DOI:10.32604/EE.2020.010493

| Energy Engineering DOI:10.32604/EE.2020.010493 |  |
| Article |
Upgrading the Quality of Solid Fuel Made from Nyamplung (Calophyllum inophyllu) Wastes Using Hydrothermal Carbonization Treatment
1Graduate Program of Vocational Teacher Education, Sebelas Maret University, Surakarta, 57126, Indonesia
2Department of Mechanical Engineering Education, Sebelas Maret University, Surakarta, 57126, Indonesia
3Department of Mechanical Engineering, Janabadra University, Yogyakarta, 55231, Indonesia
4Lee Kong Chian Faculty of Engineering and Science, UTAR, Kajang, 43000, Malaysia
*Corresponding Author: Nugroho Agung Pambudi. Email: agung.pambudi@staff.uns.ac.id
Received: 07 March 2020; Accepted: 15 June 2020
Abstract: One of the major problems faced in managing biomass waste to higher quality products is choosing the right technology. Wastes are used as an alternative fuel, with increase in the calorific value. Hydrothermal carbonization (HTC) is a biomass conversion technology, used to obtain solid fuel. This study aims to utilize of Calophyllum inophyllum as an alternative solid fuel through HTC. The calorific value and proximate of the hydrochar will be determined and analyzed to find out its quality. The experiments were carried out at temperature variations of 160°C, 190°C, and 220°C and holding times of 30 and 60 minutes. The results show that an increase in temperature and holding time causes a decline in the moisture content 1.87%, volatile matter 54.03%, and ash content 12.35%, respectively, leading to elevations in the fixed carbon at 31.75%. In addition, the highest calorific value of 4149 Kcal/Kg was produced at a temperature of 220°C, within a holding time of 60 minutes. The results showed a significant increase in the quality of solid fuels between 3500–4611 Kcal/Kg in accordance with the American Standard Testing and Materials (ASTM). Therefore, this research leads to an important finding that Calophyllum inophyllum waste through the HTC process can be used as an alternative fuel to substitute lignite coal, which is environmentally friendly.
Keywords: Hydrothermal; carbonization; solid fuel; Calophyllum inophyllum; Nyamplung
Hydrothermal Carbonization (HTC) is a process of converting biomass into solid fuels, which is often conducted at medium pressure and temperatures ranging from 180°C up to 280°C [1]. The pressure used is from 1.0 Mpa to 4.0 Mpa [2]. In addition, its benefits include the conversion of wet biomass into chemicals and liquid, solid or gaseous fuels without requiring a drying process in advance. The water contained in biomass plays the role of a solvent and reactant, therefore, it is beneficial when conversion occurs. HTC requires a relatively low temperature [2], while the Pyrolysis process requires a higher temperature. Furthermore, the biomass used for pyrolysis is first dried to prevent the water from interfering with the combustion process [3].
HTC is a promising technology in thermochemical conversions, and also in the transformation of waste biomass, attained through the use of water sub, which act as a reactant, catalyst, and solvent, and supercritical, as medium of processing. During the heating process, water is converted to steam and this reduces latent heat loss, thereby, increasing thermal efficiency. During high temperature and pressure, the water becomes supercritical, therefore, it is a beneficial organic solvent for the conversion process of biomass. During biomass conversion, sulfur and nitrogen oxide gases are easily dissolved in water. This process makes the HTC technology a promising and environmentally friendly technology, due to the reduction of harmful gas emissions. After the reaction is complete, the process is returned to normal temperature and pressure. In addition, the water is separated from other liquid organic products [2].
Brunner [4] reported that the technology termed Hydrothermal Upgrading (HTU), was introduced by Shell Oil Company in the 1980s. This method requires that the degradation occurs with the use of water, which is capable of affecting physicochemical properties. Supercritical hot water has the potential to produce more useful products due to the ability of the water to process unwanted compounds into compatible materials with the environment under review. When water is hot and supercritical, it changes from ionic to non-ionic substances. Boiling water and high temperatures are used for extraction and at moderate temperatures, ionic and polar substances are extracted. Subsequently, at high and near critical temperatures, non-polar substances are easily dissolved and extracted, with high reactivity on pressurized hot water. This is generally called a hydraulic reaction that is catalyzed by acid or arises from hydrothermal transformation. This is because dissolution of 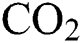 in water increases the availability of protons, while its addition catalyzes the hydrolysis reaction [5].
in water increases the availability of protons, while its addition catalyzes the hydrolysis reaction [5].
HTC is based on a simple chemical process of separation between water and carbohydrates (dehydration):
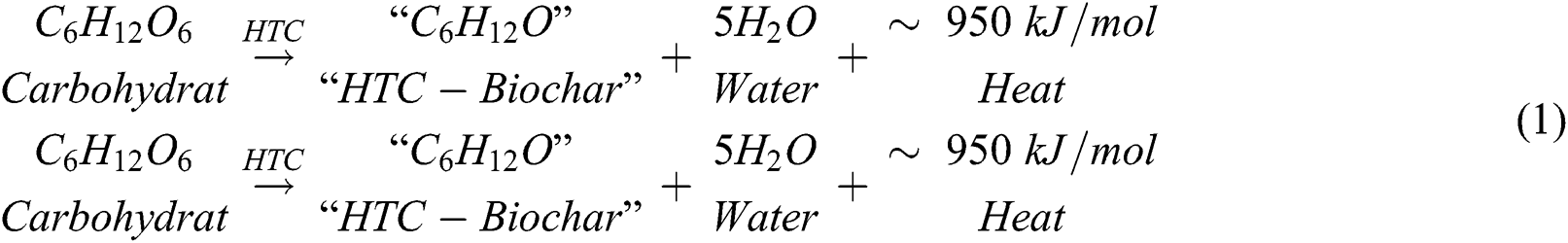
The energy balance shows it to be included as an exothermic reaction, which generates hydrochar [6,7]. During the HTC process, there was a release reaction by one third of the combustion energy stored in carbohydrates during dehydration due to the high thermodynamic stability of water in the reactor [6]. Meanwhile, within the process exists better efficiency, due to the production of higher carbon and lower moisture contents [8]. The water produced from the HTC reaction process contains dissolved organic carbons (DOC) of 5–30 gL−1 which was dependent on the biomass used and the process conditions such as temperature, pressure, holding time, and pH [9]. Moreover, the process also generates small amounts of gas containing a large quantity of carbon dioxide (CO2) which is less than 5% of the feedstock carbon [1]. All processes, production and quality of solid fuel produced from HTC are dependent on the parameters used, such as temperature, pressure, type of material, holding time, pH and the ratio of water, which served as a catalyst as shown in Fig. 1.
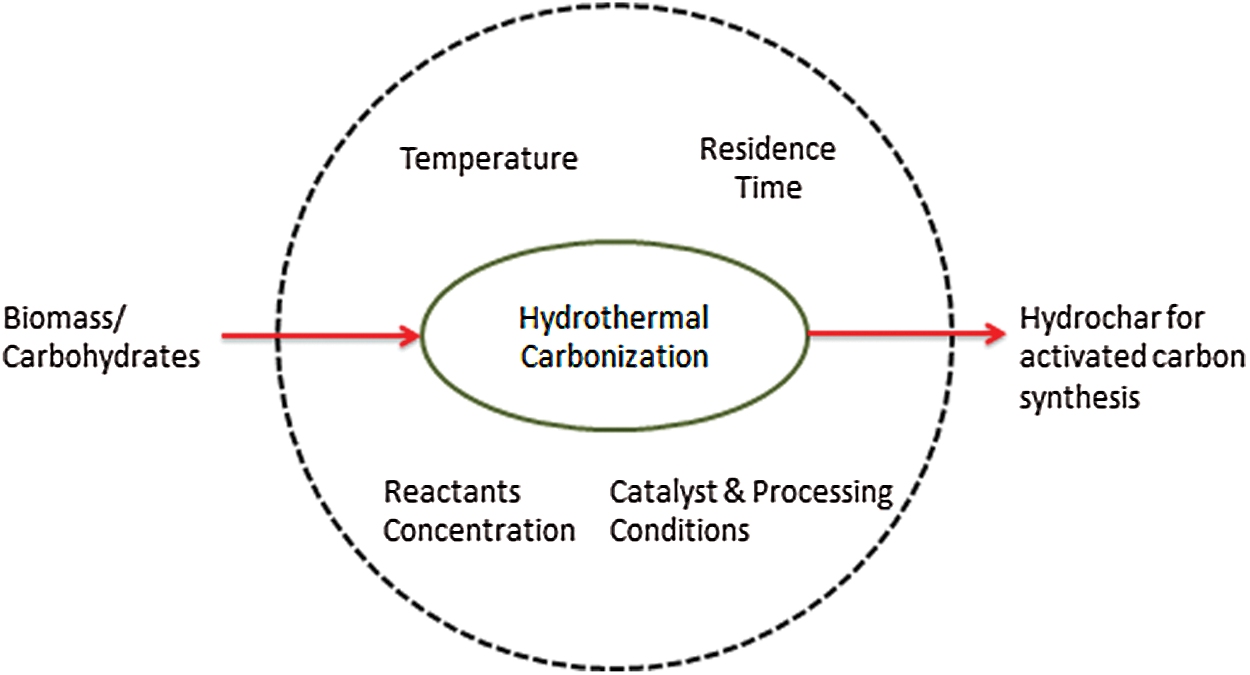
Figure 1: Parameters of HTC [10]
Gao et al. [2] reported on the influence of carbonization temperature on the physicochemical and thermal behavior of hydrocarbon products, during the HTC process. An increase in temperature, leads to a decrease in energy yield. In addition, the volatile material produced during the carbonization process leads to loss of hydrochar mass and energy. However, during hydrothermal carbonization, degradation of lignocellulose produces small pieces of hydrochar which is released as inert gas, thereby leading to energy loss.
Based on the study conducted by Cyrilla Oktaviananda [11], an elevation in temperature influences the hydrochar quality, causing a decline in amount, as well as a reduction in energy yield, moisture, ash, volatility, and potassium content. However, this elevates the ratio of energy densification, calorific value, and fixed carbon, and the process initiates the generation of hydrochar, which is equivalent to the type of lignite coal. According to the American Society for Testing and Materials (ASTM), lignite coal has moist calorific value below 4611 Kcal/Kg, which is brown coal below 3500 Kcal/Kg and lignite coal of 3500–4611 Kcal/Kg.
In addition, a research conducted by Eriska [12], showed the effect of elevated temperatures on the chemical and physical properties of some raw materials and hydrochar. This also causes an increase in the Higher Heating Value (HHV). This research shows that increase in heat and temperature, leads to a decrease in mass and energy yields. Furthermore, biomass’ cellulose and hemicellulose content are easily decomposed compared to lignin. It also has higher calorific value and carbon content in the form of cellulose.
Other studies investigating biomass and kinetic analysis also show that the rate of thermal decomposition of biomass fuels under oxidizing conditions will be faster than the inert atmosphere [13]. In addition, as the temperature increases, there is a continuous decrease in biomass mass [13,14]. Furthermore, in the process of thermal decomposition, the content of cellulose, hemiselolosa, and lignin in the biomass will produce oxygenated by-products. Thus, as the temperature increases, these by-products will achieve spontaneous ignition and the heat released will contribute to the decomposition of the remaining organic material. Biomass material which has a high cellulose content is very good for the bio-thermal conversion process, while the high lignin content is very suitable for the combustion or gasification process [13].
In the experimental conducted by Irsyad, the HTC was reported to have improved the characteristics and calorific value of empty oil palm bunches [15]. Therefore, the process reduced organic matter content, thus, producing clean fuel, which is more environmentally friendly.
The Research by Nizamuddin, also proved the preference of temperature, over holding time, and ratio of water composition in the collective impact on the hydrochar effectiveness and quality, resulting from the ability to significantly enhance porosity in crude oil shells [16].
Other studies also shows that the HTC narrows the difference in fuel properties between different biomass feedstocks. The quality of hydrochar fuel increases along with a rise in ignition and combustion temperatures. This research shows that HTC is a better conversion process for hydrochar production with enhanced fuel quality compared to raw biomass [17].
Calophyllum inophyllum is a type of forest plant, containing potential bioenergy, and is also widely distributed worldwide, including East Africa, South and Southeast Asia (Malaysia, Philippines, Thailand, Indonesia, and Papua New Guinea), the Pacific Islands, Madagascar, West Indies, and South America. Meanwhile, they are produced in Indonesia by up to 255,300 ha (Forestry Balitbang, 2008). Calophyllum inophyllum tree has a vertical height of up to 25 m, with horizontal branch ranging from 4 m to 10 m, and diameter reaching 150 cm. In addition, it has high plant seed production, of about 20 tonnes/ha/yr, which is more significant than other types of similar plants, including Jatropha curcas, of 5 tonnes/ha/yr, and palm oil, with 6 tonnes/ha/yr. According to the Research Agency of biotechnology and plant breeding of the forests in Indonesia, the Calophyllum inophyllum has some potential such as including for medical [18].
Furthermore, its seed possesses a fairly high oil yield in contrast with other plants, which is about 40% to 73%. This is higher than the Jatropha curcas of 25% to 40%, Sterculia foetida of 24% to 40%, Abrus precatorius Linn of 14% to 28%, Moringa oleifera 39% to 40%, and Schleichera oleosa 30% to 40%. Calophyllum inophyllum dried seed percentage has about 75% content in the form of oil, and 25% as waste seeds [19].
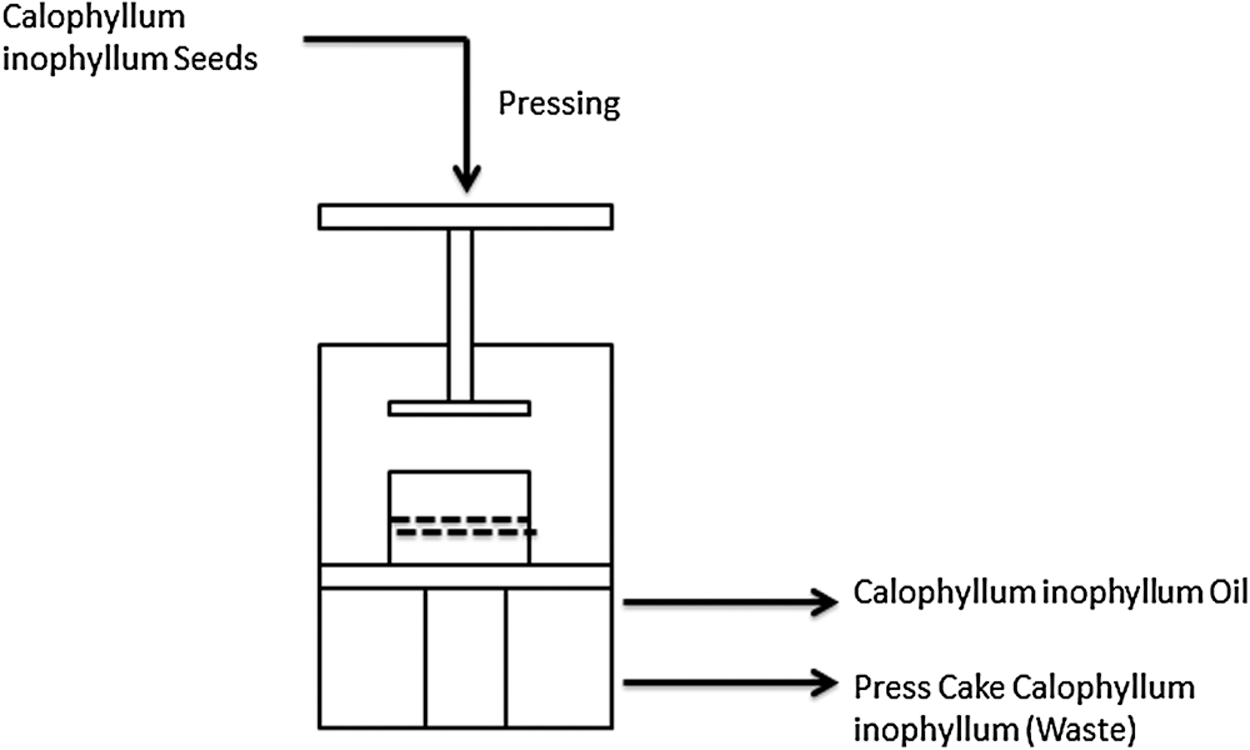
Figure 2: Calophyllum inophyllum oil design scheme
Oil production from Calophyllum inophylllum scheme is shown in Fig. 2. Indrayani reported that during the processing of oil, using porous carbon catalytic pyrolysis treatment, a liquid fuel with fraction equivalent to gasoline at 84.14%, and the main waste is cake seed was produced [20]. Samino reported the Calophyllum inophyllum cake seed produced were capable of generating large amounts of waste, which is yet to be utilized [21]. However, the proper method application is capable of developing zero-waste. The protein content of the seed cake is 22.76%, through the hydrolysis process, the total is 18.64%, where the dissolved variety was 16.44%. Therefore, the exploitation of this material is highly limited since it is only used as livestock feed, and in fertilizers.
HTC is an appropriate technology used to improve the quality and economic value of Calophyllum inophylllum as a solid fuel. This raw material possesses environmentally friendly characteristics, with heating value equivalent to coal.
The Calophyllum inophyllum tree is an Indonesian forest plant consisting of evergreen trees characterized by very high seed productivity, with oil content of above 50%, which is often used as a source of bioenergy. This experiment involves the preparation of waste, where the initial stage entails the provision of a hydrothermal process for the material, followed by data retrieval and analysis.
In this study, Calophyllum inophyllum waste used was still in wet conditions. Waste is weighed with a weight of 200 grams, then water is added with a weight ratio of 1:4 and 1:5. The HTC parameters used in this study include: temperatures of 160°C (minimum), 190°C, and 220°C (maximum), holding times of 30 and 60 minutes and the ratios of biomass to water 1:4 and 1:5 at a temperature of 190°C. Meanwhile, the selection of 190°C was conducted based on Eriska’s research which showed that it was the best in providing the highest heat value [12].
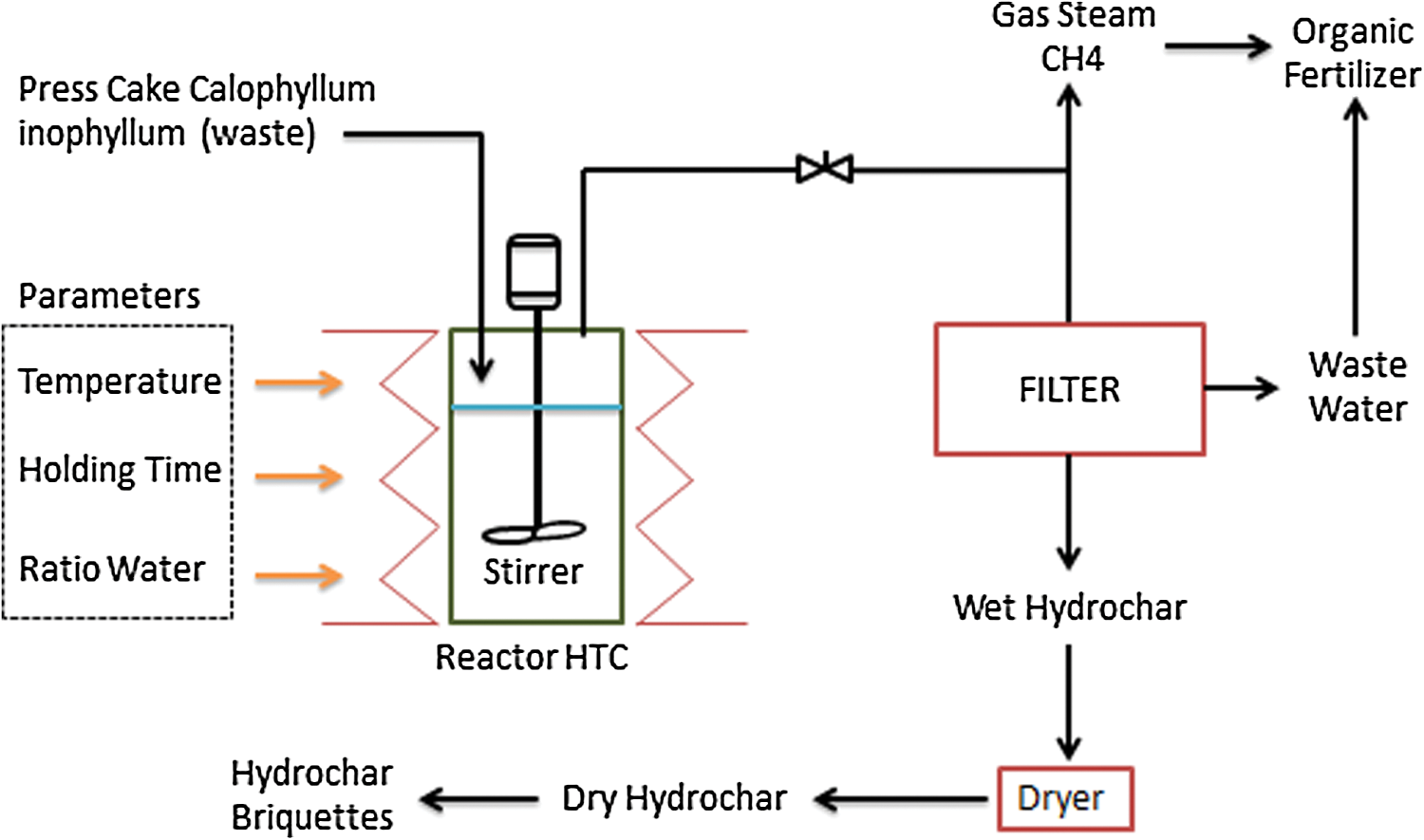
Figure 3: Calophyllum inophyllum HTC process
Hydrothermal equipment include autoclave reactor (the maximum capacity of the reactor is 1200 liters), equipped with an electric motor, which serves as a drive for the agigator, as well as a biomass and water stirrer (Fig. 3). The heater is carried out to heat the reactor according to the input given to HTC, while the Stirrer mixes the mixture in the reactor. Furthermore, steam, liquid and the hydrochar mixture were taken on completion of the process, which are possibly adopted as organic fertilizers. These were, therefore, separated through the use of a filter, and the liquid part possessed the potential of being used as an organic fertilizer, while the other was dried for application as solid fuel.
The wet hydrochar is separated from liquid, and dried with oven machine for 1 hour. After the hydrochar is completely dry, calorific value and proximate are determined using a bomb calorimeter and furnace. Furthermore, proximate evaluation involved the use of ASTM and ISO standards, while heat testing standard required ASTM D5865-13. In addition, ash content involved ASTM D3174, while moisture content uses ASTM D-3173, and volatile levels needed the ISO 562 standards.
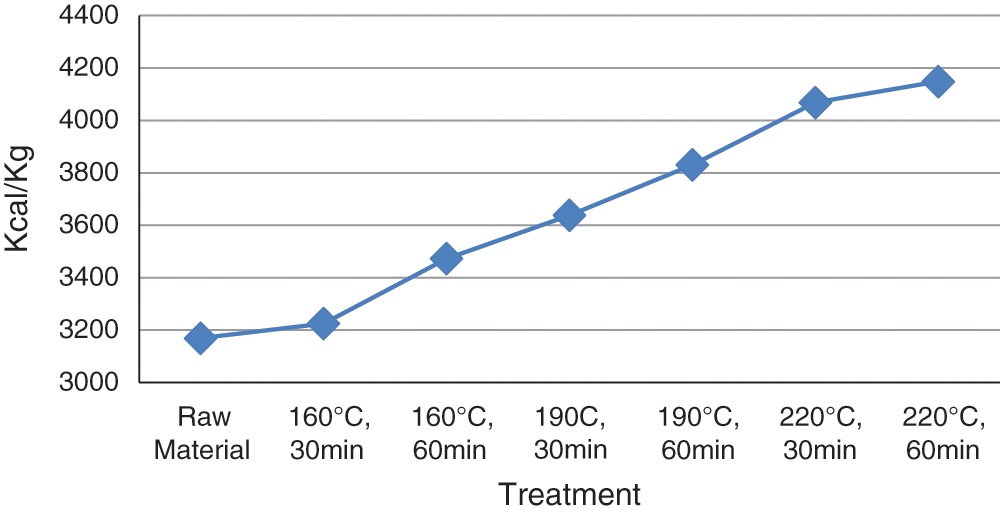
Figure 4: Calorific value
Calorific value with temperatures of 160°C, 190°C, and 220°C, and a holding time of 30 and 60 minutes are shown in Fig. 4. Meanwhile, the highest data was obtained at 220°C, using a holding time of 60 minutes, at a 4149 Kcal/Kg. In addition, the graph shows a higher temperature used affects the length of holding time, and subsequently elevate the hydrochar calorific value. This is a standard energy parameter on solid fuel, which is also dependent on water, ash and carbon content of a material.
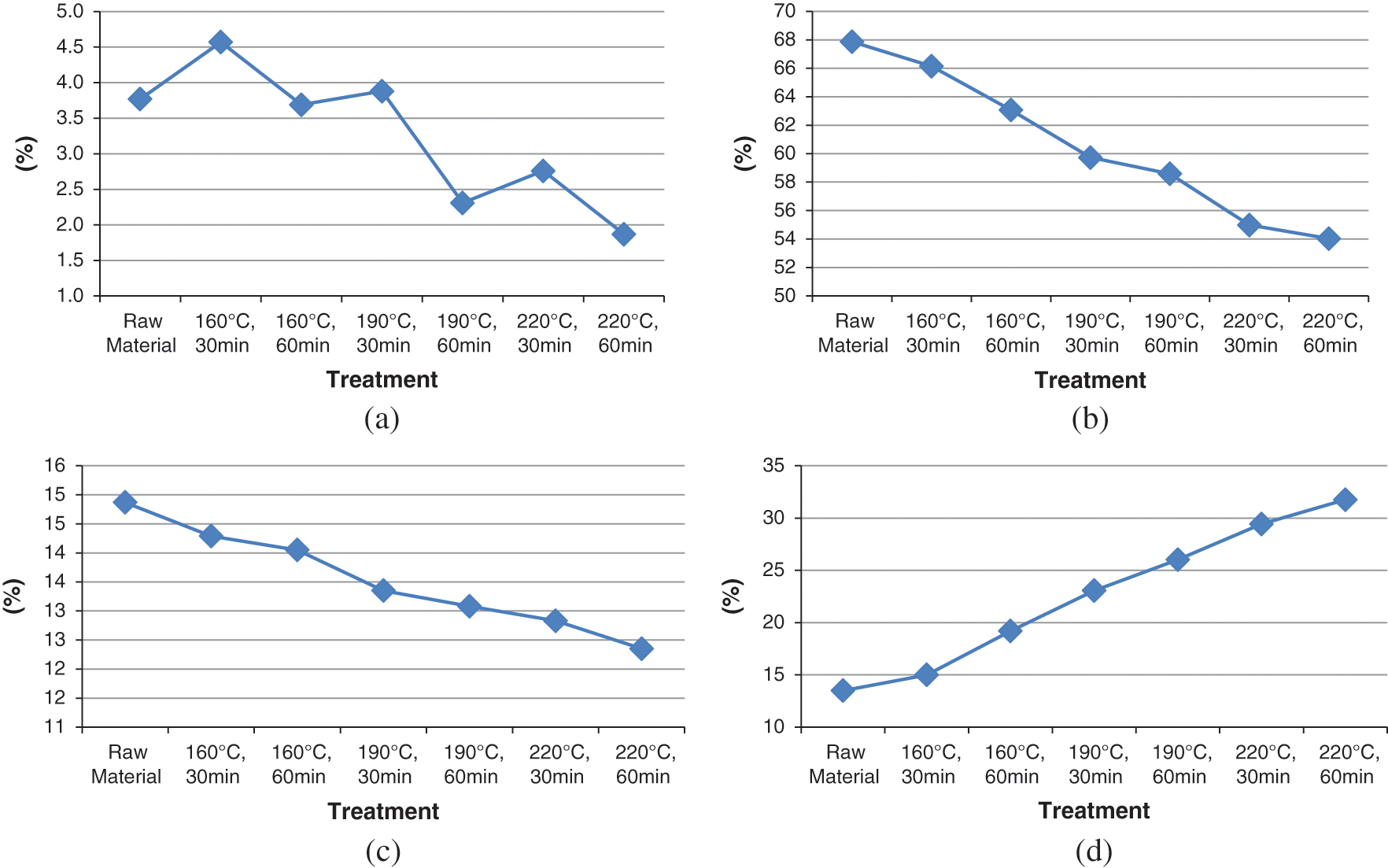
Figure 5: Proximate analysis. (a) Moisture content, (b) Volatile matter, (c) Ash content, and (d) Fixed carbon
The calorific value increases with a rise in temperature and length of holding time. However, an increase in temperature decreases the water content which is directly related to a rise in the calorific value. This is because, assuming the water content in a material is low, the amount of smoke produced decreases and the combustion reaction increases [22].
Fig. 5a shows the moisture content, where the lowest (1.87%) was observed at a temperature of 220°C, and holding time of 60 minutes, as shown in the graph.
Based on the theory of thermal decomposition, an elevation in the value for temperature causes fracturing of the material and water structure, which are exhibited as an initial product of the process [23]. The water content decreases with a rise in temperature and length of holding time. The strong quality of a fuel is greatly influenced by the water content, this is because the lower the water content, the better fuel quality [24]. Furthermore, high water content is also due to the existence of more pores on a product, further, decreases calorific value, reducing the efficiency and performance of the fuel, and also prevent subsequent combustion. Conversely, the low moisture content value tends to increase efficiency and performance, thus, accelerating the combustion process [22].
Volatile levels are shown in Fig. 5b, where the lowest occurred at a temperature of 220°C, and holding time of 60 minutes, exhibiting a percentage of 54.03%. The higher the temperature and the longer the holding time, the lower the volatile matter which affects the flame and fuel combustion rate. Fuels with high volatile matter releases the calorific value as combustion vapor, and this jeopardizes combustion efficiency.
Obernberger & Thek reported on the incidence of fuel released as vapors at high temperature [22]. Thus, the reduced content is as a result of the constituent breakdown, which was due to an elevation in temperature. Therefore, high levels of volatile compounds are caused by the incomplete decomposition of carbon compounds and H2.
Fig. 5c shows the ash content, where the lowest amount (12.35%) was found at a temperature of 220°C and at a holding time of 60 minutes, as seen in the graph. The decrease in ash content occurs with increase in temperature and holding time. A decrease in ash content shows a higher calorific value in solid fuels and vice versa.
Meanwhile, high ash content was due to the low density of raw materials, with higher percentage in fuel, which reduces the calorific value. Conversely, a decline in this percentage leads to more significant calorific values, consequently causing greater carbon content in a product. This phenomenon was influenced by the reduction in water content, and volatile substances, while carbon content was elevated, hence higher quantity of water is needed.
Furthermore, a higher pressure in the autoclave reactor during the hydrothermal process, subsequently reduces the ash content of the material.
The carbon content shown in Fig. 5d is obtained after conducting the hydrothermal process on Calophyllum inophyllum waste, at temperatures of 160°C, 190°C, and 220°C, and also the holding time of 30 and 60 minutes. Fig. 5d shows the most significant carbon content at 220°C, at 31.75%, during a holding time of 60 minutes. In addition, the graph also demonstrates a positive correlation with the carbon content of hydrochar obtained from Calophyllum inophyllum waste.
An increase in fixed carbon was inversely proportional to the biomass moisture content, resulting from the ability of the reduced moisture to increase fixed carbon. This is also affected by lower composition of volatile matter, being a result of chemical decomposition, followed by the formation of solid fuels from complex mixtures. Therefore, higher temperatures are affiliated with greater extents of decomposition, alongside a reduction in volatile compounds present in the fuel molecules, leading to an increase in fixed carbon content [25].
Table 1: Coal Calorific Value Standards according to the American Standard Testing and Material (ASTM) [26]
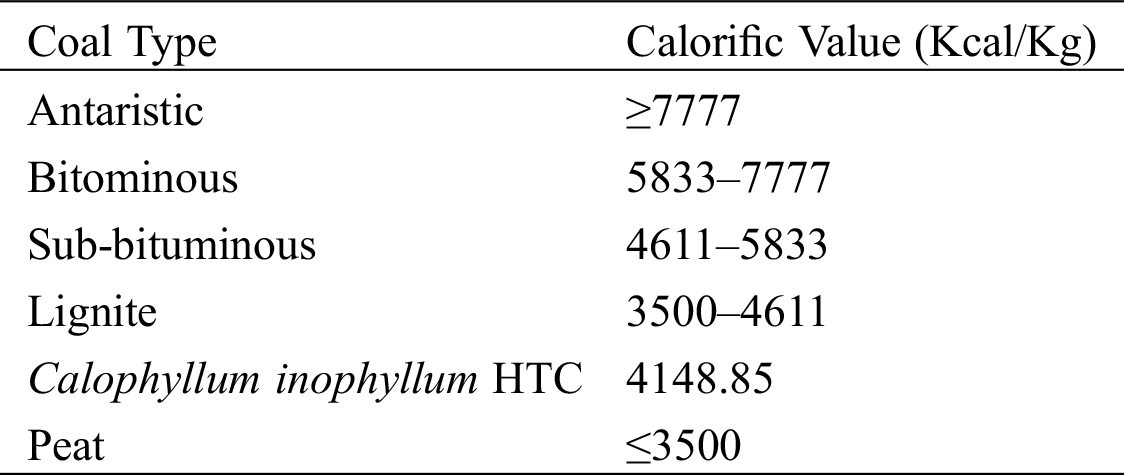
According to the research, the HTC treatment at 220°C and holding time of 60 minutes had the highest calorific value, at 4148.85 Kcal/Kg. The calorific value of Calophyllum inophyllum waste has been shown to increase through the HTC process. The value of this solid fuel is in accordance with American Standard Testing and Materials (ASTM) standards for lignite A type of coal, which is 3500–4611 Kcal/Kg (see Tab. 1).
Proximate analysis showed the following characteristics of the best outcomes: a decline in moisture content, volatile matter, and ash content to 1.89%, 54.03% and 12.35%, respectively, as well as an increase in the amount of fixed carbon to 31.75%.
Increase in temperature and holding time, decreases the water, ash content and volatile matter, with a rise in fixed carbon of solid fuels. These results indicate that the HTC process can significantly improve the quality of solid fuels from Calophyllum inophyllum waste. In addition, hydrochar produced from this waste can also be used as an alternative material to replace coal (lignite).
Therefore, the results of hydrochar from Calophyllum inophyllum waste is recommended as an alternative solid fuel to replace coal. Further studies need to be carried out on the use of pressure variations to determine the effect of pressure on the HTC process.
Funding Statement: This article has been funded from research activities entitled Increasing Geothermal Technology Education and Public Outreach for Communities as Supporters for the Development of 35 MW Geothermal Power Plants according to research contract No. 452/UN27.21/PN/2020 (Featured Research Grants) PNBP funding sources for Budget Year 2020.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Weiner, B., Baskyr, I., Poerschmann, J., Kopinke, F. D. (2013). Potential of the hydrothermal carbonization process for the degradation of organic pollutants. Chemosphere, 92(6), 674–680. DOI 10.1016/j.chemosphere.2013.03.047.
2. Gao, Y., Yu, B., Wu, K., Yuan, Q., Wang, X. et al. (2016). Physicochemical, pyrolytic, and combustion characteristics of hydrochar obtained by hydrothermal carbonization of biomass. BioResources, 11(2), 4113–4133.
3. Zhao, P., Shen, Y., Ge, S., Chen, Z., Yoshikawa, K. (2014). Clean solid biofuel production from high moisture content waste biomass employing hydrothermal treatment. Applied Energy, 131, 345–367. DOI 10.1016/j.apenergy.2014.06.038.
4. Tekin, K., Karagöz, S., Bektaş, S. (2014). A review of hydrothermal biomass processing. Renewable and Sustainable Energy Reviews, 40, 673–687. DOI 10.1016/j.rser.2014.07.216.
5. Brunner, G. (2009). Near critical and supercritical water. Part I. Hydrolytic and hydrothermal processes. Journal of Supercritical Fluids, 47(3), 373–381. DOI 10.1016/j.supflu.2008.09.002.
6. Ramke, H. G., Blöhse, D., Lehmann, H. J., Fettig, J., Höxter, S. T. (2009). Hydrothermal carbonization of organic waste. Sardinia 2009. Twelfth International Waste Management and Landfill Symposium. Symposium in S. Margherita di Pula–Cagliari, Sardinia, Italy 05–09 October 2009.
7. Titirici, M. M., Thomas, A., Antonietti, M. (2007). Back in the black: hydrothermal carbonization of plant material as an efficient chemical process to treat the CO2 problem? New Journal of Chemistry, 31(6), 787–789. DOI 10.1039/b616045j.
8. Fuertes, A. B., Arbestain, M. C., Sevilla, M., Maciá-Agulló, J. A., Fiol, S. et al. (2010). Chemical and structural properties of carbonaceous products obtained by pyrolysis and hydrothermal carbonisation of corn stover. Soil Research, 48(6–7), 618–626. DOI 10.1071/SR10010.
9. Funke, A., Zieglar, F. (2010). Hydrothermal carbonization of biomass: a summary and discussion of chemical mechanisms for process engineering. Biofuels, Bioproducts and Biorefining, 4(2), 160–177. DOI 10.1002/bbb.198.
10. Jain, A., Balasubramanian, R., Srinivasan, M. P. (2016). Hydrothermal conversion of biomass waste to activated carbon with high porosity: a review. Chemical Engineering Journal, 283, 789–805. DOI 10.1016/j.cej.2015.08.014.
11. Oktaviananda, C. (2017). Pengaruh temperatur dan komposisi campuran biomassa terhadap kualitas hydrochar pada hydrothermal treatment biomassa serbuk gergaji kayu jati dan serbuk tongkol jagung, pp. 96–97.
12. Eriska, H., Dewi, K., Darmawan Pasek, A., Damanhuri, E. (2017). Hydrothermal carbonization of biomass waste by using a stirred reactor: an initial experimental results. Reaktor, 16(4), 212. DOI 10.14710/reaktor.16.4.212-217.
13. Sher, F., Iqbal, S. Z., Liu, H., Imran, M., Snape, C. E. (2020). Thermal and kinetic analysis of diverse biomass fuels under different reaction environment: a way forward to renewable energy sources. Energy Conversion and Management, 203, 112266. DOI 10.1016/j.enconman.2019.112266.
14. Hai, I. U., Sher, F., Yaqoob, A., Liu, H. (2019). Assessment of biomass energy potential for SRC willow woodchips in a pilot scale bubbling fluidized bed gasifier. Fuel, 258, 116143.
15. Rofi, A., Prawisudha, P., Darmawan, A. (2014). Kaji eksperimental produksi bahan bakar padat ramah lingkungan dari tandan kosong kelapa sawit menggunakan proses hidrotermal. Proceeding, 13, 15–16.
16. Nizamuddin, S., Baloch, H. A., Griffin, G. J., Mubarak, N. M., Bhutto, A. W. et al. (2017). An overview of effect of process parameters on hydrothermal carbonization of biomass. Renewable and Sustainable Energy Reviews, 73, 1289–1299. DOI 10.1016/j.rser.2016.12.122.
17. Liu, Z., Balasubramanian, R. (2012). Hydrothermal carbonization of waste biomass for energy generation. Procedia Environmental Sciences, 16, 159–166. DOI 10.1016/j.proenv.2012.10.022.
18. Priyanto, A. (2012). The exploration of Nyamplung (Calophyllum inophyllum I.) on The Widelife West Kalimantan (Ketapang) for tree improvement program. Informasi Teknis, vol. 11. no. 2, September 2013, 69–78.
19. Dweck, A. C., Meadows, T. (2002). Tamanu (Calophyllum inophyllum)-The African, Asian, Polynesian and Pacific Panacea. International Journal of Cosmetic Science, 24(6), 341–348. DOI 10.1046/j.1467-2494.2002.00160.x.
20. Indrayani, N. L. (2013). Pirolisis Minyak Nyamplung Menggunakan Katalis Karbon Berpori, Sinergi 95–102, Indonesia.
21. Budiarto, A. (2012). limbah kulit biji nyamplung untuk bahan bakar briket bioarang sebagai sumber energi alternatif. Jurnal Teknologi Kimia dan Industri (JKTI1(1), 165–174.
22. Obernberger, I., Thek, G. (2004). Physical characterisation and chemical composition of densified biomass fuels with regard to their combustion behaviour. Biomass and Bioenergy, 27(6), 653–669. DOI 10.1016/j.biombioe.2003.07.006.
23. Speight, J. G. (1994). Chemical and physical studies of petroleum asphaltenes. In Developments in petroleum science, vol. 40, pp. 7–65, Elsevier.
24. Tarasov, D., Shahi, C., Leitch, M. (2013). Effect of additives on wood pellet physical and thermal characteristics: a review. ISRN Forestry, 2013(6), 1–6. DOI 10.1155/2013/876939.
25. Berkowitz, N. (1985). The chemistry of coal (coal science and technology). Amsterdam: Elsevier, pp. 513. DOI 10.1002/aic.690330128.
26. Othmer, K. (2007). Encyclopedia of chemical technology. 5th Edition, vol. 6. Chichester: Wiley-Interscience.
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |