 Open Access
Open Access
ARTICLE
Advance IoT Intelligent Healthcare System for Lung Disease Classification Using Ensemble Techniques
1 Department of Networking communications, Faculty of Engineering and Technology, SRM Institute of Science and Technology, Kattankulathur, 603203, Tamilnadu, India
2 Department of Computing Technologies, Faculty of Engineering and Technology, SRM Institute of Science and Technology, Kattankulathur, 603203, Tamilnadu, India
* Corresponding Authors: Prabakaran. Email: ,
Computer Systems Science and Engineering 2023, 46(2), 2141-2157. https://doi.org/10.32604/csse.2023.034210
Received 09 July 2022; Accepted 25 November 2022; Issue published 09 February 2023
Abstract
In healthcare systems, the Internet of Things (IoT) innovation and development approached new ways to evaluate patient data. A cloud-based platform tends to process data generated by IoT medical devices instead of high storage, and computational hardware. In this paper, an intelligent healthcare system has been proposed for the prediction and severity analysis of lung disease from chest computer tomography (CT) images of patients with pneumonia, Covid-19, tuberculosis (TB), and cancer. Firstly, the CT images are captured and transmitted to the fog node through IoT devices. In the fog node, the image gets modified into a convenient and efficient format for further processing. advanced encryption Standard (AES) algorithm serves a substantial role in IoT and fog nodes for preventing data from being accessed by other operating systems. Finally, the preprocessed image can be classified automatically in the cloud by using various transfer and ensemble learning models. Herein different pre-trained deep learning architectures (Inception-ResNet-v2, VGG-19, ResNet-50) used transfer learning is adopted for feature extraction. The softmax of heterogeneous base classifiers assists to make individual predictions. As a meta-classifier, the ensemble approach is employed to obtain final optimal results. Disease predicted image is consigned to the recurrent neural network with long short-term memory (RNN-LSTM) for severity analysis, and the patient is directed to seek therapy based on the outcome. The proposed method achieved 98.6% accuracy, 0.978 precision, 0.982 recalls, and 0.974 F1-score on five class classifications. The experimental findings reveal that the proposed framework assists medical experts with lung disease screening and provides a valuable second perspective.Keywords
A respiratory illness is about the gas exchange between the lungs and is often called a pulmonary disease. These pathological conditions influence the airways and other parts of the respiratory; some of the life-threatening lung disorders are TB, pneumonia, Covid-19, lung cancer, etc. According to the global impact of respiratory disease report, each year 2.4 million people have been dead from pneumonia, 1.4 million individuals died from TB, and lung cancer kills 1.8 million people. The Covid-19 pandemic [1] enormously spread throughout the whole world and has affected millions of people. According to the world health organization (WHO) 1.4 million individuals died from untreated or undiscovered lung diseases [2]. Preliminary detection of lung diseases is vital to slow down the mortality rate [3,4]. CT, X-ray, magnetic resonance imaging (MRI) [5], and other medical imaging techniques tend to diagnose lung disorders. For radiologists, manually examining pulmonary images is a time-consuming and tedious job [6]. Also, accurate detection in all cases is questionable. machine learning (ML)-based automated system is an effective tool for ameliorating detection accuracy and speed while simultaneously lowering the impact of healthcare worker shortages.
The IoT is a revolution that enables intelligent sensors to communicate over a network without human interaction. However, the efficacy of IoT gets restricted by low storage capacity and processing capacity. In a smart city context IoT combined with cloud technology, which has a massive storage capacity and significant processing power, has enabled efficient services such as smart healthcare [7,8]. Moreover, handling issues such as excessive latency, communication overload, and location awareness between the IoT and cloud is challenging. Fog computing [9] has emerged to bring cloud applications closer to medical IoT devices at the network edge, overcoming the aforementioned restrictions of the cloud.
The objective of the intelligent healthcare system is to provide a highly personalized and precise targeted care experience. Also, it includes a tremendous need for a smart healthcare system that delivers a seamless and speedy response with IoT-cloud connectivity. Herein Cognitive behavior and decision-making can be improved using deep learning (DL) and artificial intelligence (AI). Existing methods focused on detecting Covid-19 [10–12] from CT images, approached in [13,14] focused on Lung cancer detection, approaches in [15,16] focused on pneumonia detection, [17] focused on tuberculosis detection. Above mentioned techniques are only concentrated on single or 2 domain diseases due to the limited storage facility. The proposed method detects four types of disease as well as their severity by employing cloud and fog technologies as storage. In a smart city environment, intelligent health care provides immediate action in case of emergency without the need for qualified doctors.
In this study, an intelligent healthcare system for lung disease detection based on cloud and fog technologies has been presented. Through medical IoT devices, CT scan images are encrypted and transmitted to the edge network (fog node) to convert into processing format. Further formatted images are fetched to cloud technology for disease detection, herein ensemble transfer learning (ETL) technique classified lung diseases like Covid-19, pneumonia, Tuberculosis, and lung cancer. Followed by classified disease severity level is predicted with the help of the RNN network, and based on the result patients are advised to take the treatment. The primary purpose of the proposed model is to formulate an intelligent healthcare system that can automatically detect lung diseases and their severity levels in a smart city context.
The remaining section of this research is demonstrate as follows. The literature review is explained in Section 2. The suggested techniques were shown in Section 3, along with an explanation and the related algorithm. The performance outcome and their analysis are provided in Section 4. Conclusions and further work were completed in Section 5.
Many studies have been undertaken in the healthcare sector to formulate an effective solution for lung pathology detection using modern computer vision technology. This section describes some of the existing lung disease detection approaches and associated algorithms.
In 2018, Shabut et al. [18] created an intelligent methodology for diagnosing tuberculosis. For this ML algorithms were adopted and executed in the point-of-care (POC) platform. This intelligent model achieved 98.9% accuracy. Even though that was a good result in the mobile platform, there was a noise problem during the image processing time. In 2019, Masood et al. [19] presented a cloud-based intelligent system to identify lung cancer from CT images. Here, the 3D-CNN model was leveraged to detect the disease, and the respected features were selected with the help of the multi-region proposal network (mRPN) model. This model had the limitation of insufficient sample images and overfitting.
In 2020, Bharati et al. [20] implemented a VGG Data STN with CNN (VDSNet) to detect lung diseases. This hybrid model combines the benefits of a visual geometry group-based network (VGG), spatial transformer network (STN), and data augmentation with a convolutional neural network (CNN) to improve detection performance. This approach achieved 73% validation accuracy and was ineffective in real-time applications due to the small dataset. Valluru et al. [21] (2020) developed an intelligent lung cancer detection method by using IoT-cloud technology. To select and optimize features (GWO-GA), gray wolf optimization is combined with the evolutionary algorithm. The optimum support vector machine (SVM) was used for the identification of lung cancers. The accuracy of this model was 93.54% yet, this method was not suitable for large datasets.
In 2022, Shimpy Goyal et al. [22] established an approach for categorizing Covid-19 and pneumonia cases from X-ray scans. Feature-based recurrent neural networks with long short-term memory were used to classify lung diseases’ features (F-RNN-LSTM). The idea of detecting severity in this model did not work. In 2021 Nasser et al. [23] presented a smart healthcare system that includes IoT and cloud for the identification and monitoring of Covid-19. Firstly, the CT reports of affected individuals were transmitted to the cloud. In the cloud, the pre-trained ResNet-50 model was utilized for detecting Covid-19. This method’s generalization ability was poor due to the small dataset.
In 2021, Mukherjee et al. [24] developed an IoT-cloud-oriented intelligent system to detect Covid-19. Firstly, enhanced KNN was used to classify Covid-19. Secondly, Ant Colony Optimization (ACO) combined with an enhanced K-nearest neighbor(eKNN) approach was applied. The result shows the second mechanism provides the highest accuracy yet this method classifies a single disease. In 2021 das Chagas et al. [25] established an IoT-based novel technique to detect pneumonia in children using X-ray scans. In this model, 12 pre-trained CNN networks are chosen as feature extractors, and the approaches were integrated with classification learning models. Although the combination of VGG19 and SVM and radial basis function (RBF) has an accuracy range of 95 percent, it only detects one disease.
In 2021, Almezhghwi et al. [26] introduced an AI-based lung disease prediction system in the IoT era. Chest X-ray images were processed utilizing VGG19 and AlexNet coupled with SVM models. This model was able to classify 12 lung disorders; however, its generalization ability was questioned due to the short dataset and the fact that it may require a lot of hardware memory. In 2021, Koushikand et al. [27] developed a CNN-based method to detect respiratory diseases like pneumonia and Covid-19. The network was optimized using the Adam method, and the final precision rate was 0.95. Herein a small dataset hampered the system’s performance.
According to the literature survey, most techniques are used in a small dataset to train the model and detect one or two domain lung diseases. Their execution platforms are low storage and high computational hardware which makes them cost-effective. This study presents an intelligent healthcare system by using fog and cloud platforms to reduce storage and hardware complexity. Instead of relying on a single network, the proposed model makes use of the potential of an ensemble method, which combines weak base learners to offer an ideal prediction rate. The proposed ETL and severity detection framework provides good classification accuracy for 5-class classification compared to existing methods.
3 Proposed Intelligent Health Care System for Lung Disease Detection
The primary objective of the proposed lung disease intelligent system is to provide highly experienced and accurate diagnoses in a smart city environment. To succeed an AI-based lung disease recognition system is developed in cloud technology. Data acquired from IoT devices is preprocessed in the fog node to reduce data transfer latency between the IoT and the cloud. In the cloud, the ETL framework is introduced for lung disease classification. Finally, the Classified disease is fed to the RNN-LSTM for severity analysis. The proposed workflow of the intelligent system is shown in Fig. 1.
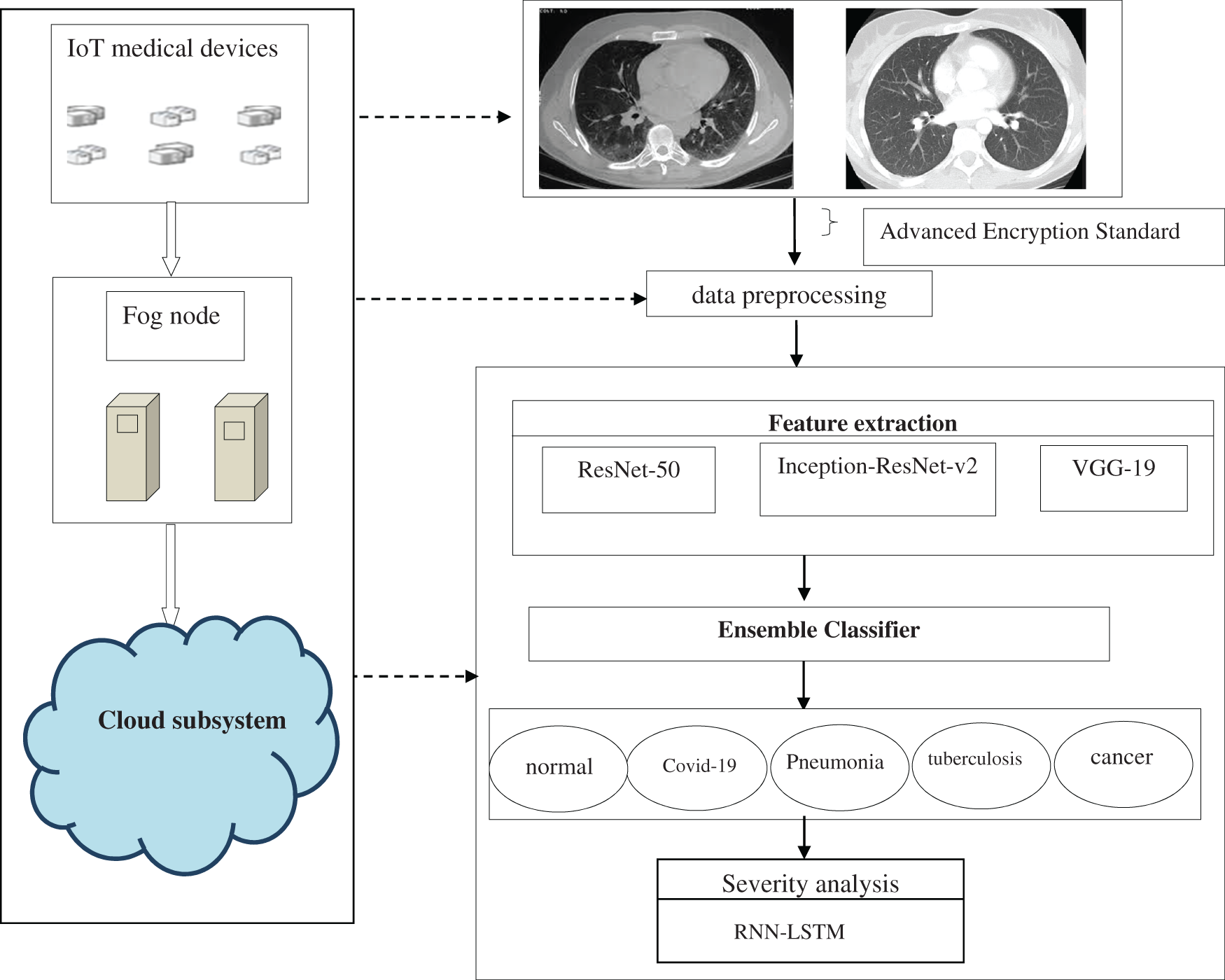
Figure 1: The workflow of the proposed intelligent health care methodology for lung disease detection
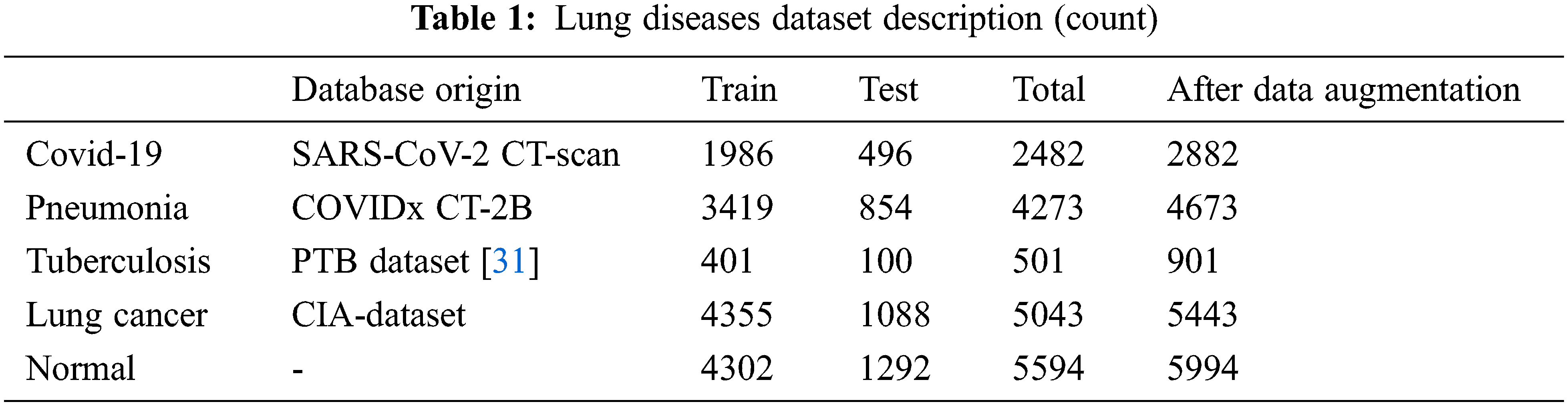
The proposed model incorporates four publicly available datasets for four disease classes and one normal class, totaling 19,893 CT scans. Images from the SARS-CoV-2 CT dataset include 2482 Covid-19 classes and 1252 non-Covid-19 classes. The pneumonia images are drawn from the COVIDx-CT collection, which contains 4273 pneumonia images as well as 1583 normal images. The tuberculosis CT imaging dataset [28] used in this study includes 501 aberrant and 501 normal images obtained from 223 patients and manually graded by two professionals. Lung cancer images are collected from the cancer imaging archive (CIA) dataset. The dataset comprises a collection of lung CT images taken from the National Cancer Institute and connected with proteomic and genetic clinical data. The collection contains 5043 CT images obtained from 48 series. Table 1 provides a full summary of the dataset utilized in this research.
3.2 Advanced Encryption Standard
The image is encrypted in a medical IoT device and transferred to a fog node using the advanced encryption standard (AES) method [29]. AES is a symmetric block cipher that transforms a 128-bit image into ciphertext by adding a secret key with a length of 128, 256 or 192 bits. The number of encryption execution rounds varies depending on the size of the cipher key, such as 10, 14, or 12. The fog node decrypts the cipher image for further processing.
A fog node is utilized to connect the cloud nearer to the medical IoT devices at the network edge. In fog node cipher format image is decrypted and transformed into standard normalization by the given process:
Data augmentation [30]: Collected data volume is enhanced with a data augmentation technique to eliminate overfitting and construct a more generalized learning model. Augmentation methods such as flipping, rotation, cropping, reflection, scaling, and blurring with a Gaussian filter are applied to produce an augmented dataset
Resize: dataset images are varying in size; they are adjusted in the size of 224 × 224 and 229 × 229 pixels respectively to fit the chosen pre-trained models.
Noise removal [31]: To reduce additive noise from CT scans, a Wiener filter is used. Noises incorporated in the images during the acquisition process assist in false detection. Therefore, the noises must be removed to accurately diagnose lung diseases. To begin, a Wiener filter is employed to remove additive noise, where the gaussian noise is added with 0 mean and σ = 30 as a standard deviation, and the kernel size is set as 9 × 9. Followed by a median filter applied with a window size of 3 × 3 to remove salt and pepper noise from the image.
3.4 Transfer Learning and Fine-Tuning DL Models
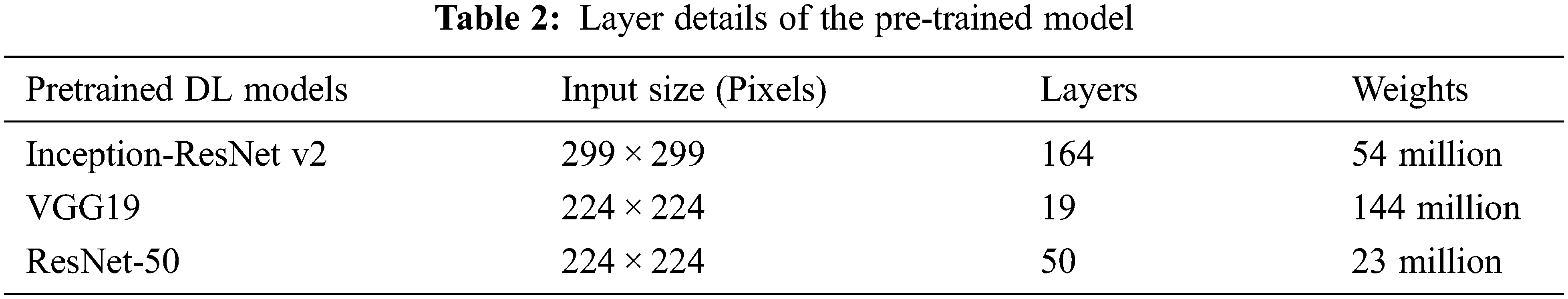
Transfer learning is an ML approach that uses a learned model from the source domain to boost generalization in a target domain. In this work Inception-ResNet-v2, ResNet-50, and VGG-19 pre-trained DL models are taken for fine-tuning. The model parameters are updated after each iteration to minimize the loss function in the training phase. The input size, parameters, and the layers detailed of the pre-trained models are shown in Table 2.
To train these models for reliable detection, a larger dataset is required, which will result in an appropriate global minimum for the cost function and it is derived as,
where the total number of training occurrences in the training set is represented by M.
In a sparser dataset, robust detection is more difficult to achieve, and the model is more prone to overfitting. So in transfer learning the initial layers weight parameters from the Inception-ResNet-v2, ResNet-50 and VGG-19 are reclaimed. The models are then fine-tuned using the acquired CT dataset by changing top layers and varied batch sizes, epochs, and learning rates. The pre-trained models’ primary layers contain general features, while the succeeding levels provide domain features. To make faster the learning of the newly added layers the learning rate of the top layers is assigned to a high value. Negative transfer does not occur in the proposed techniques due to the constrained size of the lung CT dataset. The proposed transfer learning architecture fully connected the final layer is set to 5 neurons to detect Cancer, pneumonia, tuberculosis, normal, and Covid-19. Fig. 2 depicts the fine-tuned pre-trained model. Generalizing the transfer learning model to prevent the overfitting purpose dropout 0.3 and 0.5 and batch normalization (BN) is added in the final dense layer. The softmax layer is chosen for binary classification, and its function is
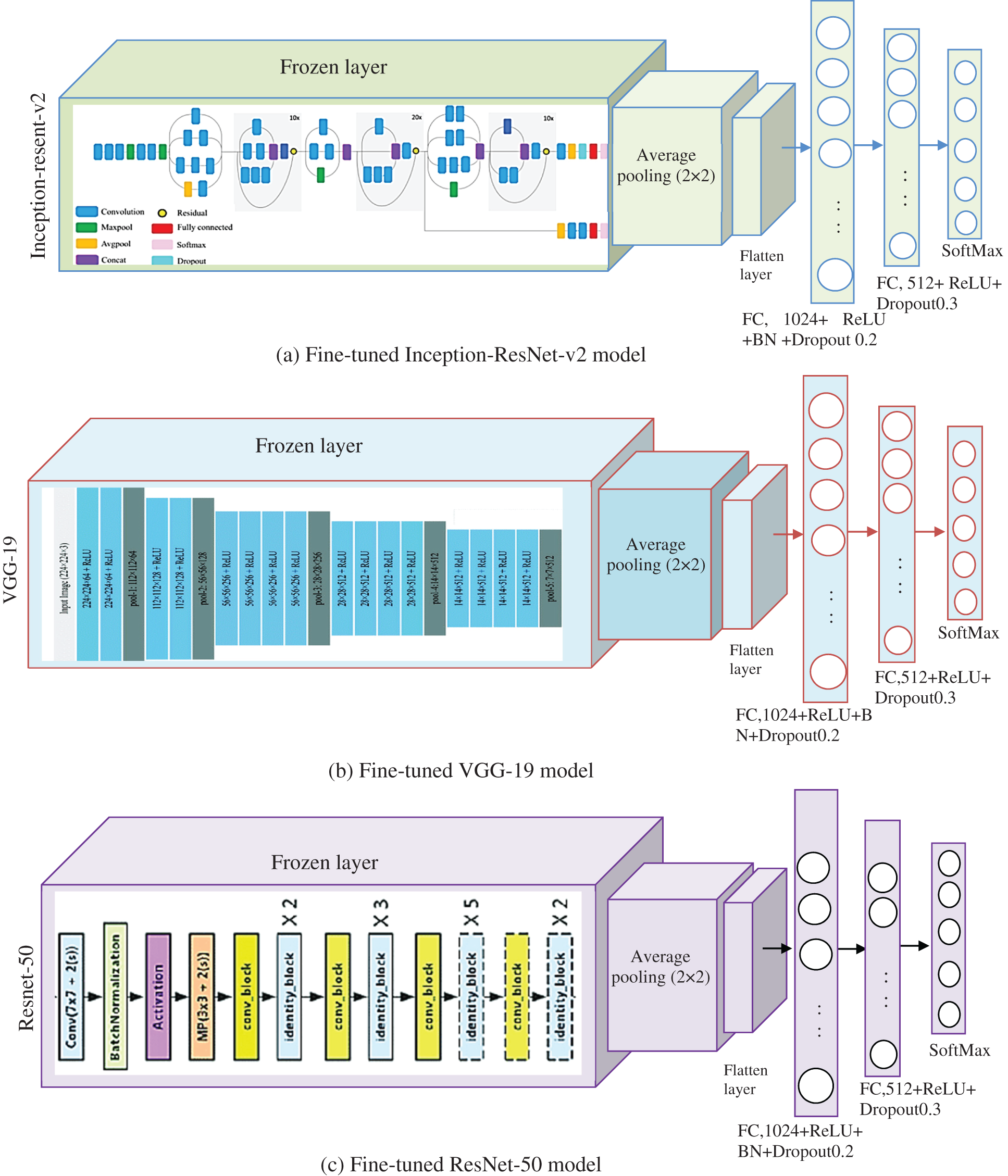
Figure 2: Fine-tuned pre-trained models (a) Inception-ResNet-v2 (b) VGG-19 and (c) ResNet-50
Here
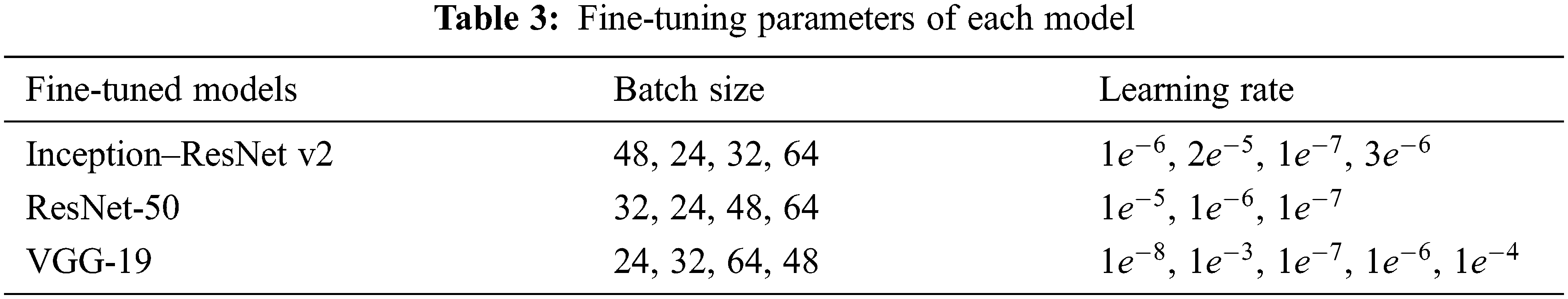
Ensemble learning [32] can considerably improve a learning system’s predictive capabilities and reduce variance by combining numerous individual models into an intelligent ensemble. In this work, the selected heterogeneous DL models (Inception-ResNet-v2, VGG-19, ResNet-50) are combined through majority voting [33], weighted averaging, and stacking to classify lung diseases. Fig. 4 depicts the proposed ensemble classifier for 5 class classification problems. In majority voting, multiple transfer learned models’ predictions are assigned as votes. Individual classifiers vote for a specific class, and the class with the most votes determine the ensemble’s output. The model’s output label is
Figure 4: Model of ensemble transfer learning
In weighted averaging the base learner’s prediction outputs are averaged with dissimilar weights as follows:
Herein Y(x) is the integrated forecasting output, and
Stacking (stacked generalization) [34] generates the output by using a meta-learner [35], that combines the individual weak learner’s prediction. Stacking uses the base learner prediction as a context and then differentially weighs these predictions to perform better than the individual base learner.
The operation of the stacking is illustrated in Fig. 3.
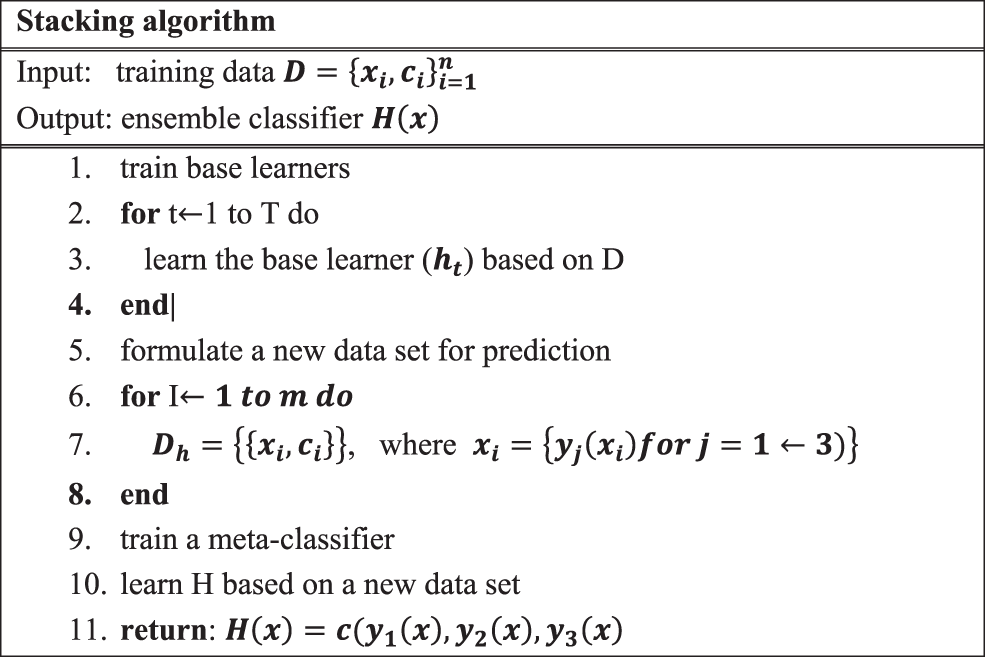
Figure 3: Stacking procedure
3.6 Severity Analysis by Using RNN-LSTM
The severity level of the classified result H(x) is detected by using RNN-LSTM. Severity detection is vital to diagnose the disease for taking appropriate treatment. The diseases Covid-19, TB, pneumonia, and lung cancer severity score levels depend on the level of disease spread over left and right lungs, and the different output scores are 1:1–25% (low), score 2:26–50% (mild), score 3:51–75% (severe), score 4: > 75% (more severe). RNN is a subset of ANN, which has a feedback loop called a recurrent loop that can process the sequence of data. RNN is differentiated by their memory, which allows them to impact contemporary input and output with the information it gathered from previous inputs. In the RNN-LSTM structure, the nonlinear function (tanh) of the RNN is replaced with LSTM which is a neuronal mechanism that performs a memory cell. The LSTM is linked to the RNN to solve the vanishing gradient problem that affects the RNN’s long-term temporal reliance. The structure of the RNN is shown in Fig. 5. RNN has 3 layers respectively Input, Recurrent hidden layer, and output layer. RNN-LSTM receives the input picture H(x) as a sequence of vectors with the time T, its weight matrix is
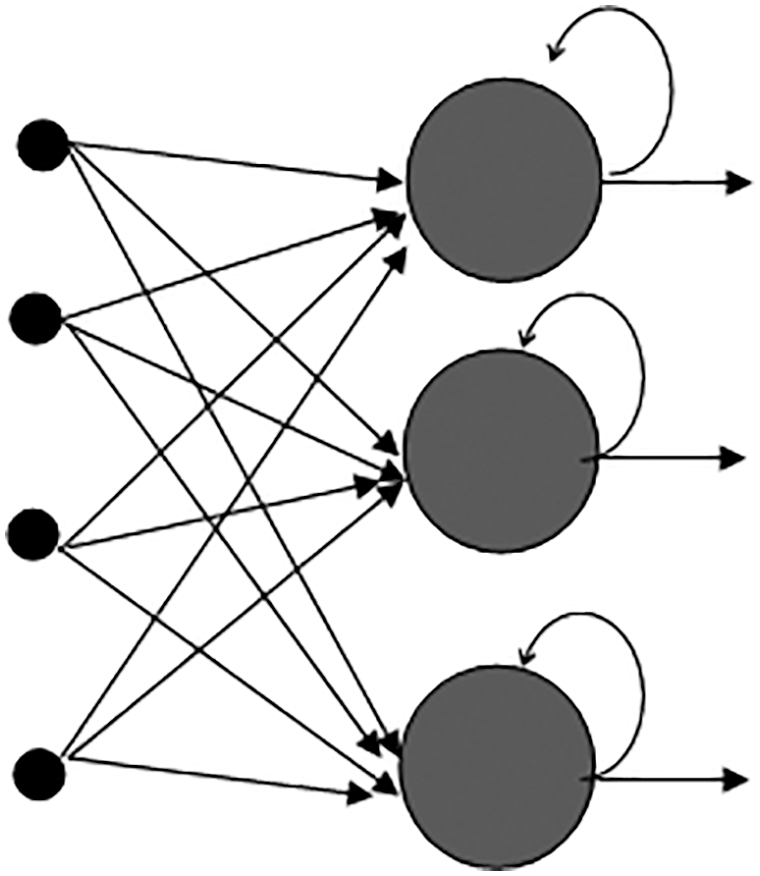
Figure 5: Structure of the recurrent neural network
Herein
RNN generated scores represent the severity level which depends on the disease spread over the left and right lung. The target score is calculated with the softmax function. softmax_cross_entropy_with_logits_v2 predicts the loss function and an SGD optimizer is used to minimize the loss. Initially, the batch size is set as 8, the training epoch of the net is 20 and the learning rate is
4 Experiment Result and Discussion
In this section performance of the proposed ensemble transfer learning-based lung diagnosis model is examined and assessed with other existing methodologies.
The proposed framework is assessed using 5-fold cross-validation. In this dataset, 80% of the data is used for training, and 20% for testing. Performance of the suggested method is measured using the following metrics:
Here True Positive (TP) is the correctly predicted lung diseases, False Negative (FN) is the casing in which the network predicts the lung disease incorrectly, True Negative (TN) is the correctly predicted non-diseases images, and False Positive (FP) are cases in which the network predicts the normal images as a disease. In the proposed framework MATLAB (2020b) is used for implementation. With 64 GB of RAM, the server system is powered by a 3.5 GHz Intel Xeon F8 CPU.
The 3 transfer learning models that are independently trained and attained accuracies for binary classification are VGG-19 (95.60%), ResNet-50 (94.4%), and Inception-ResNet-v2 (95.23%). For the proposed ETL architecture, the training time is estimated to be around 15 min and it achieved higher accuracy of 98.6% compared to the individual model. Stacking, voting, and averaging ensemble concepts are adopted for the proposed multiclass classification problem. The proposed ensemble transfer learning framework achieved 0.978 precision, 0.982 recalls, and 0.974 F1-score for predicting 5 class lung diseases, indicating that the proposed approach generalization ability is greater than existing models. The results reveal that the ETL framework achieves the greatest accuracy, implying that there is a low risk of an incorrect prediction. Table 4 contains the results of the individual learning models’ performance.
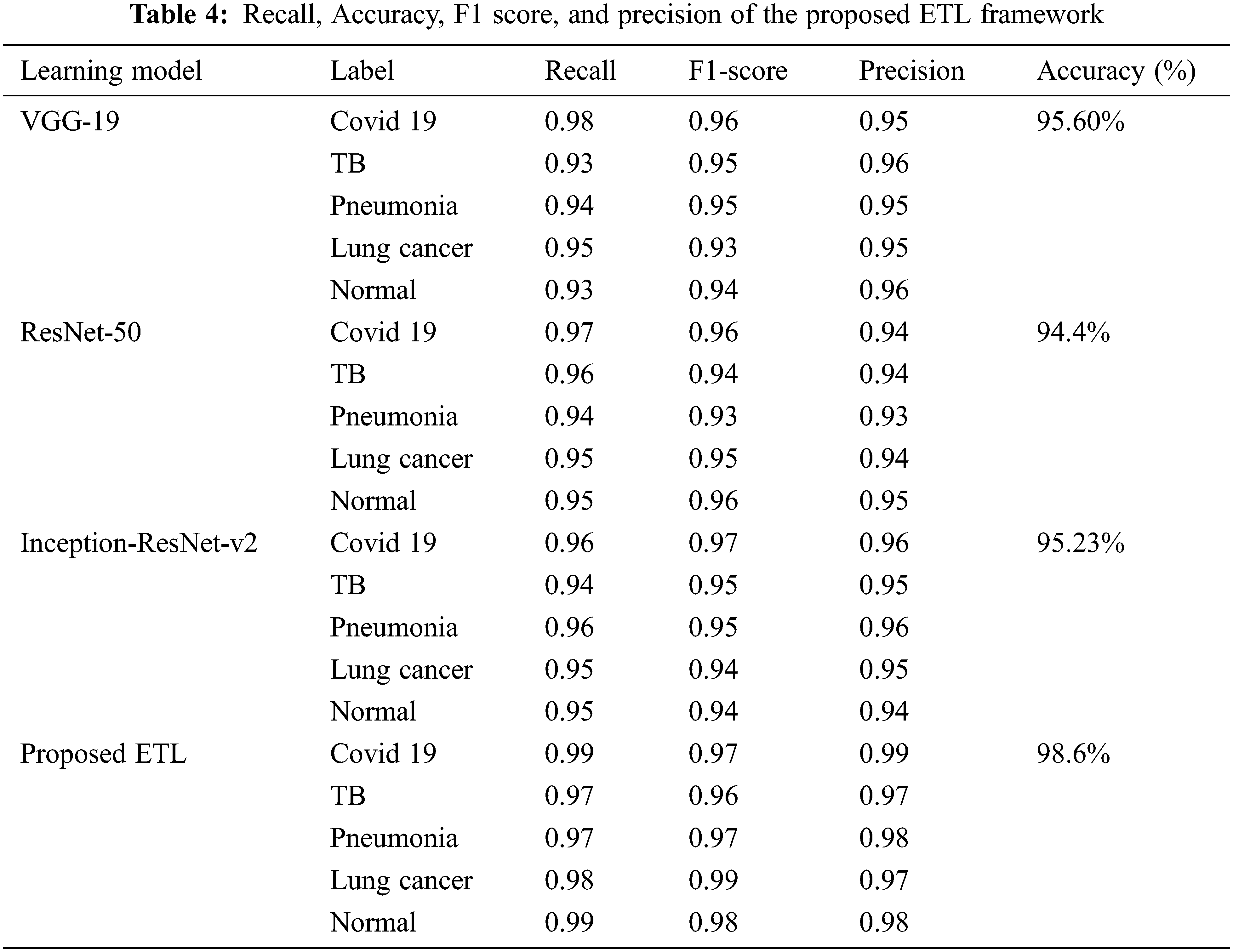
Table 5 shows the severity prediction framework RNN-LSTM model performance with specificity, sensitivity, F1 score, and accuracy, RNN model training and testing losses. The resulting probability score of the RNN-LSTM can then be evaluated with a threshold value to decide the predicted images belong to which stage of severity. The RNN-LSTM achieved 98% of severity detection. The specificity (0.97) of the RNN-LSTM showed the possibility of false prediction is significantly low so this method is effectively suitable for the severity analysis.
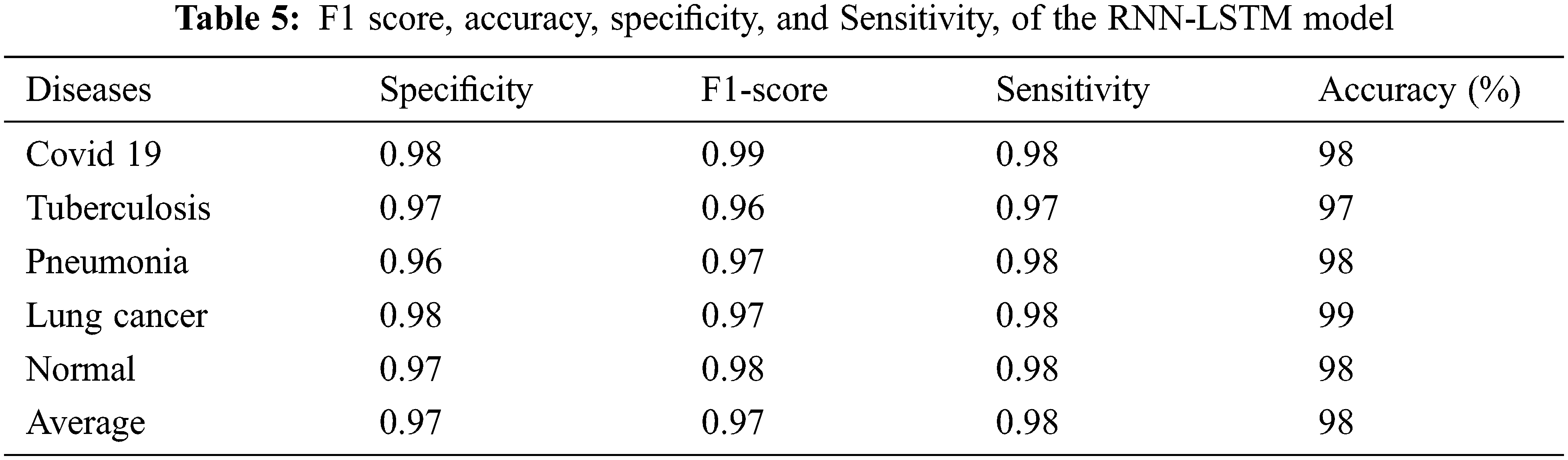
Fig. 6 depicts the proposed RNN-LSTM model’s training and testing loss curves. The x-axis shows training epochs ranging from 0 to 100, while the y-axis illustrates cross-entropy. The curve indicates that as batch size increases, the model’s loss function decreases.
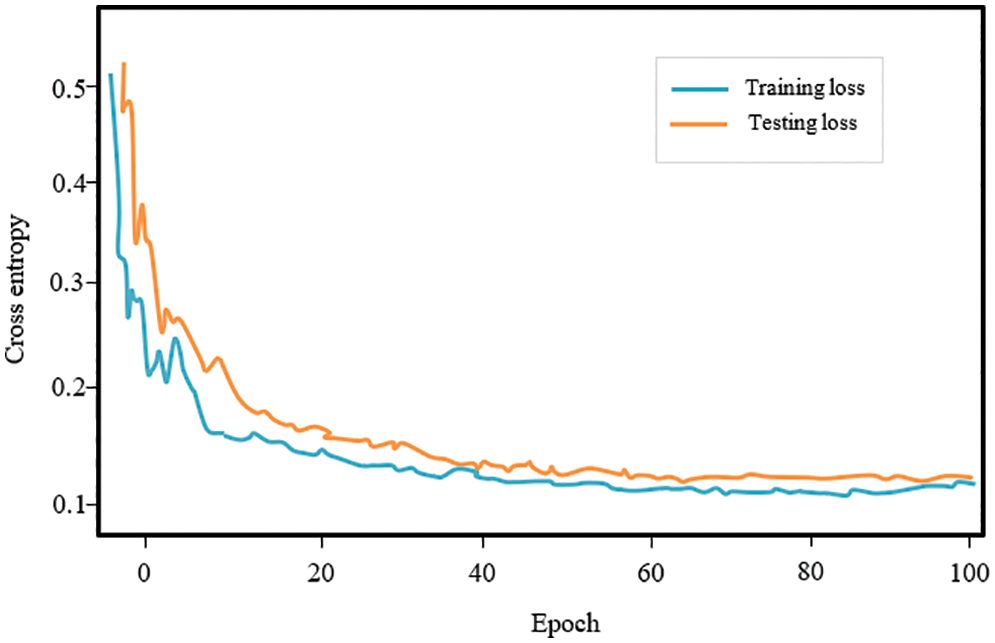
Figure 6: RNN model training and testing losses
The findings show that a proposed DL methodology may considerably aid in the identification of lung disease subjects using CT scan images, providing a low-cost and quick option for Covid-19 identification. Fig. 7 shows the accuracy of the individual learning networks for 0–100 epochs. The proposed model’s accuracy is substantially higher than other models, and it grew as the epoch size increased, as seen in the plot.
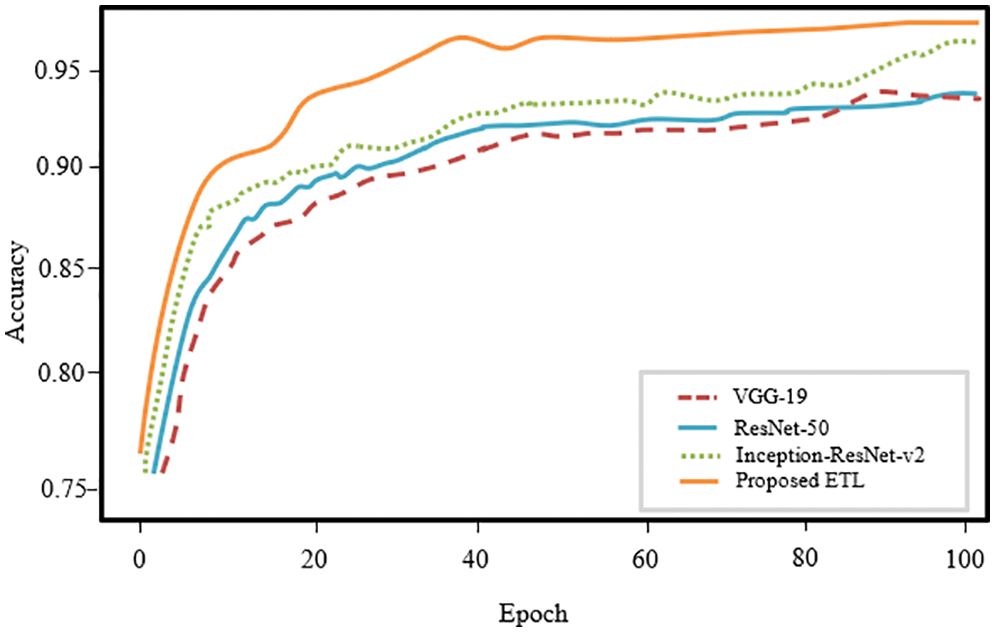
Figure 7: Accuracy of the individual model of the proposed framework for 1–100 epochs
The confusion matrix plot for the individual pre-trained model and proposed framework is illustrated in Fig. 8. Confusion matrixes visualize and describe the efficiency of a classification model. Compared to the individual models, the proposed model performed best with an overall accuracy of 98.6% and a less misclassification rate of 0.0140 for five classes. Fig. 9 depicts the receiver operating characteristic (ROC) curve for the proposed model. The ROC plot is shown vs. the true positive rate (vertical axis) and false positive rate (horizontal axis). The proposed model had a high area under the ROC curve (AUC) value, proving its capability to distinguish between classes.
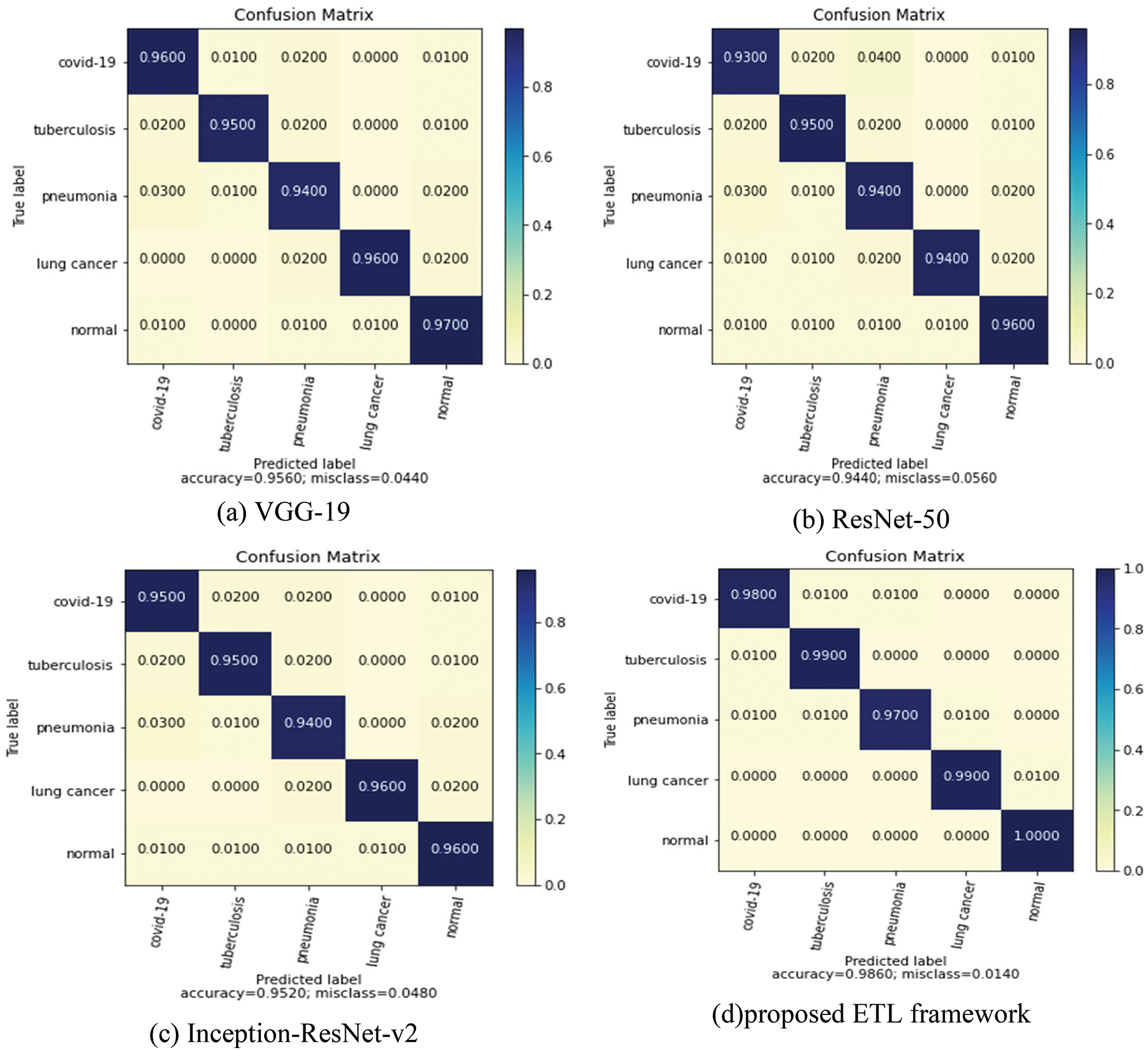
Figure 8: Confusion matrix of individual and the coupled models in the ETL framework
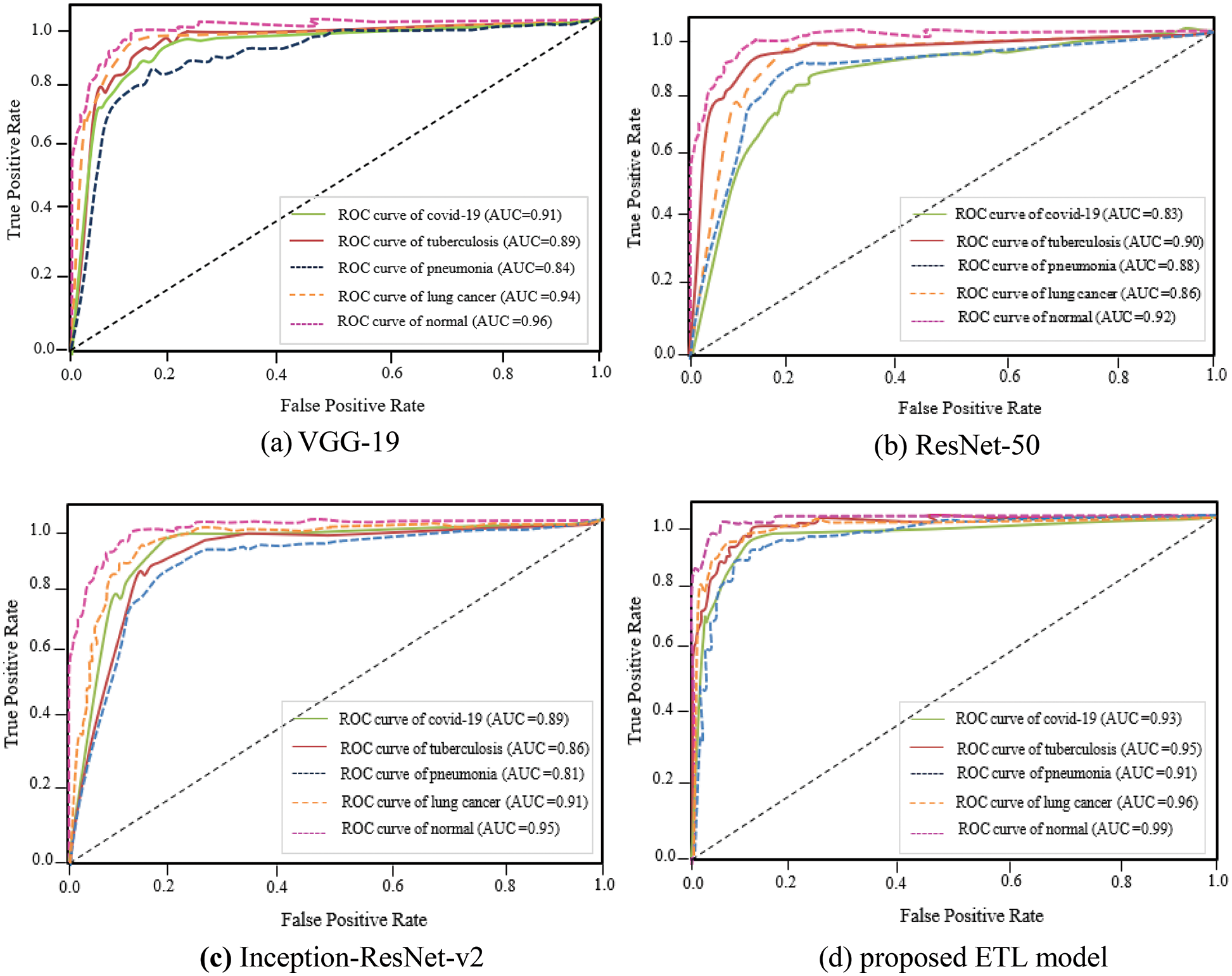
Figure 9: ROC curve of individual and the coupled models in the ETL framework
Table 6 compares the proposed multi-classification technique to existing state strategies in terms of accuracy. Although the notion in [32] closely follows the proposed technique, it only classifies two classes, which is lower than the ETL framework, and it does not provide severity analysis. Because of its low generality, autoencoder-based CNN [5] performs poorly. The accuracy of the [19,24] approaches is 0.09%, 1.1% lower than that of the proposed model. As a result, with a final classification rate of 98.6 percent, the proposed ensemble transfer learning for the multi-classification job surpassed numerous previous techniques.
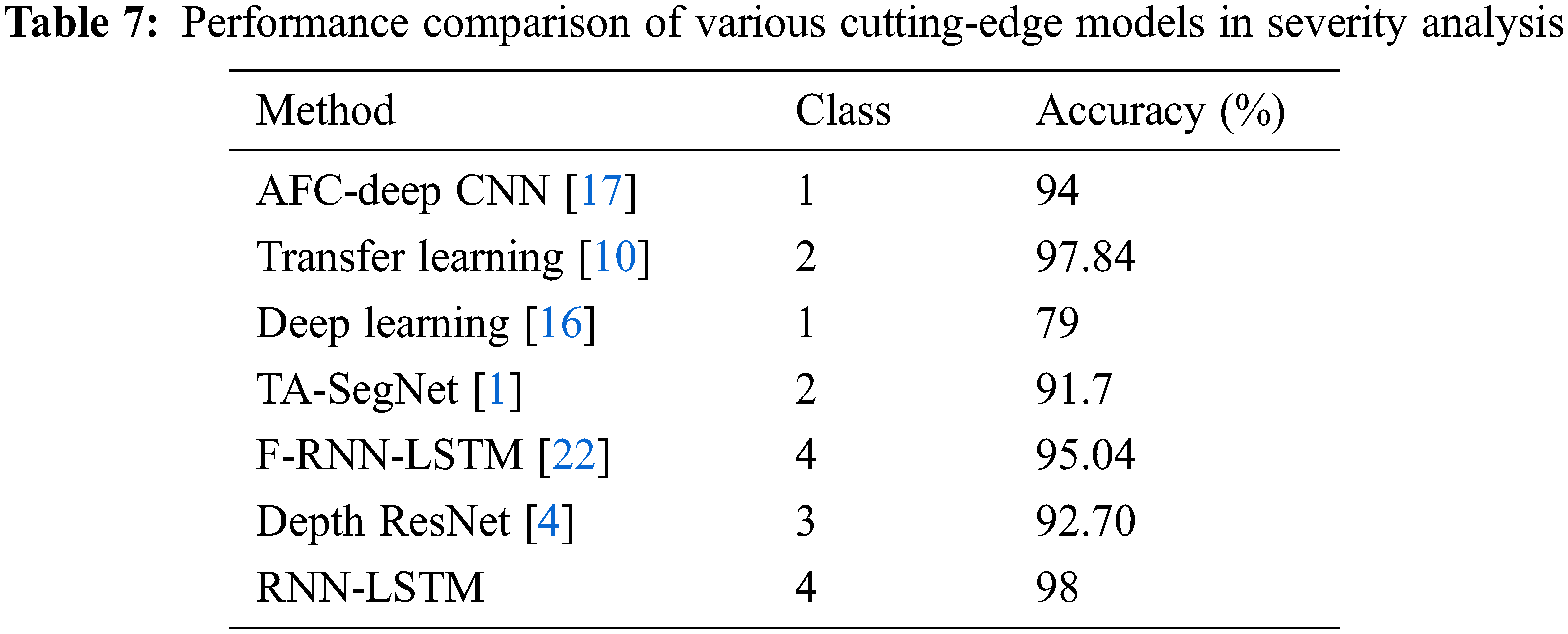
Table 7 depicts the performance evaluation of the RNN-LSTM with existing state of art models. Reference [10] used ResNet-50 and DenseNet-201 hybrid model transfer learning method which is suitable for fewer datasets but training is complex also performance is lacking by 0.16% more than the proposed framework. Reference [17] used the adaptive fractional crow-oriented CNN model to figure out lung micro bacterial disease but the training time is high compared to the RNN-LSTM framework. The proposed model outperforms the tri-level attention based SegNet model [1] by 7.3%. The proposed RNN-LSTM model offers high accuracy, specificity, and F1 score compared to the existing state-of-art severity framework; these results prove to outline the use of its severity detection in lung diseases.
In this research, an intelligent healthcare framework is provided for exploiting computer tomography (CT) pictures to diagnose lung disorders like Covid-19, TB, pneumonia, and Covid-19. Through medical Internet of Things (IoT) devices, CT scan images are collected and securely encrypted via the AES algorithm and then fed to the fog node. The core concept of the fog node is to bring the cloud near to the IoT device; through this mini cloud data preprocessing is conducted and sent to the cloud for disease detection. The cognitive behavior of cloud technology improved via the Ensemble Transfer Learning framework. Three transfer learning methods like Inception-ResNet-v2, ResNet-50, and VGG-19, were taken for feature extraction and models are combined through different ensemble techniques for final prediction. Finally, the RNN-LSTM model is adopted to detect the severity level based on the infection spread over the left and right of the lung. The proposed methodology achieved 98.6% accuracy, 0.978 precision, 0.982 recalls, and 0.974 F1-score when predicting 5 class lung diseases like normal, Covid-19, pneumonia, TB, and lung cancer. Medical diagnosis research and intelligent healthcare systems will benefit from the proposed deep learning-based approach. It will also help medical experts with lung disease screening and provide a valuable second perspective. Detecting lung diseases still have challenges due to the similar feature of the disease. Future work will focus on designing a more generalized model to predict lung disease with more accuracy for practical application. Also, the model is trained with more CT images for lung disease detection.
Acknowledgement: The authors with a deep sense of gratitude would thank the supervisor for his guidance and constant support rendered during this research.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. T. Mahmud, M. J. Alam, S. Chowdhury, S. N. Ali, M. M. Rahman et al., “CovTANet: A hybrid tri-level attention-based network for lesion segmentation, diagnosis, and severity prediction of COVID-19 chest CT scans,” IEEE Transactions on Industrial Informatics, vol. 17, no. 9, pp. 6489–6498, 2020. [Google Scholar]
2. Q. Ke, J. Zhang, W. Wei, D. Połap, M. Woźniak et al., “A neuro-heuristic approach for recognition of lung diseases from X-ray images,” Expert Systems with Applications, vol. 126, pp. 218–232, 2019. [Google Scholar]
3. S. Khobragade, A. Tiwari, C. Y. Patiland and V. Narke, “Automatic detection of major lung diseases using chest radiographs and classification by feed-forward artificial neural network,” in Proc. IEEE 1st Int. Conf. on Power Electronics, Intelligent Control and Energy Systems (ICPEICES), Delhi, India, pp. 1–5, 2016. [Google Scholar]
4. X. W. Gao, C. James-Reynolds and E. Currie, “Analysis of tuberculosis severity levels from CT pulmonary images based on enhanced residual deep learning architecture,” Neurocomputing, vol. 392, pp. 233–244, 2020. [Google Scholar]
5. L. Li, H. Huang and X. Jin, “AE-CNN classification of pulmonary tuberculosis based on CT images,” in 2018 9th Int. Conf. on Information Technology in Medicine and Education (ITME), Hangzhou, China, IEEE, pp. 39–42, 2018. [Google Scholar]
6. S. Huang, F. Lee, R. Miao, Q. Si, C. Lu et al., “A deep convolutional neural network architecture for interstitial lung disease pattern classification,” Medical &Biological Engineering & Computing, vol. 58, no. 4, pp. 725–737, 2020. [Google Scholar]
7. M. Alhussein, G. Muhammad, M. S. Hossainand and S. U. Amin, “Cognitive IoT-cloud integration for smart healthcare: Case study for epileptic seizure detection and monitoring,” Mobile Networks and Applications, vol. 23, no. 6, pp. 1624–1635, 2018. [Google Scholar]
8. M. G. Abdelhaleem Hussein, M. Ali, M. Y. Shams and T. Medhat, “Healthcare system using a medical service robot based on internet of things,” ARPN Journal of Engineering and Applied Sciences, vol. 16, no. 23, pp. 2483–2491, 2021. [Google Scholar]
9. E. M. Tordera, X. Masip-Bruin, J. Garcia-Alminana, A. Jukan, G. J. Ren et al., “What is a fog node a tutorial on current concepts towards a common definition,” arXiv preprint arXiv:1611.09193, 2016. [Google Scholar]
10. A. L. Aswathy, A. Hareendranand, V. C. SS, “COVID-19 diagnosis and severity detection from CT-images using transfer learning and back propagation neural network,” Journal of Infection and Public Health, vol. 14, no. 10, pp. 1435–1445, 2021. [Google Scholar]
11. S. Wang, B. Kang, J. Ma, X. Zeng, X. Xiao et al., “A deep learning algorithm using CT images to screen for corona virus disease (COVID-19),” European Radiology, vol. 31, no. 8, pp. 6096–6104, 2021. [Google Scholar]
12. Y. D. Zhang, S. C. Satapathy, S. Liu and G. R. Li, “A five-layer deep convolutional neural network with stochastic pooling for chest CT-based COVID-19 diagnosis,” Machine Vision and Applications, vol. 32, no. 1, pp. 1–13, 2021. [Google Scholar]
13. E. J. Hwang, J. M. Goo, H. Y. Kim, J. Yi, S. H. Yoon et al., “Implementation of the cloud-based computerized interpretation system in a nationwide lung cancer screening with low-dose CT: Comparison with the conventional reading system,” European Radiology, vol. 31, no. 1, pp. 475–485, 2021. [Google Scholar]
14. S. Makaju, P. W. C. Prasad, A. Alsadoon, A. K. Singhand, A. Elchouemi et al., “Lung cancer detection using CT scan images,” Procedia Computer Science, vol. 125, pp. 107–114, 2018. [Google Scholar]
15. H. S. Maghdid, A. T. Asaad, K. Z. Ghafoor, A. S. Sadiq, S. Mirjalili et al., “Diagnosing COVID-19 pneumonia from X-ray and CT images using deep learning and transfer learning algorithms,” in Multimodal Image Exploitation and Learning, vol. 11734, United States: SPIE Proceedings, pp. 117340E, 2021. [Google Scholar]
16. Q. Ni, Z. Y. Sun, L. Qi, W. Chen, Y. Yang et al., “A deep learning approach to characterize 2019 coronavirus disease (COVID-19) pneumonia in chest CT images,” European Radiology, vol. 30, no. 12, pp. 6517–6527, 2020. [Google Scholar]
17. R. S. Chithra and P. Jagatheeswari, “Severity detection and infection level identification of tuberculosis using deep learning,” International Journal of Imaging Systems and Technology, vol. 30, no. 4, pp. 994–1011, 2020. [Google Scholar]
18. A. M. Shabut, M. H. Tania, K. T. Lwin, B. A. Evans, N. A. Yusof et al., “An intelligent mobile-enabled expert system for tuberculosis disease diagnosis in real time,” Expert Systems with Applications, vol. 114, pp. 65–77, 2018. [Google Scholar]
19. A. Masood, P. Yang, B. Sheng, H. Li, P. Li et al., “Cloud-based automated clinical decision support system for detection and diagnosis of lung cancer in chest CT,” IEEE Journal of Translational Engineering in Health and Medicine, vol. 8, pp. 1–13, 2019. [Google Scholar]
20. S. Bharati, P. Podderand and M. R. H. Mondal, “Hybrid deep learning for detecting lung diseases from X-ray images,” Informatics in Medicine Unlocked, vol. 20, pp. 100391, 2020. [Google Scholar]
21. D. Valluru and I. Jeya, “IoT with cloud-based lung cancer diagnosis model using optimal support vector machine,” Health Care Management Science, vol. 23, no. 4, pp. 670–679, 2020. [Google Scholar]
22. Shimpy Goyal and R. Singh, “Detection and classification of lung diseases for pneumonia and covid-19 using machine and deep learning techniques,” Journal of Ambient Intelligence and Humanized Computing, pp. 1–21, 2021. http://doi.org/10.1007/s12652-021-03464-7. [Google Scholar]
23. N. Nasser, Q. Emad-ul-Haq, M. Imran, A. Ali, I. Razzak et al., “A smart healthcare framework for detection and monitoring of COVID-19 using IoT and cloud computing,” Neural Computing and Applications, pp. 1–15, 2021. http://doi.org/10.1007/s00521-021-06396-7. [Google Scholar]
24. R. Mukherjee, A. Kundu, I. Mukherjee, D. Gupta, P. Tiwari et al., “IoT-cloud based healthcare model for COVID-19 detection: An enhanced k-nearest neighbour classifier-based approach,” Computing, pp. 1–21, 2021. http://doi.org/10.1007/s00607-021-00951-9. [Google Scholar]
25. J. V. S. das Chagas, D. D. A. Rodrigues, R. F. Ivo, M. M. Hassan, V. H. C. de Albuquerque et al., “A new approach for the detection of pneumonia in children using CXR images based on an real-time IoT system,” Journal of Real-Time Image Processing, vol. 18, no. 4, pp. 1099–1114, 2021. [Google Scholar]
26. K. Almezhghwi, S. Serteand and F. Al-Turjman, “Convolutional neural networks for the classification of chest X-rays in the IoT era,” Multimedia Tools and Applications, vol. 80, pp. 29051–29065, 2021. [Google Scholar]
27. S. S. Koushikand and K. G. Srinivasa, “Detection of respiratory diseases from chest X rays using nesterov accelerated adaptive moment estimation,” Measurement, vol. 176, pp. 109153, 2021. [Google Scholar]
28. X. Li, Y. Zhou, P. Du, G. Lang, M. Xu et al., “A deep learning system that generates quantitative CT reports for diagnosing pulmonary tuberculosis,” Applied Intelligence, vol. 51, no. 6, pp. 4082–4093, 2021. [Google Scholar]
29. R. K. Sharma and A. R. Nair, “Iot-based secure healthcare monitoring system,” in 2019 IEEE Int. Conf. on Electrical, Computer and Communication Technologies (ICECCT), Coimbatore, India, IEEE, pp. 1–6, 2019. [Google Scholar]
30. V. Chandran, M. G. Sumithra, A. Karthick, T. George, M. Deivakani et al., “Diagnosis of cervical cancer based on ensemble deep learning network using colposcopy images,” BioMed Research International, vol. 2021, Article ID 5584004, 2021. https://doi.org/10.1155/2021/5584004. [Google Scholar]
31. R. R. Isnanto, Y. E. Windarto and M. V. Mangkuratmaja, “Assessment on image quality changes as a result of implementing median filtering, wiener filtering, histogram equalization, and hybrid methods on noisy images,” in 2020 7th Int. Conf. on Information Technology, Computer, and Electrical Engineering (ICITACEE), Semarang, Indonesia, IEEE, pp. 185–190, 2020. [Google Scholar]
32. T. Zhou, H. Lu, Z. Yang, S. Qiu, B. Huo et al., “The ensemble deep learning model for novel COVID-19 on CT images,” Applied Soft Computing, vol. 98, pp. 106885, 2021. [Google Scholar]
33. D. Velusamy and K. Ramasamy, “Ensemble of heterogeneous classifiers for diagnosis and prediction of coronary artery disease with reduced feature subset,” Computer Methods and Programs in Biomedicine, vol. 198, pp. 105770, 2021. [Google Scholar]
34. W. Książek, M. Hammad, P. Pławiak, U. R. Acharya and R. Tadeusiewicz, “Development of novel ensemble model using stacking learning and evolutionary computation techniques for automated hepatocellular carcinoma detection,” Biocybernetics and Biomedical Engineering, vol. 40, no. 4, pp. 1512–1524, 2020. [Google Scholar]
35. T. Akhtar, S. O. Gilani, Z. Mushtaq, S. Arif, M. Jamil et al., “Effective voting ensemble of homogenous ensembling with multiple attribute-selection approaches for improved identification of thyroid disorder,” Electronics, vol. 10, no. 23, pp. 3026, 2021. [Google Scholar]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF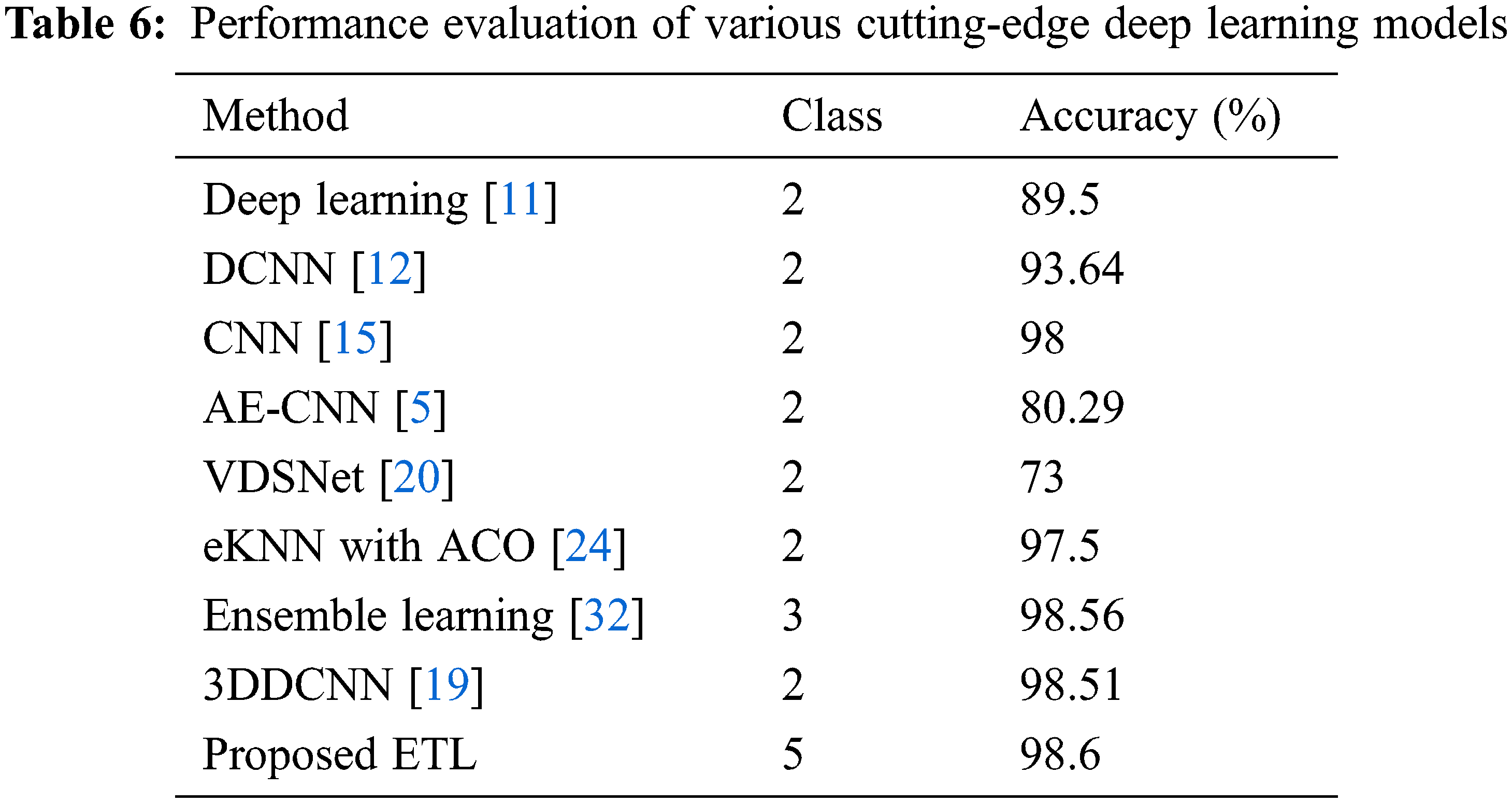
 Downloads
Downloads
 Citation Tools
Citation Tools