 Open Access
Open Access
ARTICLE
Federated Machine Learning Based Fetal Health Prediction Empowered with Bio-Signal Cardiotocography
1 Department of Computer Sciences, Bahria University Lahore Campus, Lahore, 54000, Pakistan
2 Faculty of Information Technology, Liwa College, Abu Dhabi, 00000, United Arab Emirates
3 Department of Computing, Skyline University College, Sharjah, 1797, United Arab Emirates
4 Department of Computer Science, Taibah University, Medina, 42353, Saudi Arabia
5 Riphah School of Computing and Innovation, Faculty of Computing, Riphah International University, Lahore Campus, Lahore, 54000, Pakistan
6 Department of Engineering and Development, Dubizzle Labs, Lahore, 54000, Pakistan
7 Department of Software, Faculty of Artificial Intelligence and Software, Gachon University, Seongnam-si, 13120, Korea
* Corresponding Author: Khan Muhammad Adnan. Email:
Computers, Materials & Continua 2024, 78(3), 3303-3321. https://doi.org/10.32604/cmc.2024.048035
Received 25 November 2023; Accepted 25 December 2023; Issue published 26 March 2024
Abstract
Cardiotocography measures the fetal heart rate in the fetus during pregnancy to ensure physical health because cardiotocography gives data about fetal heart rate and uterine shrinkages which is very beneficial to detect whether the fetus is normal or suspect or pathologic. Various cardiotocography measures infer wrongly and give wrong predictions because of human error. The traditional way of reading the cardiotocography measures is the time taken and belongs to numerous human errors as well. Fetal condition is very important to measure at numerous stages and give proper medications to the fetus for its well-being. In the current period Machine learning (ML) is a well-known classification strategy used in the biomedical field on various issues because ML is very fast and gives appropriate results that are better than traditional results. ML techniques play a pivotal role in detecting fetal disease in its early stages. This research article uses Federated machine learning (FML) and ML techniques to classify the condition of the fetus. This study proposed a model for the detection of bio-signal cardiotocography that uses FML and ML techniques to train and test the data. So, the proposed model of FML used numerous data preprocessing techniques to overcome data deficiency and achieves 99.06% and 0.94% of prediction accuracy and misprediction rate, respectively, and parallel the proposed model applying K-nearest neighbor (KNN) and achieves 82.93% and 17.07% of prediction accuracy and misprediction accuracy, respectively. So, by comparing both models FML outperformed the KNN technique and achieved the best and most appropriate prediction results as compared with previous studies the proposed study achieves the best and most accurate results.Keywords
There were around 213 million pregnancies globally in 2012 [1]. Pregnancies were recorded in 190 million impoverished nations (89%) and 23 million prosperous countries (23%) (11 percent). In 2013, 293,336 women were murdered by pregnancy-related complications such as maternal hemorrhage, abortion difficulties, high blood pressure, maternal infection, and obstructed labor [2]. According to the World Health Organization (WHO), more than 303,000 women died during and after pregnancy and delivery in 2015 [3], and an estimated 830 women die per day as a result of complications during pregnancy or childbirth. Birth abnormalities, genetic issues, and maternal antibody difficulties result from the late prediction of fetal health. As a result, early diagnosis of prenatal health is critical to prevent any hereditary abnormalities.
Cardiotocography is a popular technique for determining infant status. Cardiotocograms constantly monitor infant heart rate and vaginal bleeding noninvasively during childbirth [4]. Obstetricians have been diagnosing infant status for decades based on visual examinations of infant heart rate patterns in cardiotocography signals, such as acceleration, retardation, starting heart rate, and electrocardiogram. Furthermore, the temporal link between infant heart rate and uterine spasm has been generally regarded as a critical element in cardiotocograph interpretation and has been classified as earlier than normal retardation [5].
The number of infant heartbeats per minute is referred to as the infant heart rate. Cardiotocograms are used in prenatal clinical diagnosis of infant health to measure infant activity and heart rate, as well as pelvic spasms while the baby is in the womb. Doctors can use this check to determine whether the fetus is healthy before and during birth. Cardiotocogram findings help prevent precocious delivery and lower the risk of perinatal death by giving pivotal physiological and pathological information to gynecologists.
The International Federation of Obstetrics and Gynecology classifies cardiotocograph test findings into three categories: healthy, questionable, and malignant. These classifications are based on infant heart rate, fluctuation, amplitudes, and decelerations [6]. Clinicians do this, but software can also accomplish it. In the study [7], there was a considerable reduction in neonatal mortality with the use of the computerized cardiotocograph, with a relative risk of 0.20 and a confidence interval of 95 percent when compared to the conventional cardiotocography. However, because the data in this study is of moderate quality, more research is needed to examine the influence of cardiotocographs on pregnancy outcomes [7]. Fig. 1 depicts the overall image of previously proposed works to detect the fetus's health in its early stages.

Figure 1: Block diagram of previously proposed works
Bio-Signal processing technology has employed artificial intelligence in recent years to translate data from the human body into a prognosis. Oncologists are focusing on developing an automated cardiotocography interpretation, however, the findings have yet to successfully anticipate hazardous fetus abnormalities [8]. As a result, numerous academics currently researching by employing Machine learning (ML) algorithms to identify the status of the infant in the mother's stomach. So, in this research article, the overall aim of the proposed is to use the various ML techniques and Federated machine learning (FML) to diagnose the fetal status in the mother’s womb and oncologists to take the appropriate steps according to the fetal health status. Fig. 2 shows the overall process of the proposed model from data collection to predict the fetus's health enabled with blockchain storage cloud for trained model and patient data.

Figure 2: Block diagram of the proposed model
Federated machine learning poses many security risks to healthcare systems. To access information, intruders might easily break into the integrated networks. The most serious flaw in hacking attempts into multiple healthcare networks and patient registries is that they can risk patients' lives by illegally utilizing their data to send spam and phishing emails to patients. Because most network systems in healthcare organizations are centralized, their networks are frequently a target for hacker backstabbing. All of these issues are the result of centralized networks. All of these issues may be easily resolved by utilizing a secure cloud solution powered by blockchain technology. Satoshi Nakamoto created the blockchain in 2008, which was a collection of multiple time-stamped hacker evidence files protected by a network of networks. Fig. 3 illustrates the blockchain technologies architecture. It is made up of a fundamental collection of cryptographically connected components. Blockchain technology enables several responsibilities, such as transparency, decentralization, and rigidity. Students were prepared to work with embedded devices, centralized networks, virtual currency, and other technology through these three exercises.

Figure 3: Blockchain technologies block architecture
Cardiotocography is a scientific term for observing and recording infant heart rate and uterine spasms throughout pregnancy to assess infant comfort and detect a boosted possibility of pregnancy complications. This enables the early diagnosis and treatment of fetal hypoxia before it progresses to unadorned asphyxia or death [9]. The fetus's heart rate and its volatility, reactivity, and potential decelerations during uterine spasms are important indicators of infant well-being [10].
The author of [11] introduced a novel clinical verdict support system built on an enhanced adaptive genetic algorithm and an extreme machine learning algorithm in [11], and the model's concluding classification accuracy reached 94 percent. The parameters used to detect the infantile state of an Electrocardiogram (ECG) were improved in [12] by utilizing the least squares support vector machine, swarm optimization, and a binary decision tree.
The author of [13] developed a time-frequency function-based classifier that includes a cost-sensitive Support vector machine (SVM). The cardiotocography's non-stationarity and the data set's instability are corrected, and more effective findings are achieved, with a specificity of 66.1 percent, a sensitivity of 85.2 percent, and a qualitative scale of 75.0 percent.
Reference [14] used self-developed cardiotocography autonomous analytic software to extract descriptive data from cardiotocography signals and forecast delivery using a variety of modes, including AdaBoosting, random forests, J48, and gradient-boosting trees. With a prediction accuracy of 87.6 percent and an area under the curve of 93.0 percent, the random forest classification results were the best.
The authors of [15] used bespoke software to collect seventeen existing cardiotocography data parameters and classified them utilizing three machine learning algorithms i-e random forest, J48, and ada boosting decision tree, with random forest beating the others. The area under the curve for classification is more than 94.9 percent.
In [16], the authors suggested and compared a backpropagation-based duration neutrophil performance neural network framework to other algorithms such as neural network, decision tree, K-nearest neighbor (KNN), and approximation neural network, demonstrating that this framework is a solution. useful and efficient.
Using primary module analysis, receiver functioning descriptions, and International Federation of Obstetrics and Gynecology guidelines, the authors of [17] confirmed the impact of an application on delivery quality. The standard SisPorto data set and the Lagrange support vector machine were used to assess the infant's condition.
The authors contrasted 11 infant heart rate morphological analyses provided by the automated analysis approach with expert consensus [18]. Conclude that the automatic analysis approach proposed by Lu and Wei outperformed previous automatic analysis methods in terms of baseline calculation.
In [19], the authors used numerous comparative machine learning algorithms such as logistic regression, random forest, decision tree, SVM, voting classifier, and KNN. Their model random forest achieved the best prediction accuracy of 97.51%.
In [20], the authors employed a deep neural network to predict the fetal heart rate and uterine spasm with the help of numerous patterns and parameters. Their employed model achieved an area under the curve of 0.7 and a sensitivity of 89%.
In [21], the authors used a comparative machine learning approach to predict the fetal heart rate and uterine spasm and they employed five algorithms Extreme Gradient (XG) Boost, support vector machine, KNN, light Gradient Boosting Machine (GBM), and random forest. So, the random forest algorithm outperformed and achieved 98.00% prediction accuracy.
Reference [22] proposed the use of arithmetical features derived from experiential modal rottenness. The characteristics derived from the breakdown of sub-bands are classed as normal or dangerous. They got 86 percent prediction accuracy of the test data right.
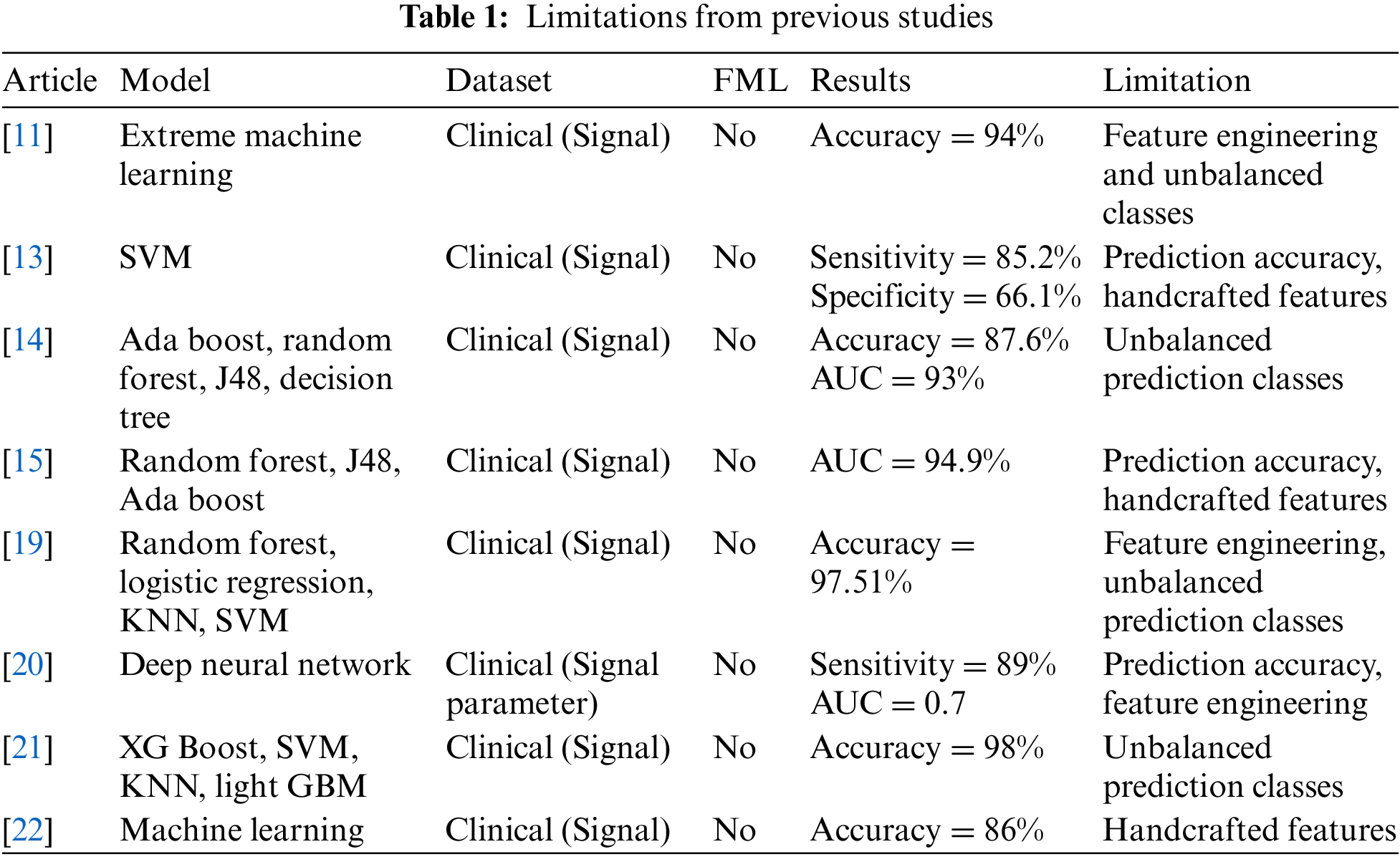
Table 1 shows the limitations of previous studies and it depicts that the [11] study used extreme machine learning with a publicly available clinical signal dataset and achieved 94% prediction accuracy with feature engineering and unbalanced prediction classes as research limitations, [13] study used support vector machine with publicly available clinical signal dataset and achieved 82.5% sensitivity and 66.1% specificity with prediction accuracy and handcrafted features as research limitation, [14] study used ada boost, random forest, Java (J) 48 and decision tree with publicly available clinical signal dataset and achieved 87.6% prediction accuracy and 93% Area Under Curve (AUC) with unbalanced prediction classes as research limitation, study [15] used random forest, J48 and ada boost with publicly available clinical signal dataset and achieved 94.9% AUC with prediction accuracy and handcrafted features as research limitation, study [19] used random forest, logistic regression, KNN and SVM with publicly available clinical signal dataset and achieved 97.51% prediction accuracy with feature engineering and unbalanced prediction classes as research limitation, study [20] used deep neural network with publicly available clinical signal dataset and achieved 89% sensitivity and 0.7 AUC with unbalanced prediction classes as research limitation, study [21] used XG boost, SVM, KNN and light GBM with publicly available clinical signal dataset and achieved 98% prediction accuracy with unbalanced prediction classes as research limitation and study [22] used machine learning with publicly available clinical signal dataset and achieved 86% prediction accuracy with handcrafted features as research limitation.
To cope with the major discrepancies of previous studies, the proposed model's major contributions are stated below:
● The proposed employed FML to cope with the prediction accuracy discrepancy.
● The proposed model used bio-signal cardiotocography data from publicly available hospitals.
● The proposed applied other machine learning techniques as well to compare with the best model.
● The proposed study used various statistical factors to gauge the model performance and validity of results.
In Fetal health prediction in a mother’s womb is very important for an infant's physical health. So, in this research, the proposed model uses federated machine learning to predict fetal health in the mother’s womb. Fig. 4 shows the overall process of the proposed model using federated machine learning to predict fetal health using bio-signal of cardiotocography in the mother’s womb. The proposed model consists of numerous layers including the training data layer, training layer, testing data layer, and testing layer. Initially, the proposed model extracts data from bio-signal cardiotocography using a cardiotocograph and employs data preprocessing techniques including fetching the overall data insights from a number of each class instance and then applying data cleaning techniques including removing the redundant data and storing data in a private cloud A empowered with blockchain technology. After the data preprocessing the proposed model initiates the training layer of the proposed model and imports data from private cloud A. The proposed model imports data into the KNN machine learning algorithm for training purposes, after the completion of the KNN training performance meter, check the learning criteria if it meets then move the trained model into private cloud Z empowered with blockchain technology otherwise start to retrain process. In a parallel phase, the proposed model imports data in Levenberg Marquardt (LM), Bayesian Regression (BR), and Stochastic Gradient Descent (SGD) machine learning algorithms and checks the learning criteria if it meets then moves trained weights into federated average process otherwise starts to retune the weights. After the federated average process again checks the learning criteria if it meets then moves the trained federated model into public cloud Z empowered with blockchain technology. In the next phase, the proposed model initiates the testing phase, in this segment the proposed model fetches data from bio-signal cardiotocography using a cardiotocograph and gives it to the testing process. In the testing layer, the proposed model imports the better resultant model from public cloud Z and starts testing, if the fetus has a normal heart rate, then move to a home, and if the fetal heart rate is pathologic and suspects then move to the hospital for further medication.

Figure 4: Proposed model for the prediction of fetal heart rate using FML empowered with blockchain technology
To calculate the proposed model testing performance, the proposed model uses numerous statistical factors [23–27] such as Prediction accuracy (PA), F1-score, sensitivity, False positive rate (FPR), Positive predicted value (PPV), False negative rate (FNR), Classification misrate (CMR), Fowlkes Mallows Index (FMI) and LPR. All statistical parameter equations are stated below.
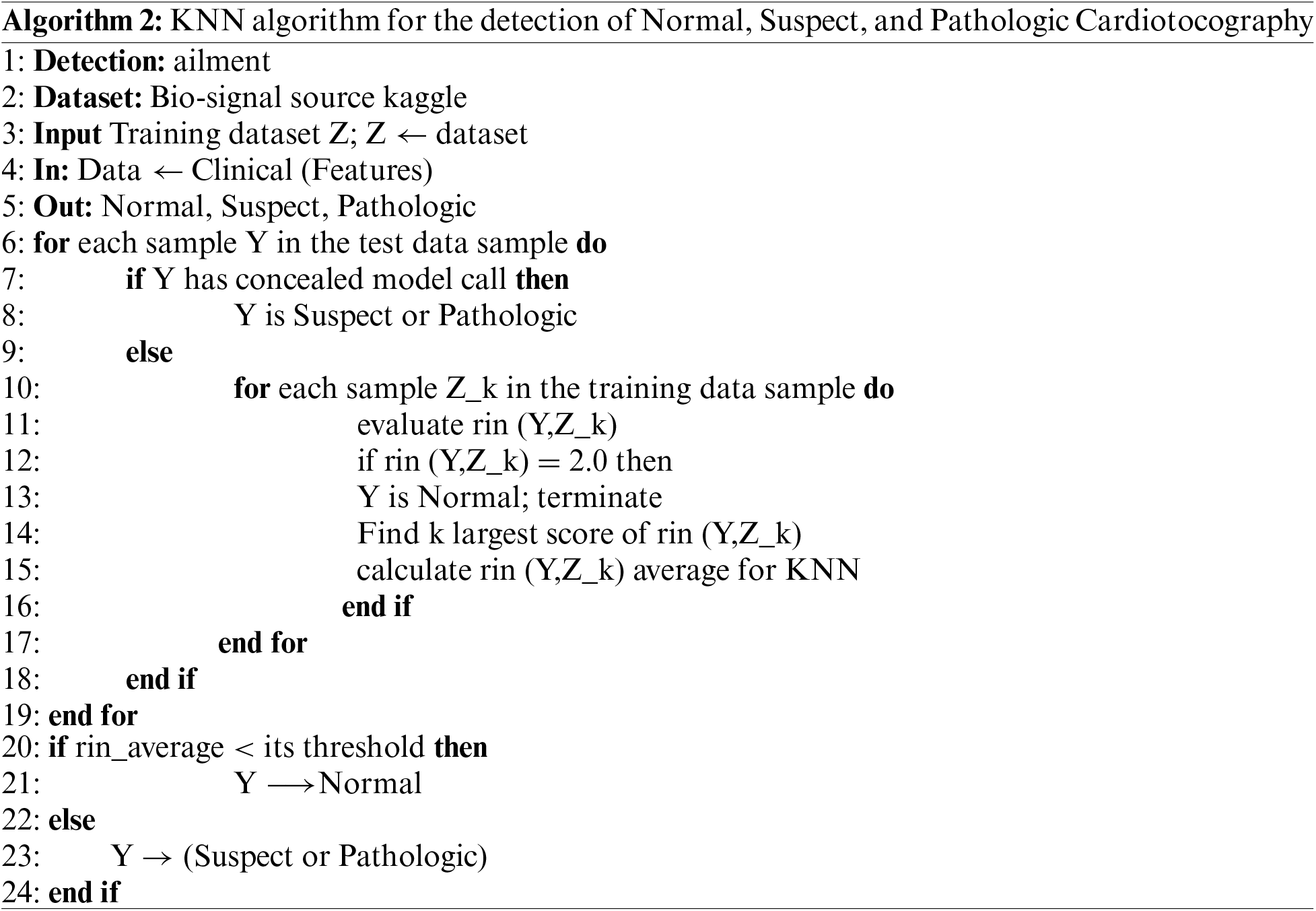
KNN and FML algorithms for the proposed model to predict fetal health in the mother’s womb empowered with blockchain technology are stated below in the descriptive form. Algorithm 1 depicts the training and testing process using FML in the client and server phases. In the server phase, the proposed model does a training of FML weights using feedforward and tuning weights (Rij and Gjp) for better performance the proposed model calculates backpropagation error, and after the successful training, the session server sends tuned weights to the client phase for aggregation. In the client, phase step 6 and step 7 do average aggregation for testing the instances. Algorithm 2 depicts the overall training and testing process using KNN, first KNN gets instances and performs training using the K score if K scores less than the expected threshold then the instance is normal else the instance is pathologic or suspect.


In this study, the proposed model uses publicly available data [28] from bio-signal cardiotocographs for the prediction of fetal health. Table 2 depicts the dataset description of all variables with chi-square values.

In this proposed study, the proposed model uses FML and KNN to predict the fetal heart rate using bio-signal cardiotocography in the mother’s womb. Training and testing simulations of the proposed model have been done on MacBook 2017 core i5 with internal graphic process unit support, 16 gigabytes of random access memory, and 512 gigabytes of a solid-state drive. The proposed model uses 2126 fetal heart rates and subdivides them into 70% of training and 30% of respectively. The training and testing performance of the proposed model has been measured by numerous statistical parameters and statistical parameters have been discussed in the previous section.
Fig. 5 shows the training performance Bayesian regression algorithm. As Fig. 5 depicts algorithms achieved the best training PA of 99.77% and 0.23% of CMR and the proposed model depicts that training is very smooth. There are minor instances that are wrongly predicted and training shows these instances out of bounds.

Figure 5: Training performance of Bayesian regression
Fig. 6 shows the training performance stochastic gradient descent algorithm. As Fig. 6 depicts algorithms achieved training PA of 95.88% and 4.12% of CMR and the proposed model depicts training as very smooth but not better than Bayesian regression. There are minor instances that are wrongly predicted and training shows these instances out of bounds.

Figure 6: Training performance of stochastic gradient descent
Fig. 7 shows the training performance Levenberg Marquardt algorithm. As Fig. 7 depicts algorithms achieved training PA of 97.20% and 2.80% of CMR and the proposed model depicts training as very smooth but not better than Bayesian regression. There are minor instances that are wrongly predicted and training shows these instances out of bound and mostly instances predicted against their cluster as well.

Figure 7: Training performance of Levenberg Marquardt
Table 3 depicts the cumulative training results of FML and it shows that Bayesian regression secures 99.77% PA and 2.3% CMR, Stochastic gradient descent secures 95.88% and 4.12% CMR, Levenberg Marquardt secures 97.20% and 2.80% CMR and at the federated average, FML secured 99.08% PA and 0.92 CMR.
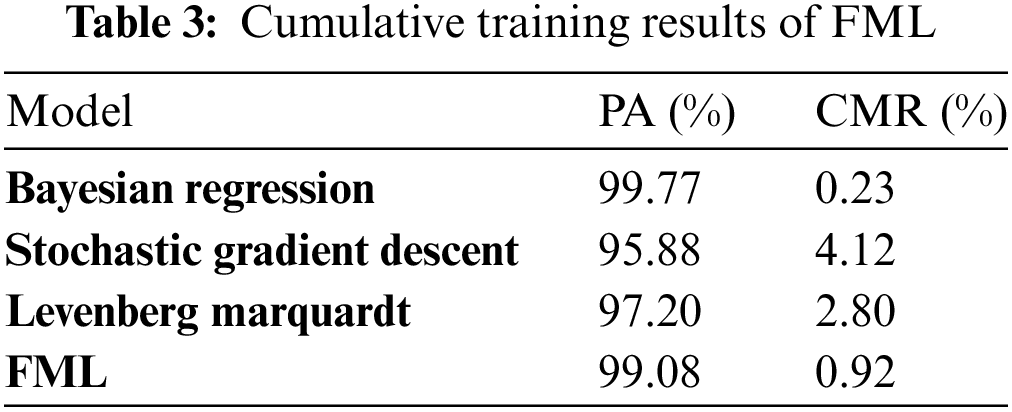
Fig. 8 shows the training performance of KNN and it depicts that KNN converged at the 18th iteration and achieved 92.60% PA and 7.4% of CMR with minimum classification error is 0.07239, distance weight squared inverse, a distance metric is a cosine and the total number of neighbors is 81.

Figure 8: Training performance of KNN
Table 4 shows the training confusion matrix of KNN and it depicts that the proposed model right positive predicted 1575 instances of all classes including normal, suspect, and pathologic. The proposed model negatively predicted 125 instances from all classes.

Table 5 illustrates the KNN testing confusion matrix and it depicts that the proposed model positively predicted 351 instances of all classes including normal, suspect, and pathologic. The proposed model negatively predicted 75 instances from all classes. The proposed model of KNN performed well in the testing phase.

Table 6 depicts the testing confusion matrix of FML and it depicts that the proposed model positively predicted 422 instances of all classes including normal, suspect, and pathologic. The proposed model negatively predicted 4 instances from all classes. The proposed model of FML outperformed KNN in the testing phase.
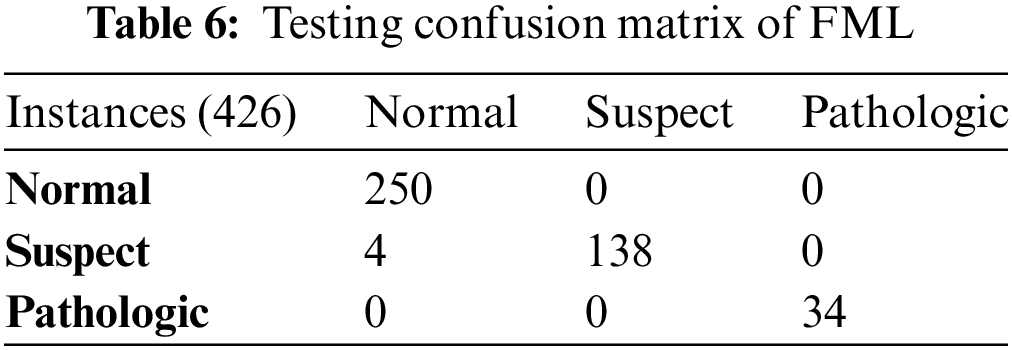
Table 7 shows the testing statistical parameter results of FML, it depicts that the proposed model of FML achieves 99.06%, 0.94%, 99.53%, 99.53%, 100%, 0.47%, 1.00, 99.53%, and 99.53% of PA, CMR, Sen, PPV, FPR, FNR, LPR, FMI and F1-score, respectively. So, FML outperformed and achieved the highest testing prediction accuracy as compared with KNN.

Table 8 illustrates the testing statistical parameter results of KNN, it depicts that the proposed model of KNN achieves 82.93%, 17.07%, 82.93%, 82.93%, 100%, 17.61%, 0.82, 82.93%, and 82.93% of PA, CMR, Sen, PPV, FPR, FNR, LPR, FMI and F1-score, respectively. Therefore, KNN did not perform well and achieved the lowest testing prediction accuracy as compared with other FML.
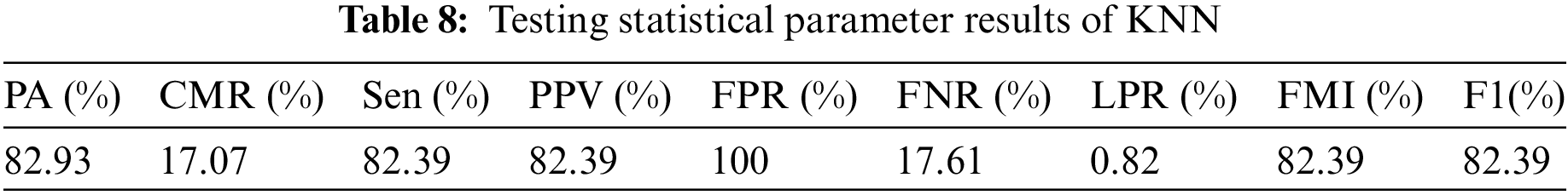
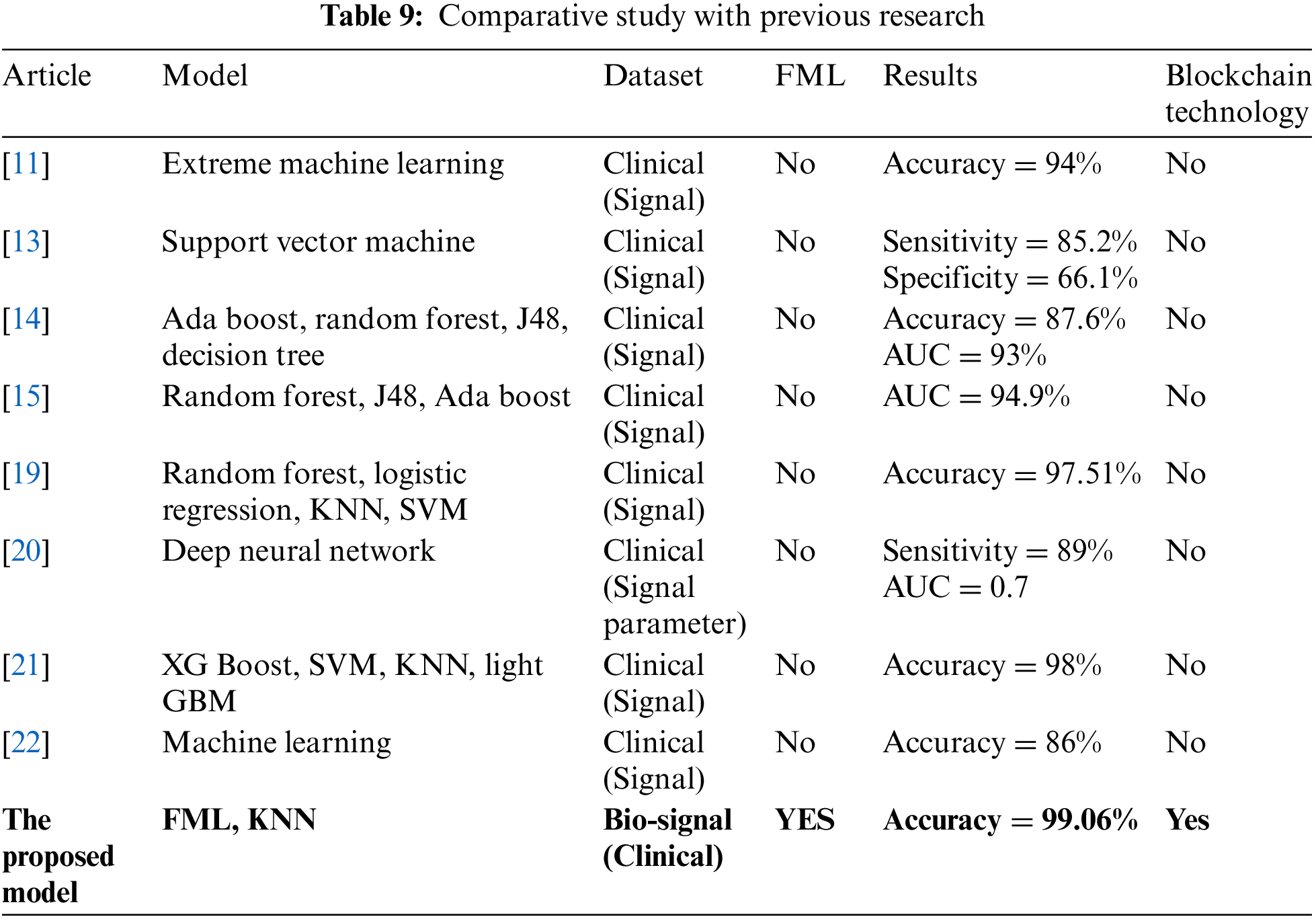
Table 9 shows the comparative analysis of the proposed model with previous studies, it depicts that study [11] used extreme machine learning with a publicly available clinical signal dataset and achieved 94% prediction accuracy and no blockchain technology, study [13] used support vector machine with publicly available clinical signal dataset and achieved 82.5% sensitivity and 66.1% specificity no blockchain technology, study [14] used ada boost, random forest, J48 and decision tree with publicly available clinical signal dataset and achieved 87.6% prediction accuracy and 93% AUC and no blockchain technology, study [15] used random forest, J48 and ada boost with publicly available clinical signal dataset and achieved 94.9% AUC and no blockchain technology, study [19] used random forest, logistic regression, KNN and SVM with publicly available clinical signal dataset and achieved 97.51% prediction accuracy no blockchain technology, study [20] used deep neural network with publicly available clinical signal dataset and achieved 89% sensitivity and 0.7 AUC and no blockchain technology, study [21] used XG boost, SVM, KNN and light GBM with publicly available clinical signal dataset and achieved 98% prediction accuracy no blockchain technology, study [22] used machine learning with publicly available clinical signal dataset and achieved 86% prediction accuracy and no blockchain technology and the proposed model use FML and KNN with bio-signal publicly available clinical signal dataset and achieve 99.06% prediction accuracy with blockchain technology. As results show the proposed model outperformed the previous studies and achieved the highest prediction accuracy.
This research depicts the proposed model of FML for fetal heart rate classification in a mother’s womb for fetal physical health empowered with blockchain technology. The proposed model employed FML for the training and testing simulation of fetal heart rate and it achieved 99.06% testing accuracy with 0.94% CMR also in parallel the proposed model employed KNN to simulate the data and it achieved 82.93% testing accuracy and 17.07 CMR. As the result shows, the proposed FML outperformed KNN. These improved results will be helpful in biotechnology and in the clinical field to improve fetal physical health. The proposed model achieves the best accuracy among the existing studies but needs improvement more using a big dataset to get more accurate and precise results. In the future, this proposed model will be expanded with the merger of a fuzzed model and fuzzed data technology empowered with the Internet of medical things and also this work will be more accurate by the implementation of a weighted federated machine learning technique.
Acknowledgement: Thanks to our families & colleagues who supported us morally.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: Study conception and design: M.U. Nasir, O.K. Khalil, M.A. Khan, M.H. Azam; data collection: K. Ateeq, M.H. Azam; analysis and interpretation of results: M.U. Nasir, M.A. Khan, B.S. Almogadwy and K.M. Adnan; draft manuscript preparation: M.U. Nasir, K. Ateeq, O.K. Khalil, B.S. Almogadwy and K.M. Adnan. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data used in this paper can be requested from the corresponding author upon request.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. G. Sedgh, S. Singh, and R. Hussain, “Intended and unintended pregnancies worldwide in 2012 and recent trends,” Stud. Fam. Plan., vol. 45, no. 3, pp. 301–314, 2014. doi: 10.1111/j.1728-4465.2014.00393.x. [Google Scholar] [PubMed] [CrossRef]
2. C. J. Murray, “Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the global burden of disease study 2013,” Lancet, vol. 385, no. 9963, pp. 117–171, 2015. doi: 10.1016/S0140-6736(14)61682-2. [Google Scholar] [PubMed] [CrossRef]
3. WHO, “World health organization, maternal mortality: Fact sheet,” [Online]. Available: http://www.who.int/mediacentre/factsheets/fs348/en/ (accessed on 05/05 2022). [Google Scholar]
4. D. Ayres-de-Campos, C. Y. Spong, and E. Chandraharan, “FIGO intrapartum fetal monitoring expert consensus panel FIGO consensus guidelines on intrapartum fetal monitoring: Cardiotocography,” Int. J. Gynecol. Obstet., vol. 131, no. 1, pp. 13–24, 2015. doi: 10.1016/j.ijgo.2015.06.020. [Google Scholar] [PubMed] [CrossRef]
5. American College of Obstetricians and Gynecologists, “Practice Bulletin No. 116: Management of intrapartum fetal heart rate tracings,” Obstet. Gynecol., vol. 116, no. 5, pp. 1232–1240, 2010. doi: 10.1097/AOG.0b013e3182004fa9. [Google Scholar] [PubMed] [CrossRef]
6. D. Ayres-De-Campos, C. Y. Spong, and E. Chandraharan, “FIGO guidelines FIGO consensus guidelines on intrapartum fetal monitoring: Cardiotocography FIGO intrapartum fetal monitoring expert consensus panel,” Int. J. Gynecol. Obstet., vol. 131, no. 1, pp. 13–24, 2015. doi: 10.1016/j.ijgo.2015.06.020. [Google Scholar] [PubMed] [CrossRef]
7. G. M. L. Gyte and R. M. Grivell, “Antenatal Cardiotocography for Fetal Assessment,” Cochrane Library, 2019, vol. 9, pp. 7863–7872. [Google Scholar]
8. D. Ayre-de-Campos, C. Costa-Santos, and J. Bernardes, “Prediction of neonatal state by computer analysis of fetal heart rate tracings: The antepartum arm of the SisPorto® multicentre validation study,” European Journal of Obstetrics & Gynecology and Reproductive Biology, vol. 118, pp. 52–60, 2005. [Google Scholar]
9. I. Ingemarsson, “Fetal monitoring during labor,” Neonatology, vol. 95, no. 4, pp. 342–346, 2009. doi: 10.1159/000209299. [Google Scholar] [PubMed] [CrossRef]
10. FIGO News, “Report of the FIGO study group on the assessment of new technology,” Int. J. Gynecol. Obstet., vol. 59, no. 2, pp. 169–173, 1997. doi: 10.1016/S0020-7292(97)00208-7. [Google Scholar] [CrossRef]
11. R. Sindhu, A. B. Jambek, M. Hariharan, and S. C. Neoh, “A novel clinical decision support system using improved adaptive genetic algorithm for the assessment of fetal well-being,” Comput. Math. Methods, vol. 15, pp. 1–11, 2015. [Google Scholar]
12. E. Yılmaz and C. Kılıkçıer, “Determination of fetal state from cardiotocogram using LS-SVM with particle swarm optimization and binary decision tree,” Comput. Math. Methods, vol. 13, pp. 487179–487188, 2013. [Google Scholar]
13. R. Zeng, Y. S. Lu, S. Long, C. Wang, and J. Bai, “Cardiotocography signal abnormality classification using time-frequency features and ensemble cost-sensitive SVM classifier,” Comput. Biol. Med., vol. 134, pp. 104466–104478, 2021. doi: 10.1016/j.compbiomed.2021.104466. [Google Scholar] [PubMed] [CrossRef]
14. G. Improta, C. Ricciardi, F. Amato, G. D. Addio, G. Cesarelli and M. Romano, “Efficacy of machine learning in predicting the kind of delivery by cardiotocography,” Mediterranean Conf. Med. Biol. Eng. Comput., vol. 76, pp. 793–799, 2019. doi: 10.1007/978-3-030-31635-8. [Google Scholar] [CrossRef]
15. C. Ricciardi, G. Improta, F. Amato, G. Cesarelli, and M. Romano, “Classifying the type of delivery from cardiotocographic signals: A machine learning approach,” Comput. Methods Programs Biomed., vol. 196, no. 5, pp. 105712–105723, 2020. doi: 10.1016/j.cmpb.2020.105712. [Google Scholar] [PubMed] [CrossRef]
16. B. Amin, A. A. Salama, I. M. El-Henawy, K. Mahfouz, and M. G. Gafar, “Intelligent neutrosophic diagnostic system for cardiotocography data,” Comput. Intell. Neurosci., vol. 2021, no. 1, pp. 1–12, 2021. doi: 10.1155/2021/6656770. [Google Scholar] [PubMed] [CrossRef]
17. M. Jeżewski, R. Czabański, and J. Łęski, “The influence of cardiotocogram signal feature selection method on fetal state assessment efficacy,” J. Med. Inform. Technol., vol. 23, pp. 1–13, 2014. [Google Scholar]
18. A. H. de l’Aulnoit et al., “Automated fetal heart rate analysis for baseline determination and acceleration/deceleration detection: A comparison of 11 methods versus expert consensus,” Biomed. Signal Process. Control., vol. 49, no. 1, pp. 113–123, 2018. doi: 10.1016/j.bspc.2018.10.002. [Google Scholar] [CrossRef]
19. M. T. Alam et al., “Comparative analysis of different efficient machine learning methods for fetal health classification,” Appl. Bionics Biomech., vol. 9, pp. 1–12, 2022. doi: 10.1155/2022/6321884. [Google Scholar] [PubMed] [CrossRef]
20. J. Ogasawara et al., “Deep neural network-based classification of cardiotocograms outperformed conventional algorithms,” Sci. Rep., vol. 11, no. 1, pp. 13367, 2021. doi: 10.1038/s41598-021-92805-9. [Google Scholar] [PubMed] [CrossRef]
21. N. Rahmayanti, H. Pradani, M. Pahalwan, and R. Vinarti, “Comparison of machine learning algorithms to classify fetal health using cardiotocogram data,” Procedia Comput. Sci., vol. 197, no. 14, pp. 162–171, 2022. doi: 10.1016/j.procs.2021.12.130. [Google Scholar] [CrossRef]
22. M. L. Huang and Y. Y. Hsu, “Fetal distress prediction using discriminant analysis, decision tree, and artificial neural network,” J. Biomed. Sci. Eng., vol. 526, no. 5, pp. 1–12, 2022. [Google Scholar]
23. M. U. Nasir et al., “Breast cancer prediction empowered with fine-tuning,” Comput. Intell. Neurosci., vol. 2022, no. 1, pp. 1–9, 2022. doi: 10.1155/2022/5918686. [Google Scholar] [PubMed] [CrossRef]
24. M. U. Nasir et al., “Single and mitochondrial gene inheritance disorder prediction using machine learning,” Comput. Mater. Contin., vol. 73, no. 1, pp. 953–963, 2022. doi: 10.32604/cmc.2022.028958. [Google Scholar] [PubMed] [CrossRef]
25. A. Rahman et al., “Histopathologic oral cancer prediction using oral squamous cell carcinoma biopsy empowered with transfer learning,” Sensors, vol. 10, no. 10, pp. 3833–3864, 2022. doi: 10.3390/s22103833. [Google Scholar] [PubMed] [CrossRef]
26. T. M. Ghazal et al., “Supervised machine learning empowered multifactorial genetic inheritance disorder prediction,” Comput. Intell. Neurosci., vol. 7, pp. 1–10, 2022. doi: 10.1155/2022/1051388. [Google Scholar] [PubMed] [CrossRef]
27. N. Taleb, S. Mehmood, M. Zubair, I. Naseer, B. Mago and M. U. Nasir, “Ovary cancer diagnosing empowered with machine learning,” in Proc. Int. Conf. on Business Analytics for Technology and Security, UAE, 2022, pp. 1–6. [Google Scholar]
28. S. Rana, “Bio-signal classification for cardiotocography,” [Online]. Available: https://www.kaggle.com/datasets/sohelranaccselab/biomedical-cardiotocography (accessed on 01/04/2022). [Google Scholar]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools