 Open Access
Open Access
ARTICLE
New Trends in the Modeling of Diseases Through Computational Techniques
1 Department of Mathematics and Statistics, College of Science, Taif University, P. O. Box 11099, Taif, 21944, Saudi Arabia
2 Department of Mathematics, Govt. Maulana Zafar Ali Khan Graduate College Wazirabad, Punjab Higher Education Department (PHED), Lahore, 54000, Pakistan
3 Baqai Medical University, Karachi, 75340, Pakistan
4 Shalamar Medical and Dental College, Lahore, 54000, Pakistan
5 Department of Automation, Biomechanics and Mechatronics, Lodz University of Technology, 1/15 Stefanowskiego St., 90-924, Lodz, Poland
6 Department of Mathematics, Faculty of Sciences and Technology, University of Central Punjab, Lahore, 54000, Pakistan
7 Department of Mathematics and Statistics, University of Lahore, Lahore, 54590, Pakistan
8 Institute of Machine Tools and Production Engineering, Lodz University of Technology, 1/15 Stefanowskiego St., 90-537, Lodz, Poland
* Corresponding Author: Ali Raza. Email:
Computer Systems Science and Engineering 2023, 45(3), 2935-2951. https://doi.org/10.32604/csse.2023.033935
Received 01 July 2022; Accepted 22 August 2022; Issue published 21 December 2022
Abstract
The computational techniques are a set of novel problem-solving methodologies that have attracted wider attention for their excellent performance. The handling strategies of real-world problems are artificial neural networks (ANN), evolutionary computing (EC), and many more. An estimated fifty thousand to ninety thousand new leishmaniasis cases occur annually, with only 25% to 45% reported to the World Health Organization (WHO). It remains one of the top parasitic diseases with outbreak and mortality potential. In 2020, more than ninety percent of new cases reported to World Health Organization (WHO) occurred in ten countries: Brazil, China, Ethiopia, Eritrea, India, Kenya, Somalia, South Sudan, Sudan, and Yemen. The transmission of visceral leishmaniasis is studied dynamically and numerically. The study included positivity, boundedness, equilibria, reproduction number, and local stability of the model in the dynamical analysis. Some detailed methods like Runge Kutta and Euler depend on time steps and violate the physical relevance of the disease. They produce negative and unbounded results, so in disease dynamics, such developments have no biological significance; in other words, these results are meaningless. But the implicit nonstandard finite difference method does not depend on time step, positive, bounded, dynamic and consistent. All the computational techniques and their results were compared using computer simulations.Keywords
In 2017, Boukhalfa et al. presented a mathematical model which describes the dynamics of visceral leishmaniasis in the dog population. They observed the effect of primary reproduction numbers and showed global stability using the Lyapunov function [1]. In 2020, Coffeng et al. predicted the impact of reduced detection delays and increased population coverage on observed visceral leishmaniasis cases using a mathematical model [2]. In 2020, Gandhi et al. discussed the delayed visceral leishmaniasis model’s dynamical characteristics and presented the steady states’ global stability [3]. Zamir et al. formulated well known mathematical model with the help of deterministic techniques [4]. In 2019, Nadeem et al. constructed a mathematical model of zoonotic cutaneous leishmaniasis, including vector populations, reservoirs, and humans. Based on sensitivity analysis of threshold number, they proposed some strategies for eliminating the disease [5]. Rutte et al. studied the infectiousness of kala-azar dermal leishmaniasis [6]. In 2019, Adamu et al. presented a mathematical model with time delay for zoonotic visceral leishmaniasis transmission dynamics. They concluded that the incubation period significantly affects the stability of the equilibrium points [7]. Zamir et al. proposed a mathematical model of visceral leishmaniasis disease with a saturated infection rate. They recommended different control strategies based on sensitivity analysis to manage the spread of this disease in the community [8]. In 2017, Shimozako et al. proposed a new model for zoonotic visceral leishmaniasis using a modified set of differential equations on Brazilian human data. They recommended that sandfly population control be prioritized to eliminate the disease in Brazil [9]. Ghosh et al. proposed a compartmental model of visceral leishmaniasis cases from South Sudan in 2013. They also performed a cost-effectiveness study and cost sensitivity analysis on different interventions [10]. In 2017, Lmojtaba et al. proposed the visceral leishmaniasis dynamics with seasonality’s effect. They applied two control, treatment, and vaccination, to the model that forces the system to be non-periodic [11]. Biswas studied disease dynamics to analyze the seasonal visceral leishmaniasis incidence data from South Sudan. He also discussed the optimal control strategy using vaccination and possible treatment of infective humans [12]. In 2017, Debroy et al. studied vector-borne diseases and collected challenges and successes related to modeling transmission dynamics of visceral leishmaniasis [13]. In 2017, Rutte et al. presented three transmission models of visceral leishmaniasis in the Indian subcontinent with structural differences regarding the disease stage [14]. In 2017, Zou et al. developed a mathematical model to study the transmission dynamics of visceral leishmaniasis considering three populations: dogs, sandflies, and humans [15]. In 2016, Siewe et al. developed a mathematical model to explain the evolution of the disease and used the model to simulate treatment by existing or potential new drugs [16]. Rock et al. presented the next-generation matrix methods of visceral leishmaniasis with clinical infection [17]. In 2015, Roy et al. considered a mathematical model of cutaneous leishmaniasis with a time delay effect in the disease transmission [18]. In 2015, Subramanian et al. developed a compartmental-based mathematical model of zoonotic visceral leishmaniasis transmission. They analyzed the model for positivity, boundedness, and stability around steady states indifferent to diseased and disease-free scenarios and derived the primary reproduction number [19]. In 2015, Medley et al. used empirical data on health-seeking behavior and health-system performance from the Indian state of Bihar, Bangladesh, and Nepal to parametrize a mathematical model [20]. Some well-known mathematical models are studied through different aspects [21–29]. The paper’s strategy is as follows: Section 2 goes to the formulation, and Section 3 states the fundamental properties of the model. Section 4 goes to the model’s numerical results, and the last quarter delivers the results, discussion, and concluding remarks.
The parameters and variables of the leishmaniasis model are described as follows:
Following non-negative initial conditions,
The system (1–5) can be normalized by subsidizing variables to avoid complications
with nonnegative initial conditions
The feasible region of the model as follows:
Theorem 1: For any time, t, the system (6–10) admits a positive solution.
Proof: By letting the system,
Theorem 2: For any time, t, the system (6–10) admits a bounded solution and lies in the feasible region. For considerable time t, the following inequality satisfies
Proof: Consider the population function as follows:
The model admits two types of equilibria as follows:
Leishmaniasis free equilibrium point
Leishmaniasis existing equilibrium point
In this section, the calculation of the infection force ratio by using the next-generation matrix method and the leishmaniasis-free equilibrium of the model as follows:
where
The largest eigenvalue of
Theorem 3: The leishmaniasis free equilibrium
Proof: The Jacobian matrix at
Consider,
where
Since
Theorem 4: The leishmaniasis existing equilibrium
Proof: The Jacobian matrix at
where
Applying Routh-Hurwitz Criterion for 3rd order,
Using the command-built software such as Matlab and simulating the system (1–6) at both equilibria of the model using scientific literature presented in Tab. 1 as follows:
The Euler method could be applied to the system (1–5) as follows:
where h is any time step size?
The Euler’s method graphs are plotted for both equilibria of the model as follows:
The Runge Kutta method could be applied to the system (1–5) as follows:
Stage I
Stage II
Stage III
Stage IV
Final Stage
where h is any time step size.
The Runge Kutta method graphs are plotted for both equilibria of the model as follows:
The NSFD method that could be applied to the system (1–5) as follows:
Like, Eq. (21),
where h is any time step size.
4.6 Stability Results of NSFD Method
Considering the function for the system (3.11–3.15),
The partial derivatives of the Jacobean matrix,
At disease free equilibrium point
For stability, to prove the following conditions as follows:
i)
ii)
iii)
iv)
Since the step size is never zero, so the condition is satisfied.
(ii)
where
Hence, this condition is also satisfied.
(iii)
where
So, condition (iii) is also satisfied, as desired.
The NSFD method graphs are plotted for both equilibria of the model as follows:
5 Results and Concluding Remarks
The simulation of the Euler method is presented in Figs. 1a–1d. Figs. 1b and Fig. 1d show that the system violates the properties of the real-world problem like positivity, boundedness, and dynamical consistency by an increase in the time step size. The Runge Kutta method of order fourth depicts the graphical representation in Figs. 2a–2d, but the technique is time-dependent and converges to the false steady state of the model. The NSFD process depicts the graphical solution of the model in Figs. 3a–3d; the NSFD method converges to proper equilibria of the model at any time step size and fulfils all the model properties. The support comparison of the numerical methods is presented in Figs. 4a–4d. The Euler and RK-4 schemes are dependent on time step size. These numerical schemes are convergent for small step sizes while divergent for large step sizes. But the nonstandard finite difference method used for communication dynamics of leishmaniasis disease is independent of the time step size. It is convergent even for any time step size like hundreds and thousands. NSFD scheme shows positivity, boundedness, and stability and behaves similarly to the behavior of the continuous model. Hence, the NSFD scheme is more efficient, fast convergent, and reliable than the remaining method. In the future, the work will extend to the field as presented in [30–33].
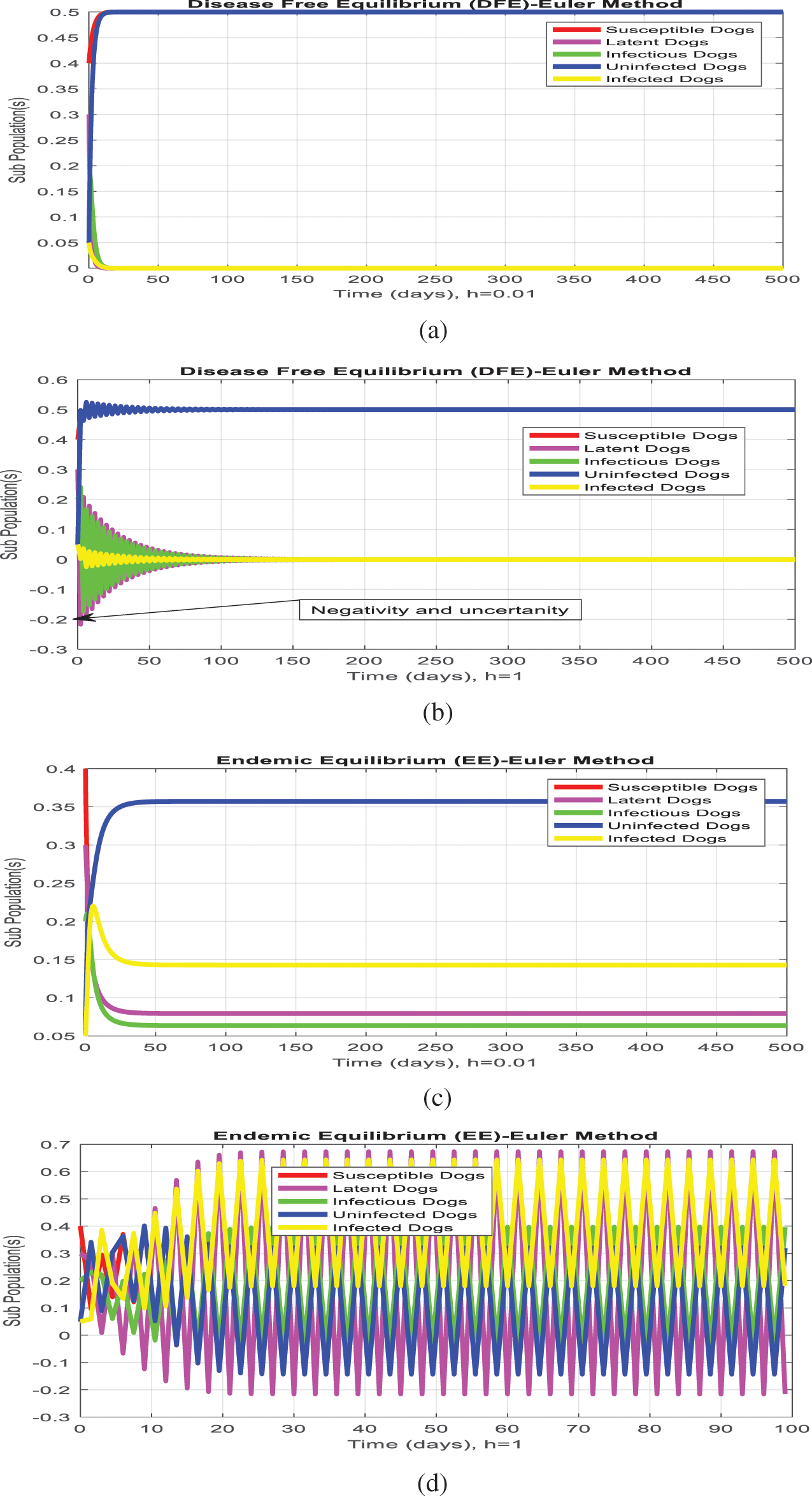
Figure 1: (Euler simulations) subpopulation at
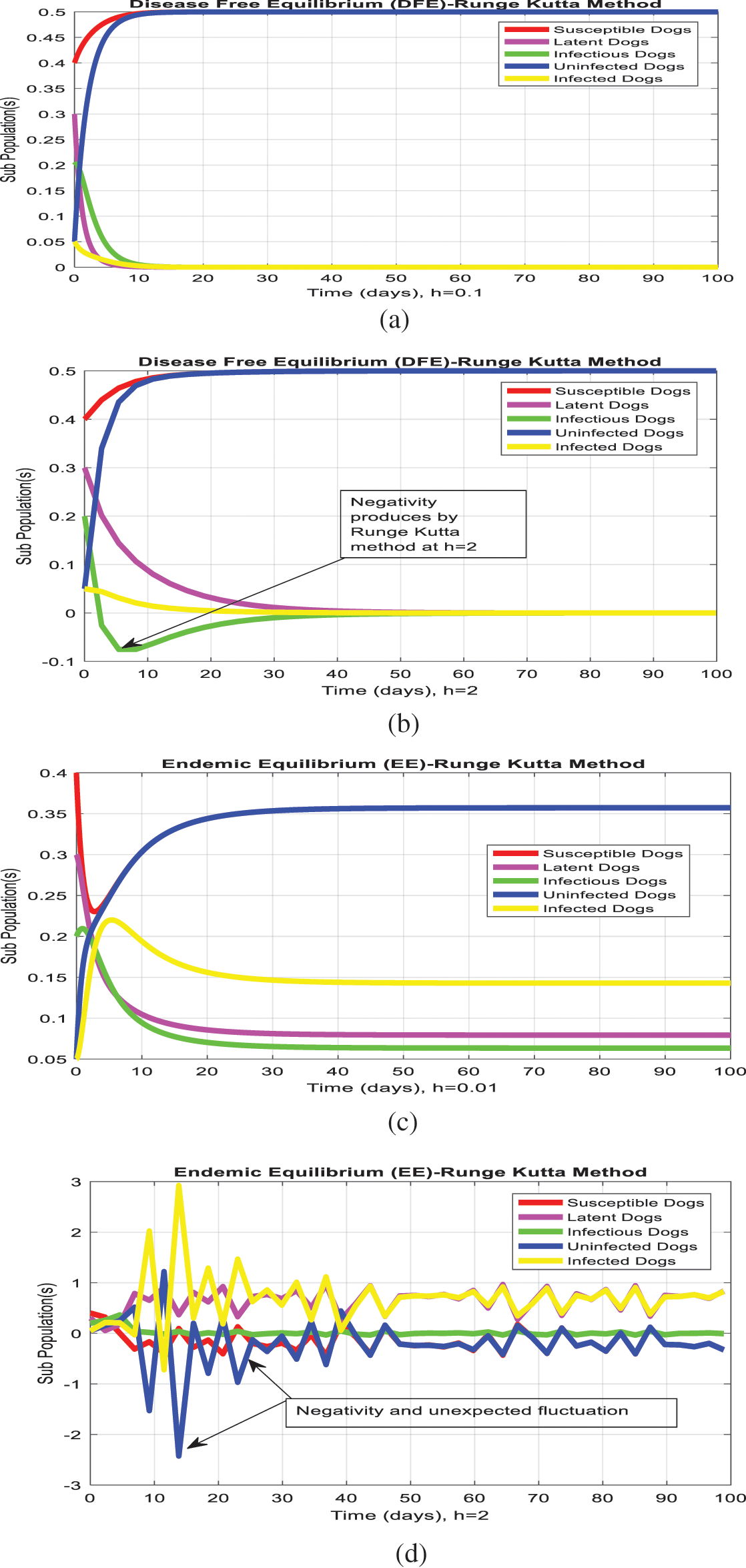
Figure 2: (Runge-Kutta simulations) (a) subpopulation at
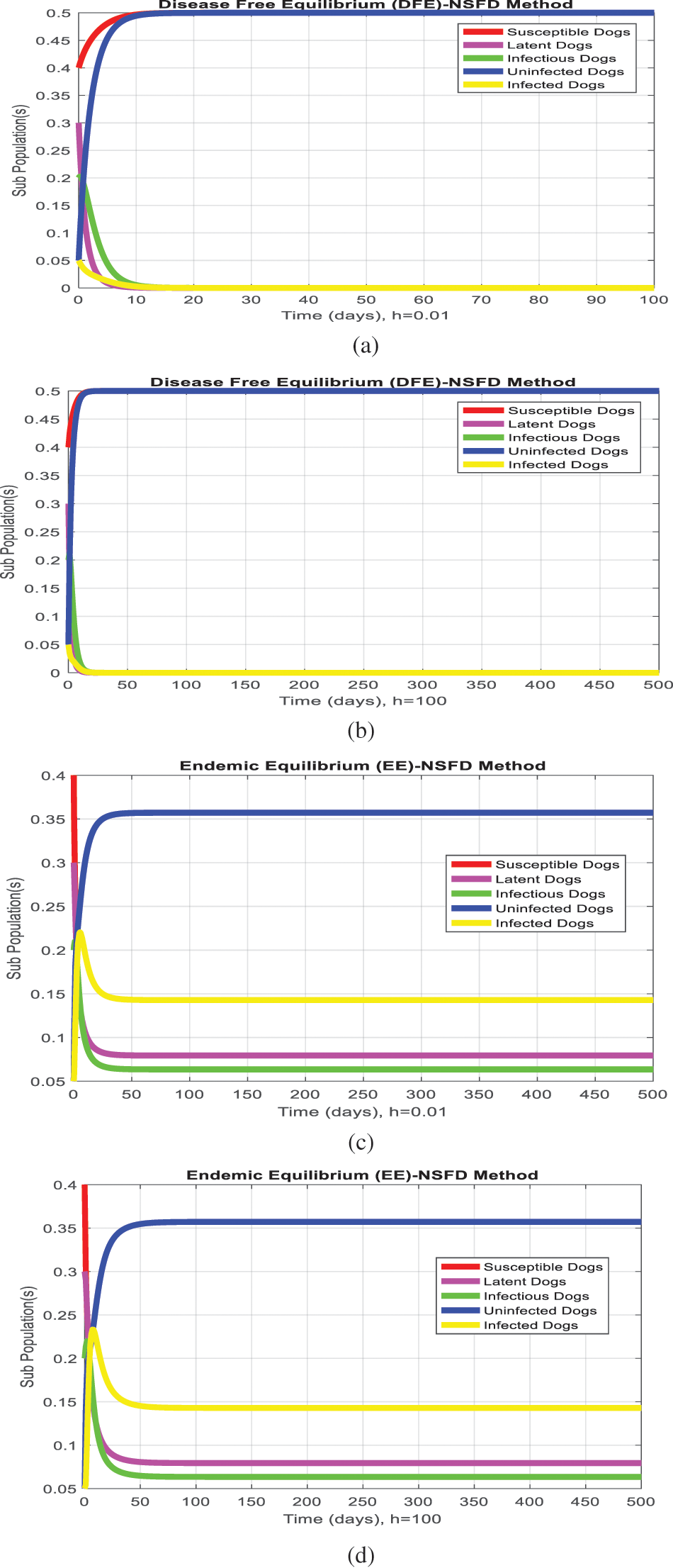
Figure 3: (NSFD simulations) subpopulation at
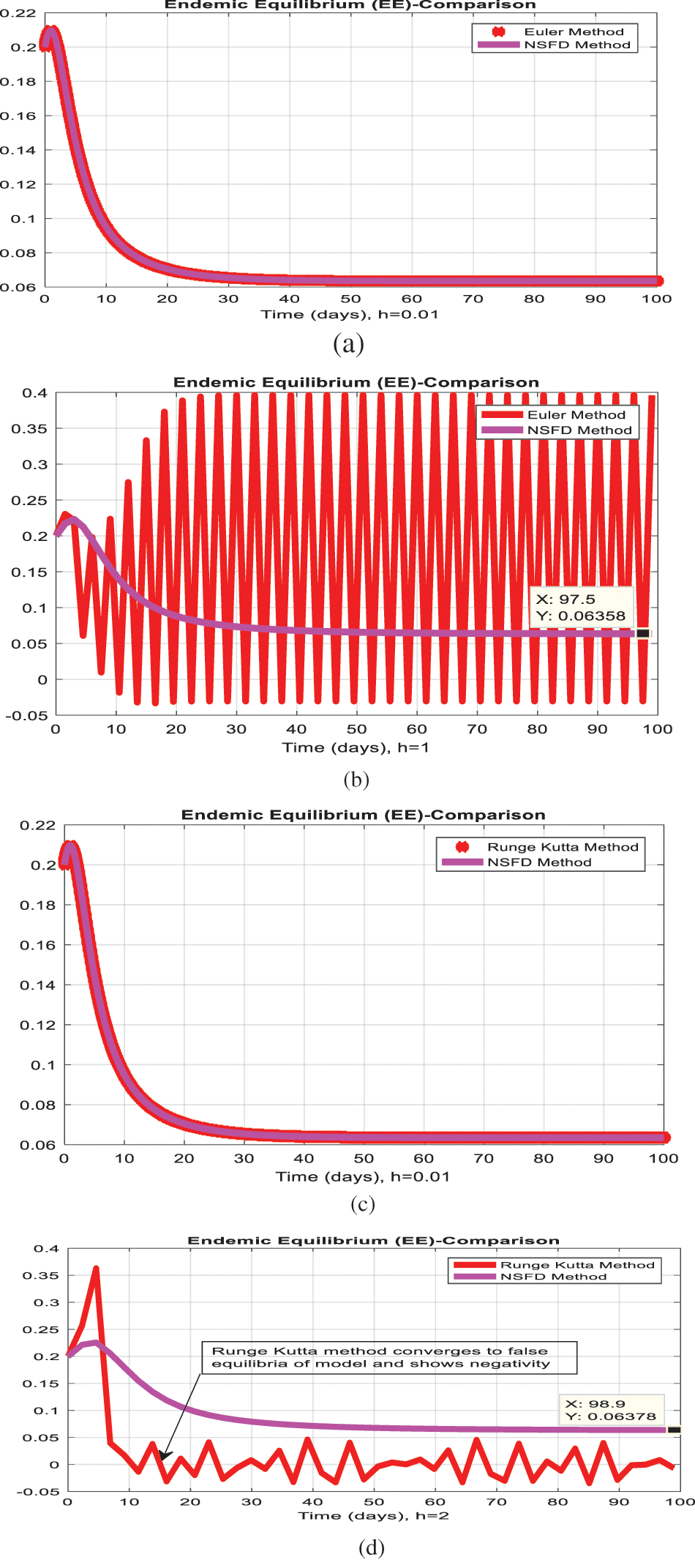
Figure 4: Comparison of methods (a) infected humans at
Acknowledgement: The authors are thankful to the Govt. of Pakistan for providing the facility to conduct the research. All Authors are grateful for the suggestions of anonymous referees to improve the quality of the manuscript.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. F. Boukhalfa, M. Helal and A. Lakmeche, “Mathematical analysis of visceral leishmaniasis model,” Research in Applied Mathematics, vol. 1, no. 1, pp. 01–06, 2017. [Google Scholar]
2. L. E. Coffeng, E. A. L. Rutte, J. Muñoz, E. R. Adams, J. M. Prada et al., “Impact of changes in detection effort on control of visceral leishmaniasis in the Indian subcontinent,” The Journal of Infectious Diseases, vol. 221, no. 5, pp. 546–553, 2019. [Google Scholar]
3. V. Gandhi, N. S. A. Salti and I. M. Elmojtaba, “Mathematical analysis of a time delay visceral leishmaniasis model,” Journal of Applied Mathematics and Computing, vol. 63, no. 1, pp. 217–237, 2020. [Google Scholar]
4. M. Zamir, F. Nadeem and G. Zaman, “Optimal control of visceral, cutaneous and post kala-azar leishmaniasis,” Advances in Difference Equations, vol. 20, no. 1, pp. 01–23, 2020. [Google Scholar]
5. F. Nadeem, M. Zamir and A. Tridane, “Modeling and control of zoonotic cutaneous leishmaniasis,” Punjab University Journal of Mathematics, vol. 51, no. 2, pp. 01–12, 2019. [Google Scholar]
6. E. A. L. Rutte, E. E. Zijlstra and S. J. D. Vlas, “Post-kala-azarkala-azar dermal leishmaniasis as a reservoir for visceral leishmaniasis transmission,” Trends in Parasitology, vol. 35, no. 8, pp. 590–602, 2019. [Google Scholar]
7. L. Adamu and N. Hussaini, “An epidemic model of zoonotic visceral leishmaniasis with time delay,” Journal of the Nigerian Society of Physical Sciences, vol. 19, no. 1, pp. 20–29, 2019. [Google Scholar]
8. M. Zamir, G. Zaman and A. S. Alshomrani, “Control strategies and sensitivity analysis of anthroponotic visceral leishmaniasis model,” Journal of Biological Dynamics, vol. 11, no. 1, pp. 323–338, 2017. [Google Scholar]
9. H. J. Shimozako, J. Wu and E. Massad, “Mathematical modelling for zoonotic visceral leishmaniasis dynamics: A new analysis considering updated parameters and notified human Brazilian data,” Infectious Disease Modelling, vol. 2, no. 2, pp. 143–160, 2017. [Google Scholar]
10. I. Ghosh, T. Sardarand and J. A. Chattopadhyay, “Mathematical study to control visceral leishmaniasis: An application to South Sudan,” Bulletin of Mathematical Biology, vol. 79, no. 5, pp. 1100–1134, 2017. [Google Scholar]
11. M. E. Lmojtaba, S. Biswas and J. Chattopadhyay, “Global analysis and optimal control of a periodic visceral leishmaniasis model,” Mathematics, vol. 5, no. 4, pp. 01–80, 2017. [Google Scholar]
12. S. Biswas, “Mathematical modeling of visceral leishmaniasis and control strategies,” Chaos, Solitons & Fractals, vol. 17, no. 104, pp. 546–556, 2017. [Google Scholar]
13. S. Debroy, O. Prosper, A. Mishoe and A. Mubayi, “Challenges in modeling complexity of neglected tropical diseases: A review of dynamics of visceral leishmaniasis in resource-limited settings,” Emerging Themes in Epidemiology, vol. 14, no. 1, pp. 01–04, 2017. [Google Scholar]
14. E. A. L. Rutte, L. A. Chapman, L. E. Coffeng, S. Jervis, E. C. Hasker et al., “Elimination of visceral leishmaniasis in the Indian subcontinent: A comparison of predictions from three transmission models,” Epidemics, vol. 17, no. 18, pp. 67–80, 2017. [Google Scholar]
15. L. Zou, J. Chen and S. Ruan, “Modeling and analyzing the transmission dynamics of visceral leishmaniasis,” Mathematical Biosciences and Engineering, vol. 14, no. 5, pp. 1500–1585, 2017. [Google Scholar]
16. N. Siewe, A. A. Yakubu, A. R. Satoska and A. Friedman, “Immune response to infection by leishmania: A mathematical model,” Mathematical Biosciences, vol. 16, no. 276, pp. 28–43, 2016. [Google Scholar]
17. K. S. Rock, R. J. Quinnell, G. F. Medley and O. Courtenay, “Progress in the mathematical modelling of visceral leishmaniasis,” Advances in Parasitology, vol. 16, no. 94, pp. 49–131, 2016. [Google Scholar]
18. P. K. Roy, D. Biswas and F. A. Basir, “Transmission dynamics of cutaneous leishmaniasis: A delay-induced mathematical study,” Journal of Medical Research and Development, vol. 4, no. 2, pp. 11–23, 2015. [Google Scholar]
19. A. Subramanian, V. Singh and R. R. Sarkar, “Understanding visceral leishmaniasis disease transmission and its control: A study based on mathematical modelling,” Mathematics, vol. 3, no. 3, pp. 913–944, 2015. [Google Scholar]
20. G. F. Medley, T. D. Hollingsworth, P. L. Olliaro and E. R. Adams, “Health-seeking behaviour, diagnostics and transmission dynamics in the control of visceral leishmaniasis in the Indian subcontinent,” Nature, vol. 528, no. 7580, pp. 102–108, 2015. [Google Scholar]
21. F. M. Allehiany, F. Dayan, F. F. Al-Harbi, N. Althobaiti, N. Ahmed et al., “Bio-inspired numerical analysis of COVID-19 with fuzzy parameters,” Computers, Materials & Continua, vol. 72, no. 2, pp. 3213–3229, 2022. [Google Scholar]
22. Z. Iqbal, N. Ahmed, D. Baleanu, W. Adel, M. Rafiq et al., “Positivity and boundedness preserving numerical algorithm for the solution of fractional nonlinear epidemic model of HIV/AIDS transmission,” Chaos, Solitons & Fractals, vol. 134, no. 1, pp. 01–19, 2020. [Google Scholar]
23. Y. T. Mangongo, J. D. K. Bukweli and J. D. B. Kampempe, “Fuzzy global stability analysis of the dynamics of malaria with fuzzy transmission and recovery rates,” American Journal of Operations Research, vol. 11, no. 6, pp. 257–282, 2021. [Google Scholar]
24. M. Jawaz, N. Ahmed, D. Baleanu, M. Rafiq and M. A. Rehman, “Positivity preserving technique for the solution of HIV/AIDS reaction-diffusion model with time delay,” Frontiers in Physics, vol. 7, no. 1, pp. 01–10, 2020. [Google Scholar]
25. N. Ahmed, M. Rafiq, W. Adel, H. Rezazadeh, I. Khan et al., “Structure preserving numerical analysis of HIV and CD4+ T-cells reaction-diffusion model in two space dimensions,” Chaos, Solitons & Fractals, vol. 139, no. 1, pp. 01–18, 2020. [Google Scholar]
26. A. Raza, J. Awrejcewicz, M. Rafiq, N. Ahmed, M. S. Ahsan et al., “Dynamical analysis and design of computational methods for nonlinear stochastic leprosy epidemic model,” Alexandria Engineering Journal, vol. 61, no. 10, pp. 8097–8111, 2022. [Google Scholar]
27. A. Raza, M. Rafiq, J. Awrejcewicz, N. Ahmed and M. Mohsin, “Stochastic analysis of nonlinear cancer disease model through virotherapy and computational methods,” Mathematics, vol. 10, no. 3, pp. 01–18, 2022. [Google Scholar]
28. A. Raza, M. Rafiq, J. Awrejcewicz, N. Ahmed and M. Mohsin, “Dynamical analysis of coronavirus disease with crowding effect, and vaccination: A study of third strain,” Nonlinear Dynamics, vol. 107, no. 4, pp. 3963–3982, 2022. [Google Scholar]
29. A. Raza, J. Awrejcewicz, M. Rafiq and M. Mohsin, “Breakdown of a nonlinear stochastic Nipah virus epidemic model through efficient numerical methods,” Entropy, vol. 23, no. 12, pp. 01–20, 2021. [Google Scholar]
30. M. A. Akbar, L. Akinyemi, S. W. Yao, A. Jhangeer, H. Rezazadeh et al., “Soliton solutions to the boussinesq equation through Sine-Gordon method and Kudryashov method,” Results in Physics, vol. 25, no. 1, pp. 01–19, 2021. [Google Scholar]
31. I. Ahmad, M. N. Khan, M. Inc, H. Ahmad and K. S. Nisar, “Numerical simulation of simulate an anomalous solute transport model via local meshless method,” Alexandria Engineering Journal, vol. 59, no. 4, pp. 2827–2838, 2020. [Google Scholar]
32. H. Ahmad and T. A. Khan, “Variational iteration algorithm-I with an auxiliary parameter for wave-like vibration equations,” Journal of Low Frequency Noise, Vibration and Active Control, vol. 38, no. 3, pp. 1113–1124, 2019. [Google Scholar]
33. H. Ahmad, T. A. Khan, P. S. Stanimirovic and I. Ahmad, “Modified variational iteration technique for the numerical solution of fifth-order KdV-type equations,” Journal of Applied and Computational Mechanics, vol. 6, no. 20, pp. 1220–1227, 2020. [Google Scholar]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools