 Open Access
Open Access
ARTICLE
Deep Learning ResNet101 Deep Features of Portable Chest X-Ray Accurately Classify COVID-19 Lung Infection
1 Basic Health Unit Sumra, Lodhran, 59320, Pakistan
2 Basic Health Unit 20-G, Chishtian, Bahawalnagar, 62300, Pakistan
3 Doctor’s Hospital, Lahore, 54590, Pakistan
4 Department of Computer Science & IT, Neelum Campus, The University of Azad Jammu and Kashmir, Athmuqam, 13230, Azad Kashmir, Pakistan
5 Department of Computer Science & IT, King Abdullah Campus, The University of Azad Jammu and Kashmir, Muzaffarabad, 13100, Azad Kashmir, Pakistan
6 College of Computer science and information technology, Shaqra University, Shaqra, 15273, Saudi Arabia
7 Faculty of Engineering and Technology, Future University in Egypt, New Cairo, 11835, Egypt
8 Department of Electrical Engineering, University of Azad Jammu and Kashmir, Chehla Campus, Muzaffarabad, 13100, Azad Kashmir, Pakistan
9 Department of Electrical Engineering, Mirpur University of Science & Technology, Mirpur, Muzaffarabad, 10250, Azad Kashmir, Pakistan
10 Children’s National Hospital, 111 Michigan AVE NW, Washington, DC, 20854, USA
* Corresponding Authors: Lal Hussain. Email: ; Amjad Aldweesh. Email:
Computers, Materials & Continua 2023, 75(3), 5213-5228. https://doi.org/10.32604/cmc.2023.037543
Received 08 November 2022; Accepted 30 January 2023; Issue published 29 April 2023
Abstract
This study is designed to develop Artificial Intelligence (AI) based analysis tool that could accurately detect COVID-19 lung infections based on portable chest x-rays (CXRs). The frontline physicians and radiologists suffer from grand challenges for COVID-19 pandemic due to the suboptimal image quality and the large volume of CXRs. In this study, AI-based analysis tools were developed that can precisely classify COVID-19 lung infection. Publicly available datasets of COVID-19 (N = 1525), non-COVID-19 normal (N = 1525), viral pneumonia (N = 1342) and bacterial pneumonia (N = 2521) from the Italian Society of Medical and Interventional Radiology (SIRM), Radiopaedia, The Cancer Imaging Archive (TCIA) and Kaggle repositories were taken. A multi-approach utilizing deep learning ResNet101 with and without hyperparameters optimization was employed. Additionally, the features extracted from the average pooling layer of ResNet101 were used as input to machine learning (ML) algorithms, which twice trained the learning algorithms. The ResNet101 with optimized parameters yielded improved performance to default parameters. The extracted features from ResNet101 are fed to the k-nearest neighbor (KNN) and support vector machine (SVM) yielded the highest 3-class classification performance of 99.86% and 99.46%, respectively. The results indicate that the proposed approach can be better utilized for improving the accuracy and diagnostic efficiency of CXRs. The proposed deep learning model has the potential to improve further the efficiency of the healthcare systems for proper diagnosis and prognosis of COVID-19 lung infection.Keywords
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), associated with COVID-19 is a single-stranded ribonucleic acid (RNA) virus was found in Wuhan, China, in December 2019 [1,2]. The highly transmissible disease quickly gained global attention and was declared a “public health emergency of international concern” by the World Health Organization (WHO) in January 2020. By March, the disease was officially declared a global pandemic [3]. As of 2nd August 2021, the disease was responsible for 4.2 million confirmed deaths and had affected more than 198 million people worldwide [4]. The virus causes pneumonia with symptoms similar to that of the seasonal flu, which includes fever, dry cough, and fatigue, amongst others.
To date, a standard of care to detect SARS-CoV-2 infection uses Reverse Transcription Polymerase Chain Reaction (RT-PCR) [4]. This method has high specificity but relatively low sensitivity and is usually performed with a nasopharyngeal swab-based sample. The results produced have turnaround times ranging from a few hours to days. Antibody tests also used to predict SARS-CoV-2 infection [1,2].
Portable chest X-ray (CXR) was one of the primary tools to triage patients associated with SARS-CoV-2 infection before RT-PCR being as reliable it is today. To date, CXR is primarily used to assess the extent of lung infection in emergency room settings as well as to evaluate the severity of lung infection in intensive care units (ICU) and to evaluate intubation tube placement in invasive mechanical ventilation. Portable CXR is still widely used in developing countries to detect and evaluate infection. The CXRs have several advantages as it is readily available, cost-effective, and have faster turnaround than other modalities. Moreover, it can provide the extent and location of SARS-CoV2 infection, amongst others. These characteristics are still useful for triaging patients promptly, on time for isolation as well as assisting in ICU care [5]. However, computed topography (CT) is more sensitive and offers more information than CXR. The CT is less assessable, more expensive, and more susceptible to cross-contamination and is not extensively utilized in COVID-19 settings, except earlier in China [1,2].
Artificial intelligence (AI) methods have widely been utilized for the diagnosis and prognosis of different cancer diseases. In this current pandemic, various counties encounter a lack of experienced health professionals for proper diagnosis of the disease. Thus, AI techniques in diagnosing COVID-19 have rapidly gained traction over the past year. The computer-aided diagnosing (CAD) frameworks use graphical processing units (GPUs) to process medical images and to provide a rapid diagnosis of COVID-19. Deep learning (DL) and CAD have been used in combination for many medical imaging applications, including the analysis of COVID-19 [5]. Furthermore, Convolutional neural networks (CNNs) are often used for processing radiological images. As a result, CNN frameworks and DL can also be promising areas of exploration for COVID-19 diagnosis.
The deep learning methods have diverse applications, including liver diseases [6], lung cancer [7], breast cancer [8], brain tumor [9], colon cancer [10], skin cancer [11], pneumonia [12], and more recently COVID-19 analysis exclusive of any human involvement. The principal reason for applying deep learning in disease diagnosis is because, unlike classical machine learning, deep learning (DL) methods learn by forming more abstract representations of data as the network continues to grow deeper. Moreover, deep learning algorithms maximize model accuracy by specifying features through different nonlinear functions organized in a combinatory fashion. Consequently, deep learning models can automatically extract features leading to higher accuracy and precision results [13]. Moreover, deep learning methods focus intensely on automatic extraction and image classification, this has conferred more accurate and efficient predictions and diagnoses [1,2]. By integrating computer-aided diagnosis (CAD) into healthcare systems, doctors will not only be able to reduce their workload, but also give a more exact diagnosis, especially in times of crisis where there will be an inevitable lack of doctors and healthcare personnel [14]. An additional limitation is that in many developing countries, radiologists might be scarce; AI has the potential to solve this issue by providing an immediate diagnostic interpretation or, at the least, offering a second opinion as well as prioritizing a reading list. This will allow for improved workflow, and accessibility Convolutional Neural Networks (CNNs) need trained on the ImageNet dataset that is comprised of images from diverse sources [15,16]. Due to prior training, transferred learning lowers the need for large dataset and, consequently, calculation costs [17].
Recently, researchers utilized different deep learning methods to predict COVID-19 using chest X-ray images [13,18–20] and to utilize CT images [14,21–35]. Some studies even used lung dataset for detecting and diagnosing COVID-19 [4,36], while others used limited dataset with CNN for detecting COVID-19 from chest X-rays, as detailed in [37,38]. Several other researchers took an interest to detect COVID-19 by differentiating it from other chest diseases like pneumonia [5,8,16,20,39]. It was also noted by authors [37] that X-ray images have little impetus in the early stages of the disease, while CT scans prove valuable even earlier symptoms appear. However, one issue associated with chest CT images and chest X-rays is the possibility of overlap in diagnosing COVID-19, chest cancer, and pneumonia. This is especially the case when the healthcare worker diagnosing the patient is inexperienced or lacks patient history files. Since classification models for classifying the three lung diseases have not been heavily explored, this allows to present a model that can automate diagnosing patients to confirm scrupulously whether a patient has one of the three diseases.
This study is aimed to improve the multiclass (COVID-19, normal, pneumonia including viral and bacterial) classification to detect COVID-19 on publicly available dataset recently used in the studies [13,14,38,39]. Though few prior studies have used two-class classification, most of which analyze CT images that are less suitable for contagious disease, our multiclass classification is robust for performance improvement by employing Residual network 101 (ResNet101) with Softmax for classification using (i) default parameters, (ii) optimized hyperparameters, (iii) extraction of in depth features from ResNet101 and fed into k-nearest neighbor (KNN) and support vector machine (SVM) classification algorithms rather than simply using Softmax for classification, from the last pooling layer of ResNet10, the images are retrained. The hyperparameters optimization enhanced the multiclass detection performance. Moreover, the high order features with machine learning classification algorithm provided excellent detection performance because multiclass chest x-ray images are first automatically enhanced learning with the deep learning feature learning approach. The in-depth features are then fed to machine learning algorithms and trained further to enhance performance.
This section below will attempt to cover ongoing attempts of utilizing CXR images to diagnose COVID-19, highlighting attempts of automatic frameworks utilizing CNN. Hemdan introduced COVIDC-Net, a unique network that analyzes normalized forces in CXR images to analyze the positivity of the COVID-19 virus. It combines seven deep convolutional neural networks, including VGG19 and the second form of Google MobileNet [40]. DenseNet and VGG19 networks were seen to detect COVID-19 automatically. Furthermore, Khan proposed CoroNet, a DCNN model with a similar goal [41]. Similarly, Li proposed Covid-MobileXpert, a mobile-based DL network framework [42]. Narin et al. proposed a deep convolutional neural network (CNN) based pre-trained model on chest X-ray images. The deep learning pre-trained models used to classify patients as normal (healthy), COVID-19 infected, bacterial, or viral pneumonia patients are as follows: ResNet50, ResNet101, ResNet152, InceptionV3, and InceptionResNetV2 [14].
Researchers recently developed Artificial intelligence (AI) based CAD tools that automated the detection of COVID-19 and improved the healthcare system. Shahin et al. [43] proposed a machine learning-based CAD system, which comprised of CT lungs screening collection, pre-processing to improve the ground glass opacities (GGOs), segmentation and modifying the machine learning algorithms. Rehman et al. [44] conducted a systematic review to summarise the machine learning and deep learning algorithms for COVID-19 classification, prediction, and detection using clinical and radiological dataset. The outcomes can be helpful for healthcare practitioners and radiologists for improved diagnosis and prognosis. Chen et al. [45] developed a method based on medical screening and isolation measures numerical methods for assessing and simulating the virus’s transmission and spread. Hou et al. [46] proposed a deep learning (DL) model with six-layers by combing batch normalization, max pooling and Adam algorithm for improving the COVID-19 detection. Agrawal et al. [47] developed deep learning model for improving COVID-19 detection. Gupta et al. [48] improved COVID-19 detection by utilizing deep learning models. Abir et al. [49] proposed a CovNet model based on Long Short-term Memory Variational Autoencoder (LSTM-VAE) to detect COVID-19 infection with wearable devices i.e., fitness trackers and smartwatches in the pre-symptomatic stage from resting heart rate (RHR).
Previously, the researchers not considered most of the factors to detect COVID-19. In this study, deep learning models were proposed to detect COVID-19 from multiclass (i.e., COVID-19, normal, pneumonia; COVID-19, normal, viral, COVID, bacterial, normal) with multi-approach. First, different pre-processing methods were utilized, such as normalization, augmentation, resizing and splitting. The ResNet101 model was pre-trained on the ImageNet dataset using the transfer learning approach, which is trained on 1000 classes. In this case, the last three layers were removed i.e., {‘fc1000’, ‘prob’, ‘ClassificationLayer_predictions’} and replaced with the new classes of the current study. Secondly, the ResNet101 features were extracted from the last average pooling layer with default parameters. Thirdly, the ResNet101 features, by optimizing the hyperparameters were computed from the last average pooling layer. Fourthly, the extracted features from ResNet101 obtained from the last average pooling layers were fed into machine learning KNN and SVM as detailed in Fig. 1. Below. The Section 2 summarizes the methodology, Section 3 describes the results, and discussions and Section 4 show the conclusions.

Figure 1: Schematic diagram to predict COVID-19 lung infection using deep convolutional neural network (CNN) ResNet101 by optimizing parameters and with deep features
This section presents the dataset used and methods utilized.
Standard datasets, such as the Italian Society of Medical and Interventional Radiology (SIRM), The Cancer Imaging Archive (TCIA), Radiopaedia and the Radiological Society of North America (RSNA), contain collections of familiar pneumonia X-ray images were utilized to differentiate COVID-19 from normal pneumonia. The COVID-19 (N = 1525) was taken from GitHub collected by Cohen et al. [50], Radiopaedia and TCIA. The pneumonia (N = 3863) and chest x-ray healthy (N = 1525) were collected from the Kaggle repository and National Institutes of Health (NIH) dataset [39]. Fig. 1. shows the workflow of our model.
2.2 Convolutions Neural Network
The CNN model has a significant role in extracting dynamic features, rather than manual features elimination. The features extracted from the activation map are also fed back to machine learning (ML) algorithms [51]. This has made the CNN models very useful compared to prior machine learning algorithms. To train the CNN models, two approaches are used: (1) train with no prior training, (2) train using transfer learning. Following the first approach, training the CNN model requires many samples for training from scratch. With limited data, as in our case, this training can prove inadequate. However, the second option addresses this issue, as the CNN models can be retrained by using a process known as transfer learning. This approach is the most effective method to adopt the knowledge [52]. In common transfer learning (TL) approach, convolutional layers (CL) are used as fixed feature extractors, and only fully connected layers (FL) are set for a new specific task [53]. The CNN comprised of convolution layer, fully connected layer and a pooling layer. In this study, we used multiclass (COVID-19, pneumonia, and normal). Thus, new fully connected layer has only three neurons.
2.3 Transfer Learning Approach
We applied the transfer learning approach; this means the networks such as ResNet101 of Convolution Neural Network were pre-trained on a large dataset. The network ResNet101 consisted of inception layers, convolution layers and fully connected layers. In this case, the ImageNet dataset consisting of 14 million images was used to pre-train the network. This initial training helps the first layer to find extremely generatable features from a more extensive datasets; later layers of the network take on specifics of smaller dataset for the adaptive model. We used ResNet101 in our study, described in the below section:
The convolutional neural networks require high computational resources as very high operations are performed for performing convolution and pooling operations for computing low-level, mid-level and high-level features, weight filters, and weight channels. For example, if we have pooling with filter 512, then memory and parameters are computed as depicted below:
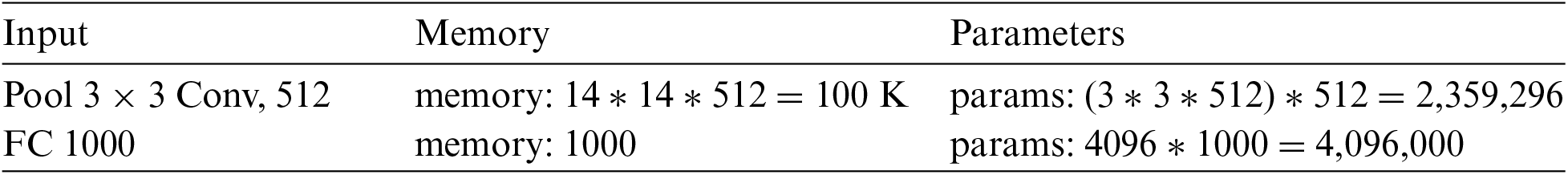
He et al., proposed the ResNet model [54] in 2016, an abbreviation of the residual network. This method is used in diverse applications, medical imaging, pattern recognition, computer vision etc. The CNN is comprised of multiple layers connected to each other in a specific manner and trained for performing various tasks [55]. There are 104 convolutional layers (CL) with 33 filters one filter for each layer, respectively. In the residual connection, 9 out of 33 layers use the previous layer output directly. The residual connections have used an operand for summation operations. The four remaining layers receive the output of the previous block as input and apply it to the convolutional layer (CL) with a filter size of 1 × 1 and a stride of 1, followed by a group of normalization layers.
2.4 Training/Testing Data Formulation
The standard 10-fold cross-validation (CV) method was utilized for testing and training data validation as detailed in [56–59].
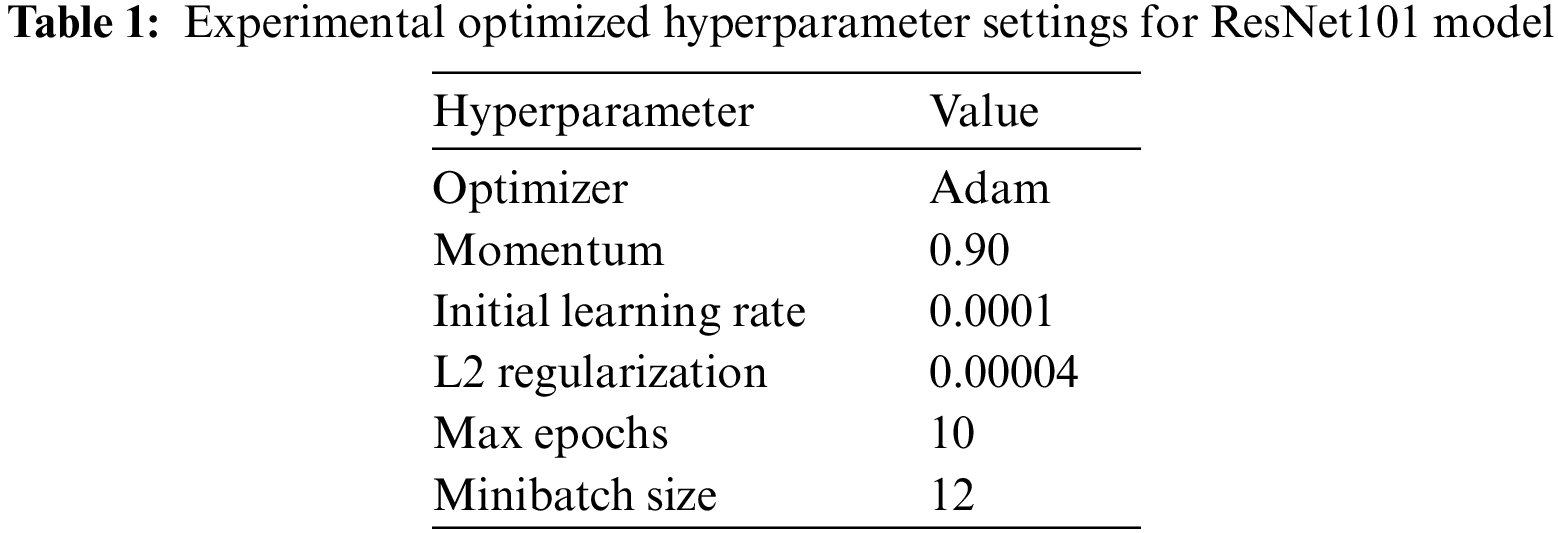
In this study, ResNet101-softmax with default and optimized parameters, ResNet101-KNN, and ResNet101-SVM were applied for multiclass COVID-19 detection. The hyperparameters of the ResNet101 model were optimized with the following parameters reflected in Table 1.
The performance was computed in terms standard performance evaluation parameters. Table 2 presents multiclass class classification (COVID, Normal, Pneumonia; Bacterial, COVID, Normal; Bacterial, Viral, Normal) results using Deep Learning ResNet101. The highest performance with default hyperparameters using ResNet101 yielded with multiclass (COVID-19, normal, pneumonia) in detecting pneumonia (99.29% accuracy, 0.99 AUC) followed by COVID-19 (98.08% accuracy, 0.98 AUC). The multiclass (Bacterial, COVID-19, normal) yield the highest accuracy to detect bacteria (98.20% accuracy, 0.98 AUC) followed by normal (97.91% accuracy, 0.98 AUC), COVID-19 (96.11% accuracy, 0.96 AUC). The multiclass (COVID-19, viral, normal) yield the highest accuracy to detect viral (99.62% accuracy, 1.00 AUC) followed by normal (96.21% accuracy, 0.96 AUC), COVID-19 (95.83% accuracy, 0.96 AUC).
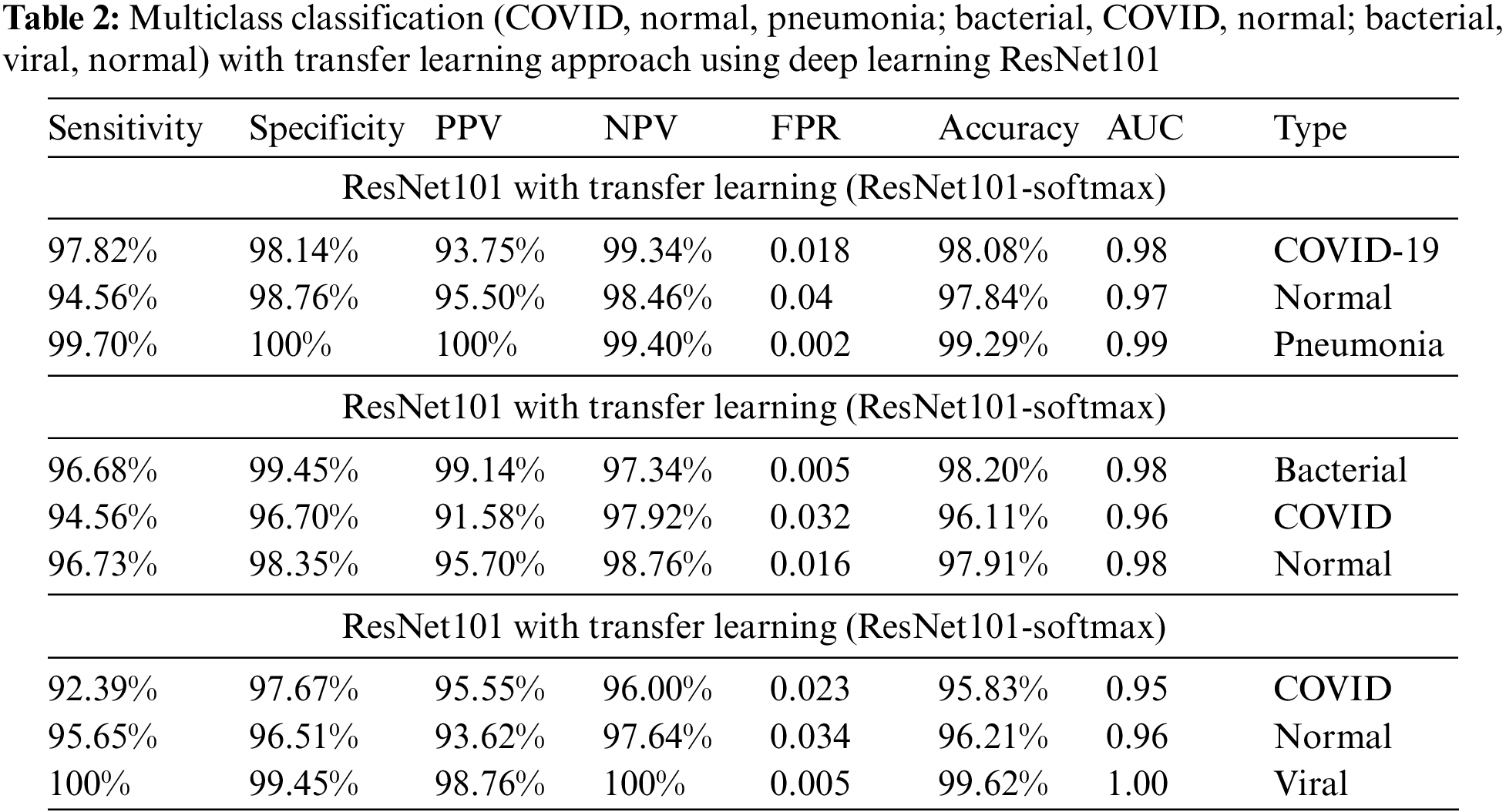
Fig. 2. reflects the confusion matrix of multiclass to predict COVID-19 using ResNet101 with 10-fold class validation.

Figure 2: Confusion matrix to detect multiclass (COVID-19, normal, pneumonia) on test data using deep learning ResNet101
Table 3 shows the results of the multiclass classification with optimized hyperparameters using transfer learning approach. A highest detection performance with optimized hyperparameters using ResNet101 yielded with multiclass (COVID-19, normal, pneumonia) to detect pneumonia (98.80% accuracy, 0.99 AUC) followed by COVID-19 (98.32% accuracy, 0.98 AUC). The multiclass (Bacterial, COVID-19, normal) yield the highest accuracy to detect bacteria (98.51% accuracy, 0.99 AUC) followed by normal (96.61% accuracy, 0.97 AUC), COVID-19 (96.71% accuracy, 0.95 AUC). The multiclass (COVID-19, normal, viral) yield the highest accuracy to detect viral (99.62% accuracy, 0.99 AUC) followed by normal (96.97% accuracy, 0.97 AUC), COVID-19 (96.59% accuracy, 0.96 AUC).
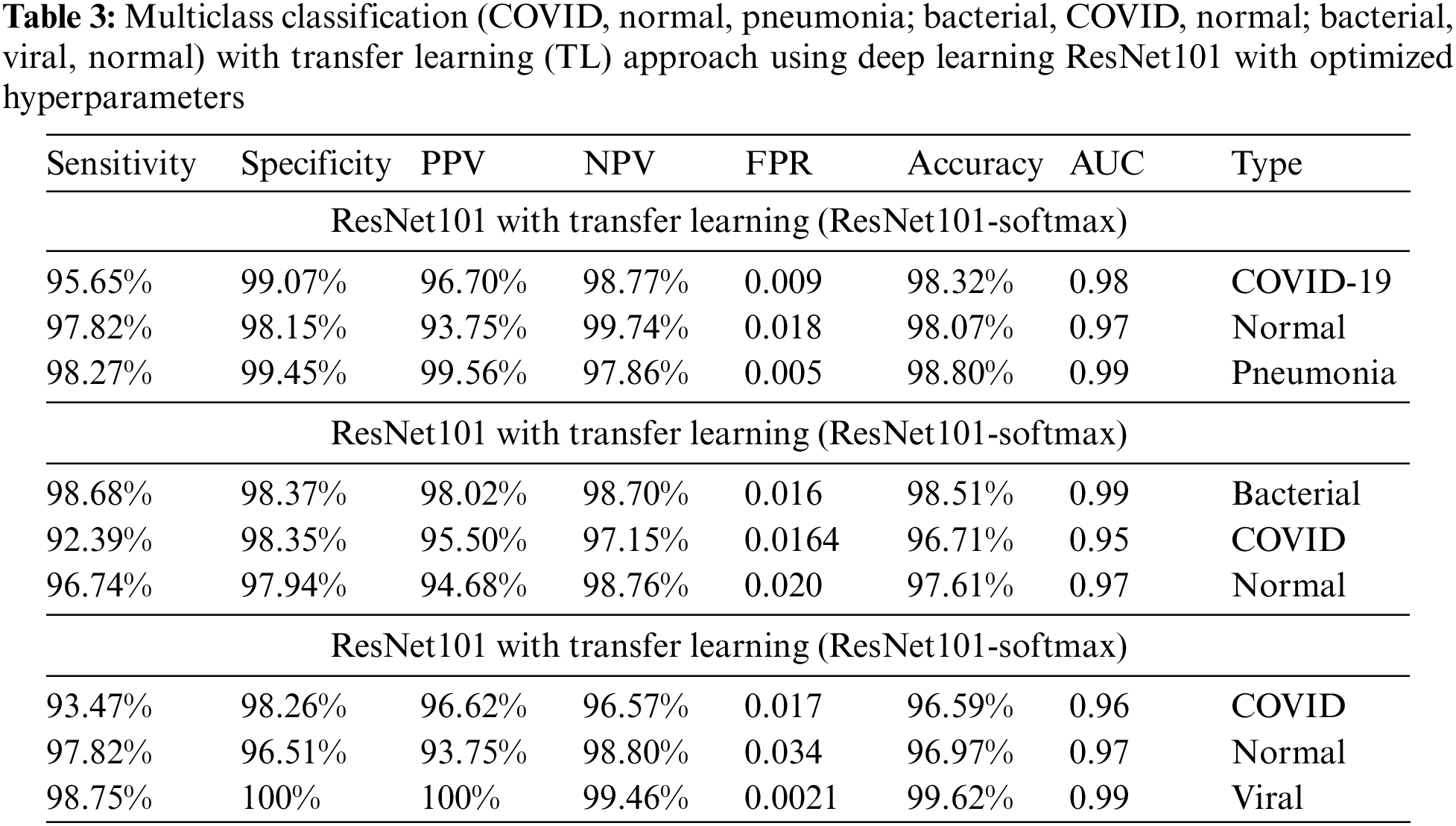
Fig. 3. reflects the accuracy and loss graph of training and validation data using ResNet101 for multiclass classification. The accuracy of training and validation data is reflected in the upper portion of the graph. At the 150th epoch, the performance was yielding (100% accuracy, 0.0004 error) and (99.03% validation accuracy, 0.0764 error).

Figure 3: Accuracy and loss plot to classify COVID-19, pneumonia and normal by reflecting validation, training accuracy and corresponding error utilizing ResNet101 with transfer learning
The Multiclass (Covid-19, normal, viral) classification using ResNet-KNN is reflected in Table 4.

The Multiclass (Covid-19, normal, viral) classification using ResNet-SVM is presented in Table 5.

Table 6 reflects the results of the multiclass (COVID-19, viral and normal) classification by extracting deep features from ResNet101 the average pooling layer and fed to machine learning algorithms such as SVM linear and KNN. The ResNet101-SVM yielded 99.84% performance to detect viral, 98.56% in detecting COVID-19, whereas the ResNet101-KNN yielded 99.46% accuracy in predicting viral.

Fig. 4. depicts the multiclass classification using deep learning ResNet101-SVM yielded AUC (0.98) to detect COVID, AUC (0.99) to detect normal, and AUC (1.00) to detect viral.

Figure 4: AUC to distinguish multiclass (normal, COVID-19, pneumonia) using deep learning ResNet101-SVM
This study aimed to optimize and employ robust deep learning ResNet101 model to detect COVID-19 from multiclass of other community pneumonia accurately to detect COVID-19 from multiclass of other community pneumonia accurately. We computed the multiclass with and without optimizing the hyperparameters. With default hyperparameters, the overall sensitivity, specificity, accuracy, and AUC were 97.82%, 98.14%, 98.08%, and 0.98, respectively, to detect COVID-19 from multiclass. With the optimized parameters, the performance was improved sensitivity, specificity, accuracy, and AUC were 95.65%, 96.70%%, 98.32%, and 0.98, respectively to detect COVID-19. With optimized hyperparameters, overall accuracy and AUC were 98.51%, 0.99 respectively, were yielded to detect COVID-19 from multiclass (Bacterial, COVID-19, normal), whereas, accuracy and AUC of 96.59%, 0.96 were obtained to predict COVID-19 from multiclass (COVID-19, normal, viral). By extracting the deep features from ResNet101 and employing machine learning algorithms, the SVM linear yielded the highest accuracy and AUC of 100% and 1.00 to predict COVID-19 from multiclass (COVID-19, normal, bacterial) and (COVID-19, viral, normal). By applying KNN for classification using deep features, the overall accuracy of 99.73%, AUC of 0.9982 yielded to detect COVID-19 from multiclass (Normal, COVID-19, bacterial). Likewise, an accuracy of 99.46%, and AUC of 0.9966 yielded to detect COVID-19 with deep features using KNN from multiclass (COVID-19, normal, viral).
We have used a multiclass approach with 03 group combinations from COVID-19, bacterial, viral pneumonia and normal to detect COVID-19, bacterial, viral pneumonia and normal. Moreover, we used the default parameters of ResNet101. We also optimized the parameters of the Adam optimizer with (learning rate = 0.0003, beta-1, beta-2, epsilon). We also extracted in-depth features from the last pooling layer of ResNet101 and retrained the machine-learning algorithms based on these features. The SVM and KNN algorithms are used for classification.
Table 7 compares AI findings used by researchers to detect COVID-19. Most of these studies show the two-class classification using machine learning or deep learning. Ghoshal et al. [60] applied CNN and obtained an accuracy of 92.9%. Zhang et al. [61] utilized ResNet and obtained sensitivity (96.0%), specificity (79.7%) and AUC (0.952). Channa et al. [62] applied CNN and obtained an accuracy of 91.67%. Sethy et al. [63] applied ResNet50-SVM and obtained an accuracy of 95.33%. Hussain et al. [39] applied machine learning with texture features and obtained a two-class classification of 100% accuracy (COVID vs. Normal). Previous studies have conducted COVID-19 multiclass studies that have resulted in high accuracy rates. Sahinbas et al. used deep-SqueezeNet to analyze normal, pneumonia, and COVID-19 X-rays and achieved 98.26% accuracy [64]. Ucar et al. used pre-trained models VGG19, InceptionV3, ResNet, DenseNet, and VGG16. The ladder with which they achieved 80% accuracy [65]. Khan et al. utilized the CoroNet Model in a multi-study (normal, pneumonia-bacterial, pneumonia viral, COVID-19) to achieve an 89.6% performance. They analyzed 290 COVID-19 data, more than most studies mentioned above [41]. Medhi worked with deep CNN networks and achieved a 93% performance [66]. On the other hand, Zhang utilized the confidence-aware anomaly detection (CAAD) model in their binary and multiclass analysis of COVID-19 data, to reach a 95.18% accuracy level [67]. Postopus used the VGG-19 CNN model to analyze multiclass (COVID-10, bacterial, normal) X-ray images. They reached a 93.48% accuracy [68].

The multiclass deep-learning ResNet101 was able to accurately discriminate lung infection caused by COVID-19 from non-COVID-19 bacterial and viral pneumonia. This study was multi-fold; first, the Deep learning ResNet101 with default and optimized parameters was employed for multiclass COVID-19 detection and yielded the highest accuracy of 99.62%. In the second approach, the deep features were computed from ResNet101, the average pooling layer and fed back to machine learning SVM and KNN. The detection performance was improved, yielding an accuracy of 99.84% and AUC of 1.00 utilizing ResNet101-SVM as the model was and learned twice using this approach. This approach can help clinicians and radiologists improve workflow, diagnose SAR-CoV2 infection earlier, track disease progression, and triage patients affordably and effectively.
Limitations
This current study has several specific limitations as the retrospective study was carried out with a small COVID-19 dataset. CXR, the principal imaging modality investigated in this paper, is not highly specific as PCR SAR-CoV2 diagnosis. Furthermore, sample sizes for this study were small and only investigated four classes (normal, COVID-19, bacterial pneumonia, and viral pneumonia). Future studies should test these algorithms with larger sets of data, as well as investigate new algorithms with additional types of lung infections. In future, hyperparameters optimization of deep learning algorithms using other Bayesian optimization methods and will apply the image fusion techniques to combine the hand-crafted and dynamics features for further improving the detection performance.
Acknowledgement: The authors would like to thank the Deanship of Scientific Research. At Shaqra University for supporting.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. C. Huang, Y. Wang, X. Li, L. Ren, J. Zhao et al., “Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China,” Lancet, vol. 395, no. 10223, pp. 497–506, 2020. [Google Scholar] [PubMed]
2. N. Zhu, D. Zhang, W. Wang, X. Li, B. Yang et al., “A novel coronavirus from patients with pneumonia in China, 2019,” The New England Journal of Medicines, vol. 382, no. 8, pp. 727–733, 2020. [Google Scholar] [PubMed]
3. S. Albahli, “Efficient GAN-based chest radiographs (CXR) augmentation to diagnose coronavirus disease pneumonia,” International Journal of Medical Sciences, vol. 17, no. 10, pp. 1439–1448, 2020. [Google Scholar] [PubMed]
4. “WHO coronavirus (COVID-19) dashboard | WHO coronavirus (COVID-19) dashboard with vaccination data,” Accessed on 25-07-2022. [Google Scholar]
5. H. Kang, L. Xia, F. Yan, Z. Wan, F. Shi et al., “Diagnosis of coronavirus disease 2019 (COVID-19) with structured latent multi-view representation learning,” IEEE Transactions on Medical Imaging, vol. 39, no. 8, pp. 2606–2614, 2020. [Google Scholar] [PubMed]
6. Z. Yao, J. Li, Z. Guan, Y. Ye and Y. Chen, “Liver disease screening based on densely connected deep neural networks,” Neural Networks, vol. 123, no. 11A, pp. 299–304, 2020. [Google Scholar] [PubMed]
7. W. Ausawalaithong, A. Thirach, S. Marukatat and T. Wilaiprasitporn, “Automatic lung cancer prediction from chest X-ray images using the deep learning approach,” in 2018 11th Biomedical Engineering Int. Conf. (BMEiCON), Chiang Mai, Thailand, IEEE, pp. 1–5, 2018. [Google Scholar]
8. D. A. Ragab, M. Sharkas, S. Marshall and J. Ren, “Breast cancer detection using deep convolutional neural networks and support vector machines,” PeerJ, vol. 7, no. 1, pp. e6201, 2019. [Google Scholar] [PubMed]
9. X. W. Gao, R. Hui and Z. Tian, “Classification of CT brain images based on deep learning networks,” Computer Methods and Programs in Biomedicine, vol. 138, no. 1, pp. 49–56, 2017. [Google Scholar] [PubMed]
10. I. Pacal, D. Karaboga, A. Basturk, B. Akay and U. Nalbantoglu, “A comprehensive review of deep learning in colon cancer,” Computers in Biology and Medicine, vol. 126, no. 1, pp. 104003, 2020. [Google Scholar] [PubMed]
11. A. Esteva, B. Kuprel, R. A. Novoa, J. Ko and S. M. Swetter, “Dermatologist-level classification of skin cancer with deep neural networks,” Nature, vol. 542, no. 7639, pp. 115–118, 2017. [Google Scholar] [PubMed]
12. N. M. Elshennawy and D. M. Ibrahim, “Deep-pneumonia framework using deep learning models based on chest X-ray images,” Diagnostics, vol. 10, no. 9, pp. 649, 2020. [Google Scholar] [PubMed]
13. D. M. Ibrahim, N. M. Elshennawy and A. M. Sarhan, “Deep-chest: Multi-classification deep learning model for diagnosing COVID-19, pneumonia, and lung cancer chest diseases,” Computers in Biology and Medicin, vol. 132, no. 2, pp. 104348, 2021. [Google Scholar] [PubMed]
14. A. Narin, C. Kaya and Z. Pamuk, “Automatic detection of coronavirus disease (COVID-19) using X-ray images and deep convolutional neural networks,” Pattern Analysis and Applications, vol. 24, no. 3, pp. 1207–1220, 2021. [Google Scholar] [PubMed]
15. K. Simonyan and A. Zisserman, “Very Deep Convolutional Networks for Large-Scale Image Recognition,” arXiv preprint arXiv:1409, pp. 1556, 2014. [Google Scholar]
16. Y. Lecun, Y. Bengio and G. Hinton, “Deep learning,” Nature, vol. 521, no. 7553, pp. 436–444, 2015. [Google Scholar] [PubMed]
17. Z. Huang, Z. Pan and B. Lei, “Transfer learning with deep convolutional neural network for SAR target classification with limited labeled data,” Remote Sensing, vol. 9, no. 9, pp. 907, 2017. [Google Scholar]
18. A. Abbas, M. M. Abdelsamea and M. M. Gaber, “Classification of COVID-19 in chest X-ray images using DeTraC deep convolutional neural network,” Applied Intelligence, vol. 51, no. 2, pp. 854–864, 2020. [Google Scholar] [PubMed]
19. S. Asif, Y. Wenhui, H. Jin and S. Jinhai, “Classification of COVID-19 from chest X-ray images using deep convolutional neural network,” in 2020 IEEE 6th Int. Conf. on Computer and Communications, ICCC 2020, Chengdu, China, pp. 426–433, 2020. [Google Scholar]
20. E. E. -D. Hemdan, M. A. Shouman and M. E. Karar, “COVIDX-Net: A framework of deep learning classifiers to diagnose COVID-19 in X-ray images,” arXiv preprint arXiv:2003, pp. 11055, 2020. [Google Scholar]
21. X. Wang, X. Deng, Q. Fu, Q. Zhou, J. Feng et al., “A weakly-supervised framework for COVID-19 classification and lesion localization from chest CT,” IEEE Transactions on Medical Imaging, vol. 39, no. 8, pp. 2615–2625, 2020. [Google Scholar] [PubMed]
22. J. Zhao, Y. Zhang, X. He and P. Xie, “COVID-CT-dataset: A CT scan dataset about COVID-19,” arXiv preprint arXiv:2003, pp. 13865490, 2020. [Google Scholar]
23. O. Gozes, M. Frid-Adar, N. Sagie, H. Zhang, W. Ji et al., “Coronavirus detection and analysis on chest CT with deep learning,” arXiv preprint arXiv:2004.02640, 2020. [Google Scholar]
24. S. Wang, B. Kang, J. Ma, X. Zeng, M. Xiao et al., “A deep learning algorithm using CT images to screen for corona virus disease (COVID-19),” European Radiology, vol. 31, no. 8, pp. 6096–6104, 2021. [Google Scholar] [PubMed]
25. L. Li, L. Qin, Z. Xu, Y. Yin, Wang et al., “Artificial intelligence distinguishes COVID-19 from community acquired pneumonia on chest CT,” Radiology, vol. 296, no. 6, pp. 200905, 2020. [Google Scholar] [PubMed]
26. J. Chen, L. Wu, J. Zhang, L. Zhang, D. Gong et al., “Deep learning-based model for detecting 2019 novel coronavirus pneumonia on high-resolution computed tomography,” Scientific Reports, vol. 10, no. 1, pp. 19196, 2020. [Google Scholar]
27. B. Wang, S. Jin, Q. Yan, H. Xu, C. Luo et al., “AI-assisted CT imaging analysis for COVID-19 screening: Building and deploying a medical AI system,” Applied Soft Computing, vol. 98, no. 10223, pp. 106897, 2021. [Google Scholar] [PubMed]
28. M. Yamac, M. Ahishali, A. Degerli, S. Kiranyaz, M. E. H. Chowdhury et al., “Convolutional sparse support estimator-based COVID-19 recognition from X-Ray images,” IEEE Transactions on Neural Networks Learning Systems, vol. 32, no. 5, pp. 1810–1820, 2021. [Google Scholar] [PubMed]
29. S. Hassantabar, M. Ahmadi and A. Sharifi, “Diagnosis and detection of infected tissue of COVID-19 patients based on lung x-ray image using convolutional neural network approaches,” Chaos, Solitons & Fractals, vol. 140, no. 7553, pp. 110170, 2020. [Google Scholar]
30. Y. Oh, S. Park and J. C. Ye, “Deep learning COVID-19 features on CXR using limited training data sets,” IEEE Transactions on Medical Imaging, vol. 39, no. 8, pp. 2688–2700, 2020. [Google Scholar] [PubMed]
31. M. Loey, F. Smarandache and N. E. M. Khalifa, “Within the lack of chest COVID-19 X-ray dataset: A novel detection model based on GAN and deep transfer learning,” Symmetry, vol. 12, no. 4, pp. 651, 2020. [Google Scholar]
32. T. Ozturk, M. Talo, E. A. Yildirim, U. B. Baloglu and O. Yildirim, “Automated detection of COVID-19 cases using deep neural networks with X-ray images,” Computers in Biology and Medicine, vol. 121, no. 7798, pp. 103792, 2020. [Google Scholar] [PubMed]
33. A. A. Ardakani, A. R. Kanafi, U. R. Acharya, N. Khadem and A. Mohammadi, “Application of deep learning technique to manage COVID-19 in routine clinical practice using CT images: Results of 10 convolutional neural networks,” Computers in Biology and Medicine, vol. 121, no. 10229, pp. 103795, 2020. [Google Scholar] [PubMed]
34. T. Yan, P. K. Wong, H. Ren, H. Wang and J. Wang, “Automatic distinction between COVID-19 and common pneumonia using multi-scale convolutional neural network on chest CT scans,” Chaos, Solitons & Fractals, vol. 140, pp. 110153, 2020. [Google Scholar]
35. N. E. M. Khalifa, M. H. N. Taha, A. E. Hassanien and S. Elghamrawy, “Detection of coronavirus (COVID-19) associated pneumonia based on generative adversarial networks and a fine-tuned deep transfer learning model using chest X-ray dataset,” in Int. Conf. on Advanced Intelligent Systems and Informatics, Cham, Springer, pp. 234–247, 2020. [Google Scholar]
36. H. S. Maghdid, A. T. Asaad, K. Z. Ghafoor, A. S. Sadiq and S. Mirjalili, “Diagnosing COVID-19 pneumonia from x-ray and CT images using deep learning and transfer learning algorithms,” in Multimodal image exploitation and learning 2021, Florida, United States: SPIE, pp. 99–110, 2021. [Google Scholar]
37. V. Perumal, V. Narayanan and S. J. S. Rajasekar, “Detection of COVID-19 using CXR and CT images using Transfer Learning and Haralick features,” Applied Intelligence, vol. 51, no. 1, pp. 341–358, 2020. [Google Scholar] [PubMed]
38. T. Goel, R. Murugan, S. Mirjalili and D. K. Chakrabartty, “OptCoNet: An optimized convolutional neural network for an automatic diagnosis of COVID-19,” Applied Intelligence, vol. 51, no. 3, pp. 1351–1366, 2021. [Google Scholar] [PubMed]
39. L. Hussain, T. Nguyen, H. Li, A. A. Abbasi, K. J. Lone et al., “Machine-learning classification of texture features of portable chest X-ray accurately classifies COVID-19 lung infection,” Biomedical Engineering Online, vol. 19, no. 1, pp. 88, 2020. [Google Scholar] [PubMed]
40. E. E. -D. E. -D. Hemdan, M. A. A. Shouman and M. E. E. Karar, “COVIDX-Net: A framework of deep learning classifiers to diagnose COVID-19 in X-Ray images,” arXiv preprint arXiv:2003.11055, 2020. [Google Scholar]
41. A. I. Khan, J. L. Shah and M. M. Bhat, “CoroNet: A deep neural network for detection and diagnosis of COVID-19 from chest x-ray images,” Computer Methods and Programs in Biomedicine, vol. 196, no. 18, pp. 105581, 2020. [Google Scholar] [PubMed]
42. L. Li, L. Qin, Z. Xu, Y. Yin, X. Wang et al., “Using artificial intelligence to detect COVID-19 and community-acquired pneumonia based on pulmonary CT: Evaluation of the diagnostic accuracy,” Radiology, vol. 296, no. 2, pp. E65–E71, 2020. [Google Scholar] [PubMed]
43. O. R. Shahin, H. H. Alshammari, A. I. Taloba and R. M. A. El-Aziz, “Machine learning approach for autonomous detection and classification of COVID-19 virus,” Computers and Electrical Engineering, vol. 101, no. 2, pp. 108055, 2022. [Google Scholar] [PubMed]
44. A. Rehman, M. A. Iqbal, H. Xing and I. Ahmed, “COVID-19 detection empowered with machine learning and deep learning techniques: A systematic review,” Applied Sciences, vol. 11, no. 8, pp. 3414, 2021. [Google Scholar]
45. Z. Chen, H. Zha, Z. Shu, J. Ye and J. Pan, “Assess medical screening and isolation measures based on numerical method for COVID-19 epidemic model in Japan,” Computer Modeling in Engineering & Sciences, vol. 130, no. 2, pp. 841–854, 2022. [Google Scholar]
46. S. Hou and J. Han, “COVID-19 detection via a 6-layer deep convolutional neural network,” Computer Modeling in Engineering & Sciences, vol. 130, no. 2, pp. 855–869, 2022. [Google Scholar]
47. T. Agrawal and P. Choudhary, “FocusCovid: Automated COVID-19 detection using deep learning with chest X-ray images,” Evolving Systems, vol. 13, no. 4, pp. 519–533, 2022. [Google Scholar]
48. V. Gupta, N. Jain, J. Sachdeva, M. Gupta and S. Mohan, “Improved COVID-19 detection with chest x-ray images using deep learning,” Multimedia Tools and Applications, vol. 81, no. 26, pp. 37657–37680, 2022. [Google Scholar] [PubMed]
49. F. F. Abir, K. Alyafei, M. E. H. Chowdhury, A. Khandakar and R. Ahmed, “PCovNet: A presymptomatic COVID-19 detection framework using deep learning model using wearables data,” Computers in Biology and Medicine, vol. 147, no. 13, pp. 105682, 2022. [Google Scholar] [PubMed]
50. J. P. Cohen, P. Morrison, L. Dao, K. Roth, T. Q. Duong et al., “COVID-19 Image Data Collection: Prospective Predictions Are the Future,” arXiv preprint arXiv:2006.11988. 2020. [Google Scholar]
51. J. Deng, W. Dong, R. Socher, L. Li and K. Li, “ImageNet: A large-scale hierarchical image database,” in 2009 IEEE Conf. on Computer Vision and Pattern Recognition, Miami, Fl, USA, pp. 248–255, 2009. [Google Scholar]
52. S. M. Salaken, A. Khosravi, T. Nguyen and S. Nahavandi, “Seeded transfer learning for regression problems with deep learning,” Expert Systems with Applications, vol. 115, no. 9, pp. 565–577, 2019. [Google Scholar]
53. C. Mazo, J. Bernal, M. Trujillo and E. Alegre, “Transfer learning for classification of cardiovascular tissues in histological images,” Computer Methods and Programs in Biomedicine, vol. 165, no. 2, pp. 69–76, 2018. [Google Scholar] [PubMed]
54. J. He, K. Zhang, X. Ren and S. Sun, “Deep residual learning for image recognition,” in Proc. of the IEEE Conf. on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, pp. 770–778, 2016. [Google Scholar]
55. C. Sun, A. Shrivastava, S. Singh and A. Gupta, “Revisiting unreasonable effectiveness of data in deep learning era,” in Proc. of the IEEE Int. Conf. on Computer Vision, Venice, Italy, pp. 843–852, 2011. [Google Scholar]
56. S. Rathore, M. Hussain and A. Khan, “Automated colon cancer detection using hybrid of novel geometric features and some traditional features,” Computers in Biology and Medicine, vol. 65, no. March, pp. 279–296, 2015. [Google Scholar] [PubMed]
57. Y. Asim, B. Raza, A. K. Malik, S. Rathor, L. Hussain et al., “A multi-modal, multi-atlas-based approach for Alzheimer detection via machine learning,” International Journal of Imaging System & Technology, vol. 28, no. 2, pp. 113–123, 2018. [Google Scholar]
58. L. Hussain, A. Ahmed, S. Saeed, S. Rathore, I. Ahmed et al., “Prostate cancer detection using machine learning techniques by employing combination of features extracting strategies,” Cancer Biomarkers, vol. 21, no. 2, pp. 393–413, 2018. [Google Scholar] [PubMed]
59. S. Iqbal, G. F. Siddiqui, A. Rehman, L. Hussain, T. Saba et al., “Prostate cancer detection using deep learning and traditional techniques,” IEEE Access, vol. 9, pp. 27085–27100, 2021. [Google Scholar]
60. B. Ghoshal and A. Tucker, “Estimating uncertainty and interpretability in deep learning for coronavirus (COVID-19) detection,” arXiv preprint arXiv:2003.10769, 2020. [Google Scholar]
61. J. Zhang, Y. Xie, Y. Li, C. Shen and Y. Xia, “COVID-19 screening on chest X-ray images using deep learning based anomaly detection,” arXiv preprint arXiv:2003.12338, 2020. [Google Scholar]
62. A. Channa, N. Popescu and R. Malik, “Robust technique to detect COVID-19 using chest X-ray images,” in 2020 Int. Conf. on e-Health and Bioengineering (EHB), Iasi, Romania, IEEE, pp. 1–6, 2020. [Google Scholar]
63. P. K. Sethy, “Detection of coronavirus disease (COVID-19) based on deep features and support vector machine,” International Journal of Mathematical, Engineering and Management Sciences, vol. 5, no. 4, pp. 643, 2020. [Google Scholar]
64. K. Sahinbas and F. O. Catak, “Transfer learning-based convolutional neural network for COVID-19 detection with X-ray images,” in Data Science for COVID-19. United States: Academic Press, pp. 451–466, 2021. [Google Scholar]
65. F. Ucar and D. Korkmaz, “COVIDiagnosis-Net: Deep bayes-squeezenet based diagnosis of the coronavirus disease 2019 (COVID-19) from X-ray images,” Medical Hypotheses, vol. 140, no. 1122–1131, pp. 109761, 2020. [Google Scholar] [PubMed]
66. H. Hirano, K. Koga and K. Takemoto, “Vulnerability of deep neural networks for detecting COVID-19 cases from chest X-ray images to universal adversarial attacks,” PLoS One, vol. 15, no. 12, pp. e0243963, 2020. [Google Scholar] [PubMed]
67. J. Zhang, Y. Xie, G. Pang, Z. Liao, J. Verjans et al., “Viral pneumonia screening on chest X-ray images using confidence-aware anomaly detection,” IEEE Transactions on Medical Imaging, vol. 40, no. 3, pp. 879–890, 2020. [Google Scholar]
68. I. D. Apostolopoulos, S. I. Aznaouridis and M. A. Tzani, “Extracting possibly representative COVID-19 biomarkers from X-ray images with deep learning approach and image data related to pulmonary diseases,” Journal of Medical and Biological Engineering, vol. 40, no. 3, pp. 462–469, 2020. [Google Scholar] [PubMed]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools