DOI:10.32604/csse.2022.018534

| Computer Systems Science & Engineering DOI:10.32604/csse.2022.018534 |  |
| Article |
On Vertex-Edge-Degree Topological Descriptors for Certain Crystal Networks
1College of Computer Sciences and Information Technology, Jazan University, Saudi Arabia
2King Abdulaziz University, College of Engineering, Nuclear Engineering Department, Jeddah, 21589, Saudi Arabia
*Corresponding Author: Ali Ahmad. Email: ahmadsms@gmail.com
Received: 11 March 2021; Accepted: 30 April 2021
Abstract: Due to the combinatorial nature of graphs they are used easily in pure sciences and social sciences. The dynamical arrangement of vertices and their associated edges make them flexible (like liquid) to attain the shape of any physical structure or phenomenon easily. In the field of ICT they are used to reflect distributed component and communication among them. Mathematical chemistry is another interesting domain of applied mathematics that endeavors to display the structure of compounds that are formed in result of chemical reactions. This area attracts the researchers due to its applications in theoretical and organic chemistry. It also inspires the mathematicians due to involvement of mathematical structures. Regular or irregular bonding ability of molecules and their formation of chemical compounds can be analyzed using atomic valences (vertex degrees). Pictorial representation of these compounds helps in identifying their properties by computing different graph invariants that is really considered as an application of graph theory. This paper reflects the work on topological indices such as
Keywords: ev-degree; ve-degree; topological indices; crystal networks
Computation of topological indices for large chemical structures becomes very challenging but still useful in depicting the structure and physico-chemical properties that are extremely important in reticular chemistry. Recently reticular Metal-organic frameworks MOFs are evolved as porous conductive solids with great applicability in fuel cells, batteries, capacitors, sensors and electronics. In MOFs covalent fibers of carbon atoms form mesh like crystals [1,2]. In reticular chemistry, the numerically representation of structural characteristics of molecules, are the topological indices, which are obtained by using the graphical methods. These indices play an important role in the area of mathematical chemistry and control theory, mainly in QSAR/QSPR investigations [3,4].
The networks that are topologically equivalent, although they exibit different labelings of distinct atoms but due to topological indices they are invariant. These indices describe the connections among the atoms and in this way they are basic invariants that show a relationship with biological activity and chemical reactivity. Topological study of a MOF means transforming the connectivity of any structure into a unique number representing an index of the metal-organic framework under consideration.
Wiener in his article [5], introduced the concept of topological index while he was studying the structural relationships to identify boiling points of paraffins. Many topological indices are used to reflect the structural arrangement of graphs. In general, they are classified into distance or degree-based topological indices along with these counting related indices of graphs have play a vital role in chemical characterization. Article [6–22] can give more deep insight as literature survey.
Let
The
The physical structure of solid materials is significant for engineering applications. It depends on the arrangements of the atoms, ions, or molecules that becomes the reason for strength of solid materials. The connectivity pattern of ions or atoms in a solid and repetitive patterns in three dimensions is known as crystal structure and material is called crystalline solid or crystalline material. Due to different crystalline structure of a materials their performance and characteristics varies. The unit cell is the basic structure that explains the crystal structure and repetition of this unit cell forms the whole crystal. Some of the examples of crystalline materials are alloys, metals, and some ceramic materials. In this paper topological indices for the bismuth tri-iodide and lead chloride are determined by mapping their crystalline structures in the form of graphs.
Bismuth tri-iodide (
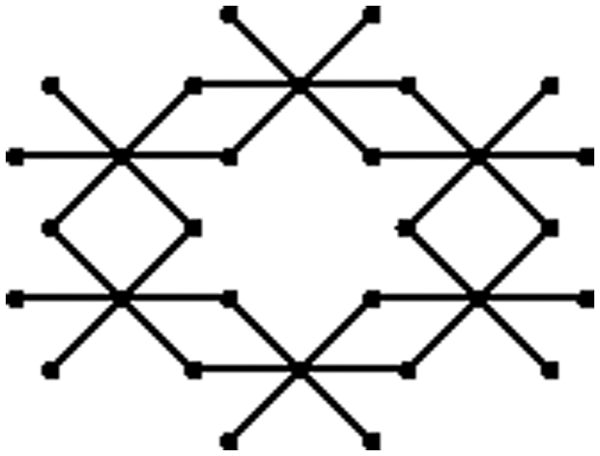
Figure 1: One unit of bismuth tri-iodide
The graph of a single unit of bismuth tri-iodide contains six 4-cycles of which two are at the bottom, two on the top and two in the middle. The unit cells of bismuth tri-iodide can be arranged either linearly or in a sheet form. A linear arrangement with

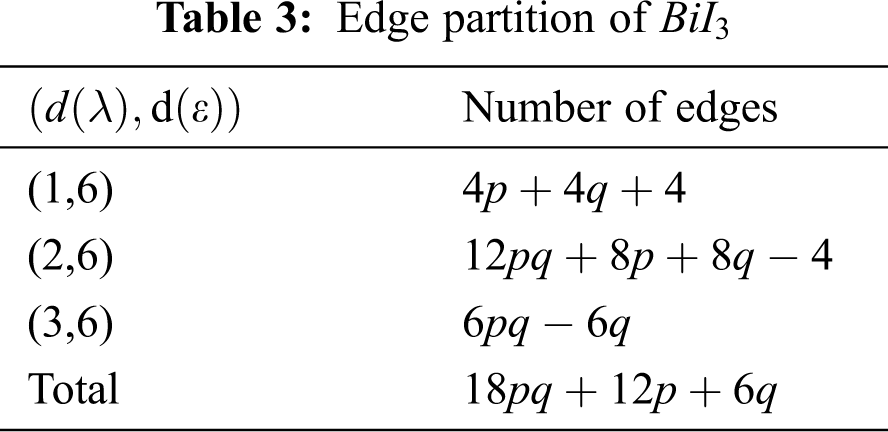
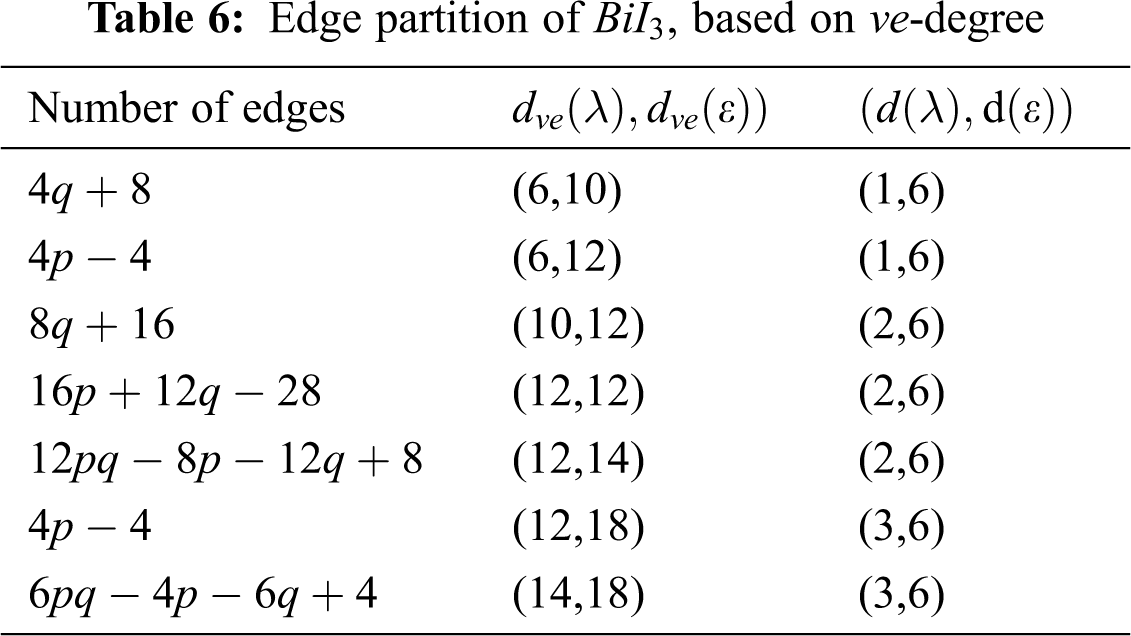
We partitioned the edges of
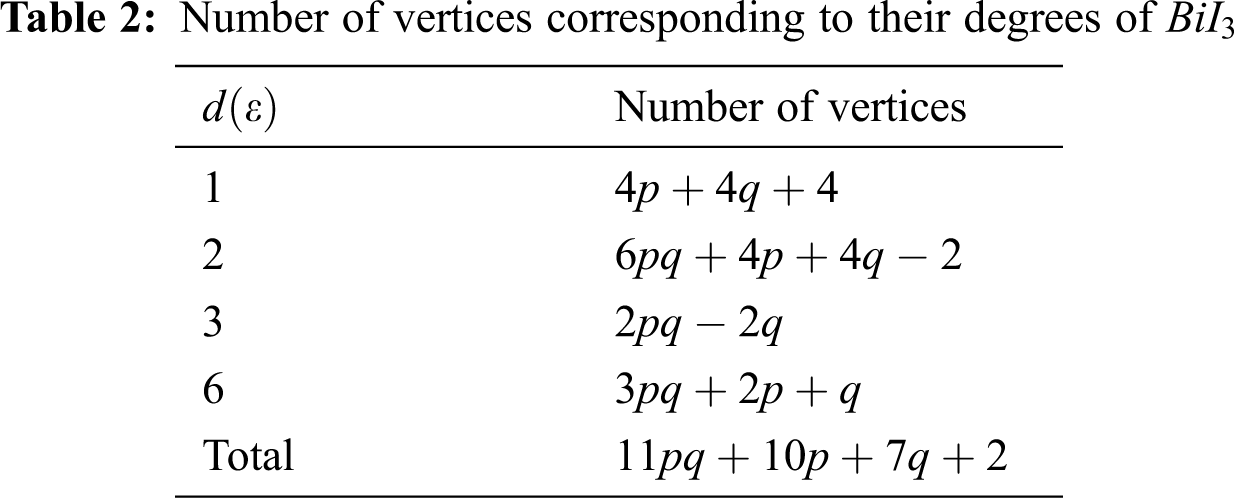
In Tab. 5, we partitioned the vertices of
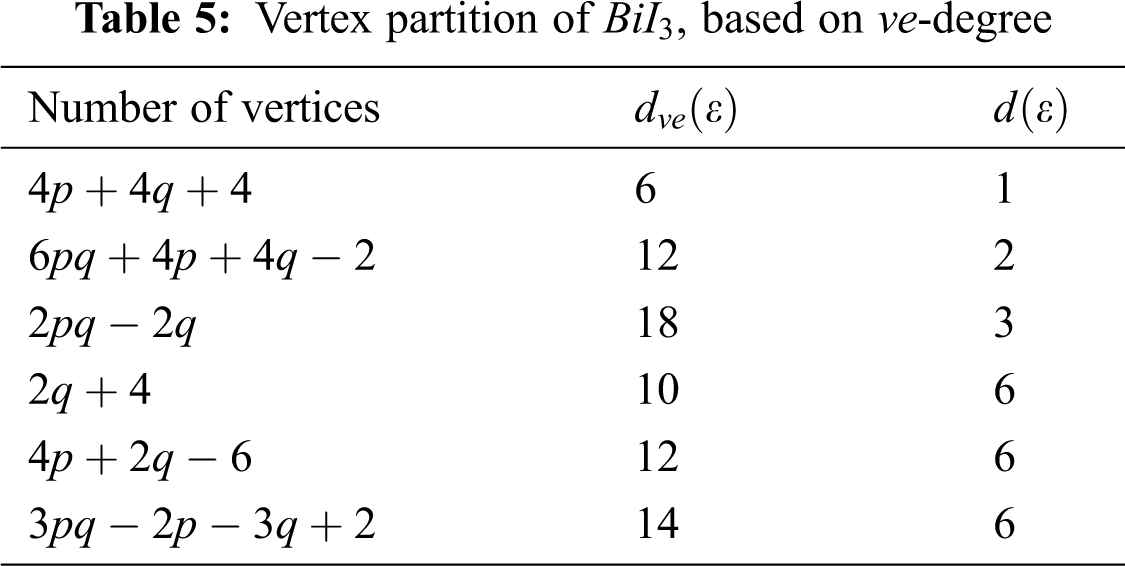
We partitioned the edge of
Now we calculated
By using
4.2 The First
Using Tab. 5 we compute the first
4.3 The First ve-degree Zagreb
Using Tab. 6 we compute the first
4.4 The second ve-degree Zagreb index
Using Tab. 6 we compute the second
Using Tab. 6 we compute the
4.6 The ev-degree Randic Index
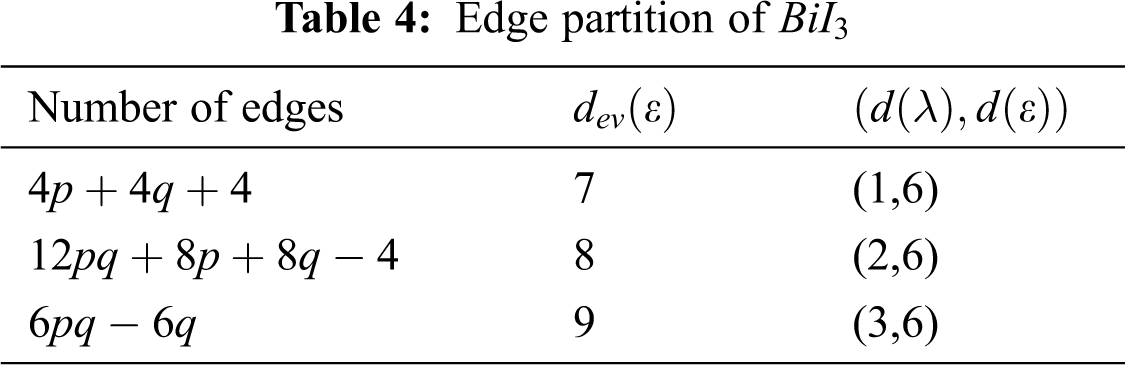
Using Tab. 4 we compute the
4.7 The ve-degree Atom-bond Connectivity Index
Using Tab. 6 we compute the
4.8 The ve-degree Geometric-arithmetic Index
Using Tab. 6 we compute the
4.9 The ve-degree Harmonic Index
Using Tab. 6 we compute the
4.10 The ve-degree Sum-connectivity Index
Using Tab. 6 we compute the
Lead chloride is a precious halide stone that usually occurs in mineral cotunnite. The structure of lead chloride is orthorhombic dipyramidal. The diagram of a solitary unit of lead chloride is obtained from bismuth tri-iodide by joining an extra vertex to only one 2-degree vertex of every one of the 4-cycles. Fig. 2 shows one unit of lead chloride.
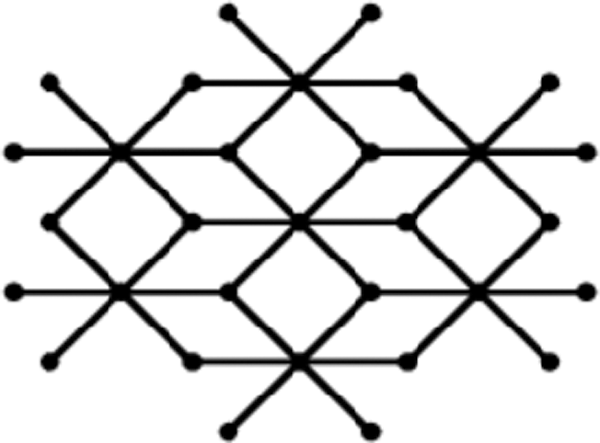
Figure 2: One unit of lead chloride
The unit cells of lead chloride can be arranged either linearly or in a sheet form. A linear arrangement with
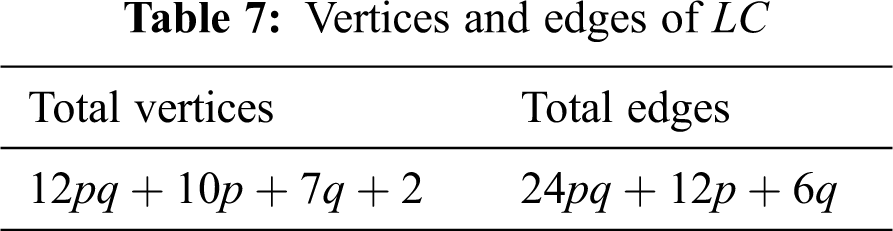
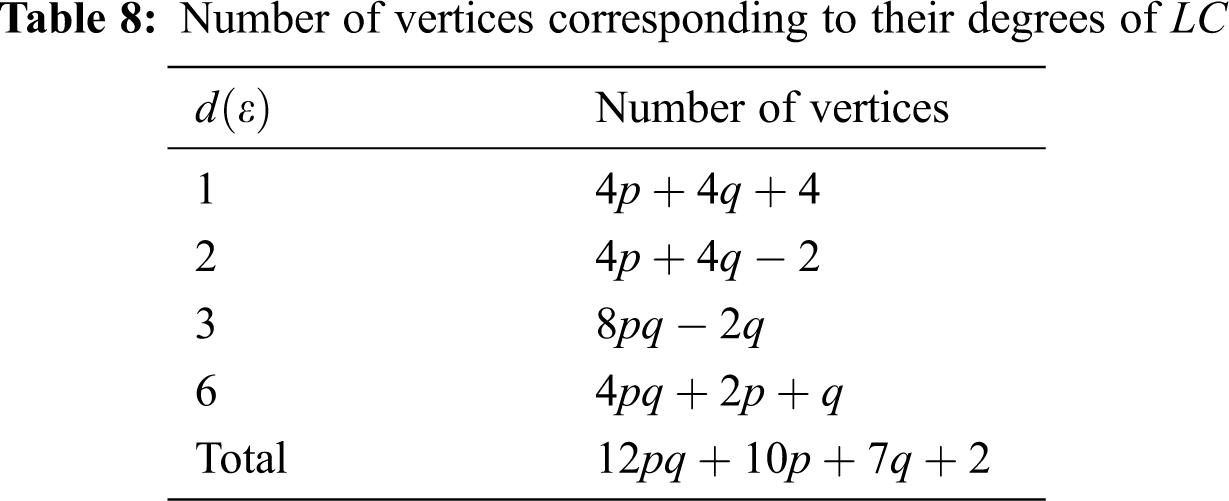
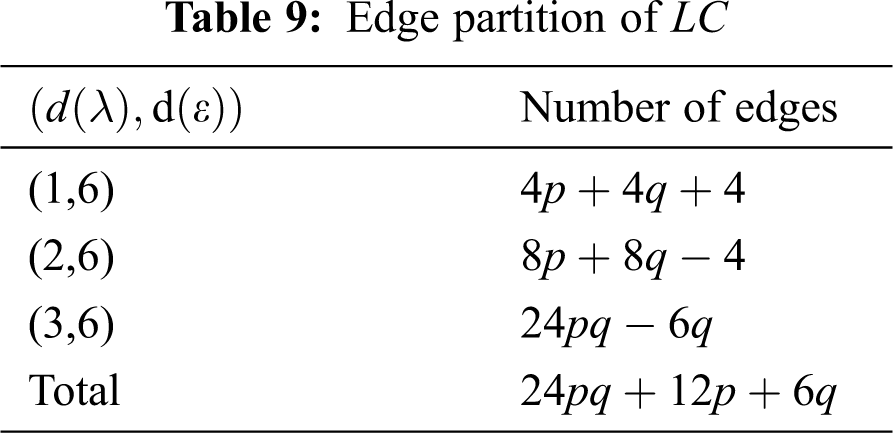
We partitioned the edges of
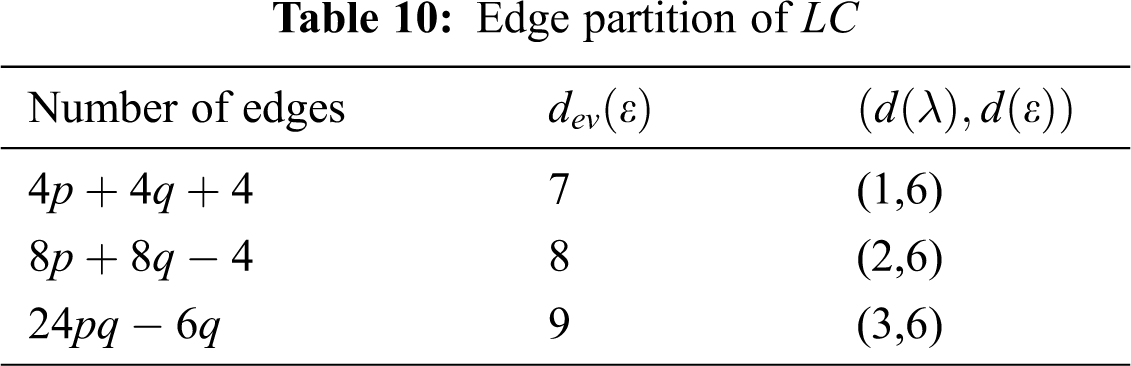
In Tab. 11, we partitioned the vertices of
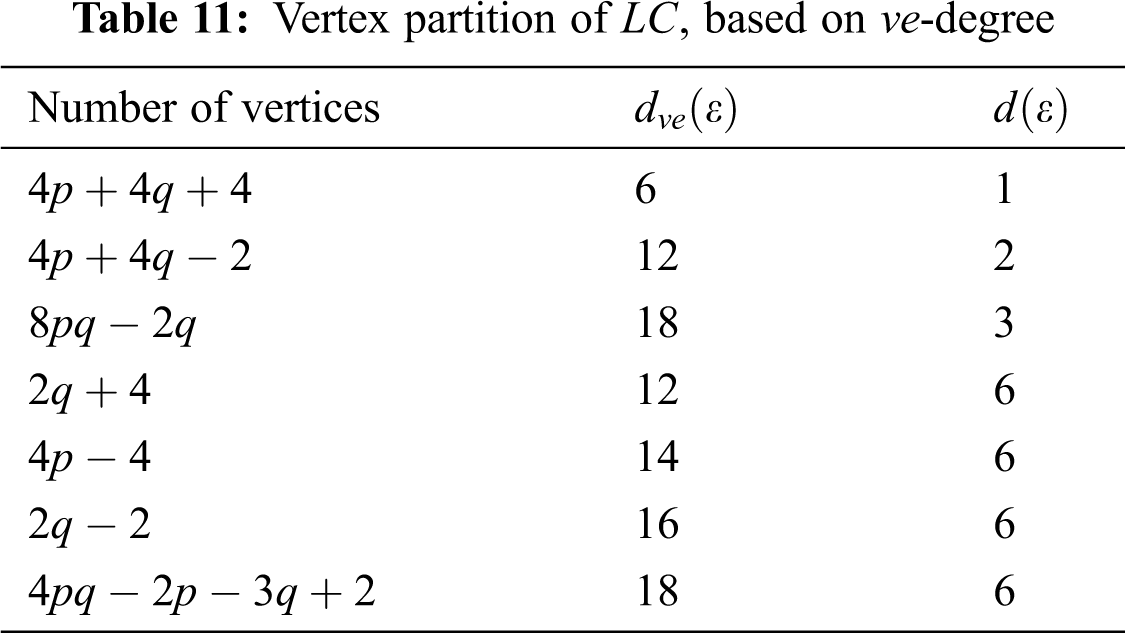
We partitioned the edge of
Now we calculated
By using
5.2 The First ve-degree Zagreb
Using Tab. 11 we compute the first
5.3 The First ve-degree Zagreb
Using Tab. 12 we compute the first
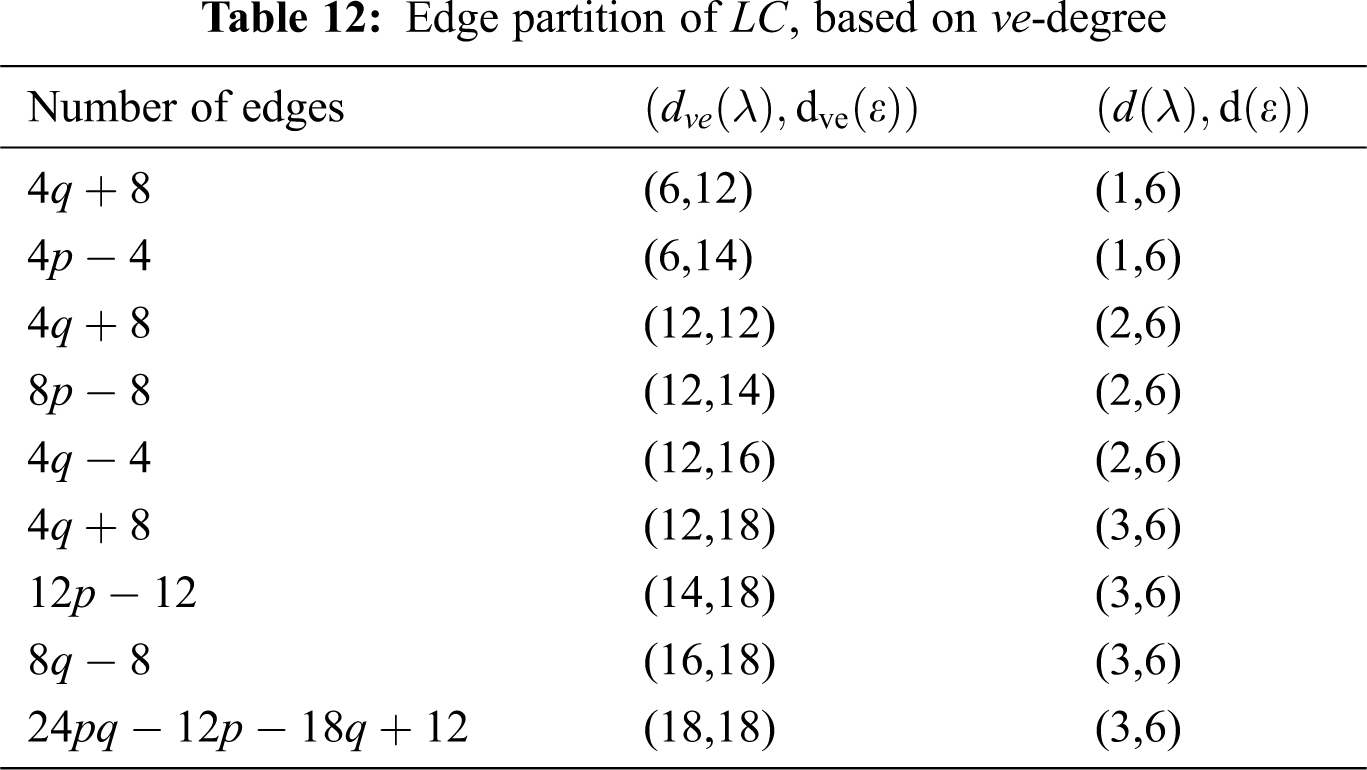
5.4 The second ve-degree Zagreb index
Using Tab. 12 we compute the second
Using Tab. 12 we compute the
5.6 The ev-degree Randic Index
Using Tab. 10 we compute the
5.7 The ve-degree Atom-bond Connectivity Index
Using Tab. 12 we compute the
5.8 The ve-degree Geometric-arithmetic Index
Using Tab. 12 we compute the
5.9 The ve-degree Harmonic Index
Using Tab. 6 we compute the
5.10 The ve-degree Sum-Connectivity Index
Using Tab. 12 we compute the
6 Graphical Representation and Discussion
Data or information visualization is becoming more popular due to ease of understanding and analysis in the modern scientific field. This section shows the graphical behavior of the above calculated topological descriptors for crystal networks (bismuth tri-iodide and lead chloride) is shown. It can be observed that by increasing the values of parameters values of topological descriptors are also increased. These graphical representations of
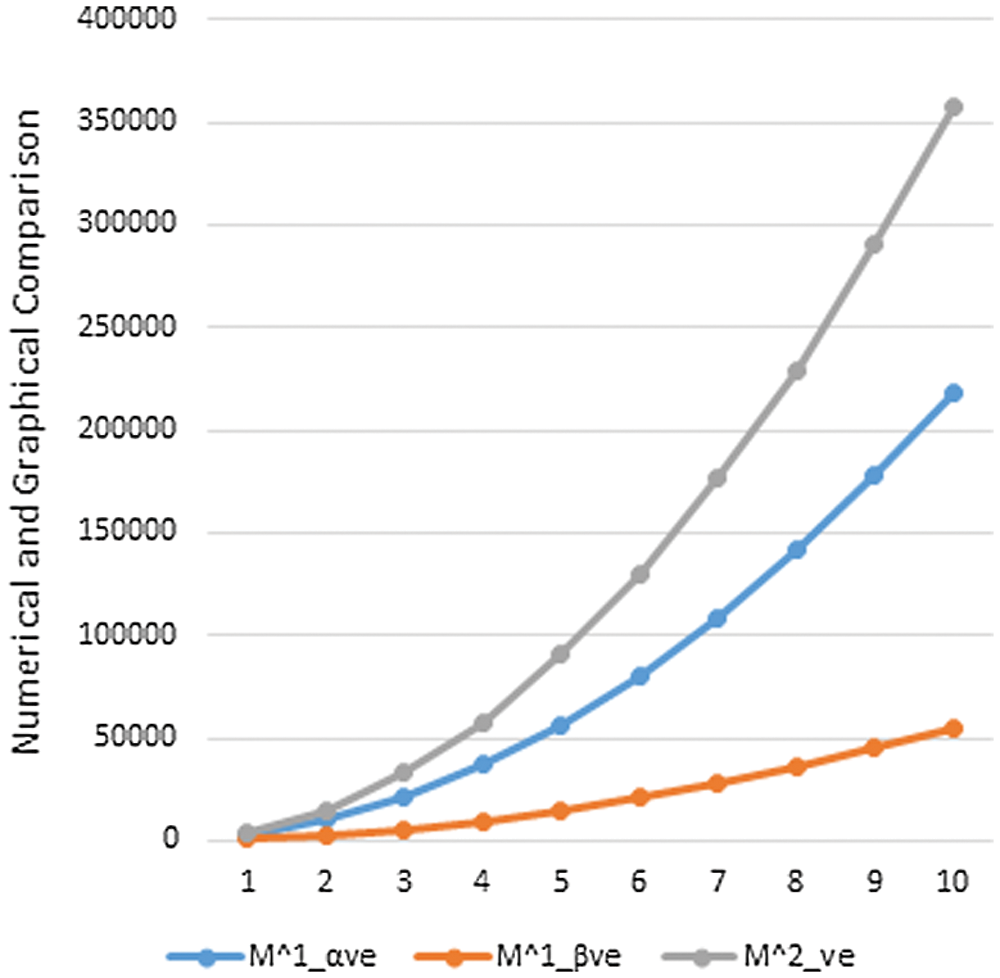
Figure 3: Graphical comparison of
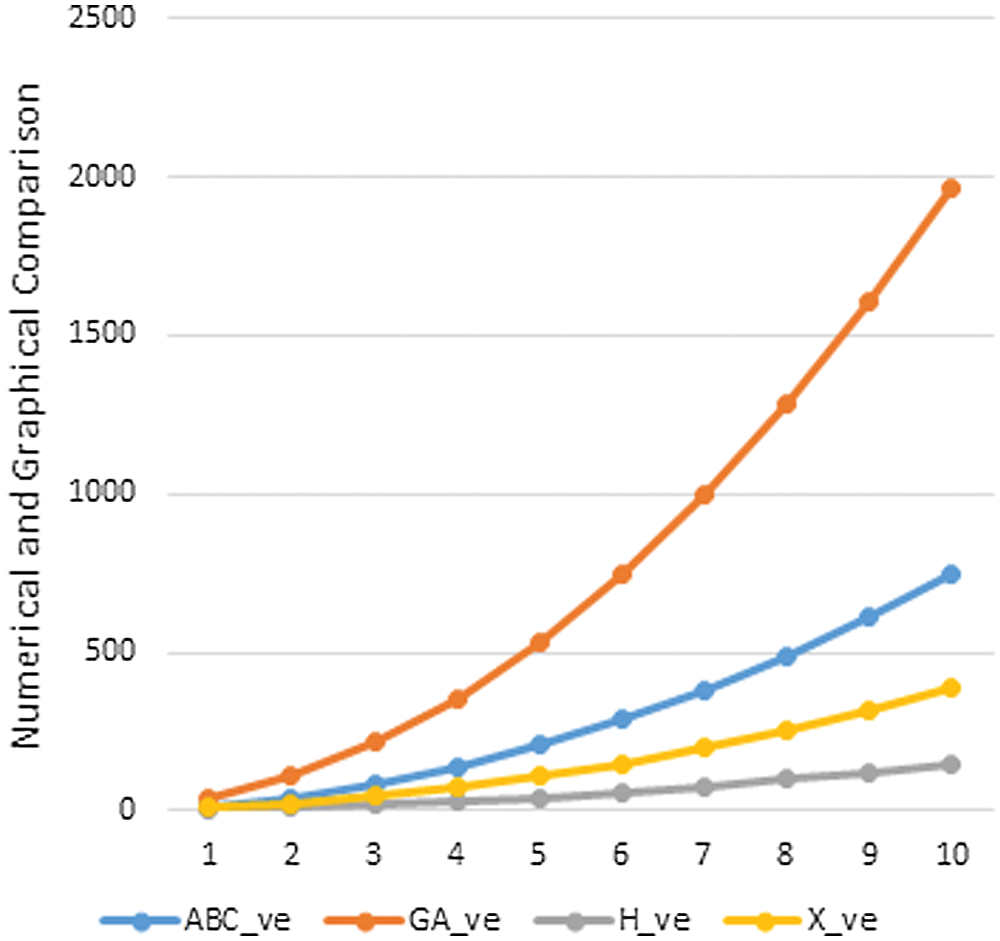
Figure 4: Graphical comparison of
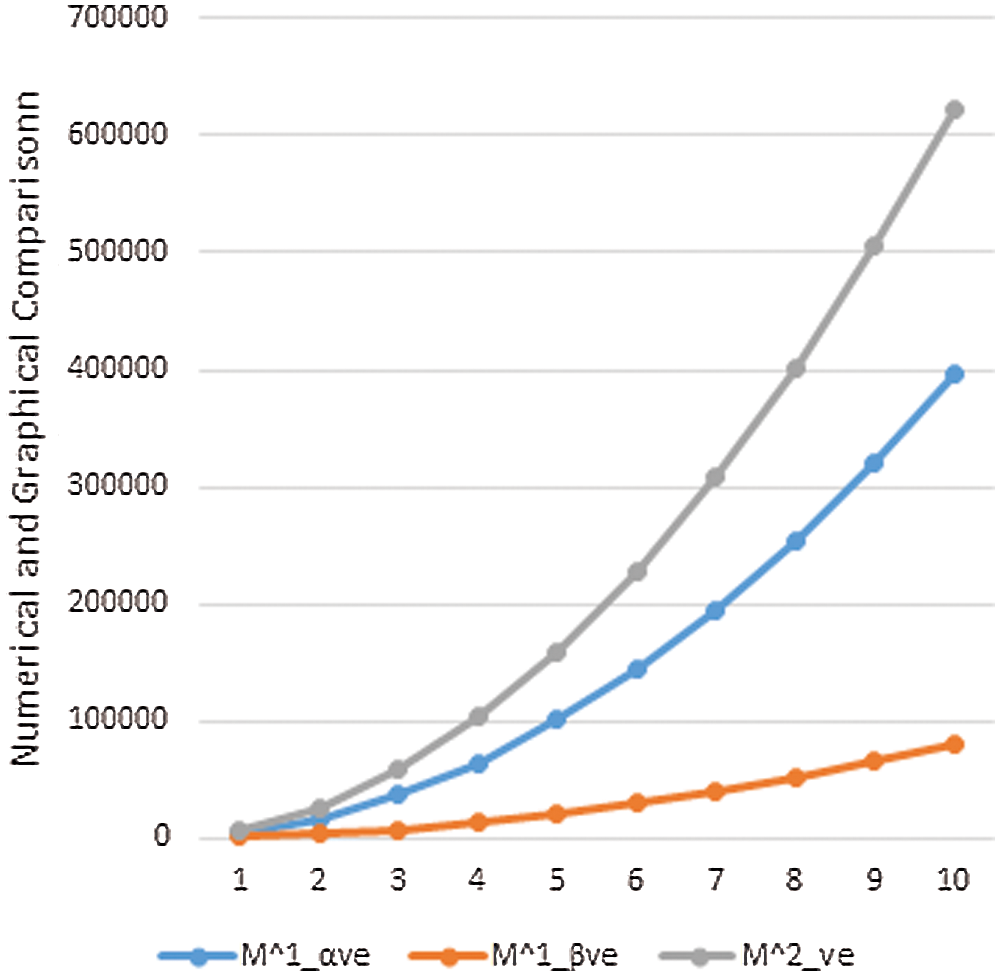
Figure 5: Graphical comparison of
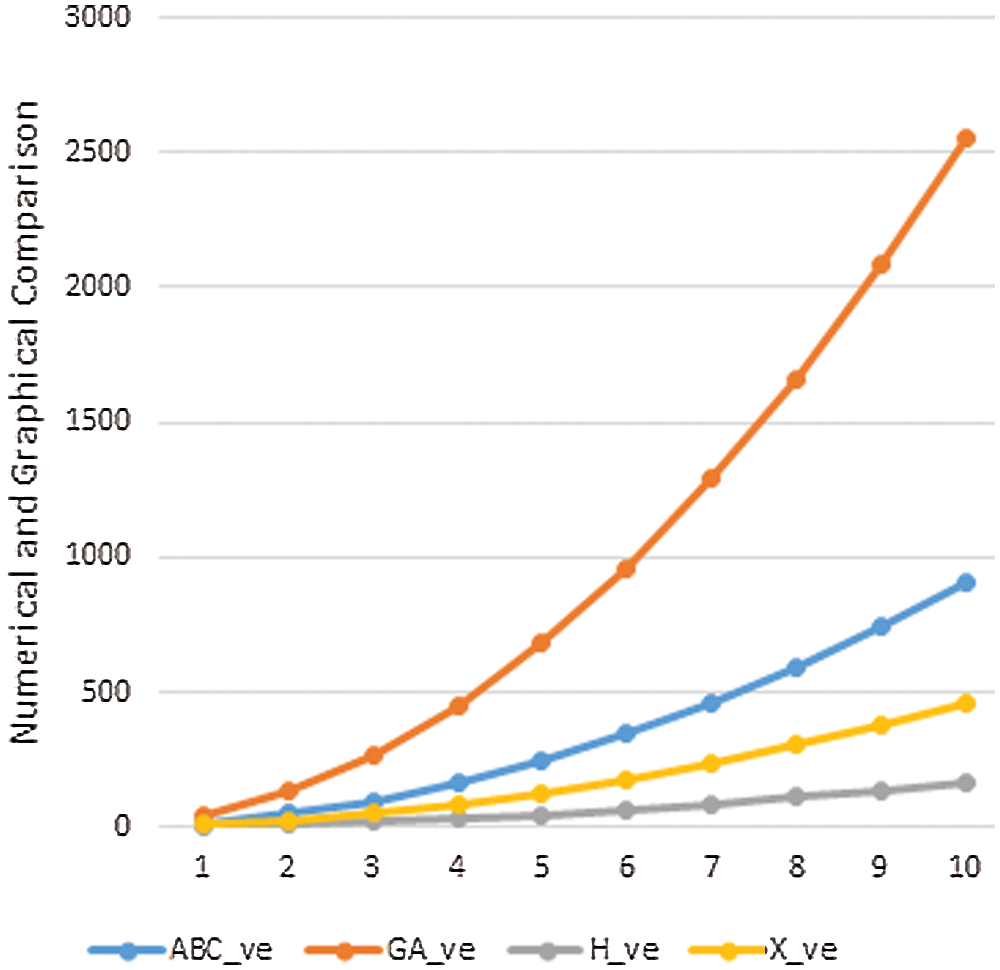
Figure 6: Graphical comparison of
In this section, we will discuss the graphical behavior of above calculated topological descriptors for crystal networks namely, bismuth tri-iodide and lead chloride. We observe that with the increase the values of parameters, the values of the defined topological descriptors are increased. The graphical representation of all above
For numerical representaion of topologies for graphs or netwroks, topological descriptors are most useful invariants. That is why such investigations are widely used in computer applications and mathematical chemistry. Calculated results in this paper for
Funding Statement: This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under Grant No. RG-29-135-38.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. J. Jiang, Y. Zhao and O. M. Yaghi, “Covalent chemistry beyond molecules,” Journal of the American Chemical Society, vol. 138, no. 10, pp. 3255–3265, 2016. [Google Scholar]
2. Y. Liu, Y. Ma, Y. Zhao, X. Sun, F. Gándara et al., “Weaving of organic threads into crystalline covalent organic frameworks,” Science, vol. 351, no. 6271, pp. 365–369, 2016. [Google Scholar]
3. M. Karelson, Molecular Descriptors in QSAR/QSPR. New York: Wiley, 2000. [Google Scholar]
4. R. Todeschini and V. Consonni, Handbook of Molecular Descriptors. Weinheim: Wiley-VCH, 2000. [Google Scholar]
5. H. Wiener, “Structural determination of paraffin boiling points,” Journal of the American Chemical Society, vol. 69, pp. 17–20, 1947. [Google Scholar]
6. A. Ahmad, “On the degree based topological indices of benzene ring embedded in P-type-surface in 2D network,” Hacettepe Journal of Mathematics and Statistics, vol. 47, no. 1, pp. 9–18, 2018. [Google Scholar]
7. M. Bača, J. Horváthová, M. Mokrišová, A. Semaničová-Feňovčíková and A. Suhányiová, “On topological indices of carbon nanotube network,” Canadian Journal of Chemistry, vol. 93, no. 10, pp. 1157–1160, 2015. [Google Scholar]
8. M. Bača, J. Horváthová, M. Mokrišová and A. Suhányiová, “On topological indices of fullerenes,” Applied Mathematics and Computation, vol. 251, pp. 154–161, 2015. [Google Scholar]
9. A. Ahmad, “Computation of certain topological properties of honeycomb networks and graphene,” Discrete Mathematics, Algorithms and Applications (DMAA), vol. 9, no. 5, Article ID: 1750064, 2017. [Google Scholar]
10. M. Ajmal, W. Nazeer, M. Munir, S. M. Kang and Y. C. Kwun, “Some algebraic polynomials and topological indices of generalized prism and toroidal polyhex networks,” Symmetry, vol. 9, no. 1, pp. 5, 2017. [Google Scholar]
11. M. R. Farahani, W. Gao, M. R. R. Kanna, R. P. Kumar and J. B. Liu, “General Randić, sum-connectivity, hyper-Zagreb and harmonic indices, and harmonic polynomial of molecular graphs,” Advances in Physical Chemistry, Article no. 2315949, 2016. [Google Scholar]
12. M. Javaid, J. B. Liu, M. A. Rehman and S. Wang, “On the certain topological indices of Titania nanotube TiO2[m,n],” Zeitschrift Für Naturforschung A, vol. 72, no. 7, pp. 647–654, 2017. [Google Scholar]
13. M. Javaid, M. U. Rehman and J. Cao, “Topological indices of rhombus type silicate and oxide networks,” Canadian Journal of Chemistry, vol. 95, no. 2, pp. 134–143, 2017. [Google Scholar]
14. M. K. Siddiqui, M. Imran and A. Ahmad, “On Zagreb indices, Zagreb polynomials of some nanostar dendrimers,” Applied Mathematics and Computation, vol. 280, pp. 132–139, 2016. [Google Scholar]
15. T. Vetrík, “Degree-based topological indices of hexagonal nanotubes,” Journal of Applied Mathematics and Computing, vol. 58, no. 1, pp. 111–124, 2018. [Google Scholar]
16. G. Hong, Z. Gu, M. Javaid, M. H. Awais and M. K. Siddiqui, “Degree-based topological invariants of metal-organic networks,” IEEE Access, vol. 8, pp. 68288–68300, 2020. [Google Scholar]
17. A. Ahmad, “Topological properties of sodium chloride,” Scientific Bulletin-University Politehnica of Bucharest, Series B, vol. 82, no. 1, pp. 35–46, 2020. [Google Scholar]
18. A. Aslam, M. F. Nadeem, Z. Zahid, S. Zafar and W. Gao, “Computing certain topological indices of the line graphs of subdivision graphs of some rooted product graphs,” Mathematics, vol. 7, no. 5, 2019. [Google Scholar]
19. S. Imran, M. K. Siddiqui, M. Imran and M. F. Nadeem, “Computing topological indices and polynomials for line graphs,” Mathematics, vol. 6, no. 8, pp. article ID: 137, 2018. [Google Scholar]
20. Z. Ahmad, M. Naseem, M. K. Jamil, S. Wang and M. F. Nadeem, “Eccentric connectivity indices of titania nanotubes TiO2 [m;n],” Eurasian Chemical Communications, vol. 2, no. 6, pp. 712–721, 2020. [Google Scholar]
21. S. Hayat and M. Imran, “Computation of topological indices of certain networks,” Applied Mathematics and Computation, vol. 240, pp. 213–228, 2014. [Google Scholar]
22. N. A. Koam and A. Ahmad, “Polynomials of degree-based indices for three-dimensional mesh network,” Computers Materials and Continua, vol. 65, no. 2, pp. 1271–1282, 2020. [Google Scholar]
23. M. Chellali, T. W. Haynes, S. T. Hedetniemi and T. M. Lewis, “On ve-degrees and ev-degrees in graphs,” Discrete Mathematics, vol. 340, no. 2, pp. 31–38, 2017. [Google Scholar]
24. M. Cancan, “On ev-degree and ve-degree topological properties of Tickysim Spiking neural network,” Computational Intelligence and Neuroscience, vol. 8, pp. 11–31, 2019. [Google Scholar]
25. S. Ediz, “A new tool for QSPR researches: ev-degree Randic index,” Celal Bayar University Journal Science, vol. 13, no. 3, pp. 615–618, 2017. [Google Scholar]
26. B. Horoldagva, K. C. Das and T. A. Selenge, “On ve-degree and ev-degree of graphs,” Discrete Optimation, vol. 31, pp. 1–7, 2019. [Google Scholar]
27. S. Ediz, “On ve-degree molecular topological properties of silicate and oxygen networks,” International Journal Computational Science Mathematics, vol. 9, no. 1, pp. 1–12, 2018. [Google Scholar]
28. B. Sahin and S. Ediz, “On ev-degree and ve-degree topological indices,” Iranian Journal of Mathematical Chemistry, vol. 9, no. 4, pp. 263–277, 2018. [Google Scholar]
29. J. Zhang, M. K. Siddiqui, A. Rauf and M. Ishtiaq, “On ve-degree and ev-degree based topological properties of single walled titanium dioxide nanotube,” Journal of Cluster Science, 2020. [Google Scholar]
30. N. Zahra, M. Ibrahim and M. K. Siddiqui, “On topological indices for swapped networks modeled by optical transpose interconnection system,” IEEE Access, vol. 8, pp. 200091–200099, 2020. [Google Scholar]
31. K. Watanabe, T. Karasawa, T. Komatsu and Y. Kaifu, “Optical properties of extrinsic two-dimensional excitons in BiI3 single crystals,” Journal of the Physical Society of Japan, vol. 55, Article ID: 897, 1986. [Google Scholar]
32. R. W. G. Wyckoff, Crystal Structures, 2nd ed., vol. 2. New York, London, Sydney: John Wiley & Sons, Inc., 1964. [Google Scholar]
33. D. Nason and L. Keller, “The growth and crystallography of bismuth tri-iodide crystals grown by vapor transport,” Journal of Crystal Growth, vol. 156, no. 3, pp. 221–226, 1995. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |