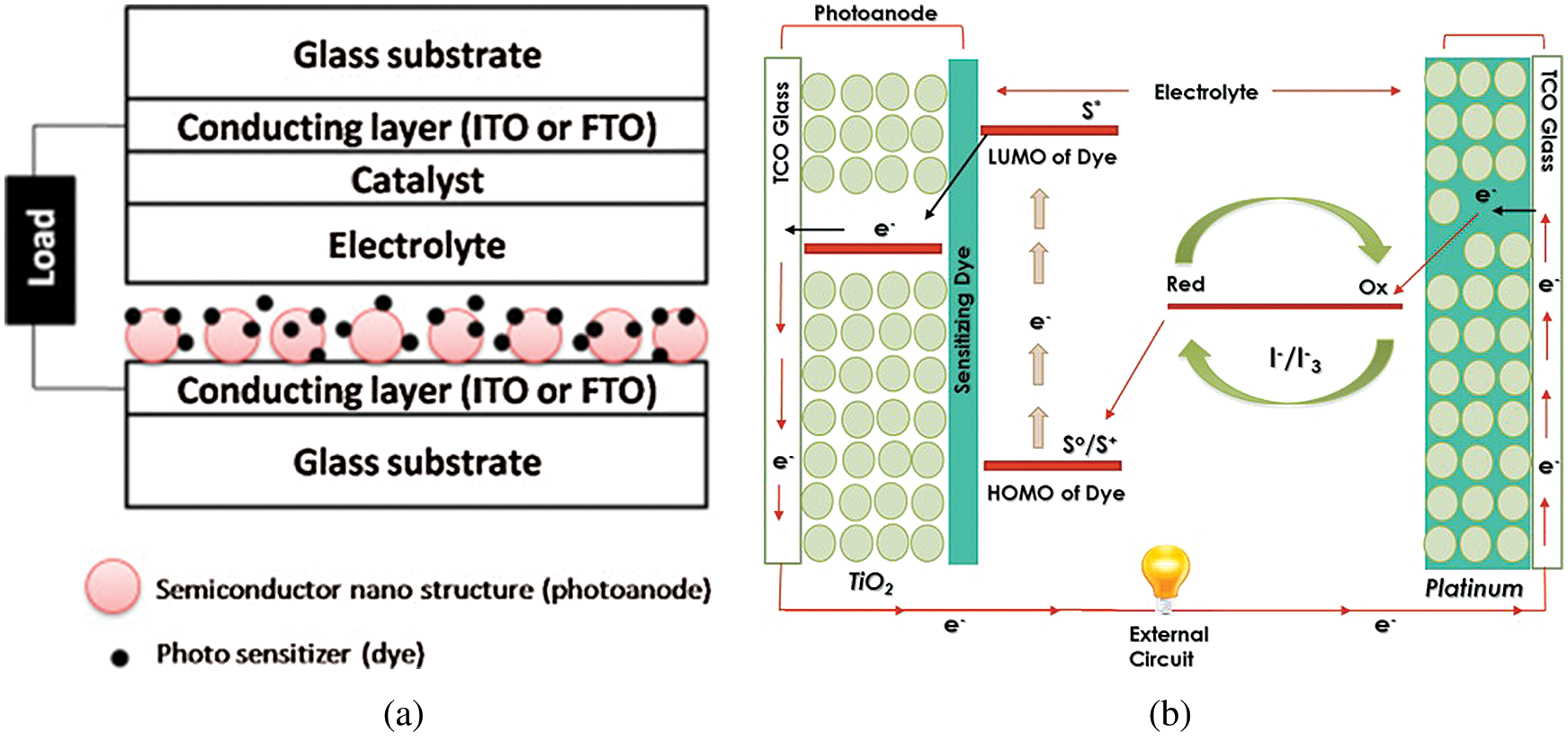
Figure 3: (a) Schematic diagram and (b) Operational principle of a Dye-sensitized solar cell [3,13] (Reprinted with permission from publisher)
The working principle of DSSC is like the photosynthesis process, as illustrated in Fig. 3b. Sunlight is incident on the surface and ejects an electron from the conductive band of dye sensitizer, leaving it in the oxidized state. The ejected electron will be gained by TiO2 photoanode due to thermodynamically suitable energy of lower unoccupied molecular orbit (LUMO). The electron passes through photoanode nanostructure and conducts to an external circuit. Whereas, electrolyte helps to reduce and stabilize the dye. Electrolytes accept an electron from the external circuit to complete the cycle. Here, the oxidized dye gets an electron from the reduced state of redox couple of electrolytes, called dye regeneration. Similarly, the oxidized state of redox couple receives an electron from counter electrode, called electrolyte regeneration [3,15]. Each component in the DSSC contributes towards power conversion efficiency, and the best design of the device is always the optimization and tradeoff between different components [13].
This review paper aims to compare the various published studies, which gives the incite of recent developments in different types of the DSSCs to highlight the bottlenecks pertaining to the improvement of overall performance and commercialization of DSSCs. This review has been divided into two main topics, i.e., (1) Different types of DSSC based on architectural assembly and physical appearance (2) limitations and challenges along with their possible improvement strategies related to the DSSC technology with the conclusion. The rest of the sections are organized as follows. The Section 2 presents the general principle of operation of various types of DSSCs. Section 3 presents the different types of DSSC. Technical challenges associated with the technology has been explained along with the improvement strategies in Section 4. Recent trends in DSSCs are described in Section 5. Finally, the conclusions obtained from the study and future recommendations are presented in Sections 6 and 7.
2 The General Principle of Operation of Various Types of DSSCs
A DSSC is a photovoltaic semiconductor-based device that directly converts natural as well as artificial radiation into electricity. Unlike traditional systems, where the semi-conductor performs both absorptions of light and charge separation and transport phenomenon, the two functions are segregated in a DSSC. Each part of the system heavily decides the cost and conversion efficiency of DSSCs. Improving the properties of absorption of light can be accomplished by optimizing the dye. In contrast, the properties of transport of charges can be enhanced by improving the semi-conductor and electrolyte composition [16].
In the category of materials of n-type, the dye absorbs light which is connected to the surface of mesoporous electrodes of n-type semi-conductor, as shown in Fig. 4a. N-type DSSCs, such as TiO2 or ZnO mesoporous films, are accumulated on top of indium-tin-oxide (ITO) or fluorine-doped tin oxide (FTO) surfaces and comprises of the photoanodes. Here, the charge separation occurs at the dye/electrode interchange by injection of an electron into the semi-conductor conductive band from the photoexcited dye [17–20].
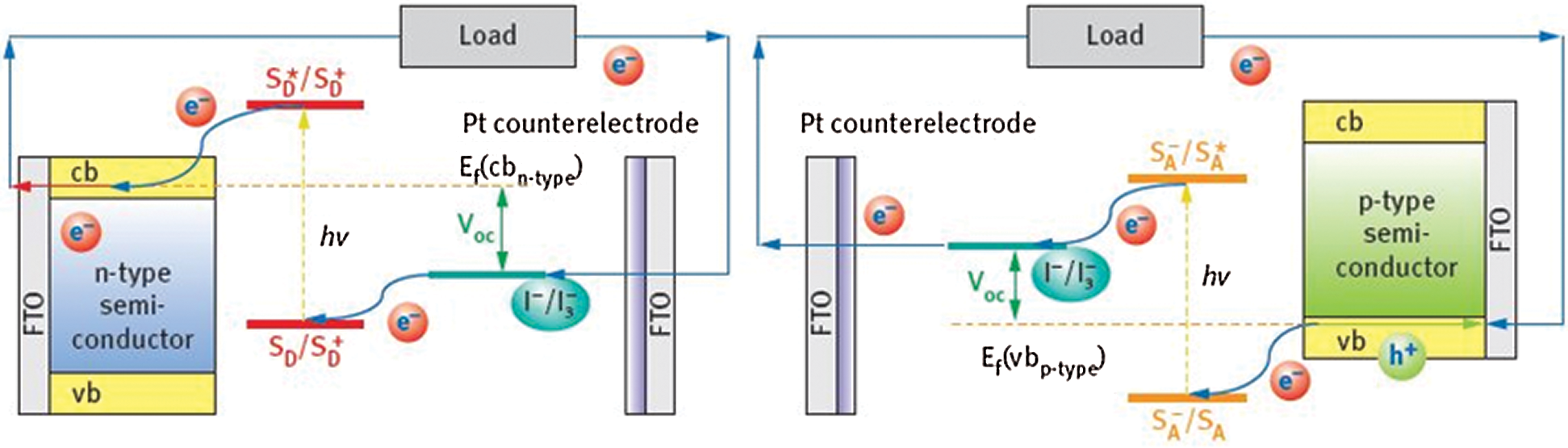
Figure 4: Operational principle of n-type (left) and p-type (right) [20] (Reprinted with permission from publisher)
In the case of p-type semi-conductor network, firstly, light is absorbed via a dye, that is placed on the surface of p-type semi-conductor mesoporous electrodes as proven in Fig. 4b. P-type DSSCs, which are especially based totally on NiO electrodes on ITO and/or FTO substrates. In this kind of DSSCs, as soon as the dye is photoexcited, charge separation injects the electrons to the photoexcited dye from the valence band of the semi-conductor [21–24]. Hence, the common phenomenon in both types discussed above is the regeneration of the oxidized or reduced dye through a redox mediating electrolyte.
Overall, to this point maximum scholars are focusing on n-type dye-sensitized solar cells (DSSCs): the usage of conventional ruthenium and zinc porphyrin dyes, power conversion efficiencies attain 12% and 13%, respectively [25,26]. Relatively, the efficiency of p-type DSSCs continuous to be low. Generally, in p-type DSSCs, a semi-conductor used is NiO with over 2.5% conversion performance which signifies the first-class file inside the area of p-DSSCs [27]. However, these dyes are not favourable for the massive-scale utility because of the excessive cost of metal dyes, tedious separation process, and environmental problems. On the other hand, mass production of this technology might be realistically possible with the development of the low-temperature synthesis of electrodes and cost-effective substances. Hence the review at the improvement of various sorts of DSSC technology for identifying low cost and higher conversion efficiency has come to be high significance within the implementation of new DSSC technologies, which is the essential focus of this review paper.
3.1 Based on Physical Appearance
In DSSC, on the one hand, the absorption of photons and on the other hand is the transfer of electrons that is mediated by two different media, i.e., dyes and n-type nanocrystalline TiO2, respectively. When the holes are passed through a substance conveying a hole which may be:
1. Liquid electrolyte
2. Solid electrolyte
3. Quasi-solid-state electrolyte
Stability and improvement in the power conversion efficiency of the DSSC device are significant for the commercialization of this technology. One of the wide research areas is focusing on the type of proper selection of electrolyte over the past decades. This section reports the recent advancements in the liquid, solid and quasi-solid-state electrolytes of the DSSC with efficiency values and current status [28,29].
3.1.1 Liquid Electrolyte Type DSSC
The electrolytes play a vital role in the power conversion efficiencies of DSSC, the foremost liquid electrolyte DSSC with an organic solvent containing an iodide/triiodide redox couple without any additive reported a 7% to 8% efficiency in 1991 [8]. The iodide-based electrolyte contains an I−/I3− redox couple and has been used since the beginning of DSSC due to its high efficiency and good stability. However, I−/I3− redox couple has several disadvantages such as corrosion effect and complex redox chemistry, therefore, recently, many electrolytic solutions with additional additives have been investigated that can achieve high conversion efficiency and also improved stability [30–32]. 4-tert-butylpyridine (TBP) is a very important additive in the iodide-based liquid electrolyte which significantly improves the VOC by decreasing the back reaction [33,34]. The structure of triiodide liquid electrolyte with the addition of TBP as shown in Fig. 5a which is a very effective electrolyte in DSSC [35]. Apart from the iodide/triiodide electrolyte, most commonly reported another redox couple for high-performance DSSC is cobalt tris-bipyridine ([Co(bpy)3]2+/3+) with Z316 an organic sensitizer [36,37] as shown in Figs. 5b and 5c Another redox couple that has unique features and extraordinary electrochemical properties is ferrocenium/ferrocene. A ferrocene/ferrocenium based redox couple with a metal-free organic sensitizer (i.e., Carbz-PAHTDTT) as shown in Fig. 5d has resulted in the conversion efficiency of more than 7.5% in DSSCs. This surpassed the efficiencies of iodide/triiodide electrolyte based DSSCs. Hence, ferrocene-based electrolytes with organic sensitizer prove to be compatible with future DSSC devices [31,38].
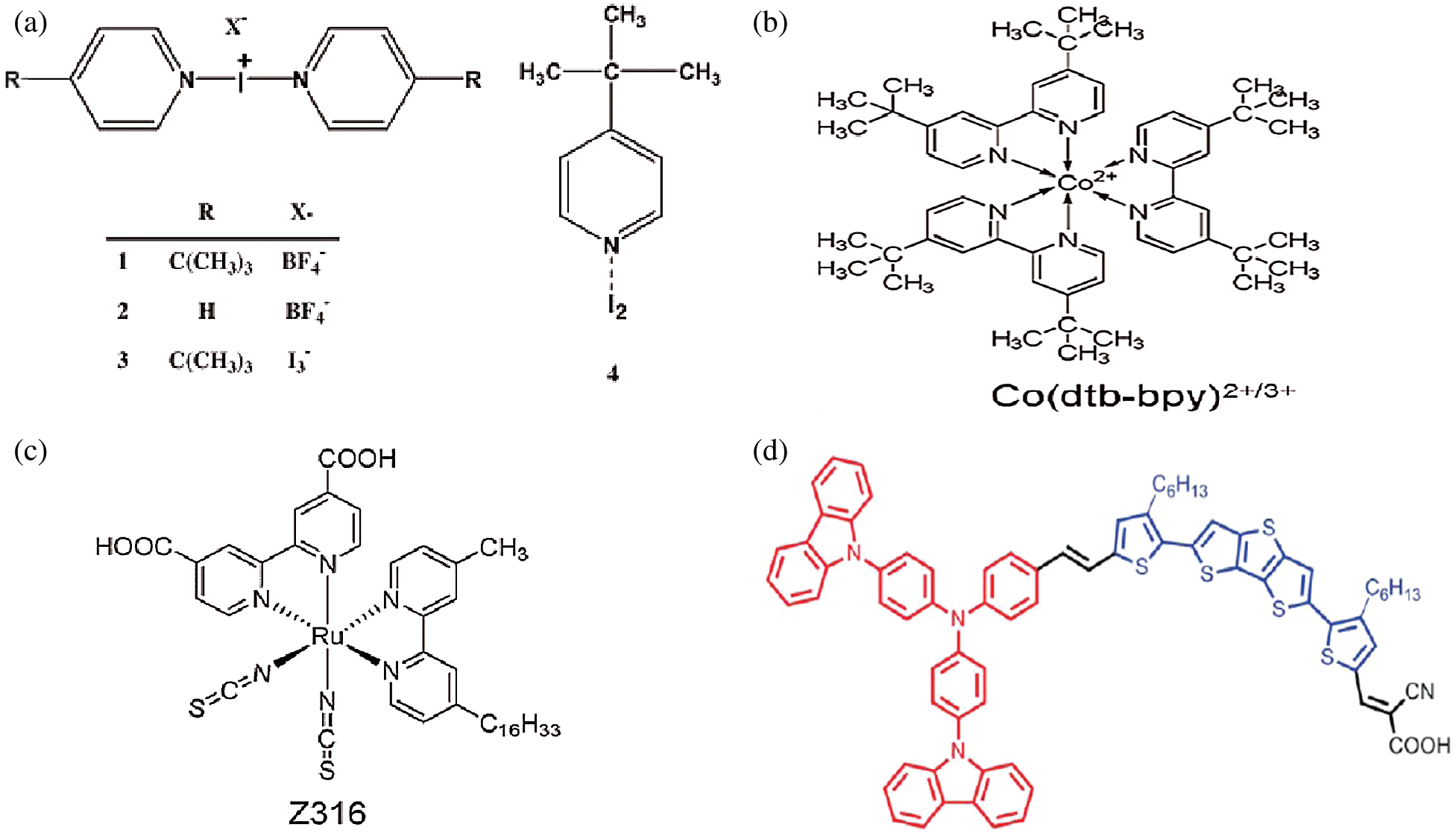
Figure 5: Structure of the compounds [35,37,38] (Reprinted with permission from publisher) (a) I3− triiodide 4-tert-butylpyridine (TBP) (b) Cobalt tris-bipyridine (c) Z316 an organic sensitizer (d) Organic sensitizer Carbz-PAHTDTT
Moreover, in liquid-based DSSC, considerable improvement within the surface region of the interface is brought by nano structuring of TiO2 that resulted in an increase of the photo-current density. The dyes undertake an excitonic separation under the action of light with the formation of a free-electron or free hole pair and as a result, electron is introduced into the TiO2 at very high speed and the holes are transported very slow to the counter-electrode through the liquid electrolyte. The process includes the re-reduction of dyes, which can be oxidized after an electron is lost. The recombination of charge carriers involved in this method is usually low that leads to an efficient charge separation [28]. It can be concluded that the electrolyte has an excessive effect on the kinetics of electrons that ultimately affects the performance of the DSSC [33]. There are many advantages and disadvantages of liquid electrolytes over other electrolytes. High conductivity, low viscosity, the outstanding connection between electrode and electrolyte and easy preparation are the advantages, while due to the low viscosity, leakage of an electrolyte from the cell is a major drawback which ultimately reduces the power conversion efficiency [39,40]. Remarkably, DSSCs with liquid electrolytes demonstrate the highest efficiency greater than 14% [12].
In previous studies, the novel porphyrin dye derivatives cosensitized with a Y123 dye obtained a conversion efficiency of 12.3% [41]. Besides, TiO2 surface remedy primarily based on various steel oxides together with Al2O3, SiO2, ZnO, MgO and many others have been used to develop the compact metallic oxide conformal layer with a view to decrease the charge recombination because of the back-electron transfer [42]. DSSCs containing noncovalent liquid crystals display up to 5.8 ± 0.2% power conversion efficiency at 30°C and 0.9 ± 0.1% at 120°C. In comparison, covalent type electrolytes solar cells display a substantial rise in conversion efficiency to 2.4 ± 0.1% at 120°C and demonstrate higher efficiency at temperatures above 90°C to noncovalent type-based systems [43,44]. Denizalti et al., have synthesized and characterized different samples of ionic liquids compounds. The synthesized butyl replaced imidazolium iodine salt showed a 5.17% power conversion efficiency [45]. While, there may be an extremely good enhancement inside the performance from 3.6% to 5.5%, which turned into in addition progressed to 7.2% through surface passivation with compact metal oxide. Also, many experiments have been performed to investigate the effects for the suppression of charge recombination and development in the photovoltaic performance [46]. Although extraordinary progress has been made in the direction of suited stable performance, however, there are many bottlenecks for the stability of these devices at a large-scale production [47]. Even if, the particularly efficient DSSC use liquid electrolyte it raises foremost technological challenges for huge scale commercial applications due to its volatilization, leakage of solvent, corrosion of electrode and airtight sealing of the cell. These challenges and their improvement strategies are discussed in detail in upcoming section in this paper.
3.1.2 Solid Electrolyte Type DSSC
Over the last decade, the focus of research is on the solid-state electrolyte to replace the liquid electrolytes. The major reason for replacement is ease of fabrication techniques, less hassle in the sealing of the device, and opportunity for monolithic connection of the cells. Consequently, these all reasons extend the overall performance and cost reduction of the DSSC device.
In one study, simulation of a solid-state dye-sensitized solar cell with the P3HT hole transport layer to solve problems related to the use of liquid electrolyte was performed and results revealed that the performance of solid-state turned into a decrease in efficiency than that of liquid electrolyte DSSC. This reduction in power conversion efficiency is because of the increase in the rate of recombination carriers [48]. Also, CsSnI3 is another promising p-type semi-conductor hole transport material, with high hole mobility, plentiful raw materials and low-cost processing. These electrolyte-based devices have an efficiency of up to 10.2% [49]. Furthermore, the process of charge transport and recombination which immediately regulate the photovoltaic properties of DSSCs. The energy conversion performance of the Ag2Se based solid-state DSSCs is 5.89% [50]. Another research with the solid-state electrolyte on dye-sensitized solar cells (DSSCs) was fabricated in 2018. The findings showed that, under 80 mW/cm2 irradiation, the conversion efficiency was 2.56%. Performance of solid-state electrolyte performance is more robust than liquid electrolyte performance [51]. Furthermore, Chung et al. defined the first example of a perovskite based DSSC. This led to an increase in collective efforts from numerous other groups as well [52].
Overall, solid electrolytes have good stability, but the power conversion efficiency is decreased by weak contact of the electrode, cell penetration and low ionic diffusion [53]. Nevertheless, the benefit of solid-state DSSCs over other devices is obvious: fewer problems in the manufacturing of the device, simpler possibility to manufacture monolithically interconnected devices, easier sealing and module encapsulation.
3.1.3 Quasi-Solid Electrolyte Type DSSC
The electrolytes are one of the key parts of DSSC devices, which could substantially alter the power conversion efficiencies. Use of polymer electrolytes, also called Quasi-solid state, are introduced to overcome the issues pertaining to the liquid and solid electrolyte based DSSC. These have good stability and high ionic conductivity than liquid and solid electrolytes. This is because the polymer matrix which could efficaciously lure the liquid solvent to prevent evaporation and, in the meantime, provide channels to increase the rate of redox couples. These electrolytes based on permeable polymer membranes are relatively favoured, that could trap a large amount of liquid electrolyte and have good contact and high conductivity with the electrodes than the solid-state [47].
Kim et al., are the pioneer of synthesizing the PMMA copolymer-based porous membrane in the polymer-based electrolyte in DSSC and attained an efficiency of 2.4% [54]. Similarly, researchers used the polymer-based electrolyte in DSSC to achieve a power conversion efficiency of 9.48%, higher than 8.84% of the DSSC with the liquid electrolyte under the same conditions [55]. Furthermore in 2019, by integrating the high-energy graded gel region with the dye-TiO2 anode and low-energy region with the Pt-Ni counter electrode, the quasi-solid-state DSSC achieved a conversion efficiency of 8.64% under one sunlight [56]. Another research in 2019 discovered that quasi-solid state DSSC have high recombination resistance and incident photon to current values, resulting in the high open-circuit voltage and the current with an energy conversion performance of 12.23% is obtained under 200 lux illuminance [57]. By integrating polyaniline nanotubes (PAniNT) into reduced graphene oxide aerogel (rGOA), Mohan et al. successfully synthesized a PAniNT/rGOA aerogel or polymer-based on graphene. The designed resulted in maximum efficiency of 5.47% [58]. The poly (3-decyl-1-vinyl imidazolium iodide) (PIL) and polyindole were synthesized and combined with PMMA in a recent study in 2020. Devices manufactured with this porous electrolyte exhibited a high conversion efficiency of 5.96% and improved stability [59]. In another recent research in 2020, it has been concluded that the structural properties of polymers performs an important role in the power conversion efficiency of quasi-solid state DSSC. Authors synthesized the polymers gelators and resulted that the overall power conversion efficiency of Polymer gel based quasi-solid state DSSC was 9.72% under AM 1.5G solar illuminance, comparable to 9.79% for liquid electrolyte DSSCs. Further, long-term stability test under 1 sun solar illuminance and 50°C proved the enhanced stability of polymer gel based quasi-solid state over liquid electrolyte DSSCs [60].
Overall, the power conversion efficiency of DSSCs produced with a certain polymer electrolyte was found to be comparable to that of liquid electrolyte based solar cells. However, there are inherent improved sealing advantages in these quasi-solid-state devices. They have lower ionic conductivity and weak pore penetration in the mesoporous matrix as compared to the liquid counterpart [28]. Therefore, for these systems, there is a deficiency of accurate long-term stability data available. However, the recent growth of laboratories is likely to create further industrial research interests in the future.
3.2 Based on the Architectural Assembly
Improving the power conversion efficiency of DSSC is a crucial importance for the commercialization of this inexpensive and environmentally friendly photovoltaic technology. One of the other widely agreed approaches is to expand light utilization spectrum as the photon-to-current conversion responses. Over the last few years, numerous works based on employing tandem systems as a new architectural assembly to clear up the above-stated problem. These designs include [61]:
1. n-n Tandem DSSC
2. p-n Tandem DSSC
3. DSSC Tandem with other solar cells
This part of the study reports the working principle, the recent advancements in the n-n and p-n tandem DSSC and tandem with other solar cells addressing their efficiency values and current status.
The photocathode comprises of a p-type semi-conductor and a dye attached as illustrated in Fig. 6a. When the photoexcitation occurs, this dye injects a hole into the conduction band of the p-type semi-conductor. It can also be considered as this hole results in an electron being injected to the dye from the semi-conductor. When this charge transmittance is completed, a redox-active electrolyte neutralizes the dye by oxidizing it back. Afterwards, this hole is transported to the external circuit via the mesoporous network of the p-type semi-conductor. Then the typical counter electrode coated with catalytic material (typically platinum) maintains the charge balance by oxidizing the reduced species in the electrolyte. In comparison, upon the photoexcitation in the n-type DSSC as illustrated in Fig. 6b, the electron is injected into the conduction band from the dye, which then transported to the external circuit via the mesoporous network. Afterwards, the electrolyte reduces the dye and again the typical counter electrode coated with catalytic material (typically platinum) reduces the electrolyte by charges at the working electrode. Additionally, in the case of tandem structure, when both of the p and n-type are stacked together as shown in Fig. 6c. As two systems are connected in series and if charge generation is balanced, the two systems will create a higher VOC [62].
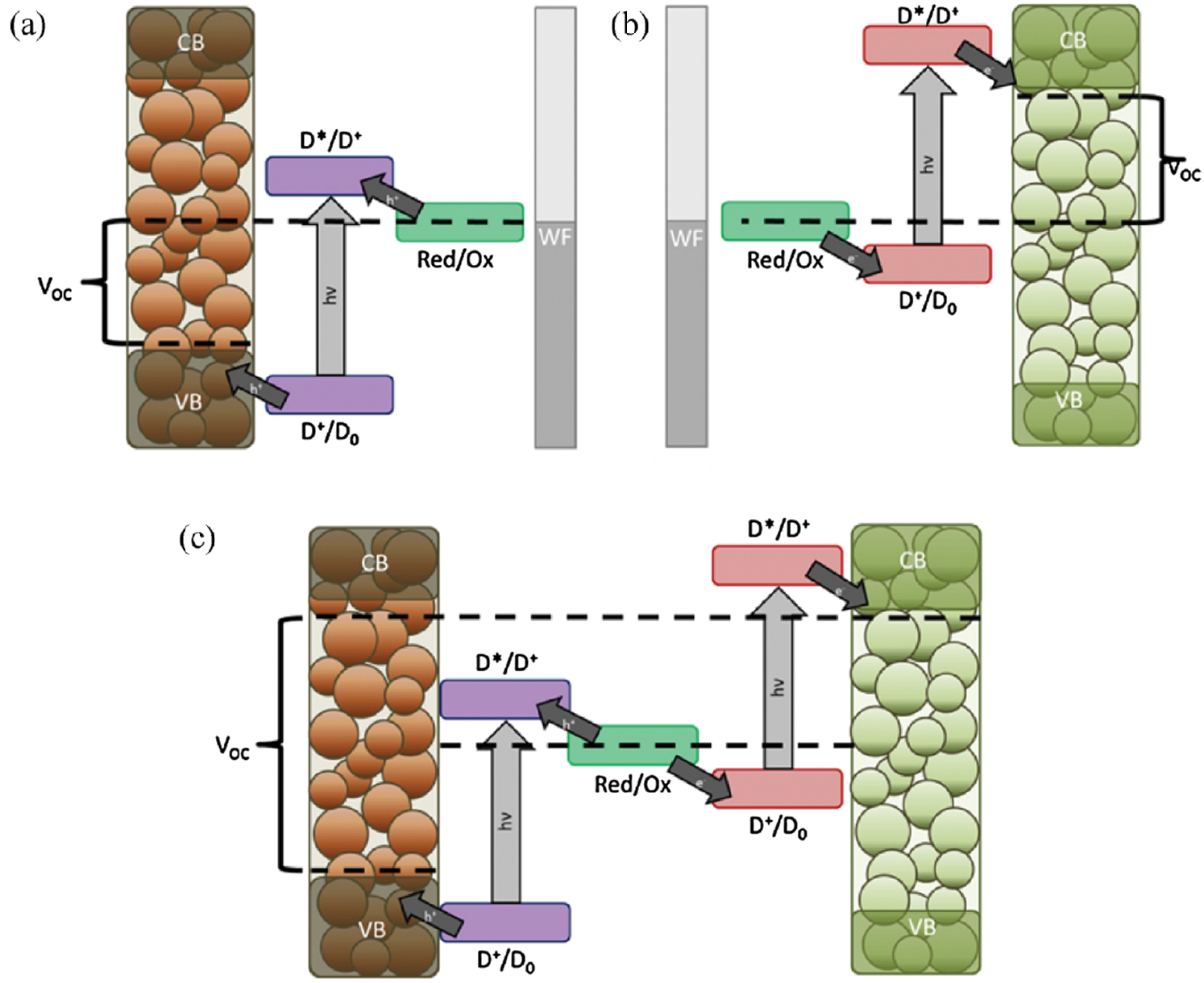
Figure 6: Working principle of (a) p-type (b) n-type and (c) tandem p-n type DSCC [62] (Reprinted with permission from publisher)
In this type of device, two separate n-type semi-conductive electrodes, called ‘n-n tandem structured DSSC’ connected in series in the tandem position that increases the device higher short-circuit photocurrent density. Firstly, a tandem structure was developed by Wataru et al., a front cell made up of N719 sensitized connected with black dye-sensitized bottom cell. He had the tandem DSSCs with PCE of 7.6% of tandem DSSCs in the first iteration, higher than each cell [63]. The tandem structure design focused on two completed cells attained improved efficiency, depending on various mixtures of dyes, more effective than near-infrared dyes being applied. Nelles et al. registered a total power conversion efficiency of up to 10.5% [64]. A series-tandem DSSCs with optimized dye combination were stated by Hironori Arakawa and collaborators. TiO2 layer obtained the higher output of 1450 mV and 10.8 mA cm−2 and 10.4% [65]. One more study in 2010, by optimizing the thickness of TiO2 films in the separate cells in terms of tandem DSSC output with different series or parallel contact modes. The integrated parallel-linked tandem DSSCs resulted in the best power conversion efficiency of 10.6% [66]. Ma and coworkers later documented a related tandem with a multilayered photoanode DSSC. The multilayered photoanode structure increased the density of the photocurrent and the maximum efficiency of 11.05% was attained [67].
Overall, the power conversion efficiency with these designs have been improved significantly in past but there is no recent reporting’s made on these systems. However, future growth is likely to create more interests in this area in future for higher performance.
In this type of device, dye-sensitized p-type and n-type electrodes are connected in series to one another which we refer as ‘p-n tandem structured DSSC’ that can generate a higher photovoltaic voltage. The efficiency of this type of solar cells has been reported minimum because of the inefficient performance of the p-DSSC. A major reason is that this type of structure cannot utilize the full spectrum of solar radiations [68]. One of the innovative approaches was developed by Sobus et al. in 2014, to exploit the energy emitted from the solar spectrum. They employed the semi-conductor of the n-type as TiO2, a p-type dye-sensitized semi-conductor as NiO, and a redox mediator between them. The p-n-DSSC designed shows improved conversion efficiency compared to n-DSSC [69]. Another benefit of serially connected tandem solar cells is the increase in photovoltage in comparison to the individual n-DSSC and p-DSSC, which results in an overall increase in efficiency. In recent years, p-DSSC research had been targeted on developing new sensitizers and redox structures and the improvement can notably growth the overall performance of the p-DSCs, frequently due to an increase inside the photovoltage [70]. Wei et al. doped metal atom into active NiO layer in 2016 to boost the p-DSSC photovoltaic efficiency [71]. Ho et al. implemented a simple technique of a compact NiO blocking layer into an active NiO photocathode network of p-DSSC and p-n DSSC in 2018 and additionally from previous research in 2016 to improve the power conversion efficiency. The designed p-n DSSC displays an improved photovoltage comparison to n-DSSC, ensuing in the conversion efficiency of 1.486% overall. This increase can be further enhanced to 1.913% by addition of an optimized NiO [72,73].
Overall, the efficiency with these designs have been improved significantly in past but again there are very limited recent reporting’s made on these designs. However, the future growth of laboratories can create more developments in the future in this area.
3.2.4 DSSC Tandem with other Solar Cells
Dye-sensitized solar cells (DSSCs) have progressed extensively during the last two decades but the total conversion efficiency of solar to electric power is still not equivalent to other types of commercial solar cells. Latest research trend towards improving conversion performance focuses on improving techniques for light-harvesting mainly panchromatic engineering and different tandem approaches, i.e., DSSC tandem with other solar cells. A few solar cells use tandem structures to enlarge the region of active absorption to a full solar spectrum [74,75].
Integrated with other thin-film solar cells, tandem cells made of DSSC demonstrate much higher performance. When these cells are either stacked using a mechanical or monolithic pattern, Top-cell transparency and photovoltaic parameters perform a significant role in the overall power conversion efficiency. A few studies recently showed a solid DSSC structure with comparable efficiency values [76]. Research on traditional repeated tandem structure with either a tunnel junction or terminal junction will be successfully performed after the optimization of these structures [77]. Tandem assembly of DSSCs can take maximum benefit from sunlight, appropriately expanding the cell absorption spectrum, resulting in increased open-circuit voltage or short circuit current than conventional single light absorber DSSC. Theoretical maximum output of these structures can reach the Schottky-Queisser limit of 33%. Thus, the DSSC tandem design is seen as a viable way to enhance DSSC bottleneck efficiency [61]. The biggest major division throughout all multi-junction solar cells is the interconnection of the sub-cells. Therefore, several system architectures and connectivity schemes which can be used for solar tandem cells [78]. Tandem architecture of DSSC with other types of solar cells have demonstrated their capacity to increase the power conversion efficiency. Numerous authors have calculated the efficiency of those tandem stacks. A good overview of these results can be found in [79]. This section presents the DSSC tandem with other CIGS and PSC addressing their efficiency values, limitations and current status.
Copper indium gallium selenide (CIGS)-DSSC tandem architecture assembly is a very helpful technique for increasing the efficiency of photoelectric conversion by optimizing the use of solar spectrum in individual cells [77]. These tandem solar cells are manufactured using the optimized DSSC and CIGS, where DSSC is used as the top cell and CIGS as the bottom cell. For the series connection, the positive DSSC electrode and the negative CIGS electrode are connected by an electrical cable, which resulted in a tandem structure with two terminal electrodes as illustrated in Fig. 7. This approach increases open-circuit voltage and ultimately improves the conversion efficiency of DSSC [80].
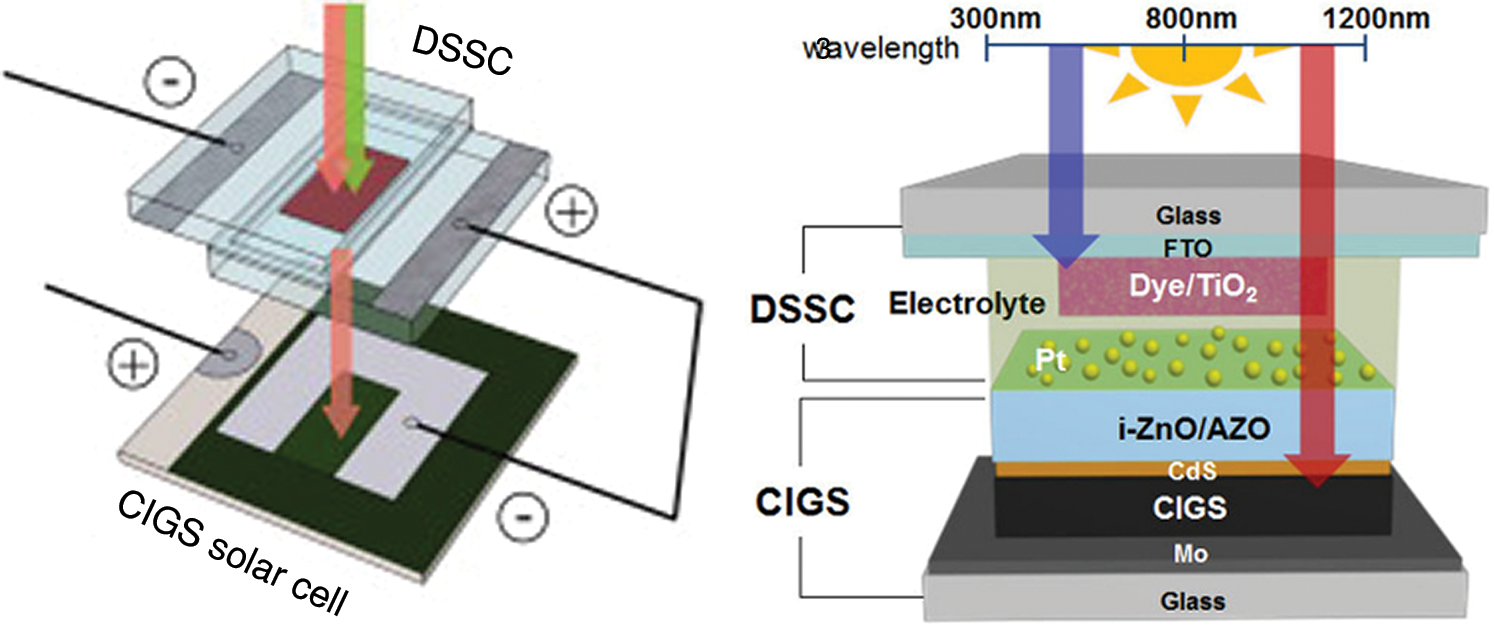
Figure 7: Structure assembly of CIGS-DSSC tandem solar cell [81] (Reprinted with permission from publisher)
Accordingly, tandem structure by DSSC series connection with low bandgap p-n junction solar cells is considered as one of the methods for improving output voltage. As DSSC are prepared as a semi-transparent device, therefore tandem cells are built by positioning a DSSC as a top cell [82]. Liska et al. prepared the first hybrid tandem cell that comprises DSSC as a top cell, and CIGS as a bottom cell. Both cells were stacked mechanically on each other and connected electrically in series. Increase in voltage and power conversion compared to single-junction solar cells were achieved. They fabricated a hybrid tandem cell consisting of a DSSC cell as the top cell for high-energy photons and a thin film CIGS as the bottom cell for photons with lower energy. The power conversion efficiency of up to 15% from a tandem solar cell was obtained. However, the assembly of CIGS film on the DSSC is very complicated and the cost of the CIGS-DSSC tandem cell is high [83]. In another study, a semi-transparent DSSC is constructed as a top cell and a CIGS solar cell as a bottom cell, where the isolated DSSC and CIGS cells demonstrate the conversion efficiency of 8.27% and 11.71%, respectively. The CIGS/DSSC tandem cell resulted in 12.35% improved conversion efficiency with 14.1 mA/cm2 photocurrent density and 1.435 V open-circuit voltage [82]. A CIGS-based tandem solar cell built with a DSSC sub-cell is very encouraging because gluing can easily assemble single-junction sub-cells made on individual glass substrates [84]. The tandem architecture was constructed particularly based on a solution process between organic DSSC and inorganic CIGS single-junction solar cells. Solar cell performance was reached to 13%, which reflects substantial improvement from individual single-joint solar cells which was 7.25% and 6.2% for DSSC and CIGS, respectively [81].
The corrosive iodide-based electrolyte is the main cause of DSSC/CIGS tandem cell instability. As such, a cobalt-based complex redox electrolyte would be a good approach as a replacement, as it is much less corrosive to single-cell DSSC metallic conductor [85,86]. In one test, the introduction of a [Co(bpy)3]2+/[Co(bpy)3]3+ redox pair and Y123 organic dye as sensitizer provided a highly stable DSSC/CIGS tandem solar cell. They also added PEDOT: PSS as the counter electrode material to the bottom CIGS cell window layer of the Al-doped zinc oxide instead of the Pt catalyst layer which provides additional stability in the tandem cell [84]. Moreover, to address the disadvantages of mechanical stacking architecture, Wenger et al. developed a monolithic architecture of a TiO2/CIGS tandem cell. DSSC was directly combined with the CIGS cell without any index matching liquid to reduce the loss of reflection and the absorption loss of free carriers. Total conversion efficiency was registered at 12.2% [85].
Wang and his collaborators pursued the DSSC/CIGS mechanical stacking process as was used by Liska. They use two different CIGS solar cells with an efficiency of 10.27% and 16.76% and stacked mechanically with the DSSC of 7% efficiency in a separate experiment to form tandem cells. Their tandem efficiencies were 10.46% and 12.45%, respectively. The authors noted that the in tandem assembly, top DSSC plays an important role in the overall conversion efficiency because even with the higher performance of the bottom CIGS, the tandem cell showed no any substantial improvement in the conversion efficiency [87]. Hao et al., reported an 8.31% overall efficiency hybrid amorphous silicon/DSSC tandem cell [88]. Ito and his colleagues reported a tandem-structured solar cell with DSSC as the top cell and a GaAs/AlXGa(1−X) as the bottom cell. The Voc of the single cells GGC and DSSC were 1.11 V and 0.76 V, resulting in a remarkable Voc of 1.85 V for the tandem cell, the efficiency of which was 7.63% [80].
Remarkable performance and economical prices of perovskite solar cells (PSCs) have been the center of the photovoltaic research field in recent years [89]. Inorganic-organic halogen perovskite has a higher absorption coefficient relative to conventional dyes, which supports its use as a sensitizer in DSSCs. As a sensitizer, nanocrystals from perovskite (CH3NH3PbI3) were first presented into DSSC in 2009 [90]. It has become evident that perovskites can act as both hole and electron conductors, and also bulk solid perovskite film can withstand charge formation and transport. However, the efficiency is not high due to the instantaneous dissolution of perovskite in a liquid electrolyte [14,91,92]. Tandem heterojunction is a novel strategy for improving the spectral response in NIR regions with long wavelengths. A perovskite tandem solar cell can be formed in three different structures, depending on how the junctions between the top and bottom cells are electrically coupled. The mechanically stacked solar cells are called a 4-terminal (4 T) tandem, a monolithic tandem solar cell called a 2-terminal (2 T) tandem solar cell, and a tandem solar cell with optical splitting as shown in Figs. 8a–8c, respectively.
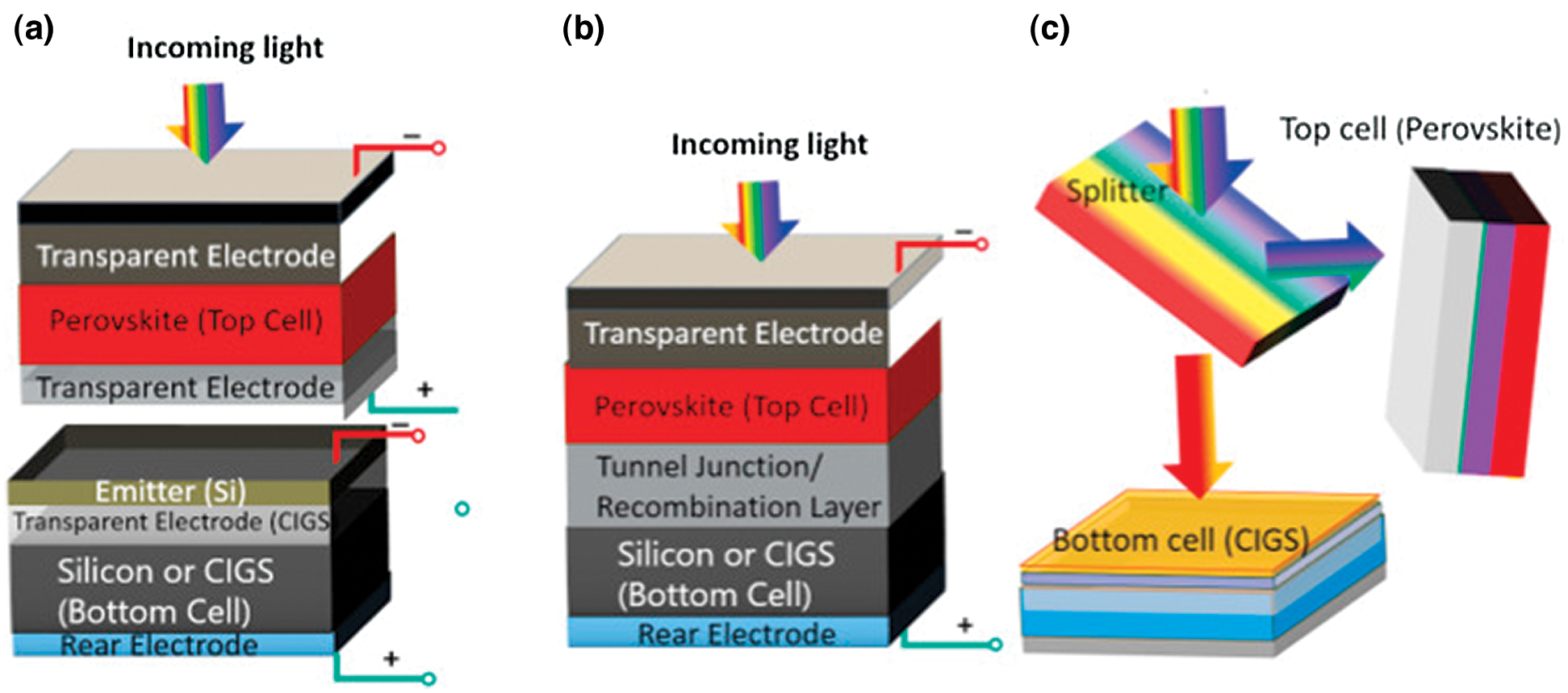
Figure 8: Structure assembly of (a) 4 T (b) 2 T and (c) Optical splitting PSC tandem cell [93] (Reprinted with permission from publisher)
Perovskite-based 4 T tandems with chalcogenide and crystalline silicon bottom cells reported conversion efficiency up to 20.5% and 22.8% [94]. Further, the conversion efficiency of 26.4% has recently been recorded for mechanically stacked perovskite-silicon solar cells in 4 T [95]. In monolithic perovskite 2 T tandem solar cell, a very high PCE ∼23.6% was obtained and the tandem device reported stable performance [96]. Similarly, the optical splitting of tandem solar cells has a benefit over the other two and increase of the efficiency value by almost 40% (relative) compared to a single cell junction is observed [97].
Researchers achieved a state-of-the-art efficiency of over 23% in one study using perovskite with a composition of CH3NH3Pb(I0.95Br0.05)3, with 18.3% from the PSC and the remainder contributing to heat transfer using the thermo-electric module [98]. One other study in which the dye-sensitized solar cell based on ruthenium is combined with a perovskite cell that harvests visible light obtained 21.5% conversion efficiency using a spectral splitting system. A mechanically stacked tandem DSSC comprises DX1 as the bottom cell NIR sensitizer and N719 as the top cell visible sensitizer has been stated to have a PCE of 12% under reduced sunlight [30]. The use of a PSC as a visible-light-absorbing cell would increase the conversion efficiency considerably. Panchromatic DX3-based DSSC ruthenium complex with 18.4% efficiency achieved 21.5% tandem conversion efficiency [99]. Researchers designed perovskite/DSSCs tandem solar cells in one recent study. The organic thioindigo based dye (C16H8O2S2) and N719 were applied as photosensitizers in the fabrication of dye-sensitized solar cells. They obtained an 8.77% and 10.54% performance tandem solar cell consisting of a top perovskite solar cell and dye-sensitized solar cells based on the organic dye and N719 respectively for the bottom cell. A tandem system with a top dye-sensitized solar cell and a bottom perovskite solar cell was manufactured for the first time from the organic dye. As predicted, the photovoltaic properties of all devices increased since Voc-tan is the addition of two Voc of single DSSCs. The tandem solar cells demonstrated a high Voc, but in a tandem configuration, photocurrent was limited by the Jsc value of DSSC bottom cell [100].
In recent years, remarkable attempts have been made to achieve a variety of tandem devices based on perovskite by optimizing certain critical parameters, such as absorber material band gaps. Overall, it can be concluded that the efficiency with these tandem structures has been improved significantly. However, future research can result in more developments in the future in this area.
Overall, in this section comparison of different types of DSSCs based on the physical appearance and architectural assembly is presented. This section can be summarized as: proper selection of electrolytes greatly affects the performance of DSSC devices, and we found from the literature that the quasi-solid and polymer gel-electrolyte, proves to be a better option because of good ionic conductivity and contact with electrode resulting in enhanced photovoltaic performances. Also, it can be summarized that tandem can achieve good performance because the performance for both individual cells could be optimized separately.
4 Technical Challenges/Limitations and Improvement Strategies
From the above discussion pertaining to the review of various types based on physical appearance and architectural assembly, this section sums up the technical limitation and their possible improvement remedies in tabular form in Tab. 1.
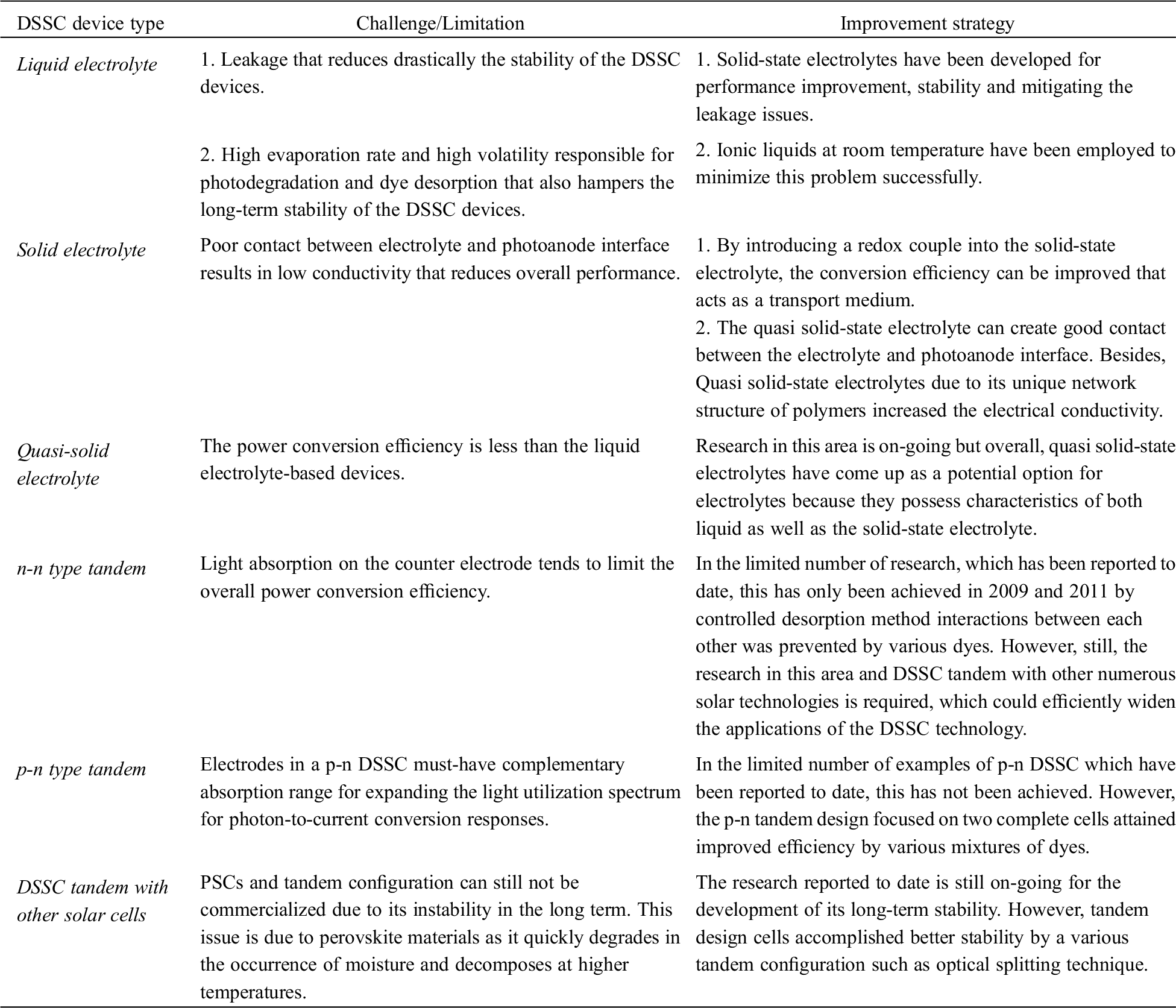
Table 1: Technical challenges and improvement remedies of different types of DSSC
The dye sensitized solar cell (DSSC) have not yet grasped the power conversion efficiency higher than 15%, because this efficiency is apposite for the commercialization of DSSCs. The primitive work on dye-sensitized solar cells was introduced by O’Regan and Gratzel in 1991 and in the last two decades the research has grown rapidly and the device efficiency has been improved from 7% in 1991 to 14.2% in 2020 as illustrated in Fig. 2. Moreover, to increase overall performance of solar cells, tandem structure are also widely being investigated. In the past decades, intensive research has been carried out on DSSC and Tandem solar cells which has been reflected in terms of large number of publications. From 2010 to 2020, an estimated number of publications of 21,619 and 21,068 of “dye sensitized solar cell” and 4,321 and 3,720 of “tandem solar cells” have been published according to Web of science and Scopus database, respectively as given in Figs. 9a and 9b.
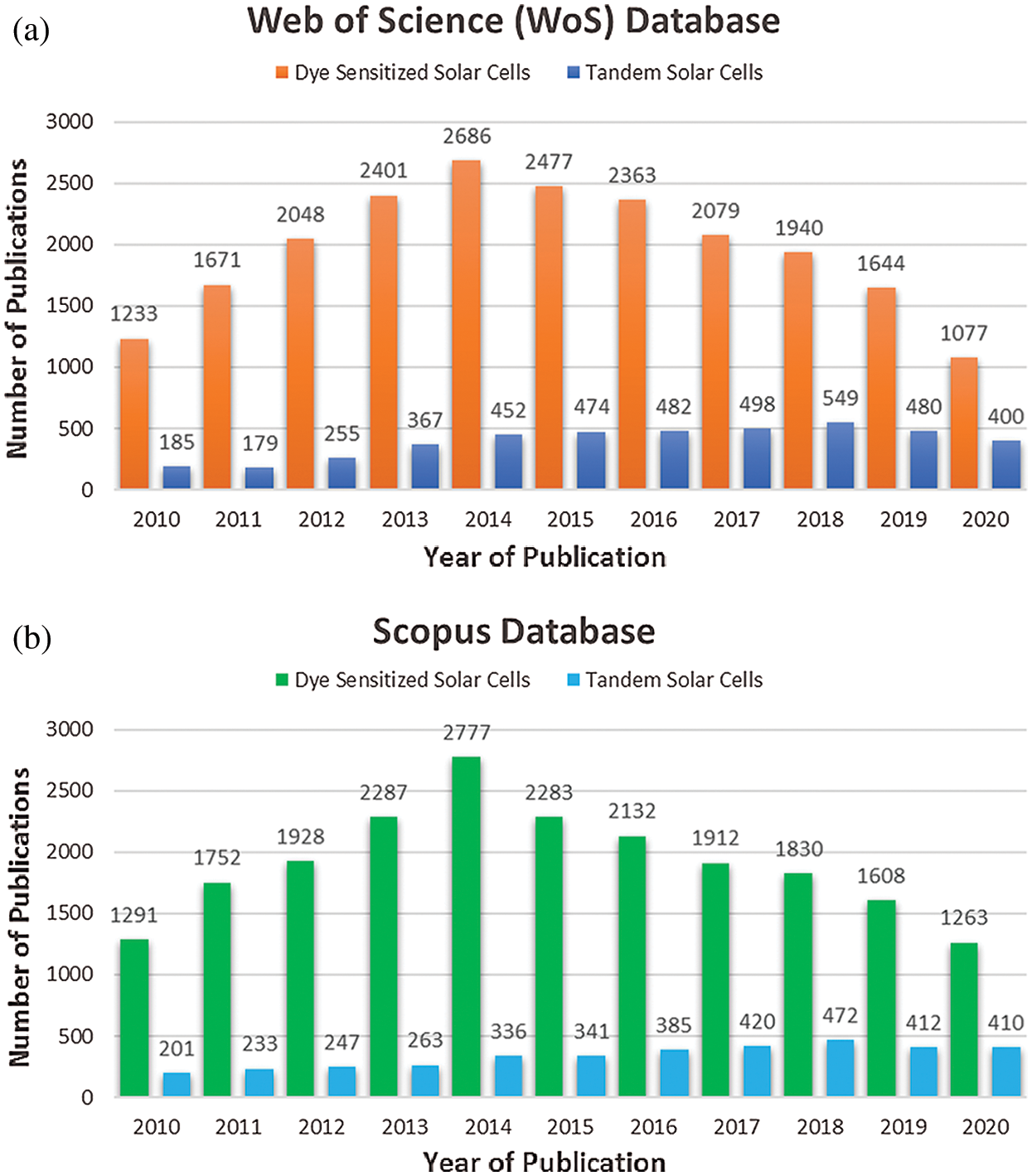
Figure 9: (a) Statistics of year wise number of publications using the key word “dye sensitized solar cells” and “tandem solar cell” (Web of Science database). (b) Statistics of year wise number of publications using the key word “dye sensitized solar cells” and “tandem solar cell” (Scopus database)
DSSCs are attractive because of low cost, colorfulness, flexibility and high efficiency in ambient light and easy fabrication. In this review paper, an attempt has been made to present the comparison of different articles published earlier, that gives the in-depth study of recent developments in different types the DSSCs based on the physical appearance such as solid, liquid and quasi-solid and architectural assembly as n-n tandem and p-n tandem. This review paper concludes that:
1. Appropriate selection of electrolytes greatly affects the performance of DSSC devices. Major problem of liquid electrolyte DSSCs are sealing and leakage due to high volatile organic solvent present in liquid electrolytes. Solid state electrolytes have poor ionic conductivity and bad contact with electrode limit photovoltaic performance. Issues pertaining to the electrolytes are high evaporation rate under the solar radiations. Changes in temperature affect the stability and cause leakage from the DSSCs. Moreover, due to the limited gap between the counter electrode and photoanode, the integration of electrolyte inside the DSSCs is another major issue. To overcome these issues, the proper selection of materials for electrolyte is important for the overall performance of DSSC. Therefore, we found in this research from the literature that combination of solid and liquid-type electrolyte, known as the quasi-solid electrolyte, proves to be a better option to mitigate these issues. Also, to overcome these problems, polymer gel electrolytes based quasi solid state-DSSCs are attractive because polymer based electrolytes have moderate ionic conductivity and contact with electrode resulting comparable photovoltaic performances.
2. It is important that the tandem structure with two tandem cells positioned can achieve good performance because the performance for both individual cells could be optimized separately. Both n-n and n-p tandem designs of DSSC aim to improve the solar cell performance. Then-n tandem structured DSSCs can be more efficient than single DSSC. Whereas the n-p tandem structured DSSCs seem to lag much behind but the progress is still needed to increase overall performance. Its further improvement relies on the development of new dyes which could utilize the more solar light spectrum. It can be anticipated that costs needed to fabricate p-n tandem structured DSSCs are less than the single n-type DSSC which makes this technology an economically viable option for the future.
3. The combination of DSSC devices with CGIS and PSC in the tandem configuration is a new approach for better performance of DSSCs. Tandem devices show greater efficiency than individual devices due to an expansion of the sunlight spectrum. PSC tandem configuration proves to be the best solution for water splitting and other applications.
DSSCs can be used as in various applications because of easy fabrication and cost-effectiveness. However, for the commercialization, research is needed to improve the stability and overall performance of the device. In this review paper, an attempt has been made to highlight the recent developments in different types the DSSCs and issues pertaining to the stability and performance. Based on the issues, this review paper recommends that:
1. It is to be recommended that for the better stability of the device, research on the combination of solid and liquid-type electrolyte i.e., quasi-solid and polymer gel electrolytes is needed.
2. It is recommended that research in tandem DSSC with other solar technologies is required, which could efficiently widen the applications and cost-effectiveness of the DSSC technology.
Acknowledgement: The authors would like to thank Sunway University for the research facilities provided.
Funding Statement: The authors would like to thank Universiti Malaysia Pahang (UMP) for the financial support under Grant RDU192205 and RDU192403.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Pandey, A., Tyagi, V., Jeyraj, A., Selvaraj, L., Rahim, N. et al. (2016). Recent advances in solar photovoltaic systems for emerging trends and advanced applications. Renewable and Sustainable Energy Reviews, 53, 859–884. DOI 10.1016/j.jplph.2005.10.006. [Google Scholar] [CrossRef]
2. McJeon, H., Edmonds, J., Bauer, N., Clarke, L., Fisher, B. et al. (2014). Limited impact on decadal-scale climate change from increased use of natural gas. Nature, 514(7523), 482–485. DOI 10.1111/jac.12288. [Google Scholar] [CrossRef]
3. Ahmed, U., Alizadeh, M., Rahim, N. A., Shahabuddin, S., Ahmed, M. S. et al. (2018). A comprehensive review on counter electrodes for dye sensitized solar cells: A special focus on Pt-TCO free counter electrodes. Solar Energy, 174, 1097–1125. DOI 10.1071/CP19510. [Google Scholar] [CrossRef]
4. Panwar, N., Kaushik, S. and Kothari, S. (2011). Role of renewable energy sources in environmental protection: A review. Renewable and Sustainable Energy Reviews, 15(3), 1513–1524. DOI 10.1093/jxb/34.2.177. [Google Scholar] [CrossRef]
5. Raj, C. C., Prasanth, R. (2016). A critical review of recent developments in nanomaterials for photoelectrodes in dye sensitized solar cells. Journal of Power Sources, 317, 120–132. DOI 10.1016/j.jplph.2004.03.006. [Google Scholar] [CrossRef]
6. Fan, K., Yu, J., Ho, W. (2017). Improving photoanodes to obtain highly efficient dye-sensitized solar cells: A brief review. Materials Horizons, 4(3), 319–344. DOI 10.1016/j.eja.2007.07.003. [Google Scholar] [CrossRef]
7. Green, M. A., Dunlop, E. D., Levi, D. H., Hohl-Ebinger, J., Yoshita, M. et al. (2019). Solar cell efficiency tables (version 54). Progress in Photovoltaics: Research Applications, 27(7), 565–575. DOI 10.1104/pp.112.210740. [Google Scholar] [CrossRef]
8. Gratzel, M. (2005). Solar energy conversion by dye-sensitized photovoltaic cells. Inorganic Chemistry, 44(20), 6841–6851. DOI 10.3390/agronomy10040488. [Google Scholar] [CrossRef]
9. Li, G., Sheng, L., Li, T., Hu, J., Li, P. et al. (2019). Engineering flexible dye-sensitized solar cells for portable electronics. Solar Energy, 177, 80–98. DOI 10.1104/pp.103.021790. [Google Scholar] [CrossRef]
10. Ji, J.-M., Zhou, H., Eom, Y. K., Kim, C. H., Kim, H. K. (2020). 14.2% efficiency Dye-sensitized solar cells by Co-sensitizing novel thieno[3,2-b]indole-based organic dyes with a promising porphyrin sensitizer. Advance Energy Materials, 10(15), 2000124. DOI 10.1007/s11033-013-2921-8. [Google Scholar] [CrossRef]
11. Zhou, H., Ji, J. M., Kang, S. H., Kim, M. S., Lee, H. S. et al. (2019). Molecular design and synthesis of D–π–a structured porphyrin dyes with various acceptor units for dye-sensitized solar cells. Journal of Materials Chemistry C, 7(10), 2843–2852. DOI 10.1111/j.1469-8137.2004.00974.x. [Google Scholar] [CrossRef]
12. Kakiage, K., Aoyama, Y., Yano, T., Oya, K., Fujisawa, J. I. et al. (2015). Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chemical Communications, 51(88), 15894–15897. DOI 10.1111/j.1744-7348.1980.tb03959.x. [Google Scholar] [CrossRef]
13. Ahmad, M. S., Pandey, A. K., Rahim, N. A. (2017). Advancements in the development of TiO2 photoanodes and its fabrication methods for dye sensitized solar cell (DSSC) applications. A review. Renewable and Sustainable Energy Reviews, 77, 89–108. DOI 10.1016/j.plantsci.2004.02.018. [Google Scholar] [CrossRef]
14. Gong, J., Sumathy, K., Qiao, Q., Zhou, Z. (2017). Review on dye-sensitized solar cells (DSSCsAdvanced techniques and research trends. Renewable and Sustainable Energy Reviews, 68, 234–246. DOI 10.1007/s11099-005-5134-0. [Google Scholar] [CrossRef]
15. Fayaz, H., Ahmad, M. S., Pandey, A., Rahim, N. A., Tyagi, V. (2020). A novel nanodiamond/Zinc nanocomposite as potential counter electrode for flexible dye sensitized solar cell. Solar Energy, 197, 1–5. DOI 10.1111/j.1365-3040.1995.tb00565.x. [Google Scholar] [CrossRef]
16. Cavallo, C., Di Pascasio, F., Latini, A., Bonomo, M., Dini, D. (2017). Nanostructured semiconductor materials for dye-sensitized solar cells. Journal of Nanomaterials, 2017, 1–31. DOI 10.1155/2017/5323164. [Google Scholar] [CrossRef]
17. Barnes, P. R., Miettunen, K., Li, X., Anderson, A. Y., Bessho, T. et al. (2013). Interpretation of optoelectronic transient and charge extraction measurements in dye-sensitized solar cells. Advanced Materials, 25(13), 1881–1922. DOI 10.1002/adma.201201372. [Google Scholar] [CrossRef]
18. Listorti, A., O’Regan, B., Durrant, J. R. (2011). Electron transfer dynamics in dye-sensitized solar cells. Chemistry of Materials, 23(15), 3381–3399. DOI 10.1007/s11120-013-9849-7. [Google Scholar] [CrossRef]
19. Costa, R. D., Guldi, D. M. (2014). Carbon nanomaterials as integrative components in dye-sensitized solar cells. Solar Cells, 11, 12. DOI 10.1104/pp.116.2.571. [Google Scholar] [CrossRef]
20. Feihl, S., Costa, R. D., Pflock, S., Schmidt, C., Schönamsgruber, J. et al. (2012). Nickel oxide nanostructured electrodes towards perylenediimide-based dye-sensitized solar cells. RSC Advances, 2(30), 11495–11503. DOI 10.2135/cropsci2002.1919. [Google Scholar] [CrossRef]
21. Gibson, E. A., Le Pleux, L., Fortage, J., Pellegrin, Y., Blart, E. et al. (2012). Role of the triiodide/iodide redox couple in dye regeneration in p-type dye-sensitized solar cells. Langmuir, 28(15), 6485–6493. DOI 10.1007/BF00384257. [Google Scholar] [CrossRef]
22. Le Pleux, L., Smeigh, A. L., Gibson, E., Pellegrin, Y., Blart, E. et al. (2011). Synthesis, photophysical and photovoltaic investigations of acceptor-functionalized perylene monoimide dyes for nickel oxide p-type dye-sensitized solar cells. Energy and Environmental Science, 4(6), 2075–2084. DOI 10.1039/C1EE01148K. [Google Scholar] [CrossRef]
23. Qin, P., Wiberg, J., Gibson, E. A., Linder, M., Li, L. et al. (2010). Synthesis and mechanistic studies of organic chromophores with different energy levels for p-type dye-sensitized solar cells. The Journal of Physical Chemistry C, 114(10), 4738–4748. DOI 10.3923/ijb.2008.309.314. [Google Scholar] [CrossRef]
24. Gibson, E. A., Smeigh, A. L., Le Pleux, L., Fortage, J., Boschloo, G. et al. (2009). A p-type NiO-based dye-sensitized solar cell with an open-circuit voltage of 0.35 V. Angewandte Chemie, 48(24), 4402–4405. DOI 10.1002/anie.200900423. [Google Scholar] [CrossRef]
25. Chiang, C. C., Hung, C. Y., Chou, S. W., Shyue, J. J., Cheng, K. Y. et al. (2018). Ptcofe nanowire cathodes boost short-Circuit currents of Ru (II)-Based Dye-Sensitized solar cells to a power conversion efficiency of 12.29%. Advanced Functional Materials, 28(3), 1703282. DOI 10.1002/adfm.20170328. [Google Scholar] [CrossRef]
26. Mathew, S., Yella, A., Gao, P., Humphry-Baker, R., Curchod, B. F. et al. (2014). Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nature Chemistry, 6(3), 242–247. DOI 10.1093/aob/mcm047. [Google Scholar] [CrossRef]
27. Perera, I. R., Daeneke, T., Makuta, S., Yu, Z., Tachibana, Y. et al. (2015). Application of the tris (acetylacetonato) iron (III)/(II) redox couple in p-Type Dye-Sensitized solar cells. Angewandte Chemie, 54(12), 3758–3762. DOI 10.1002/anie.201409877. [Google Scholar] [CrossRef]
28. Upadhyaya, H. M., Senthilarasu, S., Hsu, M. H., Kumar, D. K. (2013). Recent progress and the status of dye-sensitised solar cell (DSSC) technology with state-of-the-art conversion efficiencies. Solar Energy Materials and Solar Cells, 119, 291–295. DOI 10.2478/s11756-007-0111-7. [Google Scholar] [CrossRef]
29. Ye, M., Wen, X., Wang, M., Iocozzia, J., Zhang, N. et al. (2015). Recent advances in dye-sensitized solar cells: From photoanodes, sensitizers and electrolytes to counter electrodes. Materials Today, 18(3), 155–162. DOI 10.1016/j.mattod.2014.09.001. [Google Scholar] [CrossRef]
30. Harikisun, R., Desilvestro, H. (2011). Long-term stability of dye solar cells. Solar Energy, 85(6), 1179–1188. DOI 10.1111/tpj.13390. [Google Scholar] [CrossRef]
31. Iftikhar, H., Sonai, G. G., Hashmi, S. G., Nogueira, A. F., Lund, P. D. (2019). Progress on electrolytes development in Dye-sensitized solar cells. Materials, 12(12), 1998. DOI 10.1104/pp.73.3.555. [Google Scholar] [CrossRef]
32. Al-Alwani, M. A. M., Mohamad, A. B., Ludin, N. A., Kadhum, A. A. H., Sopian, K. (2016). Dye-sensitised solar cells: Development, structure, operation principles, electron kinetics, characterisation, synthesis materials and natural photosensitisers. Renewable and Sustainable Energy Reviews, 65, 183–213. DOI 10.1007/s11099-013-0021-6. [Google Scholar] [CrossRef]
33. Wang, N., Lin, H., Li, X., Lin, C., Zhang, L. et al. (2006). Enhanced exchange current density and diffusion coefficient of iodide-based liquid electrolyte by layered α-zirconium phosphate. Electrochemistry Communications, 8(6), 946–950. DOI 10.1007/s00344-019-10018-x. [Google Scholar] [CrossRef]
34. Peter, L. M., Wijayantha, K. G. U. (2000). Electron transport and back reaction in dye sensitised nanocrystalline photovoltaic cells. Electrochimica Acta, 45(28), 4543–4551. DOI 10.1016/S0013-4686(00)00605-8. [Google Scholar] [CrossRef]
35. Hansen, P. E., Nguyen, P. T., Krake, J., Spanget-Larsen, J., Lund, T. (2012). Dye-sensitized solar cells and complexes between pyridines and iodines. a NMR, IR and DFT study. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 98, 247–251. DOI 10.1046/j.0031-9317.2001.1140108.x. [Google Scholar] [CrossRef]
36. Nusbaumer, H., Moser, J. E., Zakeeruddin, S. M., Nazeeruddin, M. K., Grätzel, M. (2001). CoII(dbbip)22+ complex rivals Tri-iodide/Iodide redox mediator in Dye-sensitized photovoltaic cells. The Journal of Physical Chemistry B, 105(43), 10461–10464. DOI 10.3390/plants9010088. [Google Scholar] [CrossRef]
37. Yanagida, S., Yu, Y., Manseki, K. (2009). Iodine/Iodide-free Dye-sensitized solar cells. Accounts of Chemical Research, 42(11), 1827–1838. DOI 10.1093/jxb/50.333.543. [Google Scholar] [CrossRef]
38. Tian, H., Sun, L. (2011). Iodine-free redox couples for dye-sensitized solar cells. Journal of Materials Chemistry, 21(29), 10592–10601. DOI 10.1039/C1JM10598A. [Google Scholar] [CrossRef]
39. Wang, P., Zakeeruddin, S. M., Moser, J. E., Humphry-Baker, R., Comte, P. et al. (2004). Stable New sensitizer with improved light harvesting for nanocrystalline Dye-sensitized solar cells. Advanced Materials, 16(20), 1806–1811. DOI 10.1111/j.1365-3040.1995.tb00538.x. [Google Scholar] [CrossRef]
40. Kato, N., Higuchi, K., Tanaka, H., Nakajima, J., Sano, T. et al. (2011). Improvement in long-term stability of dye-sensitized solar cell for outdoor use. Solar Energy Materials and Solar Cells, 95(1), 301–305. DOI 10.1016/j.solmat.2010.04.019. [Google Scholar] [CrossRef]
41. Yella, A., Lee, H. W., Tsao, H. N., Yi, C., Chandiran, A. K. et al. (2011). Porphyrin-sensitized solar cells with cobalt (II/III)–based redox electrolyte exceed 12 percent efficiency. Science, 334(6056), 629–634. DOI 10.1126/science.1209688. [Google Scholar] [CrossRef]
42. Merazga, A., Al-Subai, F., Albaradi, A. M., Badawi, A., Jaber, A. Y. et al. (2016). Effect of sol–gel MgO spin-coating on the performance of TiO2-based dye-sensitized solar cells. Materials Science in Semiconductor Processing, 41, 114–120. DOI 10.1016/j.mssp.2015.08.026. [Google Scholar] [CrossRef]
43. Högberg, D., Soberats, B., Yatagai, R., Uchida, S., Yoshio, M. et al. (2016). Liquid-crystalline dye-sensitized solar cells: Design of two-dimensional molecular assemblies for efficient ion transport and thermal stability. Chemistry of Materials, 28(18), 6493–6500. DOI 10.1021/acs.chemmater.6b01590. [Google Scholar] [CrossRef]
44. Hao, Y., Saygili, Y., Cong, J., Eriksson, A., Yang, W. et al. (2016). Novel blue organic dye for dye-sensitized solar cells achieving high efficiency in cobalt-based electrolytes and by co-sensitization. ACS Applied Materials Interfaces, 8(48), 32797–32804. DOI 10.1021/acsami.6b09671. [Google Scholar] [CrossRef]
45. Denizalti, S., Ali, A. K., Ela, Ç., Ekmekci, M., Erten-Ela, S. (2018). Dye-sensitized solar cells using ionic liquids as redox mediator. Chemical Physics Letters, 691, 373–378. DOI 10.1016/j.cplett.2017.11.035. [Google Scholar] [CrossRef]
46. Pradhan, A., Kiran, M. S., Kapil, G., Hayase, S., Pandey, S. S. (2019). Wide wavelength photon harvesting in dye-sensitized solar cells utilizing cobalt complex redox electrolyte: Implication of surface passivation. Solar Energy Materials and Solar Cells, 195, 122–133. DOI 10.1016/j.solmat.2019.03.013. [Google Scholar] [CrossRef]
47. Shih, C. M., Wu, Y. L., Wang, Y. C., Kumar, S. R., Tung, Y. L. et al. (2018). Ionic transport and interfacial interaction of iodide/iodine redox mechanism in agarose electrolyte containing colloidal titanium dioxide nanoparticles. Journal of Photochemistry and Photobiology A: Chemistry, 356, 565–572. DOI 10.1016/j.jphotochem.2018.01.034. [Google Scholar] [CrossRef]
48. Mehrabian, M., Dalir, S. (2018). Numerical simulation of highly efficient dye sensitized solar cell by replacing the liquid electrolyte with a semiconductor solid layer. Optik, 169, 214–223. DOI 10.1016/j.ijleo.2018.05.059. [Google Scholar] [CrossRef]
49. Chung, I., Lee, B., He, J., Chang, R. (2012). PH & kanatzidis, MG. Nature, 485, 486–489. DOI 10.1038/nature11067. [Google Scholar] [CrossRef]
50. Zhang, Z., Yang, Y., Gao, J., Xiao, S., Zhou, C. et al. (2018). Highly efficient ag2se quantum dots blocking layer for solid-state dye-sensitized solar cells: Size effects on device performances. Materials Today Energy, 7, 27–36. DOI 10.1016/j.solener.2020.08.091. [Google Scholar] [CrossRef]
51. Santhaveesuk, S., Pukird, S., Kahattha, C. (2018). A development nanocrystalline TiO2 based on dye sensitized solar cells with solid state electrolyte. Materials Today: Proceedings, 5(6), 14086–14090. DOI 10.1016/j.matpr.2018.02.067. [Google Scholar] [CrossRef]
52. Lee, B., He, J., Chang, R. P., Kanatzidis, M. G. (2012). All-solid-state dye-sensitized solar cells with high efficiency. Nature, 485(7399), 486–489. DOI 10.1038/nature11067. [Google Scholar] [CrossRef]
53. Bella, F., Popovic, J., Lamberti, A., Tresso, E., Gerbaldi, C. et al. (2017). Interfacial effects in solid–liquid electrolytes for improved stability and performance of dye-sensitized solar cells. ACS Applied Materials, 9(43), 37797–37803. DOI 10.1021/acsami.7b11899. [Google Scholar] [CrossRef]
54. Kim, D. W., Jeong, Y. B., Kim, S. H., Lee, D. Y., Song, J. S. (2005). Photovoltaic performance of dye-sensitized solar cell assembled with gel polymer electrolyte. Journal of Power Sources, 149, 112–116. DOI 10.1016/j.jpowsour.2005.01.058. [Google Scholar] [CrossRef]
55. Dong, R. X., Shen, S. Y., Chen, H. W., Wang, C. C., Shih, P. T. et al. (2013). A novel polymer gel electrolyte for highly efficient dye-sensitized solar cells. Journal of Materials Chemistry A, 1(29), 8471–8478. DOI 10.1039/C3TA11331K. [Google Scholar] [CrossRef]
56. Liu, L., Wu, Y., Chi, F., Yi, Z., Wang, H. et al. (2019). An efficient quasi-solid-state dye-sensitized solar cell with gradient polyaniline-graphene/PtNi tailored gel electrolyte. Electrochimica Acta, 316, 125–132. DOI 10.1016/j.electacta.2019.05.115. [Google Scholar] [CrossRef]
57. Venkatesan, S., Liu, I. P., Hung, W. N., Teng, H., Lee, Y. L. (2019). Highly efficient quasi-solid-state dye-sensitized solar cells prepared by printable electrolytes for room light applications. Chemical Engineering Journal, 367, 17–24. DOI 10.1016/j.cej.2019.02.118. [Google Scholar] [CrossRef]
58. Mohan, K., Bora, A., Roy, R. S., Nath, B. C., Dolui, S. K. (2019). Polyaniline nanotube/reduced graphene oxide aerogel as efficient counter electrode for quasi solid state dye sensitized solar cell. Solar Energy, 186, 360–369. DOI 10.1016/j.solener.2019.05.030. [Google Scholar] [CrossRef]
59. Thomas, M., Rajiv, S. (2020). Porous membrane of polyindole and polymeric ionic liquid incorporated PMMA for efficient quasi-solid state dye sensitized solar cell. Journal of Photochemistry and Photobiology A: Chemistry, 394, 112464. DOI 10.1016/j.jphotochem.2020.112464. [Google Scholar] [CrossRef]
60. Masud Kim, K. M., Kim, H. K. (2020). Polymer Gel electrolytes based on PEG-functionalized ABA triblock copolymers for quasi-solid-state Dye-sensitized solar cells: Molecular engineering and key factors. ACS Applied Materials & Interfaces, 12(37), 42067–42080. DOI 10.1021/acsami.0c09519. [Google Scholar] [CrossRef]
61. Xiong, D., Chen, W. (2012). Recent progress on tandem structured dye-sensitized solar cells. Frontiers of Optoelectronics, 5(4), 371–389. DOI 10.1007/s12200-012-0283-9. [Google Scholar] [CrossRef]
62. Nattestad, A., Perera, I., Spiccia, L. (2016). Developments in and prospects for photocathodic and tandem dye-sensitized solar cells. Journal of Photochemistry and Photobiology C: Photochemistry Reviews, 28, 44–71. DOI 10.1016/j.jphotochemrev.2016.06.003. [Google Scholar] [CrossRef]
63. Kubo, W., Sakamoto, A., Kitamura, T., Wada, Y., Yanagida, S. (2004). Dye-sensitized solar cells: Improvement of spectral response by tandem structure. Journal of Photochemistry and Photobiology A: Chemistry, 164(1–3), 33–39. DOI 10.1016/j.jphotochem.2004.01.024. [Google Scholar] [CrossRef]
64. Dürr, M., Bamedi, A., Yasuda, A., Nelles, G. (2004). Tandem dye-sensitized solar cell for improved power conversion efficiencies. Applied Physics Letters, 84(17), 3397–3399. DOI 10.1063/1.1723685. [Google Scholar] [CrossRef]
65. Yamaguchi, T., Uchida, Y., Agatsuma, S., Arakawa, H. (2009). Series-connected tandem dye-sensitized solar cell for improving efficiency to more than 10%. Solar Energy Materials and Solar Cells, 93(6–7), 733–736. DOI 10.1016/j.solmat.2008.09.021. [Google Scholar] [CrossRef]
66. Yanagida, M., Onozawa-Komatsuzaki, N., Kurashige, M., Sayama, K., Sugihara, H. (2010). Optimization of tandem-structured dye-sensitized solar cell. Solar Energy Materials and Solar Cells, 94(2), 297–302. DOI 10.1016/j.solmat.2009.10.002. [Google Scholar] [CrossRef]
67. Miao, Q., Wu, L., Cui, J., Huang, M., Ma, T. (2011). A New type of Dye-Sensitized solar cell with a multilayered photoanode prepared by a film-Transfer technique. Advanced Materials, 23(24), 2764–2768. DOI 10.1002/adma.201100820. [Google Scholar] [CrossRef]
68. Wood, C. J., Summers, G. H., Clark, C. A., Kaeffer, N., Braeutigam, M. et al. (2016). A comprehensive comparison of dye-sensitized NiO photocathodes for solar energy conversion. Physical Chemistry Chemical Physics, 18(16), 10727–10738. DOI 10.1039/C5CP05326A. [Google Scholar] [CrossRef]
69. Sobuś, J., Ziółek, M. (2014). Optimization of absorption bands of dye-sensitized and perovskite tandem solar cells based on loss-in-potential values. Physical Chemistry Chemical Physics, 16(27), 14116–14126. DOI 10.1039/C4CP01937G. [Google Scholar] [CrossRef]
70. Maufroy, A., Favereau, L., Anne, F. B., Pellegrin, Y., Blart, E. et al. (2015). Synthesis and properties of push–pull porphyrins as sensitizers for NiO based dye-sensitized solar cells. Journal of Materials Chemistry A, 3(7), 3908–3917. DOI 10.1039/C4TA05974C. [Google Scholar] [CrossRef]
71. Wei, L., Jiang, L., Yuan, S., Ren, X., Zhao, Y. et al. (2016). Valence band edge shifts and charge-transfer dynamics in Li-doped NiO based p-type DSSCs. Electrochimica Acta, 188, 309–316. DOI 10.1016/j.electacta.2015.12.026. [Google Scholar] [CrossRef]
72. Ho, P., Thogiti, S., Cheruku, R., Ahn, K. S., Kim, J. H. (2018). Enhanced efficiency via blocking layers at photocathode interfaces in cobalt-mediated tandem dye-sensitized solar cells. Solar Energy, 161, 9–16. DOI 10.1016/j.solener.2017.12.035. [Google Scholar] [CrossRef]
73. Ho, P., Ahn, K. S., Cheruku, R., Kim, J. H. (2016). P-Type dye-sensitized solar cells: Enhanced performance with a NiO compact blocking layer. Synthetic Metals, 217, 314–321. DOI 10.1016/j.synthmet.2016.04.006. [Google Scholar] [CrossRef]
74. Lü, S., Qu, X. (2011). Algaas/GaAs tunnel junctions in a 4-j tandem solar cell. Journal of Semiconductors, 32(11), 112003. DOI 10.1088/1674-4926/32/11/112003. [Google Scholar] [CrossRef]
75. Shahrjerdi, D., Bedell, S. W., Ebert, C., Bayram, C., Hekmatshoar, B. et al. (2012). High-efficiency thin-film inGaP/InGaAs/Ge tandem solar cells enabled by controlled spalling technology. Applied Physics Letters, 100(5), 053901. DOI 10.1063/1.3681397. [Google Scholar] [CrossRef]
76. Kim, H. S., Lee, C. R., Im, J. H., Lee, K. B., Moehl, T. et al. (2012). Lead iodide perovskite sensitized All-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Scientific Reports, 2(1), 591. DOI 10.1038/srep00591. [Google Scholar] [CrossRef]
77. Balasingam, S. K., Lee, M., Kang, M. G., Jun, Y. (2013). Improvement of dye-sensitized solar cells toward the broader light harvesting of the solar spectrum. Chemical Communications, 49(15), 1471–1487. DOI 10.1039/C2CC37616D. [Google Scholar] [CrossRef]
78. Todorov, T. K., Bishop, D. M., Lee, Y. S. (2018). Materials perspectives for next-generation low-cost tandem solar cells. Solar Energy Materials and Solar Cells, 180, 350–357. DOI 10.1016/j.solmat.2017.07.033. [Google Scholar] [CrossRef]
79. Brown, A. S., Green, M. A. (2002). Detailed balance limit for the series constrained two terminal tandem solar cell. Physica E: Low-Dimensional Systems and Nanostructures, 14(1–2), 96–100. DOI 10.1016/S1386-9477(02)00364-8. [Google Scholar] [CrossRef]
80. Ito, S., Dharmadasa, I. M., Tolan, G. J., Roberts, J. S., Hill, G. et al. (2011). High-voltage (1.8 V) tandem solar cell system using a gaAs/AlXGa(1−X) as graded solar cell and dye-sensitised solar cells with organic dyes having different absorption spectra. Solar Energy, 85(6), 1220–1225. DOI 10.1016/j.solener.2011.02.024. [Google Scholar] [CrossRef]
81. Moon, S. H., Park, S. J., Kim, S. H., Lee, M. W., Han, J. et al. (2015). Monolithic DSSC/CIGS tandem solar cell fabricated by a solution process. Scientific Reports, 5(1), 8970. DOI 10.1038/srep08970. [Google Scholar] [CrossRef]
82. Jeong, W. S., Lee, J. W., Jung, S., Yun, J. H., Park, N. G. (2011). Evaluation of external quantum efficiency of a 12.35% tandem solar cell comprising dye-sensitized and CIGS solar cells. Solar Energy Materials and Solar Cells, 95(12), 3419–3423. DOI 10.1016/j.solmat.2011.07.038. [Google Scholar] [CrossRef]
83. Liska, P., Thampi, K., Grätzel, M., Bremaud, D., Rudmann, D. et al. (2006). Nanocrystalline dye-sensitized solar cell/copper indium gallium selenide thin-film tandem showing greater than 15% conversion efficiency. Applied Physics Letters, 88(20), 203103. DOI 10.1063/1.2203965. [Google Scholar] [CrossRef]
84. Chae, S. Y., Park, S. J., Joo, O. S., Jun, Y., Min, B. K. et al. (2016). Highly stable tandem solar cell monolithically integrating dye-sensitized and CIGS solar cells. Scientific Reports, 6(1), 30868. DOI 10.1038/srep30868. [Google Scholar] [CrossRef]
85. Wenger, S., Seyrling, S., Tiwari, A. N., Grätzel, M. (2009). Fabrication and performance of a monolithic dye-sensitized TiO2/Cu (In, Ga) Se 2 thin film tandem solar cell. Applied Physics Letters, 94(17), 173508. DOI 10.1063/1.3125432. [Google Scholar] [CrossRef]
86. Miettunen, K., Saukkonen, T., Li, X., Law, C., Sheng, Y. K. et al. (2012). Do counter electrodes on metal substrates work with cobalt complex based electrolyte in dye-sensitized solar cells? Journal of the Electrochemical Society, 160(2), H132–H137. DOI 10.1149/2.074302jes. [Google Scholar] [CrossRef]
87. Wang, W. L., Lin, H., Zhang, J., Li, X., Yamada, A. et al. (2010). Experimental and simulation analysis of the dye sensitized solar cell/Cu(in,Ga)Se2 solar cell tandem structure. Solar Energy Materials and Solar Cells, 94(10), 1753–1758. DOI 10.1016/j.solmat.2010.05.041. [Google Scholar] [CrossRef]
88. Hao, S., Wu, J., Sun, Z. (2012). A hybrid tandem solar cell based on hydrogenated amorphous silicon and dye-sensitized TiO2 film. Thin Solid Films, 520(6), 2102–2105. DOI 10.1016/j.tsf.2011.08.061. [Google Scholar] [CrossRef]
89. Wali, Q., Iftikhar, F. J., Elumalai, N. K., Iqbal, Y., Yousaf, S. et al. (2020). Advances in stable and flexible perovskite solar cells. Current Applied Physics, 20(5), 720–737. DOI 10.1016/j.cap.2020.03.007. [Google Scholar] [CrossRef]
90. Lu, H., Sun, J., Zhang, H., Lu, S., Choy, W. C. H. (2016). Room-temperature solution-processed and metal oxide-free nano-composite for the flexible transparent bottom electrode of perovskite solar cells. Nanoscale, 8(11), 5946–5953. DOI 10.1039/C6NR00011H. [Google Scholar] [CrossRef]
91. Olaleru, S. A., Kirui, J. K., Wamwangi, D., Roro, K. T., Mwakikunga, B. (2020). Perovskite solar cells: The new epoch in photovoltaics. Solar Energy, 196, 295–309. DOI 10.1016/j.solener.2019.12.025. [Google Scholar] [CrossRef]
92. Anaya, M., Lozano, G., Calvo, M. E., Míguez, H. (2017). ABX3 perovskites for tandem solar cells. Joule, 1(4), 769–793. DOI 10.1016/j.joule.2017.09.017. [Google Scholar] [CrossRef]
93. Wali, Q., Elumalai, N. K., Iqbal, Y., Uddin, A., Jose, R. (2018). Tandem perovskite solar cells. Renewable and Sustainable Energy Reviews, 84, 89–110. DOI 10.1016/j.rser.2018.01.005. [Google Scholar] [CrossRef]
94. Werner, J., Dubuis, G., Walter, A., Löper, P., Moon, S. J. et al. (2015). Sputtered rear electrode with broadband transparency for perovskite solar cells. Solar Energy Materials and Solar Cells, 141, 407–413. DOI 10.1016/j.solmat.2015.06.024. [Google Scholar] [CrossRef]
95. Duong, T., Wu, Y., Shen, H., Peng, J., Fu, X. et al. (2017). Rubidium multication perovskite with optimized bandgap for perovskite-silicon tandem with over 26% efficiency. Advanced Energy Materials, 7(14), 1700228. DOI 10.1002/aenm.201700228. [Google Scholar] [CrossRef]
96. Bush, K. A., Palmstrom, A. F., Yu, Z. J., Boccard, M., Cheacharoen, R. et al. (2017). 23.6%-efficient monolithic perovskite/silicon tandem solar cells with improved stability. Nature Energy, 2(4), 17009. DOI 10.1038/nenergy.2017.9. [Google Scholar] [CrossRef]
97. Shen, H., Walter, D., Wu, Y., Fong, K. C., Jacobs, D. A. et al. (2020). Monolithic perovskite/Si tandem solar cells: Pathways to over 30% efficiency. Advanced Energy Materials, 10(13), 1902840. DOI 10.1002/aenm.201902840. [Google Scholar] [CrossRef]
98. Zhou, Y., Yin, X., Zhang, Q., Wang, N., Yamamoto, A. et al. (2019). Perovskite solar cell-thermoelectric tandem system with a high efficiency of over 23%. Materials Today Energy, 12, 363–370. DOI 10.1016/j.mtener.2019.03.003. [Google Scholar] [CrossRef]
99. Kinoshita, T., Nonomura, K., Joong Jeon, N., Giordano, F., Abate, A. et al. (2015). Spectral splitting photovoltaics using perovskite and wideband dye-sensitized solar cells. Nature Communications, 6(1), 8834. DOI 10.1038/ncomms9834. [Google Scholar] [CrossRef]
100. Hosseinnezhad, M. (2019). Enhanced performance of dye-sensitized solar cells using perovskite/DSSCs tandem design. Journal of Electronic Materials, 48(9), 5403–5408. DOI 10.1007/s11664-019-07272-w. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |