 Open Access
Open Access
ARTICLE
Analyze the Performance of Electroactive Anticorrosion Coating of Medical Magnesium Alloy Using Deep Learning
1 Advanced Functional Materials Laboratory, Zhengzhou Railway Vocational and Technical College, Zhengzhou, 450000, China
2 School of Materials Science and Engineering, Zhengzhou University, Zhengzhou, 450000, China
* Corresponding Author: Jing’an Li. Email:
Computers, Materials & Continua 2024, 79(1), 263-278. https://doi.org/10.32604/cmc.2024.047004
Received 21 October 2023; Accepted 05 January 2024; Issue published 25 April 2024
Abstract
Electroactive anticorrosion coatings are specialized surface treatments that prevent or minimize corrosion. The study employs strategic thermodynamic equilibrium calculations to pioneer a novel factor in corrosion protection. A first-time proposal, the total acidity (TA) potential of the hydrogen (pH) concept significantly shapes medical magnesium alloys. These coatings are meticulously designed for robust corrosion resistance, blending theoretical insights and practical applications to enhance our grasp of corrosion prevention mechanisms and establish a systematic approach to coating design. The groundbreaking significance of this study lies in its innovative integration of the TA/pH concept, which encompasses the TA/pH ratio of the chemical environment. This approach surpasses convention by acknowledging the intricate interplay between the acidity and pH levels within the coating formulation, thereby optimizing metal-phosphate-based conversion coatings and transforming corrosion mitigation strategies. To authenticate the TA/pH concept, the study comprehensively compares its findings with existing research, rigorously validating the theoretical framework and reinforcing the correlates among TA/pH values and observed corrosion resistance in the coatings. The influence of mutations that occur naturally in the detergent solution on persistent phosphorus changes is shown by empirical confirmation, which improves corrosion resistance. This realization advances the field of materials and the field’s knowledge of coated generation, particularly anticorrosion converter layers.Keywords
Magnesium (Mg) metals, lightweight industrial metals, offer exceptional mechanics and physiological traits, including low densities, great flexibility, great tensile power, electrical reliability, and biodegradable properties. The railroads, auto, aviation, and biological industries would likely adopt Mg materials. Despite that, mg is an extremely reactive metal that readily destroys in water or humidity. Magnesium-based alloys are not suitable in practical ways. Because of this, improving corrosion resistance is crucial for mg composites [1,2]. Certain finishes have been used for aluminum alloys to increase their ability to withstand deterioration. Hydrophobic materials have received much attention recently because of their significance for basic study, prospective commercial applications, and the ability to protect the atmosphere [3]. Hydrophilic coverings can act as reliable obstacles to prevent fluid, humidity, and air from accessing the outside layers of metallic materials. Making hydrophilic surfaces is one method that humans could gain from flora along with additional elements of nature. Various techniques were devised to create hydrophilic movies, including hydrothermal, depositing, physical or chemical etching, etc. [4]. Due to its benefits, including ease of oversight, affordability, simplicity, and ability, electrode position has recently become a competitive method for fabricating hydrophilic surfaces [5]. Coatings made of aluminum alloys are strengthened by levels of protection to combat erosion and improve general toughness. Acting like obstacles, those safeguards protect the fundamental alloys from elements that may cause oxidation. The idea is to use cutting-edge coating methods, including thin coatings or coatings made from nanoparticles, to provide a strong barrier to corrosive substances. This method increases the longevity of metallic magnesium alloys. It maintains their strength, making them healthier for various uses, including aviation metals and vehicle parts, as Fig. 1 depicts the coatings on the magnesium alloys.

Figure 1: Magnesium alloy surfaces with protective layers
Aluminum alloys generated a super hydrophobic, the element cerium microstate deposit on the exterior of an Mg metal, or instance. By lowering corrosive electron density, these coatings significantly improved corrosive behavior, yet the anodized polarization contours lacked quiet areas [6]. Electroactive anticorrosion technologies represent a significant advancement in the ongoing battle against the degradation of metallic materials caused by the corrosive effects of their environment. Corrosion, a natural process driven by electrochemical reactions, leads to the deterioration of structures, equipment, and infrastructure, incurring substantial economic and safety implications [7]. Traditional methods of combating corrosion, such as protective coatings and sacrificial anodes, have shown durability, efficiency, and environmental impact limitations. In response, electroactive anticorrosion has emerged, harnessing innovative electrochemical principles to actively manage and mitigate the corrosion process. By integrating Electroactive materials and controlled electrical currents, these technologies aim to disrupt the corrosion mechanisms at the molecular level, preserving the integrity and extending the lifespan of metallic components in diverse applications ranging from aerospace and automotive industries to maritime and infrastructure sectors. Utilizing the concepts of electricity charged with corrosion-resistant coatings is a state-of-the-art technique that reduces erosion and increases the longevity of magnesium-based healthcare products. This new covering engages in electrical interactions to prevent rusting on the metal’s surfaces and serve as an inhibitor from acids and bases. Because of its advantageous strength and longevity, magnesium alloys have many applications in medicine, especially in implants for orthopedic surgery.
Nonetheless, a significant obstacle linked to alloys made from magnesium is their ability to erode in physiological settings, potentially jeopardizing the implant’s solidity as a structure and impeding its recovery. Novel ways to address this issue, like electrically charged anticorrosion coverings, show promise in improving the anticorrosion properties of magnesium alloys used in healthcare applications. This introduction explores the fundamental principles, benefits, and potential applications of Electroactive anticorrosion, offering a glimpse into a promising frontier for more effective and sustainable corrosion management [8,9]. Magnesium alloys are lightweight materials with potential applications in the medical field due to their biocompatibility and mechanical properties. However, these alloys are susceptible to corrosion, affecting their structural integrity and biocompatibility over time. Deep learning technologies are applied in developing electroactive anticorrosion coatings for medical magnesium surfaces. These technologies optimize coating formulations, predict corrosion behavior, and contribute to enhanced durability, making magnesium implants more reliable in medical applications.
The study presents the innovative TA/pH concept, marking its first proposal. This concept goes beyond traditional approaches by considering the combined impact of acidity and pH levels within the coating formulation. The meticulous engineering of metal-phosphate-based conversion coatings based on the TA/pH concept results in coatings with robust corrosion resistance and shapes the medical magnesium alloys. The combinations of TA/pH cover the formulations of magnesium materials used in healthcare applications that aim to provide an inhibitor of acidic substances with extra layers of protection that prevent corroding. This understanding elucidates the dynamic function that regulates the creation of corrosion-resistant phosphate conversion coatings. The study advances our understanding of corrosion prevention by bridging theoretical insights with practical applications.
The study [10] suggested that the basic foundation for creating a super hydrophobic covering can be found within the coral architecture generated through a reaction of Magnesium alloys with a deeply electrostatic fluid. The anti-corrosion capabilities provided by the AZ31B Mg alloys are greatly improved through the super hydrophobic converter coatings’ protective impact, according to the findings of electrical tests. The study [11] evaluated the high hyperhydrophobic and self-cleaning properties exhibited by the S.Z.P.L. double-layered coating has been shown, which may avoid electrolyte solutions ingress. Coverings present a novel technique for producing superior anticorrosive coverings over metallic surfaces with extremely high hydrophobic and cleaning capabilities. The study [12] discovered that a single-step electrode position process was used to generate calcite myristate coatings of cerium myristate covering and calcareous/cerium myristate mixture coatings on the outermost layer of AZ31 mg alloys. The outcomes suggested that there was relatively little variation in each coverings’ hydrophilic properties. The article [13] examined the creation of intelligent multipurpose coats facilitated by current studies in mimicry elements. The present analysis is based on a summary of papers released on both inorganic and organic superhydrophilic and slick coverings that are bioinspired toward the avoidance of rust on steel substrates. The study [14] performed the conception of Electroactive polymers (EAP), a sophisticated class of polymeric that may alter their form in response to electrical excitement, have attracted attention. Since all of those EAPs experience surface conversations, tri-biological research is being done regarding their physical connections. These kinds of relationships may shorten their usefulness, necessitating intensive study to prolong them. According to the article [15], the corrosion-related harm of natural coverings is primarily caused by the existence of coating flaws. The flaw’s dimensions, quantity, and location in the finish are among the most important pieces of data to estimate corrosion-related harm to a coated system. The study [16] demonstrated that with the small elastic strength (near to that of connective material) and minimal toxic effects, medicinal titanium alloy has garnered significant interest. As a consequence of the effective involvement of graphene oxide, also known as GO, in the covering creation procedure, certain tiny holes and fractures were filled in attenuated, and the granularity of the surface was decreased.
The research [17] described powerful, potential polymer-based nanotechnology strengthened by the substance nanostructures. This produces nanoarchitecture that could be used for delivering drugs via functioning within the nanotube lumens and directed adherence via the functioning of the outside layer. The study [18] examined the in-depth analysis of contemporary ceramic paints for barrier defense vs. wear from mechanical and attack by chemicals present in this piece. Numerous crucial elements, such as different coatings features, system topologies, treatment impacts, materials courses, and prospective potential for building powerful ceramic coatings, are highlighted. The study [19] stated that the protective surfaces have increased crack-free along with adhesion characteristics to endure more difficult and dangerous situations. In order to accomplish excellent wearing and corrosion-resistant coats and a variety of uses, this summary seeks to provide a quick overview of traditional covering to intelligent functioning covering substances, mechanisms, and surface design. The study [20] assigned the description and evaluation for High-entropy alloy (HEA) microscopy logical approaches over maximizing HEA. Catalysts for electricity, such as measurable planning, part oversight, pressure technology, imperfections technology, and computational techniques, are presented in this overview. Particular attention is given to nitrogen-fixing, the process. The study [21] determined that aluminum alloys have outstanding characteristics, including outstanding toughness, less weight, and good longevity. They are widely used in aviation, science, and vehicles. The evaluation of polymeric and metallic platting of corrosion resistance of magnesium metals. The research [22] explored the substance’s growing outstanding qualities; magnesium-based alloys have been employed more and more in airplane parts where less weight is essential. It is anticipated that the alloys made from magnesium will be utilized in an increasing number of aircraft parts due to the enhancement of their construction qualities, the creation of exterior security, and multifunctional technologies.
The rest of the section is allocated as follows: Section 2 explains methodology, Section 3 accomplishes the result, and Section 4 explains the conclusion.
2.1 Anticorrosion Performance of Electroactive Anticorrosion Coating (EACC)
The anticorrosion efficiency of each of the aforementioned five typical coats is assessed using the potentiodynamic polarized approach and neutrality salt-sprayed testing in Fig. 2. The capacity of the covering to release along with restructuring its inner distributed charge in reaction to stimuli from the outside is analogous to the psychological significance of polarization in Electroactive anticorrosion coating (EACC). This allows the covering to fight back and mitigate underlying acidic mechanisms that could normally erode the magnesium-based alloys. These paints stand out from passive safeguards due to their electrically charged nature, which provides a flexible and adaptable rust barrier in demanding medical conditions. The divided electrical covering effectively serves as a protective level, receiving the acidic assault and maintaining the strength of the underneath alloys through engagement in the electrolytic typically generates dissolution. About a quarter of less corrosive currently exists compared to it. It is possible to see a clear analogous passive area in the anodized sector, which suggests that the inclusion of EACC could greatly improve the magnesium substrate’s resistance to corrosion. The numerical terms EACC-F22, EACC-F25, EACC-F26, and EACC-F36 indicate the grade of chemical composition. Additionally, EACC-F1 has a simpler design and is stronger against corrosion than EACC-F22, EACC-F25, EACC-F26, and EACC-F36 due to its much lower active current concentration. It has a characteristic polarity that improves its anticorrosion properties by proactively affecting electrical reactions occurring at the surface where it is applied. The following distinctive divides in the conduct assist in regulating and reducing rust responses, offering a barrier of defense against corrosion-causing substances. By using its unique polarisation properties, EACC is able to improve the general efficacy of anticorrosion methods, guaranteeing longevity and dependability in a range of operations.
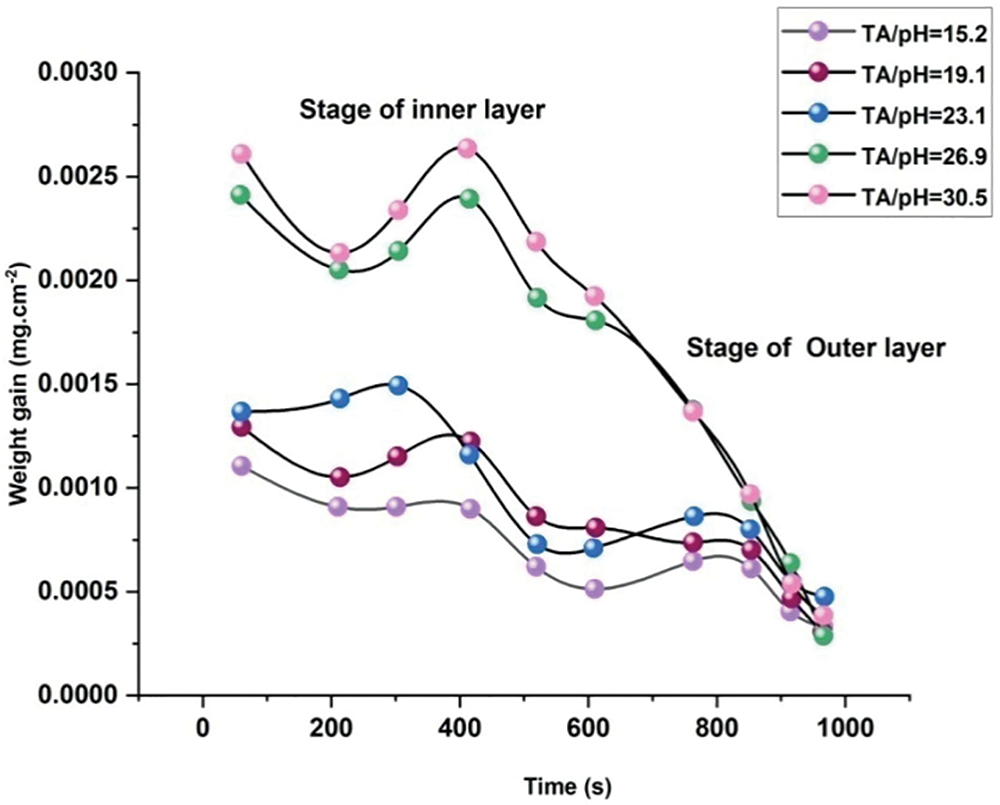
Figure 2: EACC exhibits distinctive polarization characteristics in the performance
Fig. 3 reveals five typical coverings with the presence of MgHPO4 and MnHPO4 spikes, in addition to a rise in peak strength. Additionally, the spectrum is found to show peaks for Mg and Mg17Al12, proving that the radiation passed through the small layer and reached the substrates. The processes of erosion through recognizing the by-products of erosion and the alterations in the crystal’s makeup that result from weathering. The data is useful in creating corrosive mitigation measures that work. Therefore, offering information on the configuration of molecules in the protective substance helps to optimize the manufacturing process of anticorrosive coats. This aids in the composition’s modification for improved protection by scientists.

Figure 3: XRD patterns analyzing with the five representations
2.3 Supplies with Techniques for Making Phosphorus-Converting Mixtures Using Multiple Total Acidity (TA)
Genuine AZ91D Mg alloys have been utilized in the current research. Through the use of induction-coupled plasma-atomic emissions spectroscopy, the mineral makeup of these alloys has been investigated. These alloys were then ground with rough paperwork over onto 2,000 teeth and then polished to dimensions of 21, 21, and 6 mm. Before making the EACCs, the samples were dried under pressurized air that was cold. Different concentrations of MnSO4, NaNO3, NaH2PO4, and EDTA4Na have been included in the acid-converting liquids. At ambient temperatures, the compounds were dispersed in ionized water. To investigate the effect of the TA on the corrosive characteristic of the EACCs, converter coats were developed using 37 treatments with various TA. The pH level of the mixtures proved to be preserved at roughly three, and the corresponding proportion of TA/pH was determined to be in an interval of 15.2 percent to 30. Before the further characters, the samples were sterilized for ten minutes at sixty degrees Celsius, washed using deionizer drinking water, evaporated utilizing a chilly breeze movement, and along with matured in gas for about half a day.
The mixtures with stages in particular molecule systems were determined utilizing the equilibrium-state synthesis constants with the MEDUSA program. While MEDUSA typically performs calculations at ambient humidity, the preset editing feature allows for personal modification of any variables at the consumer’s convenience.
2.3.2 EACC’s Mixture of Phases and Architecture
Field-emission scanning electron microscopy combined with energetic dispersive spectroscopy (E.D.S.) was employed to investigate the EACC surfaces as well as cross-sectional morphology. This is how a specimen for cross-sectional scanning was made: Firstly, a chosen magnesium alloy AZ91D specimen using EACCs coating was put upright in a silicone molding and stabilized using adhesives. After that, a 5:1 weight proportion of adhesive and hardening agent was put into the mold, which remained at ambient temperature for more than 1 day to cure. Secondly, the incorporated samples were continuously processed utilizing metallographic grinders with just under a hundred revolutions per minute utilizing 2000-grit silicon-carbon sheets. The coated/metal interaction remained perpendicular against the tangential orientation along the grinder discs throughout the grinder operation to prevent structural fracturing of the transformation of coatings brought on by the stress caused by shear. At last, a two-and-a-half m aluminum slush water solution was used to polish the surface of the specimens. In aluminum alloys, the term massive gains typically translates to the heaviness rise that the alloys undergo as a result of oxidization or other outside causes. Because of their reputation for being thin, magnesium alloys have become popular for a variety of uses, particularly surgical devices. But with time, being susceptible to corroding may cause weight benefit, jeopardizing the metal’s efficiency and strength. Comprehending and minimizing this weight increase is essential to guarantee the durability of medical products made with aluminum over a prolonged period. Obtaining required qualities like depth, homogeneity, and adherence requires oversight of the paint creation procedure. Scholars use an array of methodologies, such as sophisticated alteration procedures and electricity, to customize the properties of the coated in accordance with particular use demands. The resultant covering mitigates the impacts of weathering in medical settings by acting like a barrier to keep hostile ions from accessing the exterior of the alloying material. By using X-ray crystallography with a Cu K1 irradiation of 1.5404 under accelerating voltages of forty kilowatts and thirty mA, the phase compositions of both sample applications are determined in Figs. 4 and 5. The statistics have been collected utilizing an imaging stride of 0.04°/40 s alongside an accuracy range of 10°–70°. The Juniper 6:00 application was used to examine and identify the diffraction stages. Efficient anticorrosion tactics may be used in magnesium-alloyed constructions by adding weight via coatings or combining techniques. The addition of corrosive-elements to the alloy or the placement of heavier safeguards is made possible by the increased bulk. This additional substance functions as an inhibitor, delaying the rate of erosion and giving the iron base longer-lasting shielding. As a result, the extra mass contributes to the long-term reliability of metal parts, particularly in corrosion-prone areas. The creation of coated treatments is essential to the effective use of anti-corrosion techniques. The complex biochemistry required to make these fluids affects how well they prevent rusting on objects. As an illustration, it is essential to comprehend all the parts of the solution that coat, and they react. Reliable and durable coverings are developed based on knowledge regarding the moderating function provided by the solution and the effects of spontaneous mutations. Essentially, implementing dependable anticorrosion tactics requires a well-designed covering system as a coating solution.

Figure 4: Measurement of mass gains in magnesium alloy

Figure 5: Formations of coatings solution with the chemical interactions
2.3.3 Effectiveness of Converting Coverings against Corroding
An electrical machine was employed to evaluate the polarization potential of both the uncoated and coating-coated specimens in a 3.5-weight percent solution of sodium chloride above ambient temperatures. The coatings were employed for the electrodes that functioned in standard three-electrode cells, which also included an electrode made from saturated calomel for the baseline electrodes with a platinum-selling aluminum foil provided the opposing conductor. Following a 10-min period of stability at the potential of an open circuit (OCP), the test specimens are polarized in anionic as well as anodized directions at a scanning speed of 0.334 mV/S. The ability to withstand corrosion associated with the coverings was evaluated using a 48-h ambient temperature hydrogen oxidation test. The total amount of hydrogen (or H2) bursts produced by the corrosion of the underlying magnesium components in 3.6-weight percent sodium chloride solutions was gathered via a tube placed across the samples. The H2 air then went down the hole in the funnel and into a small tube that was hooked up to the distal end of the channel. Depending on the ASTM B118-04 norm, a 48-h salt-spraying testing occurred to gauge how well the finishes resisted oxidation. Applying a digital imaging device, the micro topology within the foundation’s underlying coverings was documented. Concerning repeatability, every single measurement was carried out at the smallest five periods above 30°C.
2.4 Development Rate for the Coverings for Phosphorus Conversions
To determine a covering rate of progress, the weight gains on the objects have been tracked throughout the coatings creation phase. Having a precision of 0.0001 g, thirty fragments had their weights taken on a Sartorius CP225D balanced before being submerged in a specific coatings pool for a period of ten minutes at 60 degrees Celsius. To be able to determine the median increase in mass within the instances, two of them were simultaneously taken out of the bathtub at specified involvement periods (6, 11, 16, 31, 61, 121, 181, 241, and 601 s). The mass difference between the duplicate platters preceding and following the soak operation was measured to determine the median mass increase at each reading period.
2.5 Data Mining by Convolutional Neural Network (CNN)
Convolutional neural networks (CNNs) are a type of deep neural network (DNN) that is commonly used. With these kinds of networks, every layer creates attribute maps, an increasingly more complex approximation of the source material that retains vital but distinctive characteristics. Newer CNNs obtain enhanced efficiency by adopting an extremely deeper level structure. CNNs are implemented for picture analysis of medicinal magnesium materials with electrically charged anti-corrosion coatings. These connections aid in evaluating coating homogeneity, finding flaws, and guaranteeing a reliable as well as homogeneous protecting covering. By improving the monitoring of quality, this sort of use ensures endurance and dependability for the manufacturing of healthcare mg parts immune to deterioration. CNN algorithms have been used in an array of industries, such as picture comprehension, word recognition, gaming robots, and so on. In the starting stimulation, so, me layers are arranged as a set of 2-D input maps of features during this computation. CNNs have revolutionized the field of computer vision and have achieved remarkable performance in tasks like image classification, object detection, facial recognition, and more. CNNs have shown exceptional performance in various computer vision tasks due to their ability to automatically learn relevant features from raw data.
Eq. (1) represents the corresponding matrix. They can handle different image sizes, orientations, and positions of objects within the image, making them versatile tools for image analysis.
2.6 EACCs’ Development of Morphology during the Development Procedure
Within 120 s of submersion, everything is nuclei-free. However, there are a few fairly significant nuclei scattered throughout. Most of the already present MnHPO4 nuclei developed into flakes, and other ones expanded into spheres. When the soaking period grows, the numerical densities decrease. There is a small rise in flake-like fragments as these fragments develop more quickly and according to a favored direction. In its longitude at the completion of any submersion, the flake-like components are around 30–50 m close. Conversely, the sphere-shaped nanoparticles’ nucleus volume rapidly rises while a large number of small nuclei expand until their movements are constrained by the incidence of the neighboring nucleus. When the sphere-shaped fragments collide, the development there has likely stopped, and then the protective layer’s interaction takes place. Upon the regions that are possibly completely devoid of the nucleus or have scattered nucleus small, scattered nuclei. When the TA/pH rises (23.1, Preliminary soaking (ten seconds) results in numerous MgHPO4 ions. Nanoparticles atop the magnesium matrix. Groupings have enhanced coverage of the outside. Compared to what would occur with a small TA/pH. In the MgHPO4, upon its magnesium emergence, nanoparticles collide to create homogeneous internal layers. Suppose submerged for an additional 10–120 s. The facts are apparent: the expansion of TA/pH caused the beneath layer to thicken. Yet, the disparity depth of the beneath underlying layers’ fractures’ is wider when a TA/pH level is greater. Compared to when the TA and pH are low, denoting a decrease in the innermost layer’s ability to resist rusting by expanding TA/pH. Significantly lower than in a low-TA/pH shower. The flake-like nanoparticles’ diameter is approaching roughly 120–150 m following decompression for 600 s have passed. However, many regions are still exposed to sphere-like surface particulates. In a nutshell, TA/pH significantly influences the development of the rise in TA/pH, or EACCs, allowing for the formation of flakes-like particles while delaying the nucleation of the dense sphere-like elements, which results in a pore-filled, uneven morphology.
2.7 EACC Gained Weight throughout the Development Stage
The weight increment of an EACC is displayed as an average soaking duration in solutions with various TA/pH ratios. When the lowermost layer was growing, the weight gains varied little, increasing time spent immersed (120 s), showing the interior level’s depth was not as small as the outer layers. This conclusion is consistent with our longitudinal shape findings. The creation of the outermost coating is what causes the weight gains for the samples to rise dramatically and eventually attain their optimum level. When coatings are created using fluids with an elevated TA/pH ratio, the weight gain is greater than when an adequate TA/pH ratio is used.
2.8 Frequency of Hydrogen’s Transformation throughout the Procedure of Covering Development
The transformation of hydrogen plays a significant role in the process of covering development, particularly in the context of Electroactive anticorrosion coatings on the surface of medical magnesium alloys. The change is essential since it changes the paint’s effectiveness and longevity in avoiding corroding. The importance of the hydrogen transformations throughout the process of developing the exterior layer will be briefly discussed throughout this beginning, along with how it may affect the general efficiency of the covered aluminum alloys. Magnesium alloys have a lot of potential for use in healthcare equipment because of their biodegradability and thinness. Their vulnerability to corroding, nevertheless, makes their useful implementation difficult. Electroactive corrosion inhibitors are becoming a possible remedy for this problem. These coatings are used to enhance the usefulness of alloys made of magnesium in medical devices by providing a layer of battle vs. corrosion. A critical aspect of these coatings is the transformation of hydrogen, which occurs throughout the development procedure and significantly influences the coating’s efficacy. Hydrogen transformation is an intricate process that evolves as the Electroactive anticorrosion coating is applied and forms on the surface of the magnesium alloy. This transformation is intertwined with various stages of the covering development procedure, including coating deposition, curing, and integration onto the alloy’s surface.
The electrolytic response, ionizing response, and chemistry reaction all contribute to the development of an EACC that affects AZ91 Magnesium alloys within the phosphorus solutions.
Various inferences may be drawn from the combination of this XRD analysis. Firstly, an elevated TA/pH suggests greater rainfall of magnesium phosphoric salts; this is not sufficient for the creation of a homogeneous covering. Secondly, this TA/pH primarily controls the development rates along with nucleus concentration in addition to the dynamics of how the outermost coating forms.
3.1 Development Concentration in the MnHPO4 Crystals Represents the Process Underlying the TA/pH Interaction
Eq. (4) states that the decreased quantity of H+ and ionizing rates for the acidic radicals within the metal/solution interaction is implied by higher pH values and a smaller TA. As a result, it is demonstrated that HER formation reduces when TA/pH falls. The protective property and convective impact are the primary causes of the gas hydrogen evolution’s considerable impact on the methods of coated development. Nucleation sites appear on the external layer inside the Magnesium alloys throughout the synthesis phase. Regarding the energetic ions as well, the connection between the carbon dioxide bursts may create an exhausted area. Further, the metal surfaces disappear when the TA/pH rises due to the water-bubbling digestion, suggesting a reduction in the region where MnHPO4 can form. Additionally, the appearance of bubbles made up of hydrogen causes the mixture to convict and likewise has detrimental consequences in the emergence of EACCs. Ventilation speeds up the speeds at which the magnesium dioxide, aluminum ions, Mn2+, as well as
3.2 The Rapid Development of the MnHPO4 Crystals Provides the Process Underlying the TA/pH Interaction
The physical makeup of a solution crystal border can be broken into three parts: a fluid area, a transitioning area, and a crystal area based on the bonds of chemicals theory that underlies the formation of crystals. Hydrogen peroxide (3- groupings and Mn2+ ions are compounds that create ions that hydrate (
The hydrogen-bonded connections among
They can communicate alongside others thanks to hydrogen bonds. This increases the development velocity of the MnHPO4 crystals. Furthermore, this low-pH solution displays a substantial TA/pH ratio. The chemical connection concept states that a small acidity decrease accelerates the rate of hydrogen coupling among HPO4, which is 2– and water becomes disrupted beforehand (
3.3 Compared to Reported Information
Several previously published studies on EACC in magnesium-based alloys are examined and juxtaposed using the present research to verify the tentative theories’ generality. An indication of each solution’s content and focused attention, the transformation treatment’s procedure variables (they are trying and temperatures), the degradation mediums, TA/pH, and corrosive Effectiveness. It is recognized because analyzing indicators of success from publicly available information, for instance, like HER or fat loss percentage, is a significant problem. That is made feasible by the variety of the test substrate’s solutions (such as Saline remedy, simulating body fluids, and Hank’s mixture) and the examination settings’ (such as physiological microenvironment as well as room temperatures). In order to assess the anticorrosion capabilities of these finishes, we selected improved effectiveness Internet Explorer that displays a dimensional format as well as can be defined in the following fashion:
where are the deterioration rates of the naked magnesium substrates and EACC-coated magnesium alloys, respectively? AZ31 magnesium material was examined for barite EACC coatings. While the acidity level rose between 2.3 and 2.6, TA/pH reduced from 4.45 to 3.79. The indicator value rose from 7.4 to 39.6 at the same time that the EACC Grew even more, which is in line with our findings that corrosive efficiency that homogeneity inside the EACC improved following the decline of its TA/pH. The same outcomes showed up whenever TA/pH dropped about 7.83 corrosive capability. Most of these findings show that TA/pH affects the structural characteristics as well as corrosion-resistant abilities of EACCs significantly.
3.4 Constraints of the TA/pH Concept
The findings of this study show that EACC’s corrosive resistance improves when TA/pH decreases. Yet, without water that is pure (TA/pH below 0), it is not feasible to create a covering that has excellent rust resistance. The critical number of TA/pH, known as a turning point, ought to correspond to the theoretical limit of TA/pH. Above the crucial worth, the EACC’s anticorrosion efficacy improves when TA/pH decreases. Alternatively, a drop in either the TA/pH causes the EACC’s anticorrosion efficacy to deteriorate. According to principle, the frequency of MnHPO4’s rainfall operations is significantly correlated with its frequency for its deposition products; therefore, a greater value of MnHPO4 denotes a greater rate of MnHPO4 precipitate. As the ratio of TA/pH drops, the amount of
To create EACCs with the desired corrosive efficiency, the TA/pH hypothesis was applied. The standard had been determined to be Bath-F1. To lower the amount of liberated Mn2+, an agent that complexes had previously been applied. Secondly, 0.1 M NaHCO3 solution was added to bring the TA/pH ratio lower from 15.3 to 12.8. According to an explanation provided in 3.4, the inclusion was meant to prevent the baths from failing to achieve a sustained drop in their TA/pH ratio. Fig. 6 illustrates the EACC aids in TA/pH depicts the frequency as well as particle dimensions in the produced EACC had clearly enhanced while the anticorrosion property of the EACC-coated Magnesium alloys was increased by over twofold in contrast to that displayed. While this research has concentrated on phosphorus converter showers, its findings offer a great deal of room to be broadened to include additional buffer structures that could help in the creation of novel converter pools, including converter coats having great resistance to corrosion. The combined approach offers an opportunity to advance the area of healthcare implants since it takes into account merely the electrical elements of rusting as well as the covering’s tolerability for reactivity. Through meticulous pH regulation within a preferred range, scientists may augment the chemical’s absorption and facilitate its efficient coating onto the magnesium-based alloys. In order to ensure the barrier of protection remains constant throughout the substrate, an intact and homogeneous coating must develop with this carefully regulated pH condition.
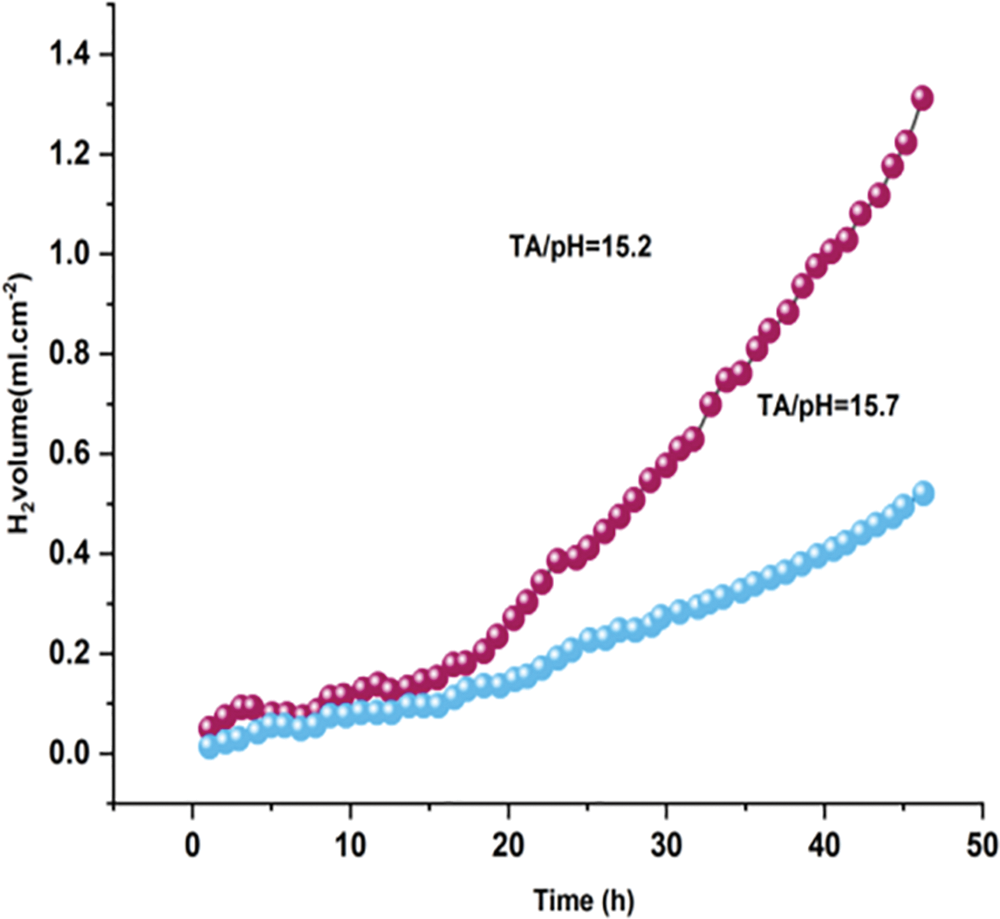
Figure 6: EACC aids in TA/pH
The study’s innovative notion of TA potential and its relationship to pH in determining the corrosion resistance of magnesium alloys represent, in our opinion, a groundbreaking step forward in the field of corrosion prevention and the medical field. It improves our understanding of corrosion prevention processes and creates a systematic approach to coating design thanks to the careful design of coatings used in this study, which combines theoretical insights with real-world applications. The novel use of the TA/pH concept, which takes into consideration the complex interaction between acidity and pH levels inside the coating formulation, is what makes this work so groundbreaking and shapes medical magnesium alloys. This strategy revolutionizes corrosion mitigation techniques while improving metal-phosphate-based conversion coatings. The investigation rigorously confirms the theoretical framework and strengthens the relationship between TA/pH values and observed corrosion resistance in the coatings in order to justify the TA/pH concept. The practical applicability of the idea in the realm of corrosion prevention is highlighted by this empirical confirmation. Furthermore, this research provides a great understanding of the function performed by naturally occurring chemical fluctuations in the development of constant phosphorus modifications in coatings. This dynamic process sheds light on a component of corrosion protection that was previously ignored by highlighting the interaction between solution dynamics and coating development. In essence, this study not only gives a novel strategy for preventing corrosion but also offers a thorough grasp of the variables affecting corrosion resistance in magnesium alloys. The study could face problems scaling up and being put into practice, which would need more testing and customization for use in practical settings. The TA/pH idea has been creatively integrated into the study, and this has the potential to advance corrosion prevention strategies and systematic coating design.
Manufacturing expenses may rise primarily as a result of the creation and use of sophisticated coverings, particularly those alongside electrically charged qualities. It is critical to strike a balance between expense and higher efficiency, particularly in healthcare settings wherein price-effectiveness plays a big role. Therefore, if the raw material is available through an affordable process, this issue can be tolerated easily. In order to guarantee that the coatings are well-absorbed through human beings, additional research might concentrate on enhancing their term. The objective was going to be to develop coverings that enhance their interaction with tissues of life while simultaneously providing anti-corrosion properties. Increased durability, resistance to rust, and survival may result from experimenting with new substances, nanotechnology, or nanoparticles in the finish formulations.
Acknowledgement: The authors would like to express their gratitude for the valuable feedback and suggestions provided by all the anonymous reviewers and the editorial team.
Funding Statement: This study is funded by the below mentioned organisation: 1. Key Research and Development Special Project of Henan Provincial Science and Technology (222102230025); 2. Key Research and Development Special Project of Henan Provincial Science and Technology (232102231015); 3. Key Research and Development Special Project of Henan Provincial Science and Technology (232102231011); 4. Natural Science Foundation of Henan Province (No. 004053100); 5. Major Science Research Project of High Education of Henan Province (No. 23B430016).
Author Contributions: Conceptualization, Yashan Feng and Yafang Tian; Data curation, Yongxin Yang; Formal analysis, Yugang Zhang; Investigation, Haiwei Guo; Methodology, Jian’an Li; Software, Yashan Feng; Validation, Yongxin Yang; Visualization, Yufang Zhang; Writing–original draft, Haiwei Guo. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data is available in the openly source journals and can be viewed through a simple google search.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. G. S. Arora, K. K. Saxena, K. A. Mohammed, C. Prakash, and S. Dixit, “Manufacturing techniques for Mg-based metal matrix composite with different reinforcements,” Crystals, vol. 12, no. 7, pp. 945, Jul. 2022. doi: 10.3390/cryst12070945. [Google Scholar] [CrossRef]
2. V. S. Saji, Advances in Corrosion Control of Magnesium and Its Alloys, 1st ed. Boca Raton, USA: CRC Press, vol. 1, pp. 1–470, 2023. [Google Scholar]
3. N. E. Sataeva, L. B. Boinovich, K. A. Emelyanenko, A. G. Domantovsky, and A. M. Emelyanenko, “Laser-assisted processing of aluminum alloy for the fabrication of superhydrophobic coatings withstanding multiple degradation factors,” Surf. Coat. Technol., vol. 397, no. 2, pp. 125993, Sep. 2020. doi: 10.1016/j.surfcoat.2020.125993. [Google Scholar] [CrossRef]
4. L. Li et al., “Hydrophobic and stable MXene-polymer pressure sensors for wearable electronics,” ACS Appl. Mater. Interfaces, vol. 12, no. 13, pp. 15362–15369, Mar. 2020. doi: 10.1021/acsami.0c00255 [Google Scholar] [PubMed] [CrossRef]
5. Y. Bai, Z. Hu, J. Jiang, and F. Huang, “Hydrophilic conjugated materials for photocatalytic hydrogen evolution,” Chem. Asian J., vol. 15, no. 12, pp. 1780–1790, May 2020. doi: 10.1002/asia.202000247 [Google Scholar] [PubMed] [CrossRef]
6. K. An et al., “Eco-friendly superhydrophobic coupling conversion coating with corrosion resistance on magnesium alloy,” Langmuir, vol. 39, no. 18, pp. 6355–6365, Apr. 2023. doi: 10.1021/acs.langmuir.3c00025 [Google Scholar] [PubMed] [CrossRef]
7. I. Milošev, “Contemporary modes of corrosion protection and functionalization of materials,” Acta Chim. Slov., vol. 66, no. 3, pp. 511–533, Sep. 2019. doi: 10.17344/acsi.2019.5162. [Google Scholar] [CrossRef]
8. Y. Yusran, Q. Fang, and V. Valtchev, “Electroactive covalent organic frameworks: Design, synthesis, and applications,” Adv. Mater., vol. 32, no. 44, pp. 2002038, Jul. 2020. doi: 10.1002/adma.202002038 [Google Scholar] [PubMed] [CrossRef]
9. J. Zheng et al., “Nonvolatile electrically reconfigurable integrated photonic switch enabled by a silicon PIN diode heater,” Adv. Mater., vol. 32, no. 31, pp. 2001218, Jun. 2020. doi: 10.1002/adma.202001218 [Google Scholar] [PubMed] [CrossRef]
10. D. Jiang, X. Xia, J. Hou, G. Cai, X. Zhang and Z. Dong, “A novel coating system with self-reparable slippery surface and active corrosion inhibition for reliable protection of Mg alloy,” Chem. Eng. J., vol. 373, no. 5, pp. 285–297, Oct. 2019. doi: 10.1016/j.cej.2019.05.046. [Google Scholar] [CrossRef]
11. X. Yin, P. Mu, Q. Wang, and J. Li, “Superhydrophobic ZIF-8-based dual-layer coating for enhanced corrosion protection of Mg alloy,” ACS Appl. Mater. Interfaces, vol. 12, no. 31, pp. 35453–35463, Jul. 2020. doi: 10.1021/acsami.0c09497 [Google Scholar] [PubMed] [CrossRef]
12. S. Tang, Y. Zhang, H. San, and J. Hu, “Hydrophobic surface contained Ca and/or Ce myristate fabricated on AZ31 by one-step electrodeposition for corrosion protection in NaCl,” Appl. Surf. Sci., vol. 496, no. 5, pp. 143627, Dec. 2019. doi: 10.1016/j.apsusc.2019.143627. [Google Scholar] [CrossRef]
13. J. S. George, P. Poornima Vijayan, A. T. Hoang, N. Kalarikkal, P. Nguyen-Tri and S. Thomas, “Recent advances in bio-inspired multifunctional coatings for corrosion protection,” Prog. Org. Coat., vol. 168, no. 3, pp. 106858, Jul. 2022. doi: 10.1016/j.porgcoat.2022.106858. [Google Scholar] [CrossRef]
14. H. Rahman et al., “Recent progress on electroactive polymers: Synthesis, properties and applications,” Ceramics, vol. 4, no. 3, pp. 516–541, Sep. 2021. doi: 10.3390/ceramics4030038. [Google Scholar] [CrossRef]
15. G. L. Song and Z. Feng, “Modification, degradation and evaluation of a few organic coatings for some marine applications,” Corros. Mater. Degrad., vol. 1, no. 3, pp. 408–442, Dec. 2020. doi: 10.3390/cmd1030019. [Google Scholar] [CrossRef]
16. J. Wang et al., “Modification effect of graphene oxide on oxidation coating of Ti-3Zr-2Sn-3Mo-25Nb near-β titanium alloy,” J. Alloys Compd., vol. 901, no. 6, pp. 163561, Apr. 2022. doi: 10.1016/j.jallcom.2021.163561. [Google Scholar] [CrossRef]
17. J. R. Beryl and J. R. Xavier, “Halloysite for clay-polymer nanocomposites: Effects of nanofillers on the anti-corrosion, mechanical, microstructure, and flame-retardant properties—A review,” J. Mater. Sci., vol. 58, no. 27, pp. 10943–10974, Jun. 2023. doi: 10.1007/s10853-023-08710-1. [Google Scholar] [CrossRef]
18. D. E. Wolfe et al., “A comprehensive review of modern engineered ceramics coatings for optimised resistance to wear and corrosion,” Adv. Appl. Ceram., vol. 122, no. 3–4, pp. 81–100, May 2023. doi: 10.1080/17436753.2023.2235497. [Google Scholar] [CrossRef]
19. S. Polat, Y. Sun, E. Çevik, H. Colijn, and M. E. Turan, “Investigation of wear and corrosion behavior of graphene nanoplatelet-coated B4C reinforced Al-Si matrix semi-ceramic hybrid composites,” J. Compos. Mater., vol. 53, no. 25, pp. 3549–3565, Apr. 2019. doi: 10.1177/0021998319842297. [Google Scholar] [CrossRef]
20. Y. Zhang, D. Wang, and S. Wang, “High-entropy alloys for electrocatalysis: Design, characterization, and applications,” Small, vol. 18, no. 7, pp. 2104339, Nov. 2021. doi: 10.1002/smll.202104339 [Google Scholar] [PubMed] [CrossRef]
21. B. Li, S. Xue, P. Mu, and J. Li, “Robust self-healing graphene oxide-based superhydrophobic coatings for efficient corrosion protection of magnesium alloys,” ACS Appl. Mater. Interfaces, vol. 14, no. 26, pp. 30192–30204, Jun. 2022. doi: 10.1021/acsami.2c06447 [Google Scholar] [PubMed] [CrossRef]
22. S. V. S. Prasad, S. B. Prasad, K. Verma, R. K. Mishra, V. Kumar and S. Singh, “The role and significance of magnesium in modern day research–A review,” J. Magnes. Alloy, vol. 10, no. 1, pp. 1–61. doi: 10.1016/j.jma.2021.05.012. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools