 Open Access
Open Access
ARTICLE
Integrated Approach of Brain Disorder Analysis by Using Deep Learning Based on DNA Sequence
1 Computer Sciences Department, College of Computer and Information Sciences, Princess Nourah Bint Abdulrahman University, Riyadh, P.O. Box 84428, 11671, KSA
2 School of Computer Scence and Engineering, Vellore Institute of Technology, Chennai, 600127, India
3 Department of Computer Science, CHRIST (Deemed to be University), Bangalore, 560029, India
* Corresponding Author: Ahmed Zohair Ibrahim. Email:
Computer Systems Science and Engineering 2023, 45(3), 2447-2460. https://doi.org/10.32604/csse.2023.030134
Received 18 March 2022; Accepted 25 May 2022; Issue published 21 December 2022
Abstract
In order to research brain problems using MRI, PET, and CT neuroimaging, a correct understanding of brainfunction is required. This has been considered in earlier times with the support of traditional algorithms. Deep learning process has also been widely considered in these genomics data processing system. In this research, brain disorder illness incliding Alzheimer’s disease, Schizophrenia and Parkinson’s diseaseis is analyzed owing to misdetection of disorders in neuroimaging data examined by means fo traditional methods. Moeover, deep learning approach is incorporated here for classification purpose of brain disorder with the aid of Deep Belief Networks (DBN). Images are stored in a secured manner by using DNA sequence based on JPEG Zig Zag Encryption algorithm (DBNJZZ) approach. The suggested approach is executed and tested by using the performance metric measure such as accuracy, root mean square error, Mean absolute error and mean absolute percentage error. Proposed DBNJZZ gives better performance than previously available methods.Keywords
When any such gene is found to be pretentious and prone to some kind of ailment, it is denoted as genetic disorder. Genetic disorder diseases such as Alzheimer and Parkinson directly affects the human being by disrupting the brain functionality [1–3]. The medical imaging has been an essential parameter to present several modalities of images such as X-ray, MRI and CT [4]. Storage of delicate form of information regarding medical images and other associated data in a secure manner also holds a prominent role in medical field. Some of the conventional form of DNA based molecular cryptography scheme and its inscription methods to hoard its image in a secure form has become an interesting area of research. One such main issue seen in traditional means of approaches is that it could not resist the instinctive force attack and hence, this research work executes DNA sequence based JPEG ZIG ZAG encryption system.
Conventional use of computational neuro-scientific systems have been known extraordinarily promising in comprehensive mental health trials. This multidisciplinary field of study could model the genetic systems prevailing the dynamic and diseased environments of the human brain and map these practices into identifiable clinical descriptions. In the previous age, the rapid rise in high-volume biomedical datasets, simultaneous with the advancements in machine learning, has unconstrained inventive prospects for assessment and prediction of neurodegenerative and neuropsychiatric complaints. From a computational viewpoint, this modern extension has fashioned the advance of tools that assimilate abundant patient-specific elucidations into assessments and rise the clinical effects of patients anguish from such circumstances. The crucial purpose of these neuro technical techniques is to magnify the introductory associate and complete the supervision impression of entities in high threat of Alzheimer’s disease and alzheimer-related cognitive failure [5].
For the purposes quantified above, existing studies have engrossed on beginning enormously capable approaches that use ML systems to develop the examination of AD. The usage of intuitive structures proficient of discriminating extreme cases from normal cases centered on their magnetic resonance imaging scans will donate massively to the primary analysis of AD. This study examines notable elements that identify AD and use MRI data, ML and Deep Learning schemes with unbounded AD datasets.
Several kinds of algorithms and systems has been presented earlier for detecting the brain disorder and the limitations seen in them includes: pre-processign and feature extraction were not evidently described and ineffective manner of handling complicated form of genomic data [6–10].
The key contribution of current research is given as follows:
1. Analysis of brain disorder could be executed with the support of deep learning approach and describes how to hoard sensitive form of data in image setup by incorporating DNA based encryption system.
2. Evaluates accurateness of pre-processing of image with respect to image registration, image enhancement, normalization filtering and smoothening.
This research is structured as follows: Section 1 defines introduction of analysis of brain disorder. Section 2 mentions literature review of the research topic. Section 3 presents the outline for classification of status of water quality. Section 4 confers about the investigated outcomes and Section 5 determines the research work with forthcoming research guidelines.
In current era of rapid advancements in several tools and technologies, brain disorder disease and its analysis is becoming more effective. There exists several methods to tackle these anxieties and deep learning is the most popular methodology these days. Some of the brain related illness terms are Alzheimer, schizophrenia and Parkinson.
Current research work attempts to introduce DBN feature extraction of the disorder image for brain related illness and to progress overall value of performance. In Alzheimer disease, the database is collected from the ADNI dataset. This could validate and process the data of MRI, CT and PET images [11]. Deep learning approach is mainly deployed to identify Parkinson disease with the use of SPECT image dataset [12]. A new DBN based architecture model is considered which could discover statistical form of values from the perceived data and certainly identifies the pretentious area [13]
To enhance the speed level of processing geome data, DNA sequencing is mainly suggested [14] and the classification of DNA sequence is accomplished with the aid of machine learning algorithm. Here, the features are extracted and stored as vector format. Supervised form of learning is executed here and the drawback found is that: it could not read by machine and moreover high dimensionality of data occurs [15].
Tab. 1. displays summary of assessment on exploration of brain disorder by incorporating deep learning process. Alzheimer is an advanced form of neuro degenerative ailment which could account for 70% dementia cases all wide and the predictions estimate that with 2030, 75 million of cases might exist worldwide. Even though there is no such cure for AD, the primary analysis could assist in helping to progress quality of life of patients and their relations . There are several drugs and medicines which are under research stage. It is expected that their effectiveness is confirmed once it is managed all through h the initial stage of the disease. These days, Alzheimer is treated with the aid of medical documents and appropriate inspection of the mental status. For suggestion based entities, the judgment is maintained by biomarkers resultant from cerebrospinal fluid and neuroimaging methods such as magnetic resonance imaging. During earlier times, several methods has described 3 major effects of Alzheimer and its progressive nature on rsEEG signals, explicitly, decay in synchronization and condensed intricacy. Moreover, modern form of tentative sign has suggested a neuro modulatory discrepancy in rsEEG signals, which might have its basis in the deduction of neurotransmitters owed to devastation in brain pathways prompted by alzheimer. To define these neuro-modulatory debits, amplitude modulation examination of rsEEG signals were projected lately. While these 4 effects were regularly perceived independently, they all developed from the loss of neurons that intrude anatomical brain connectivity at the level of functional networks. Stating these perceptions, several rsEEG biomarkers have been advised to either explore alzheimer to display disease progress.
Accurate means of diagnosis of Alzheimer shows significant changes in patient treatment during initial phase of disease as the threat alert alertness endures the patients to carry out protective approaches even afore incidence of irretrievable brain obliteration. Even though more than a few modern studies have used computers to detect alzheimer, extreme machine detection methodologies were controlled by inherited observations. Alzheimer could be diagnosed-but not predicted-at its primary phases, as prediction were only suitable formerly the disease manifests itself. Deep Learning has become a communal system for the principal analysis of alzheimer. At this point, briefly analyzes a number of the substantial works on alzheimer and determine how deep learning could help investigators ascertain the pattern at its initial points are described in Fig. 1.
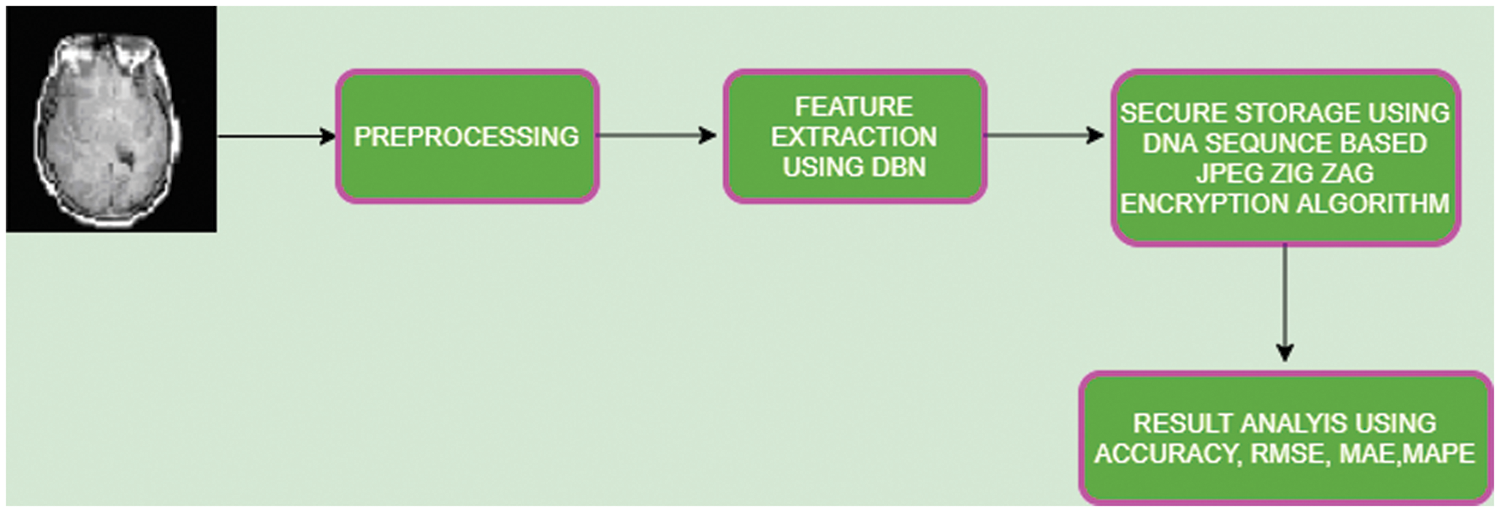
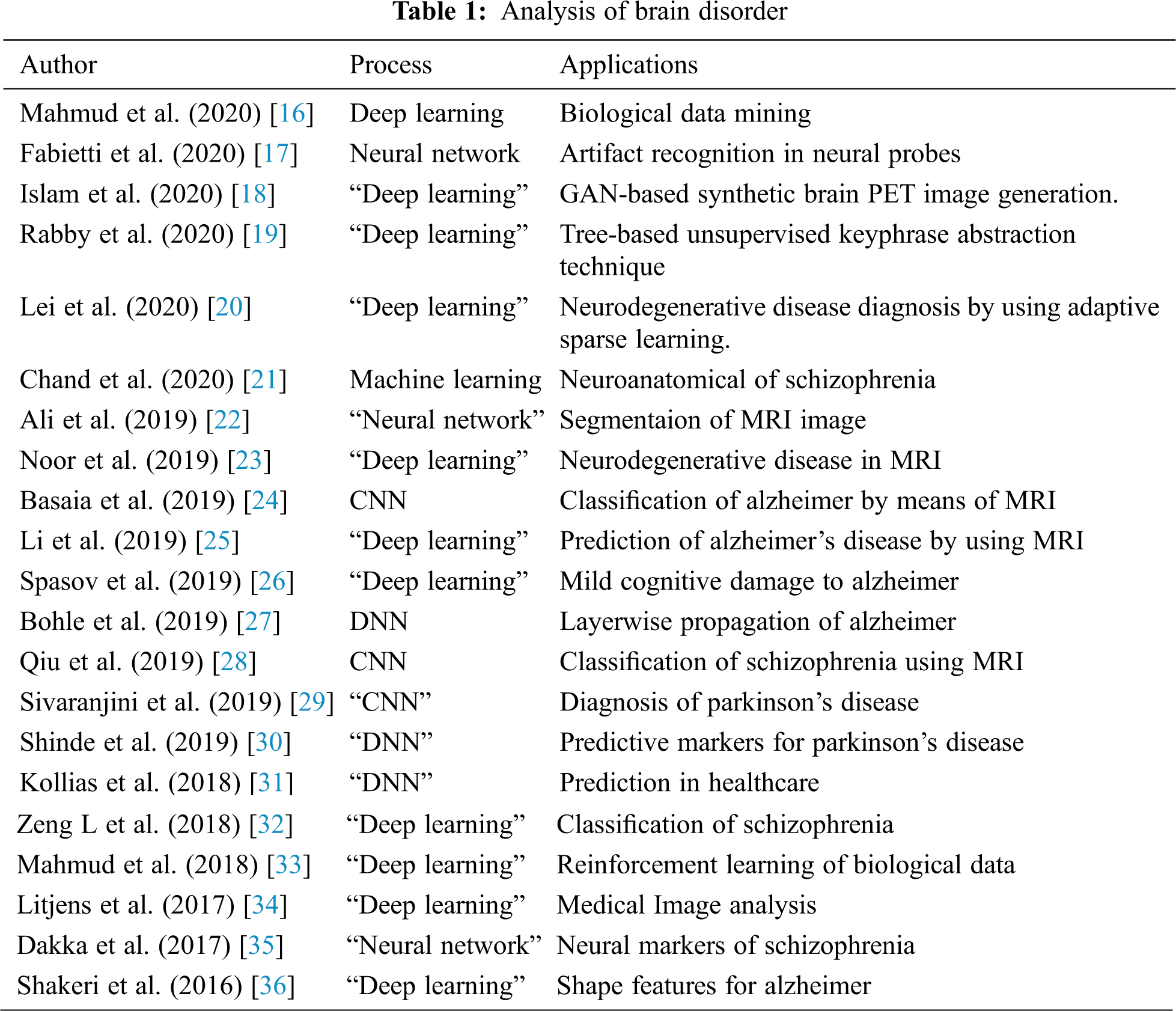
Figure 1: Workflow of DBNJZZ
Medical imaging is a way of essential tool to treat any disease and for examining brain disorder, this research work executes deep learning technique of DBN. Proposed approach contains 2 modules such as:
First Module: Pre-processing phase
Second Module: Feature Extraction of disorder image and DNA Sequence centered privacy Storage of Brain image using proposd method.
CT, MRI, PET are the neuroimaging based modalities of the brain images and pre-processing is carried out to enhance the quality of images. Phases tangled in pre-processing steps are described in Fig. 2.
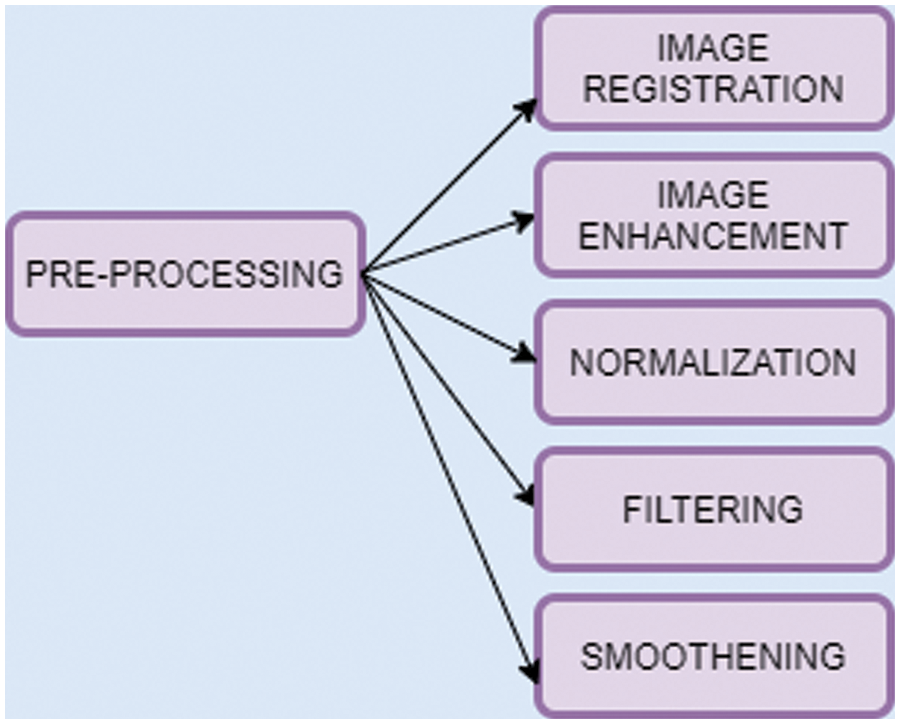
Figure 2: Pre-processing
This is the form of acquiring 2 or more similar image features along with diverse variation time structure into distinct explanatory image. The linear regression based system is mainly deployed for image registration which could entail function of image in rotation, transformation, scaling on x, y and z axes. The spatial correlation of image gets aligned in all such angles and generally, the image registration is specified by
where,
The image enhancement step expands quality of images by means of filtering with “contrast limited adaptive histogram equalization (CLAHE)”. It could increase the brightness of image with its contextual background to enhance perceptibility.
Normalization is method of alignment of image with respect to dimension and shape to infer them according to usual topographies of the image. It could map dat appoint which is attained from discrete space rate to reference space value.
With the support of Weiner filtering, it could remove the unnecessary fratures of image and reducing the noise of image.
where,
k denotes low frequency value of wiener filter. High pass filter assessment is mainly deployed for blurry form of images.
Smoothening is the criteria of minimizing noise from images and here, the spatial smoothing is executed which estimates average value of pixels from neighboring pixel features. This improves Signal-to-noise ratio value and decreases spatial resolution value.
3.2 Feature Extraction of Disorder Image and DNA Sequence
In proposed approach, Deep Belief Network could be deployed to abstract feature type of biomarkers of image. The biomarker could act as a specified tool for analytical purpose and it is mainly used for identifying anomalous state of image. DBN is mainly based on restricted boltzman machines design [37–40]. DBN is an unsupervised fearure extractor which could extact major features of image for enhancing performance level [41–43]. This could abstract the typical structure topographies from brain image to recognize disorder of brain disease. Fig. 3 indicates that workflow of commending biomarkers.
Figure 3: Work-flow of getting biomarker
The structural design of DBN is mainly composed of stacks RBM which contains a discernible layer and several hidden layers. Here, every layer comprises of nodes and association between input and hidden layers were by allocating weight value. Once after adjusting the weight vector value, the network gets trained and the arrangement of DBN is described in Fig. 4. The structural design of RBM is specified in the Fig. 5.
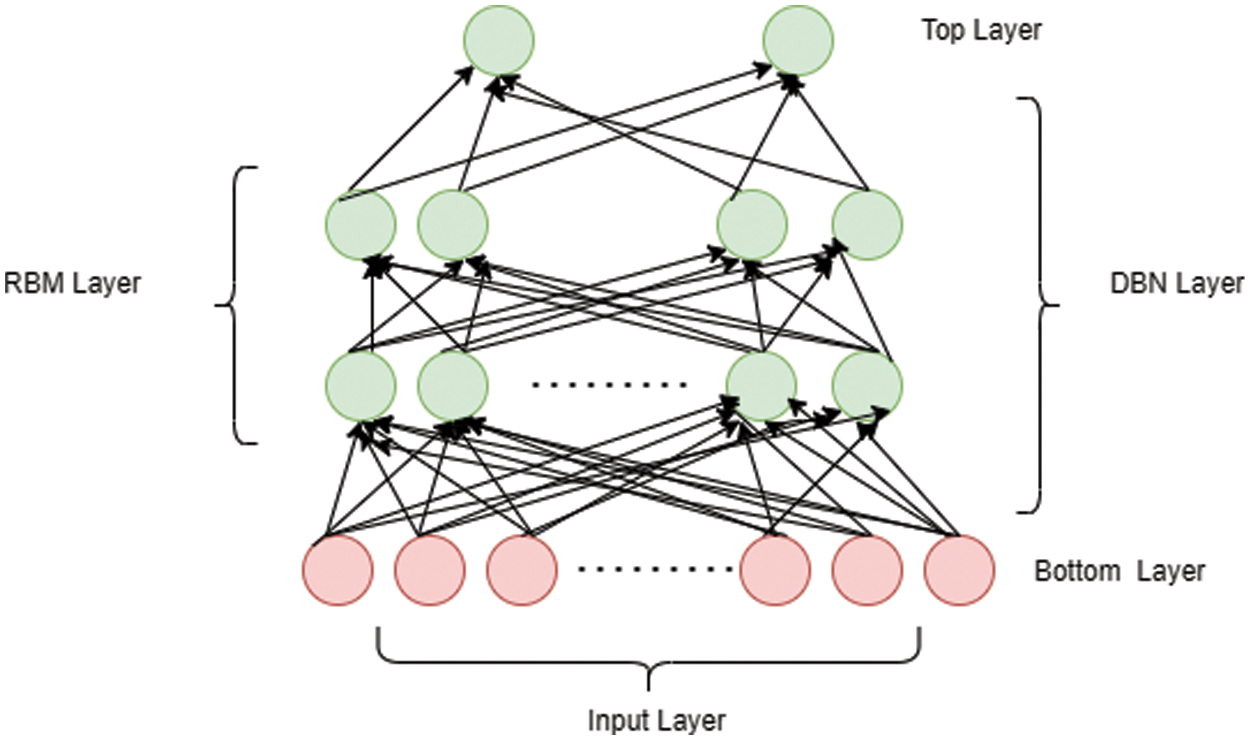
Figure 4: DBN layer
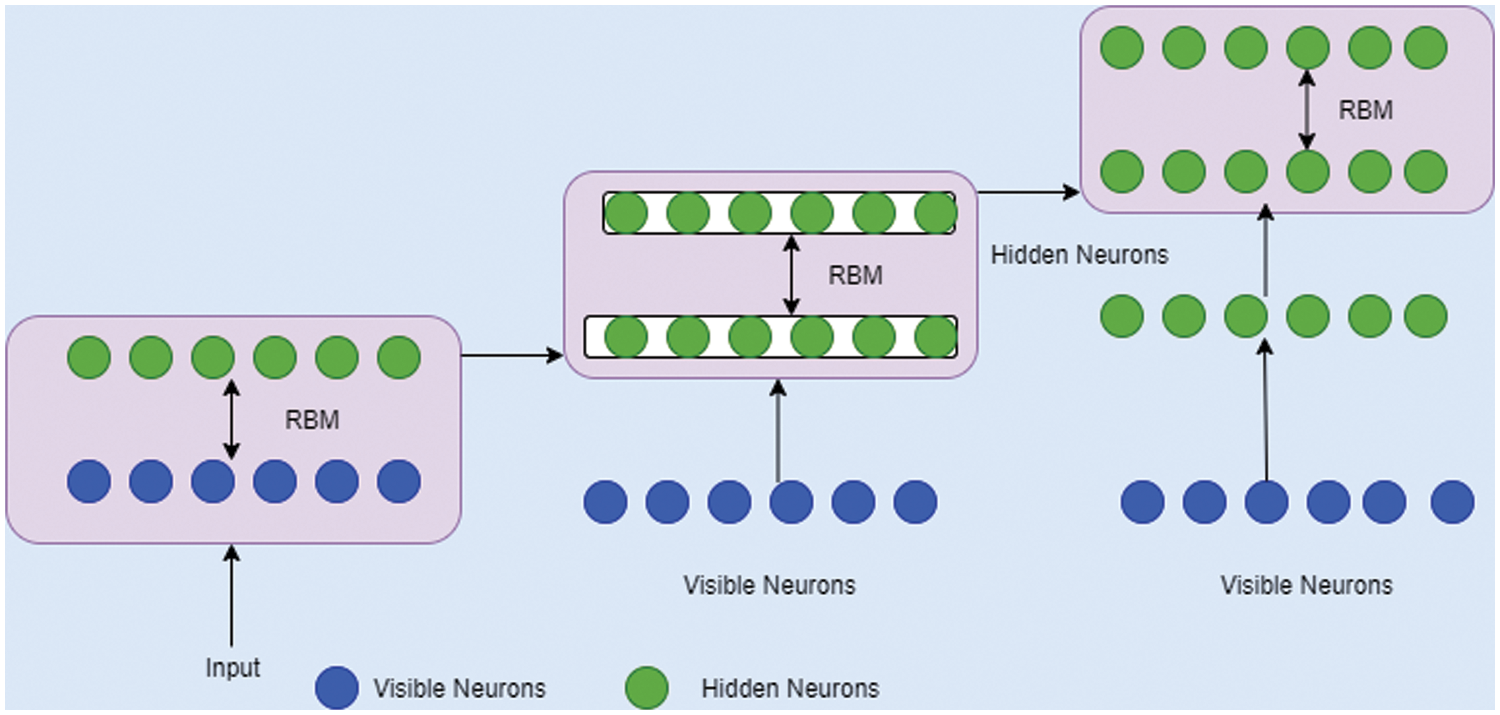
Figure 5: RBM design
The process involved for training DBN is mentioned as follows:
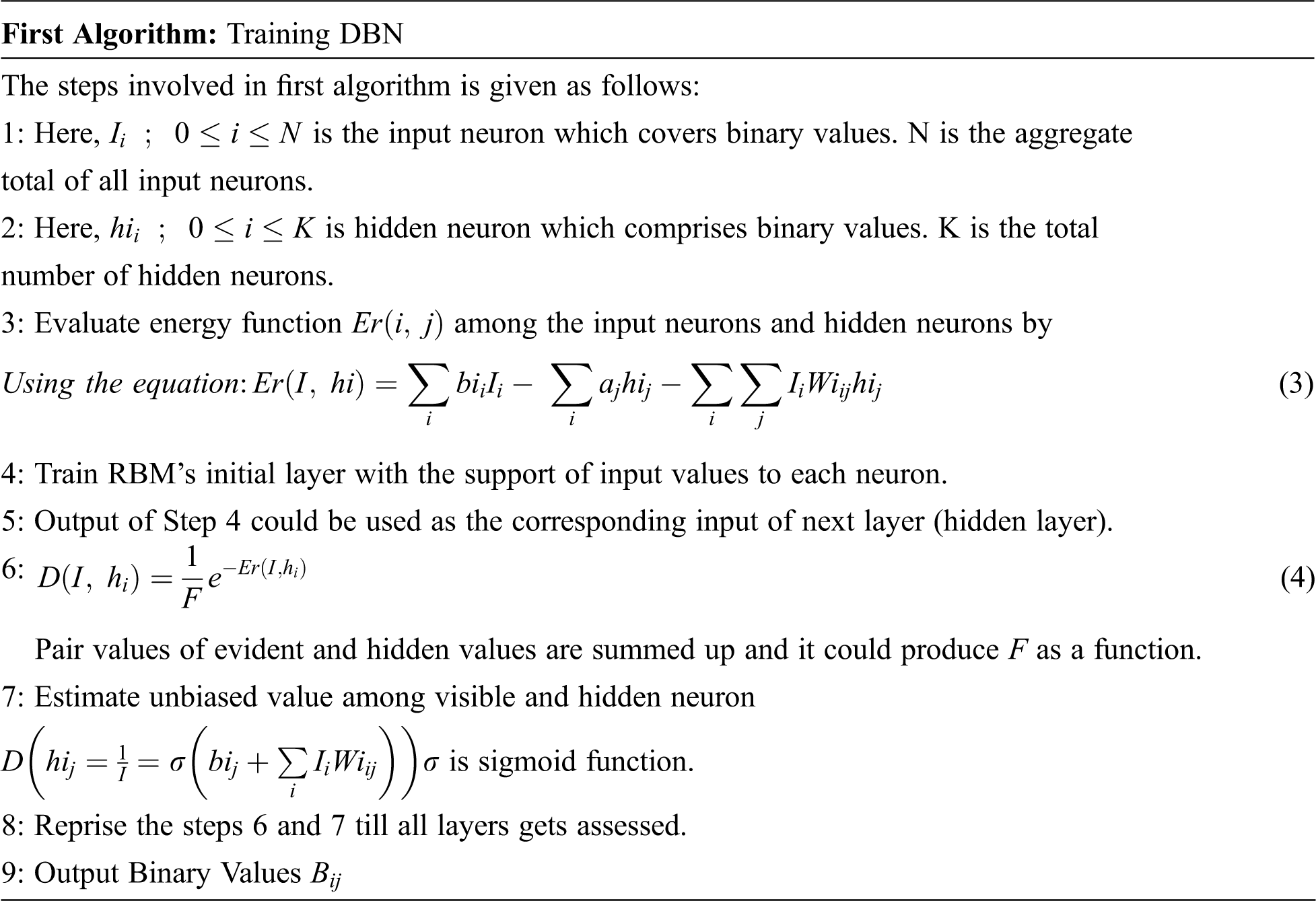
The first algorithm trained the image to perceive the disorder of the image. Lastly, it could be stowed in a secured manner by using DNA sequence-based JPEG Zig Zag Encryption method.
DNA sequence-based JPEG Zig Zag Encryption algorithm:
DNA (Deoxyribonucleic acid) is made up of monomers in a polymer configuration and is known as Deoxyribonucleotides. The elementary constituents of nucleotide are phosphate, deoxyribosesugar, and nitrogenous base. Afterward execution of first system, output values were in the matrix arrangement and 4 base variable of DNA sequences are A, C, T, Gnucleotides. The encoded value of A is [1, 0, 0, 0], C encoded value is [0, 1, 0, 0], T encoded value is [0, 0, 1, 0] and G’s encoded value is [0, 0, 0, 1] and hence, disorder image value could be signified as corresponding DNA sequence of code [38]. The encryption key DNA sequence-based JPEG Zig Zag is demarcated by the Fig. 6.
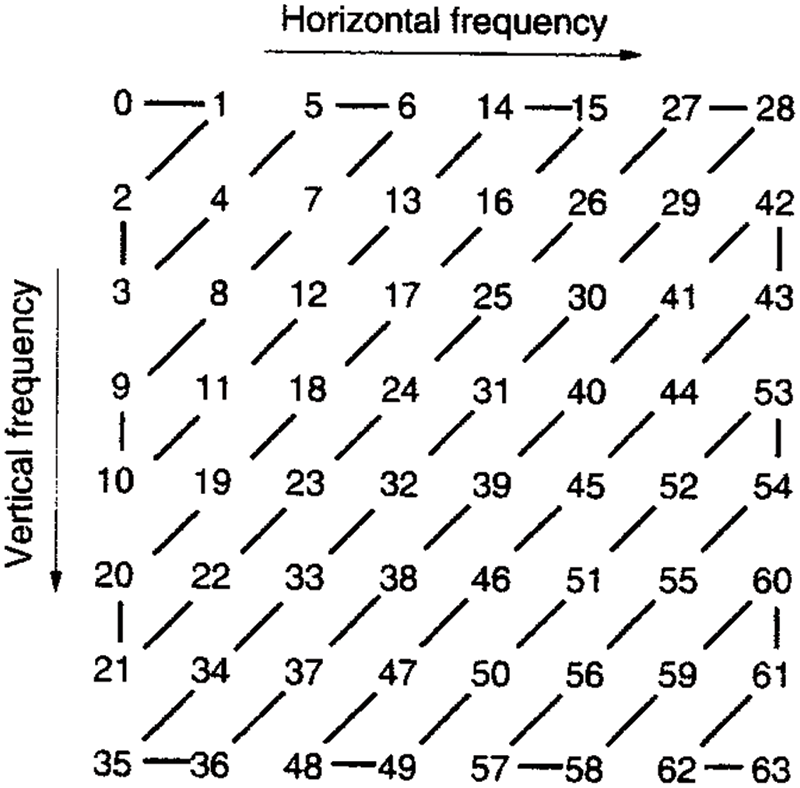
Figure 6: JPEG Zig Zag format
Currently, gene binary sequence value is measured as encrypted image and stowed in a secure manner. The encrypted DNA sequence JPEG Zig Zag process is mentioned below as follows.


Equally the sender and receiver are ought to have identical gene sequence value and stowed it as a binary format. For every DNA nucleotide sequence in B quadruple value, select it casually from the binary format file and substitute it by image. Fig. 7 displays consequence of storage of image by using JPEG Zig Zag pattern.
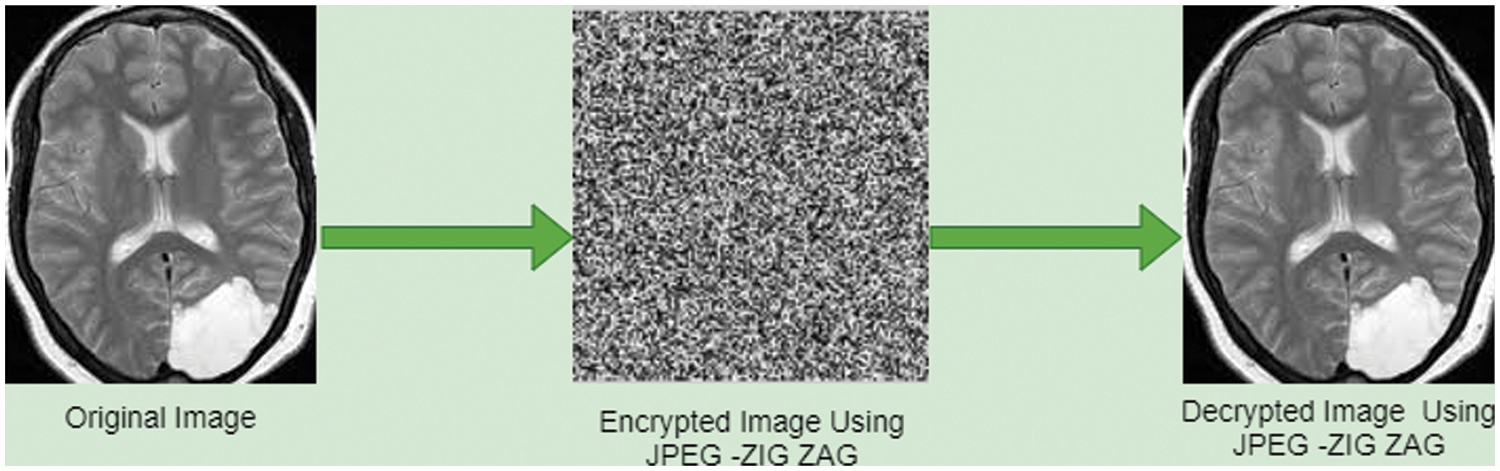
Figure 7: JPEG zig zag encryption algorithm
To estimate exploration of DBNJZZ in perceiving disorder image, four performance metric measure is used such as root mean square error, Mean absolute error, Accuracy and mean absolute percentage error.
where,
Tab. 2 displays diverse disorder disease and its dataset.
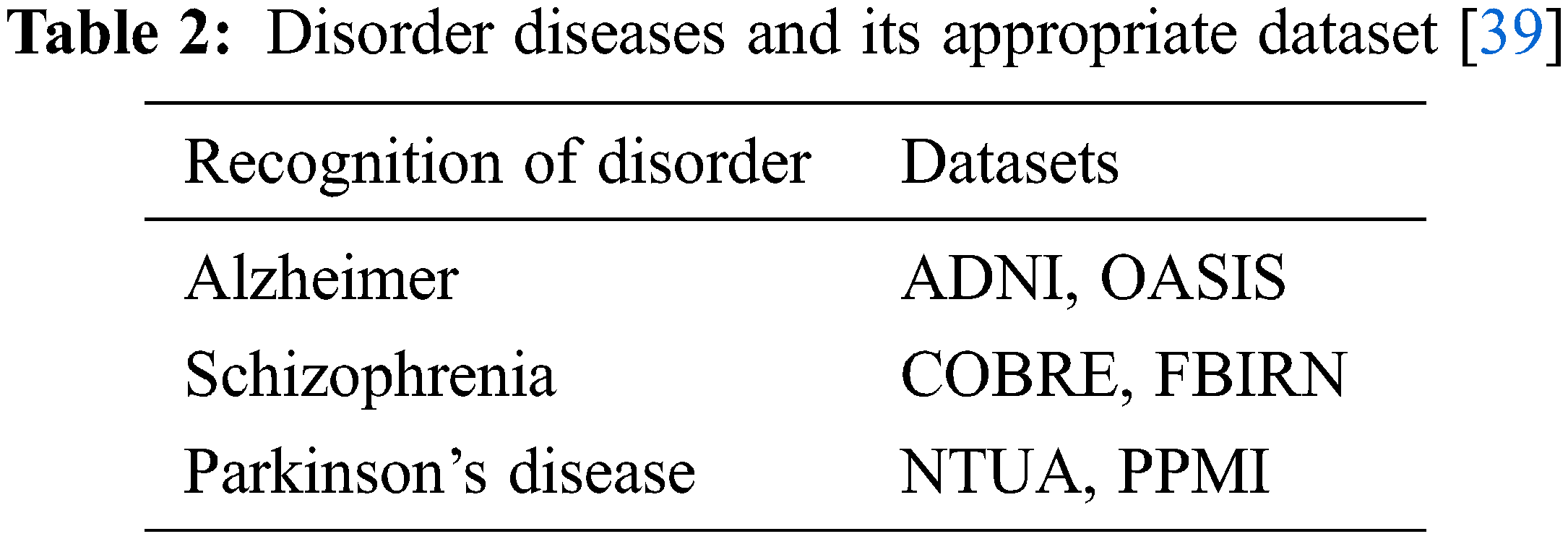
In neurological disorder of Alzheimer, it is exaggerated to older people. It will degrade them emotionally and makes disorder function of brain in specific area. Using Eq. (8) accuracy actions for pre-processing actions of Alzheimer are mentioned in the Tab. 3 by using Tab. 2 datasets.
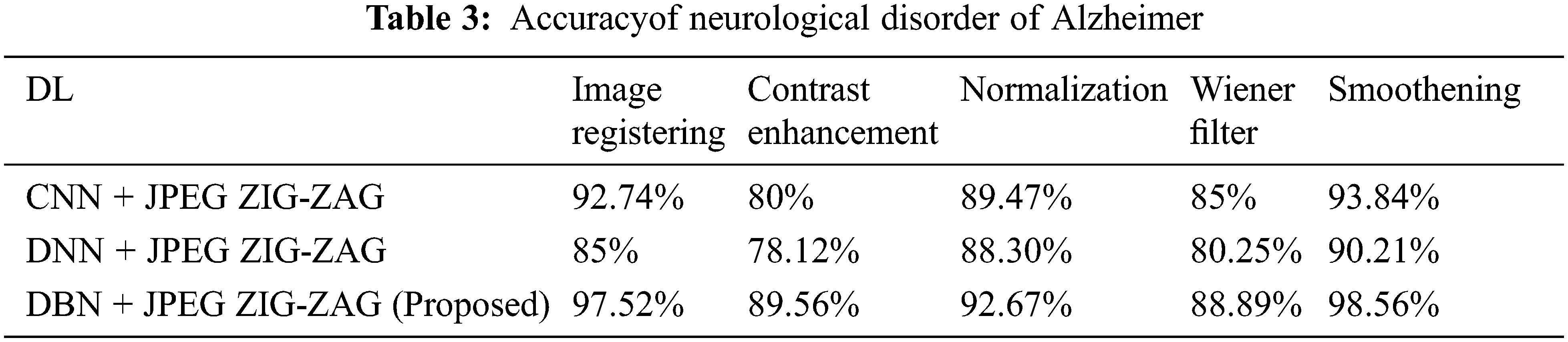
In the above Tab. 3, displays that proposed work has created enhanced performance. Using Eq. (8) Tab. 4 depicts that accuracy for neurological disorder of Schizophrenia disease. It is a psychiatric ailment and it deviates patient’s behaviour corresponding to emotion and cognition.
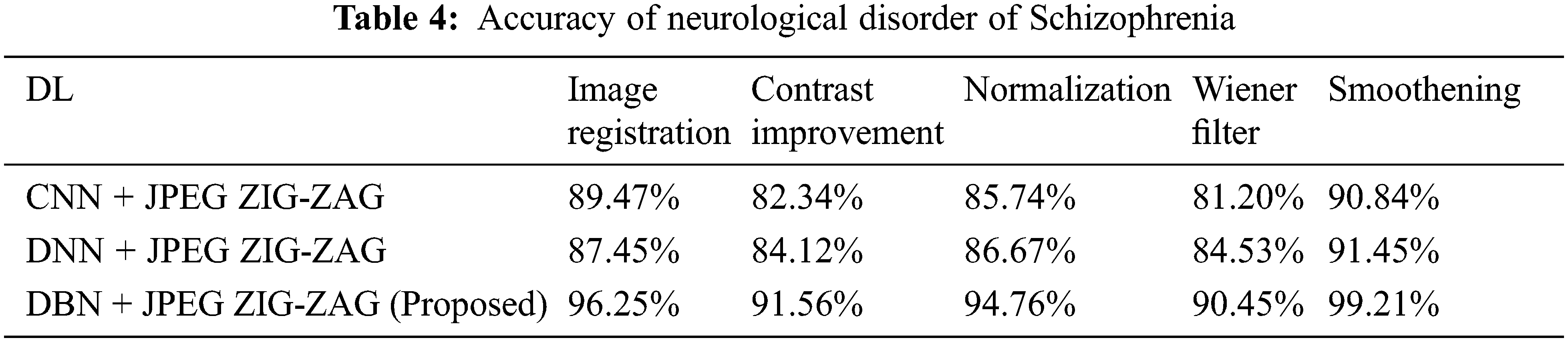
In the above Tab. 4, shows that proposed research has fashioned a superior performance. Using Eq. (8) Tab. 5 displays the accuracy for neurological disorder of Parkinson’s disease. It is a disorder in neurodegenerative and affects voluntary movements.
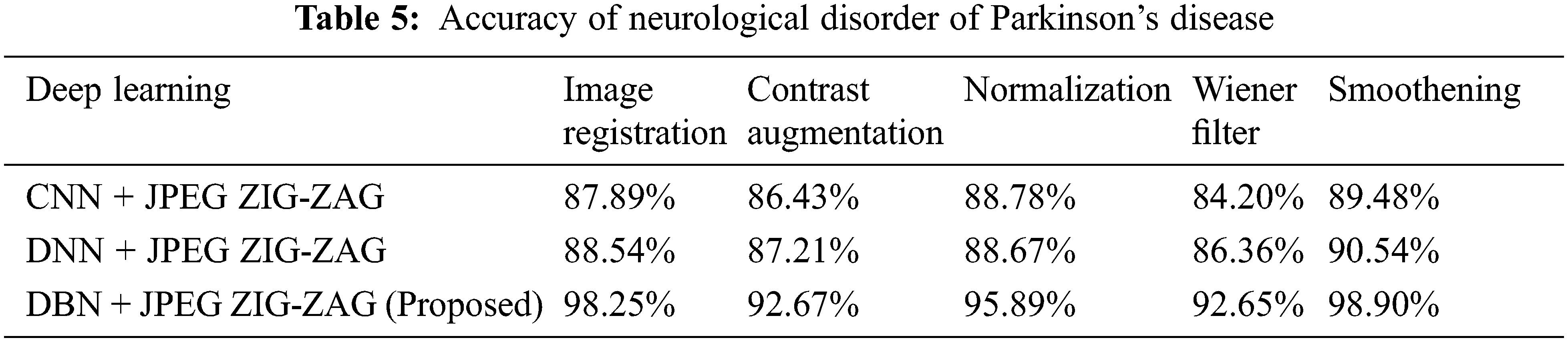
The experimental outcome of Tab. 5 displays that in accuracy metric measures for Parkinson’s disease, suggested research by incorporating DBN gives better performance. By using Eqs. (5) & (6) root mean square error, Mean absolute error is given in the Figs. 8, 9 and 10. Eq. (5) is intended as square root of mean of the squared variances among actual results and expectations. Eq. (6) is total difference between the actual or true values and the values that are anticipated. In the total difference, if outcome has an adverse sign, it is unnoticed.
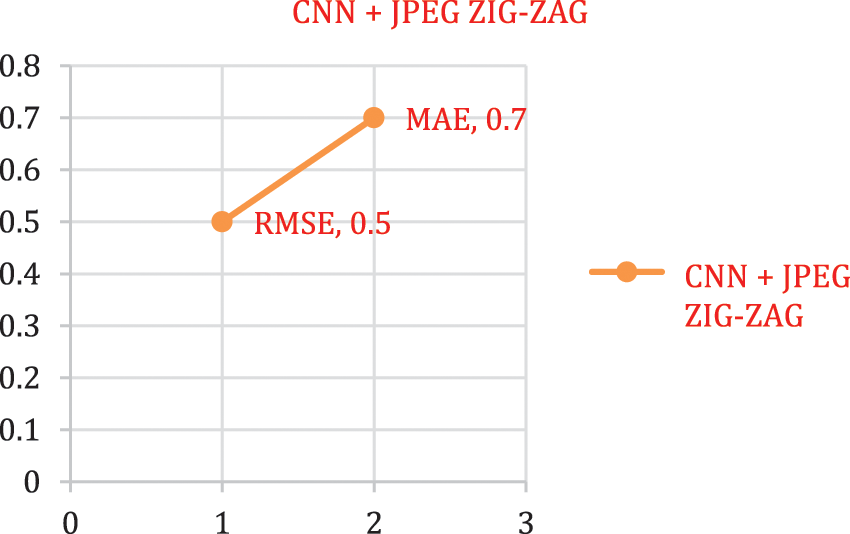
Figure 8: Error rate-CNN + JPEG ZIG-ZAG
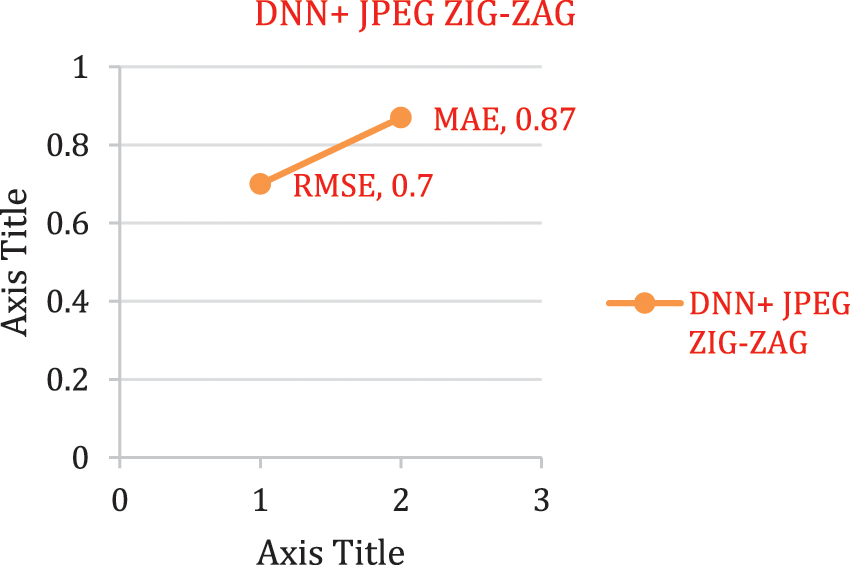
Figure 9: Error rate using DNN + JPEG ZIG-ZAG
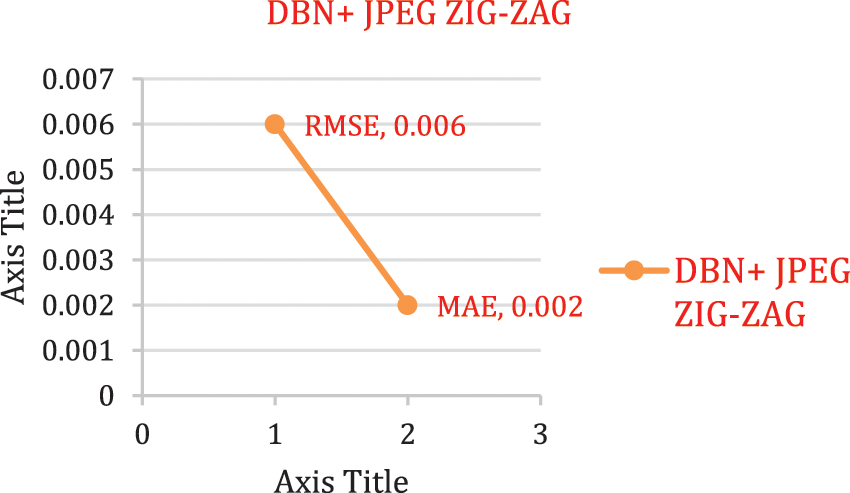
Figure 10: Error rate using DBN + JPEG ZIG-ZAG
The experimental results shown in the Figs. 8–11 implies that the suggested work DBN + JPEG ZIG-ZAG achieves enhanced prediction performance than associated with other prevailing algorithm in deep learning criteria. In the current research analysis, the accuracy metric is deployed for estimating brain disorder diseases of Alzheimer, Schizophrenia, Parkinson’s disease with deep learning classification algorithms. Recommended research work exposes improved performance in the accuracy metric.
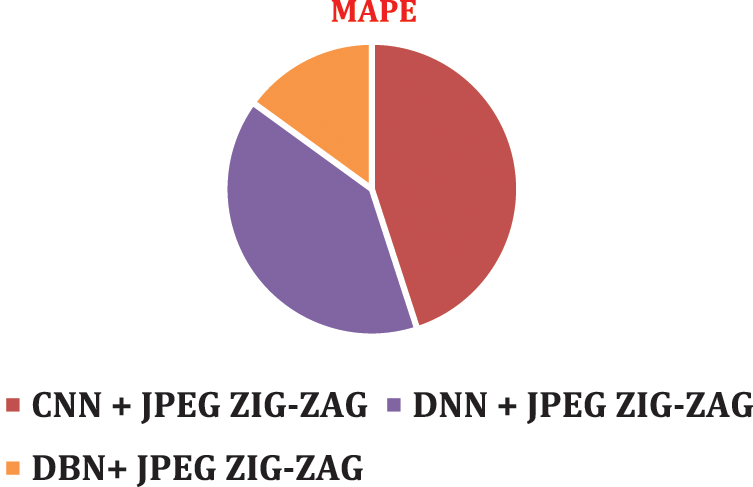
Figure 11: MAPE using DBN + JPEG ZIG-ZAG
To visualize performance impact of systems with respect to error rate, lower error rate of DBN system and results were predicted correctly.
The proposed research incorporated deep learning technique along with the CNN, DNN. DBN is estimated by using secure means of stowage of image in JPEG Zig Zag encryption system. When coming to unsupervised form of feature extractor for extracting biomarker of disorder image and tested on brain disorder of Alzheimer’s disease, Schizophrenia and Parkinson’s disease, DBN is predominantly suggested.
This DBNJZZ system could fulfil the features of image and shared this image in a safe manner. This could also be capable of providing prompt prediction of disorder of images. This current research focused on 3 diseases and the future work could examines more case studies with diverse DNA sequence.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. E. Tolosa, G. Wenning and W. Poewe, “The diagnosis of Parkinson’sdisease,” The Lancet Neurol, vol. 5, no. 1, pp. 75–86, 2006. [Google Scholar]
2. A. Danielyan and H. A. Nasrallah, “Neurological disorders in schizophrenia,” Psychiatric Clinics, vol. 32, no. 4, pp. 719–757, 2009. [Google Scholar]
3. J. Islam and Y. Zhang, “Brain MRI analysis for Alzheimer’s disease diagnosisusing an ensemble system of deep convolutional neural networks,” Brain Informatics, vol. 5, pp. 1–14, 2018. [Google Scholar]
4. A. Heidenreich, F. Desgrandschamps and F. Terrier, “Modern approach of diagnosis and management of acute flank pain: Review of all imaging modalities,” European Urology, vol. 41, pp. 351–362, 2002. [Google Scholar]
5. M. W. Weiner, “Alzheimer’s disease neuroimaging initiative: A decade of progress in Alzheimer’s disease,” Alzheimer’s & Dementia, vol. 11, no. 7, pp. 727–729, 2015. [Google Scholar]
6. Network TMR COBRE MR data. https://bit.ly/2Qdrjsd Accessed 2019–12–26, 2012. [Google Scholar]
7. OASIS Brains Dataset. https://www.oasis-brains.org/ Accessed 2019–12–26, 2007. [Google Scholar]
8. D. B. Keator, “The function biomedical informatics researchnetwork data repository,” Neuro Image, vol. 1, pp. 1–7, 2015. [Google Scholar]
9. P. T. Katsiaris, P. K. Artemiadis and K. J. Kyriakopoulos, “Relating posturalsynergies to lowd muscular activations: Towards bio-inspired controlof robotic hands,” IEEE BIBE, vol. 12, pp. 245–250, 2012. [Google Scholar]
10. J. Michael, “Fox foundation for Parkinson’s research (MJFF) : PPMIdataset,” https://www.ppmi-info.org/access-data-specimens/download-data/ Accessed, 2019–12–26, 2002. [Google Scholar]
11. S. G. Mueller, M. W. Weiner, L. J. Thal, R. C. Petersen, C. Jack et al., “The Alzheimer’s disease neuroimaging initiative,” Neuroimage Clinics, vol. 15, no. 4, pp. 869–877, 2005. [Google Scholar]
12. A. Ortiz, F. J. Martínez-Murcia, M. J. García Tarifa, F. Lozano, J. M. Górriz et al., “Automated diagnosis of parkinsonian syndromesby deep sparse filtering-based features,” in Int. Conf. on Innovation in Medicine and Healthcare (Puerto de la Cruz: Springer), Puerto de la Cruz, Spain, vol. 24, pp. 249–258, 2016. [Google Scholar]
13. W. H. Pinaya, A. Mechelli and J. R. Sato, “Using deep autoencoders toidentify abnormal brain structural patterns in neuropsychiatric disorders:A large-scale multi-sample study,” Human Brain Mapping, vol. 40, pp. 944–954, 2019. [Google Scholar]
14. M. Watson, “Illuminating the future of DNA sequencing,” Genome Biology, vol. 15, no. 2, pp. 108–115, 2014. [Google Scholar]
15. S. Saptarshi, S. SanchitaBasak, S. PallabiSaikia, P. Sayak Paul, T. Vasilios et al., “A review of deeplearning with special emphasison architectures, applications and recent trends,” IEEE Transaction Neural Networks and Learning Systems, vol. 29, no. 6, pp. 2063–2079, 2019. [Google Scholar]
16. M. Mahmud, M. S. Kaiser and A. Hussain, “A deep learning in miningbiological data,” IEEE Transaction Neural Networks and Learning Systems, vol. 29, no. 6, pp. 1089–1098, 2020. [Google Scholar]
17. M. Fabietti, M. Mahmud, A. Lotfi, A. Averna, D. Guggenmo et al., “Neural network-based artifact detection in localfield potentials recorded from chronically implanted neural probes,” Proc. Advances in Computational Intelligence Systems, vol. 13, pp. 288–299, 2020. [Google Scholar]
18. J. Islam and Y. Zhang, “GAN-Based synthetic brain PET image generation,” Brain Informatics, vol. 7, no. 1, pp. 34–49, 2020. [Google Scholar]
19. G. Rabby, S. Azad, M. Mahmud, K. Z. Zamli and M. M. Rahman, “Teket: Atree-based unsupervised keyphrase extraction technique,” Cognitive Computation, vol. 12, no. 4, pp. 811–833, 2020. [Google Scholar]
20. B. Lei, “Adaptive sparse learning using multi-template forneurodegenerative disease diagnosis,” Medical Image Analysis, vol. 5, pp. 352–368, 2020. [Google Scholar]
21. G. B. Chand, D. B. Dwyer, G. Erus, A. Sotiras and E. Varol, “Two distinct neuroanatomicalsubtypes of schizophrenia revealed using machine learning,” Medical Image Analysis, vol. 143, no. 3, pp. 1027–1038, 2020. [Google Scholar]
22. K. M. Ali, M. S. Kaiser and M. Mahmud, “Application of convolutionalneural network in segmenting brain regions from mri data,” in Int. Conf. on Brain Informatics, Nottingham Trent University, UK, pp. 136–146, 2019. [Google Scholar]
23. M. B. T. Noor, N. Z. Zenia, M. S. Kaiser, M. Mahmud and S. Mamun, “Detecting neurode generative disease from mri: A brief review on a deeplearning perspective,” in Int. Conf. on Brain Informatics, Nottingham Trent University, UK, pp. 115–125, 2019. [Google Scholar]
24. S. Basaia, “Automated classification of Alzheimer’s disease andmild cognitive impairment using a single mri and dnn, Neuro Image Clinical, vol. 22, pp. 101448–101457, 2019. [Google Scholar]
25. H. Li, M. Habes, D. A. Wolk and Y. Fan, “A deep learning model forearly prediction of Alzheimer’s disease dementia based on hippocampalmagnetic resonance imaging data,” Alzheimer’s Dementia, vol. 15, no. 8, pp. 1059–1070, 2019. [Google Scholar]
26. S. Spasov, “A Parameter-efficient dl approach to predictconversion from mild cognitive impairment to Alzheimer’s disease,” Neuro Image Clinical, vol. 189, pp. 276–287, 2019. [Google Scholar]
27. M. A. Bohle, “Layer-wise relevance propagation for explainingdnn decisions in mri-based Alzheimer’s disease classification,” Front Aging Neuroscience, vol. 11, pp. 194, 2019. [Google Scholar]
28. Y. Qiu, “Classification of schizophrenia patients and healthycontrols using ica of complex-valued fmri data and convolutionalneural networks,” in Proc. ISNN, Springer, Russian Federation, Moscow, pp. 540–547, 2019. [Google Scholar]
29. S. Sivaranjini, C. M. Sujatha, “Deep learning-based diagnosis ofparkinson’s disease using convolutional neural network,” Multimedia Tools and Applications, vol. 79, pp. 15467–15479, 2020. [Google Scholar]
30. S. Shinde, “Predictive markers for Parkinson’s disease usingdeep neural nets on neuromelanin sensitive MRI,” Neuro Image Clinical, vol. 22, pp. 101748–101758, 2019. [Google Scholar]
31. D. Kollias, “Deep neural architectures for prediction in healthcare,” Complex Intellect System, vol. 4, no. 2, pp. 119–131, 2018. [Google Scholar]
32. L. L. Zeng, “Multi-site diagnostic classification of schizophreniausing discriminant deep learning with functional connectivity mri,” E-Bio Medicine, vol. 30, pp. 74–85, 2018. [Google Scholar]
33. M. Mahmud, M. S. Kaiser, A. Hussain and S. Vassanelli, “Applications ofdeep learning and reinforcement learning to biological data,” IEEE Transaction Neural Network Learn System, vol. 29, no. 6, pp. 2063–2079, 2018. [Google Scholar]
34. G. Litjens, “A survey on deep learning in medical imageanalysis,” Medical Image Analong, vol. 42, pp. 60–88, 2017. [Google Scholar]
35. J. Dakka, “Learning neural markers of schizophrenia disorderusing recurrent neural networks,” Computer Vision and Pattern Recognition, vol. 23, pp. 345–356, 2017. [Google Scholar]
36. M. Shakeri, “Deep spectral-based shape features for Alzheimer’sdisease classification,” Proceeding Spectral and Shape Analysis in Medical Imaging, vol. 19, pp. 15–24, 2016. [Google Scholar]
37. G. E. Hinton, S. Osindero and Y. W. Teh, “A Fast-learningalgorithm for deep belief nets,” Neural Computation, vol. 18, pp. 1527–1554, 2006. [Google Scholar]
38. G. Cui and L. Qin, “Information security technology based on DNA computing,” in IEEE Int. Workshop on Anti-Counterfeiting Security, Xiamen, China, pp. 288–291, 2007. [Google Scholar]
39. N. Manan Binth Taj, Z. Nusrat Zerin, M. Shamim Kaiser, A. I. Shamim and M. Mamunand Mufti, “Application of deep learning in detectingneurological disorders from magneticresonance images: A survey on the detectionAlzheimer’s disease, Parkinson’s diseaseand schizophrenia,” Brain Information, springer, vol. 23, pp. 341–359, 2020. [Google Scholar]
40. M. Prince, A. Wimo, M. Guerchet, G. -C. Ali, Y. -T. Wu et al., “World Alzheimer report 2015, the global impact of dementia: An analysis of prevalence, incidence, cost and trends,” Alzheimer’s Disease Int, pp. 87, 2015. [Google Scholar]
41. J. Cummings and N. Fox, “Defining disease modifying therapy for Alzheimer’s disease,” J. Prevention Alzheimers Disease, vol. 4, pp. 109–115, 2017. [Google Scholar]
42. S. Al-Shoukry, T. H. Rassem and N. M. Makbol, “Alzheimer’s diseases detection by using deep learning algorithms: A mini-review,” IEEE Access, vol. 8, pp. 77131–77141, 2020. [Google Scholar]
43. N. Bhagwat, ‘‘Prognostic applications for Alzheimer’s disease using magnetic resonance imaging and machine-learning,” Ph.D. dissertation, Graduate Dept. Inst. Biomater. Biomed. Eng., Univ. Toronto, Toronto, ON, Canada, 2018. [Google Scholar]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools