 Open Access
Open Access
ARTICLE
Deep Learning Enabled Intelligent Healthcare Management System in Smart Cities Environment
1 Department of Information Systems, College of Computer and Information Sciences, Princess Nourah bint Abdulrahman University, P. O. Box 84428, Riyadh, 11671, Saudi Arabia
2 Department of Computer Science, College of Computers and Information Technology, Tabuk University, Tabuk, 71491, Saudi Arabia
3 Department of Information Systems, College of Computing and Information System, Umm Al-Qura University, Saudi Arabia
4 Department of Computer Science, College of Computer and Information Sciences, King Saud University, Riyadh, 11543, Saudi Arabia
5 Department of Information Systems, College of Science and Arts at Muhayel, King Khalid University, Saudi Arabia
6 Department of Information Technology, College of Computers and Information Technology, Taif University, P.O. Box 11099, Taif, 21944, Saudi Arabia
7 Department of Electrical Engineering, Faculty of Engineering & Technology, Future University in Egypt, New Cairo, 11845, Egypt
8 Department of Computer Science, College of Sciences and Humanities-Aflaj, Prince Sattam bin Abdulaziz University, Saudi Arabia
* Corresponding Author: Mesfer Al Duhayyim. Email:
Computers, Materials & Continua 2023, 74(2), 4483-4500. https://doi.org/10.32604/cmc.2023.032588
Received 23 May 2022; Accepted 13 July 2022; Issue published 31 October 2022
Abstract
In recent times, cities are getting smart and can be managed effectively through diverse architectures and services. Smart cities have the ability to support smart medical systems that can infiltrate distinct events (i.e., smart hospitals, smart homes, and community health centres) and scenarios (e.g., rehabilitation, abnormal behavior monitoring, clinical decision-making, disease prevention and diagnosis postmarking surveillance and prescription recommendation). The integration of Artificial Intelligence (AI) with recent technologies, for instance medical screening gadgets, are significant enough to deliver maximum performance and improved management services to handle chronic diseases. With latest developments in digital data collection, AI techniques can be employed for clinical decision making process. On the other hand, Cardiovascular Disease (CVD) is one of the major illnesses that increase the mortality rate across the globe. Generally, wearables can be employed in healthcare systems that instigate the development of CVD detection and classification. With this motivation, the current study develops an Artificial Intelligence Enabled Decision Support System for CVD Disease Detection and Classification in e-healthcare environment, abbreviated as AIDSS-CDDC technique. The proposed AIDSS-CDDC model enables the Internet of Things (IoT) devices for healthcare data collection. Then, the collected data is saved in cloud server for examination. Followed by, training and testing processes are executed to determine the patient’s health condition. To accomplish this, the presented AIDSS-CDDC model employs data pre-processing and Improved Sine Cosine Optimization based Feature Selection (ISCO-FS) technique. In addition, Adam optimizer with Autoencoder Gated Recurrent Unit (AE-GRU) model is employed for detection and classification of CVD. The experimental results highlight that the proposed AIDSS-CDDC model is a promising performer compared to other existing models.Keywords
Smart cities contribute towards the incorporation of conventional urban architectures with the support of Information Technology (IT), including Internet of Things (IoT) sensors. This phenomenon allows the cities to prosper economically and socially and provide sustainable and high-quality urban services [1,2]. For this purpose, smart cities need cooperation between private and public sectors to implement and deploy IT platforms that can collect and analyze vast quantities of data required for automated and intellectual processes. The significance of telemedicine and e-health is increasing among healthcare providers, patients, governments, citizens, and other shareholders [1]. Telemedicine has the ability to improve accessibility to services, decrease costs, and enhance self-management. Further, it can permit underserved populations earlier, to gain access to modern services without any hindrance. While the health system is developing, its technology and substructure too are increasing [2]. Since the significance of better health systems is projected amongst physicians, such healthcare models demands intellectual systems that can handle huge volumes of databases and offer improved medical treatments. The speedy advancements in the existing digitized healthcare data have brought innovative difficulties for scientific research society. Artificial Intelligence (AI), big data, and healthcare management have its own complications in terms of efficiency and social effects [3]. However, they play a progressive and vital role in guiding doctors across the facets of medicine. Healthcare organizations generally experience an issue i.e., to provide quality healthcare services at a fair cost. An inaccurate clinical prognosis and low quality medication might result in insufficient results. Healthcare organizations can employ Decision Support Systems (DSSs) as a mechanism for cost reduction [4].
In general, healthcare models involve a huge volume of patient reports, disease prognoses databases, source management, and so on. The prevalent expansion of IoT and its application in medicinal research enhanced the efficiency of distant health monitoring systems [5]. IoT is regarded as a compendium of several physical materials to detect the physical events without any interruptions [6]. By utilizing IoT technology, Cardiovascular Disease (CVD) monitoring system can collect and transfer the physical variables of a patient to a distant healthcare facility center on real-time basis. In human body, heart is one of the highly significant organs [7] while CVD specifically affects the heart’s functioning. In clinical research, CVD diagnosis is a main and difficult problem to overcome. It can be diagnosed through observation of numerous indications such as cold sweats, chest pain, blood pressure, chest congestion, and shortness of breath [8].
In order to assist in prognosis and predict CVD, IoT sensor values are considered as input values. The prognosis of CVD has been often made by examining the patient’s medical history, physical inspection records, and scrutiny of disturbed indications by a doctor [9]. However, the outcomes attained from this prognosis methodology may not be precise in recognizing whether a patient has CVD or not. Furthermore, it becomes affluent and computationally tough for analysis using such huge volumes of data [10]. In this background, there exists a need to develop a non-invasive prognosis system on the basis of classifications of Machine Learning (ML) so as to resolve the above-discussed issues. An expert decision system, on the basis of ML classifiers and the implementation of fuzzy logic (FL), can successfully help in CVD prognosis. This in turn reduces the death ratio [11].
The execution of ML system can be raised, when balanced datasets are utilized for testing and training the system. Additionally, the analytical abilities of the model can be advanced through appropriate and relevant features extracted from the data. Thus, Feature Selection (FS) and data balancing are significant steps in the development of the proposed model [12]. In the studies published earlier, several prognosis methods has been suggested by numerous authors. However, such methods could not provide a well-defined prognosis for CVD. Conversely, an appropriate ML system is essential for decent outcomes. An ML technique can be segregated as good one when it produces good performance not only with the data provided during training time (or else an ML method can easily study the training data), but also with hidden data [13].
The current study develops Artificial Intelligence enabled Decision Support System for CVD disease detection and classification in smart city e-healthcare environment abbreviated as AIDSS-CDDC technique. The proposed AIDSS-CDDC model enables the IoT devices for collect healthcare data which is then saved in cloud server for examination purposes. In addition, the presented AIDSS-CDDC model employs data pre-processing and Improved Sine Cosine Optimization (ISCO) based Feature Selection (FS) named ISCO-FS technique. In addition, Adam optimizer with Autoencoder Gated Recurrent Unit (AE-GRU) model is employed for detection and classification of CVD. The proposed AIDSS-CDDC model was experimentally validated using benchmark dataset.
Rest of the paper is organized as follows. Section 2 offers the information on related work, Section 3 introduces the proposed model, Section 4 provides experimental validation, and Section 5 concludes the study.
Li et al. [14] suggested an accurate and efficient system for prognosis of heart disease while the system depends upon ML approaches. The model could further suggested ‘original rapid conditional mutual information FS method’ to resolve the FS issue. FS methods are utilized for feature selection so as to increase the accuracy of classification and reduce the performance period of classification method. In literature [15], a difficult mission was achieved i.e., the selection of serious features from a huge data set with existing features and prognosis for heart disease. FS is one of the broadly-employed pre-processing levels in classification. An altered Differential Evolution (DE) methodology was utilized to perform FS for cardiac disease and optimization of particular features.
Zhang et al. [16] suggested an effective and Privacy Preserving Disease Prediction (PPDP) model. In PPDP, historical medical data of the patients was encoded and outsourced to cloud server so that it can use it for training the estimation systems through Single-Layer Perceptron learning technique in privacy preserving means. Kumar et al. [17] intended to provide an improved healthcare structure with the advantages of big data analytics. The massive medical data was managed efficiently in this study with the help of several analytical methods and in-depth insights were achieved from the data. The prevailing structures use methods like Artificial Neural Networks (ANN), Logistic Regression (LR), and fuzzy-related methods. In literature [18], HeartFog, an intelligent real-time decision support system was developed based on suitable scrutiny and IoT so as to achieve distant recognition of heart disease and increase the accuracy of prognosis upon unsolved data. The presented work was assessed in relation to execution time, test accuracy, training accuracy, power consumption, arbitration time, and latency.
Haq et al. [19] recommended a prognosis system with the help of ML systems for diagnosis of diabetes mellitus. The presented methodology was tested on diabetes data sets i.e., a clinical dataset sourced from clinical history of the patients [20]. Internet of Medical Things (IoMT) outline was used for the prognosis of Cardiovascular Disease with the help of Adaptive Neuro-Fuzzy Inference System (ANFIS) while Modified Salp Swarm Optimization (MSSO) was suggested. The suggested MSSO-ANFIS advanced the searching ability by means of Levy Flight (LF) process.
In this study, a novel AIDSS-CDDC model has been developed for disease detection and classification of CVD in e-healthcare environment. The proposed AIDSS-CDDC model enables the IoT devices to collect healthcare data. Besides, CVD classification process encompasses a series of processes namely, data pre-processing, ISCO-FS based feature subset selection, AE-GRU classification, and Adam optimizer. Fig. 1 shows the overall block diagram of AIDSS-CDDC technique.
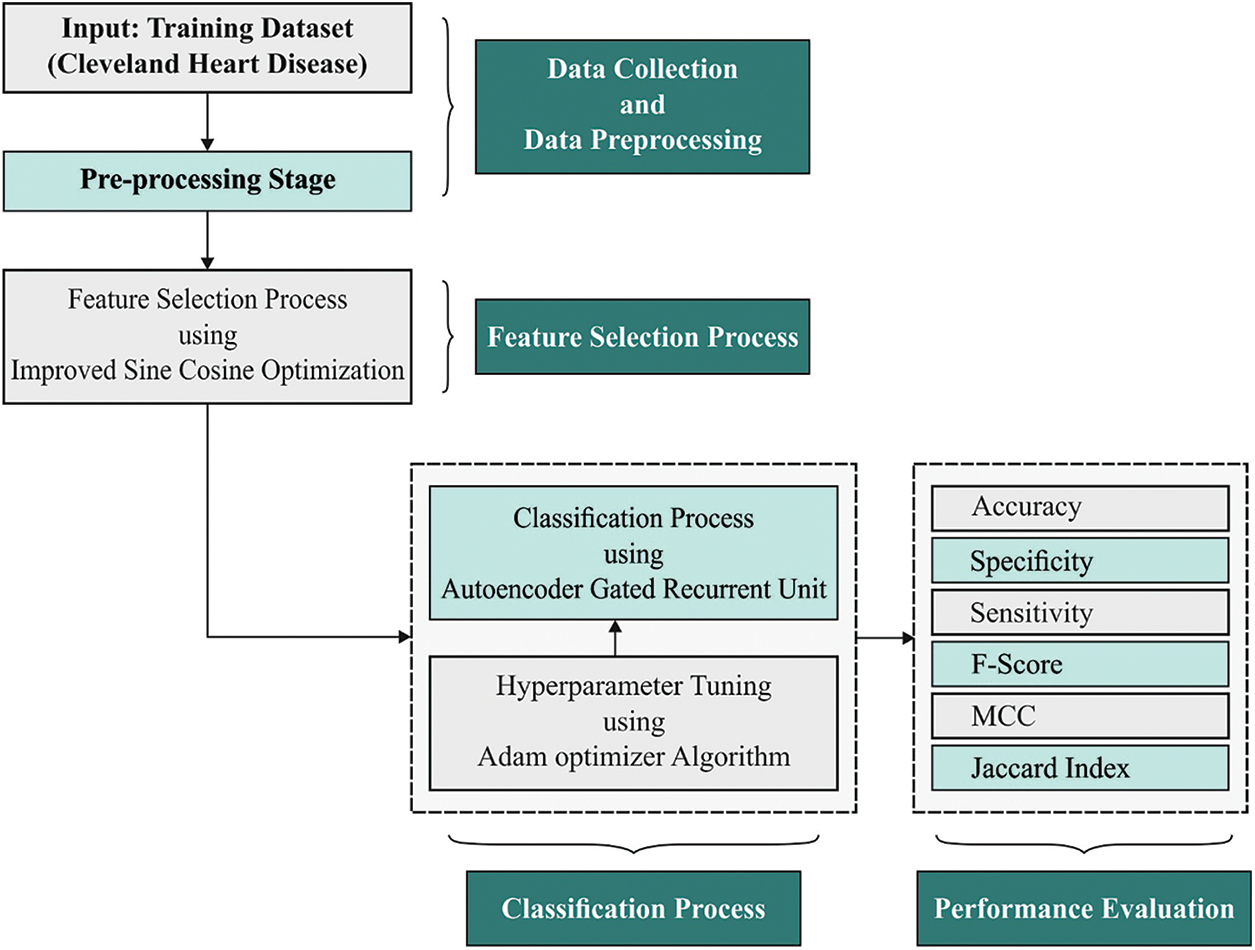
Figure 1: Block diagram of AIDSS-CDDC approach
In current study, the data undergoes scaling process prior to moving onto ISCO-FS model. This is done so to ensure that every feature gets upgraded at a concurrent rate. Here, Min-Max scaler is applied to scale the dataset at an interval between 0 and 1 to assure rapid convergence for the gradient learning procedure of Deep Learning (DL) model. It can be defined as follows.
where
3.2 Design of ISCO-FS Technique
Once the input data is pre-processed, ISCO-FS technique is exploited to elect the feature subsets. Similar to other Swarm Intelligence (SI) techniques, a primary population is created arbitrarily in the provided solution space during the initial phase of Sine Cosine Optimization (SCO) algorithm. Next, the optimum solution is attained in the primary population. The following steps are repeated until the end criteria are attained. Primarily, the adaptive parameter
Here, a implies a value equivalent to 2 whereas t and
whereas
Eventually, the cross-border procedure is executed to guarantee that every agent exists in the potential area. In case of an agent being superior to the optimum solution, then the optimum solutions are upgraded by this agent. In order to avoid a decline due to local optima and define a further potential area, a search approach called Disperse Foraging Strategy (DFS) is projected based on disperse foraging performance of wolves at the time of food shortage. The original SCO algorithm inclines to decrease towards local optima if higher dimension and multi-dimension optimization problems are resolved. In order to tackle this issue, an enhanced technique termed ISCO is presented in this case to establish DFS as SCO algorithm. In the presented DFSCA, DFS process forces all the agents to achieve ‘expanded searching space’ so as to define further potential solutions. This approach not only utilizes them to jump out of local optima with superior chance, but also allows the definition of further potential solutions. This scenario is highly important to enhance the efficiency of optimization. The stages involved in the execution of ISCO algorithm are reprised from literature [22]:
Step 1: Initialize the appropriate parameters of ISCO algorithm such as the maximal fitness computation time is MaxFES, the existing fitness computation time
Step 2: Arbitrarily initialize the population and estimate the primary population, attain the optimum solution P with optimum agents from the primary population,
Step 3: If the
Step 3.1: Upgrade the place of agents in the population. Modify the place of all the agents in the boundary. Estimate the existing population. Upgrade the optimum solution P.
Step 3.2: Create the candidate place of searching agent by DFS. Modify the candidate’s place in the boundary. When the candidate place is superior to the existing place of searching agents, replace the existing place of searching agents with candidate place; or else keep the present place of searching agent unaffected.
Step 3.3: Estimate the existing population. Upgrade the optimum solution P.
Step 4: Return the optimum solution,
The fitness function of ISCO-FS technique is derived to attain a proper tradeoff between the chosen feature count in every solution and classifier accuracy that is attained via elected features. The fitness function for evaluation solution is defined below.
where
3.3 CVD Classification Process
Next, Adam optimizer with AE-GRU model is employed for detection and classification of CVD. GRU is a particular case of Long Short Term Memory (LSTM) that is established for the reduction of long training time of LSTM [23]. GRU is far easier than LSTM as it contains only two gates such as reset and update gates that control the flow of data inside the unit. The alteration function amongst the neurons of GRU is provided as follows
whereas
1. Historical clinical information is encoded with the help of encoding side of AE.
2. The encoded clinical information is split into training as well as testing sets. A small percentage of the trained data is conserved for validation.
3. All the testing, training, and validating datasets are efficient as to chunk or window which demonstrates the amount of historical preceding days (window size).
4. The presented method is trained and simultaneously validated with the help of windows of historically-encoded clinical information and equivalent power.
5. The presented method was verified on testing set which is nothing but the rearranged chunks of preceding instances.
6. At last, the presented AE-GRU method is a great ML approach used in the prediction of data for a provided chunk of preceding clinical information.
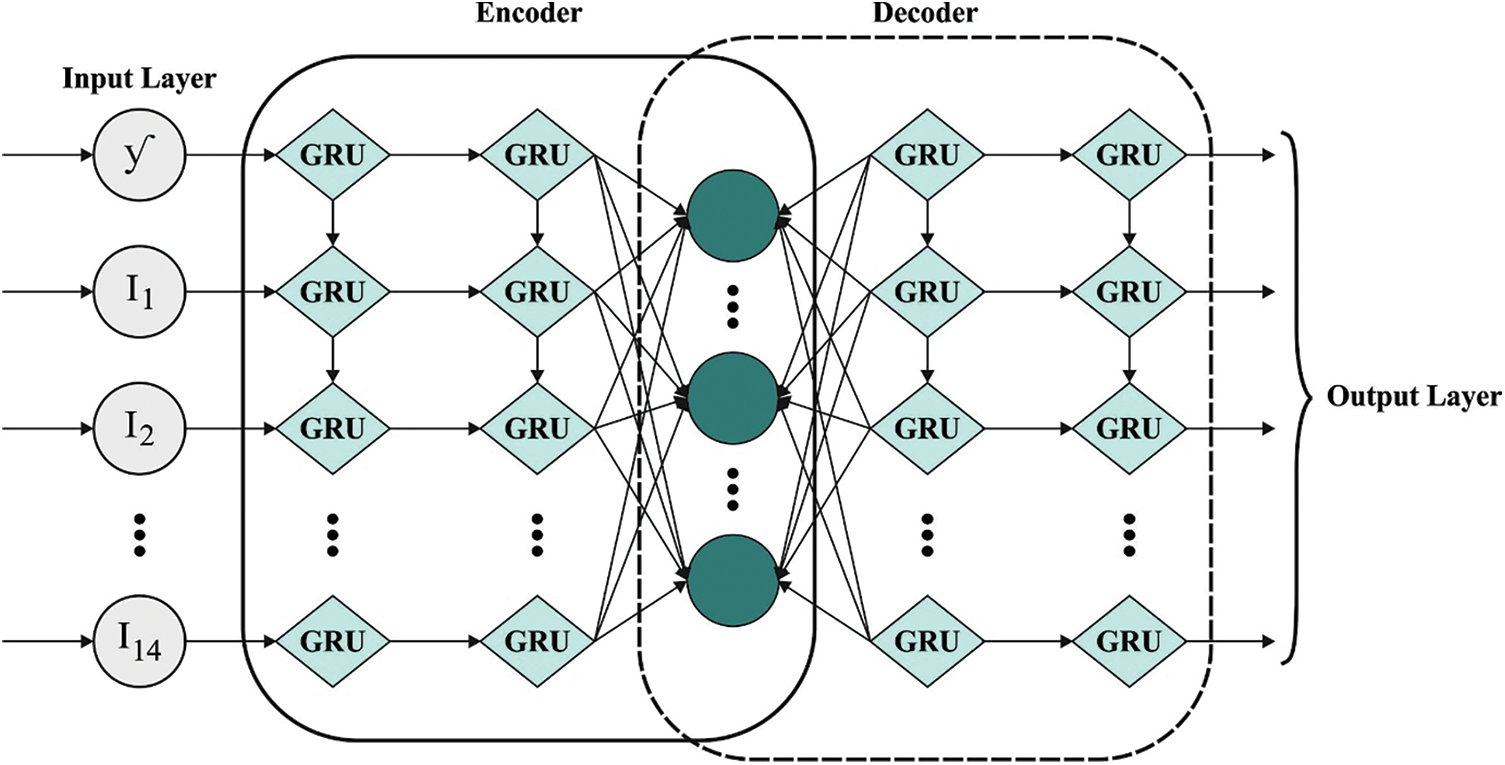
Figure 2: Structure of AE-GRU
In order to fine-tune the hyperparameters of AE-GRU technique, Adam optimizer is exploited. Adam model is a commonly applied technique that alters the learning rate adaptively for every parameter. It can be a group of distinct gradient optimization techniques. It exponentially decays the average of the past-squared gradient, i.e., Root Mean Square propagation (RMSprop) and Adadelta. Further, it takes the above-mentioned gradientssimilar to Momentum.
whereas
Then, the updated value of Adam optimizer is resolved as follows.
The gradient part of
Here, it is confirmed that every function is based on the previous gradient of current parameter that has no relation with learning rate. So, Adam has an operational outcome via learning rate model.
In current section, the proposed AIDSS-CDDC model was experimentally validated using a benchmark dataset [24] that comprises of 303 samples with 14 features as depicted in Table 1. The proposed AIDSS-CDDC model has a total of 8 features. The proposed model was simulated using Python 3.4.5 tool.
Fig. 3 depicts the correlation matrices generated by AIDSS-CDDC technique on test data with distinct attributes. The confusion matrices generated by the proposed AIDSS-CDDC model on test data under distinct Cross Validation (CV) are illustrated in Fig. 4. On CV-1, AIDSS-CDDC model categorized 162 samples under absence class and 135 samples under presence class. Afterward, on CV-3, the proposed AIDSS-CDDC methodology recognized 163 samples as absence class and 134 samples as presence class.
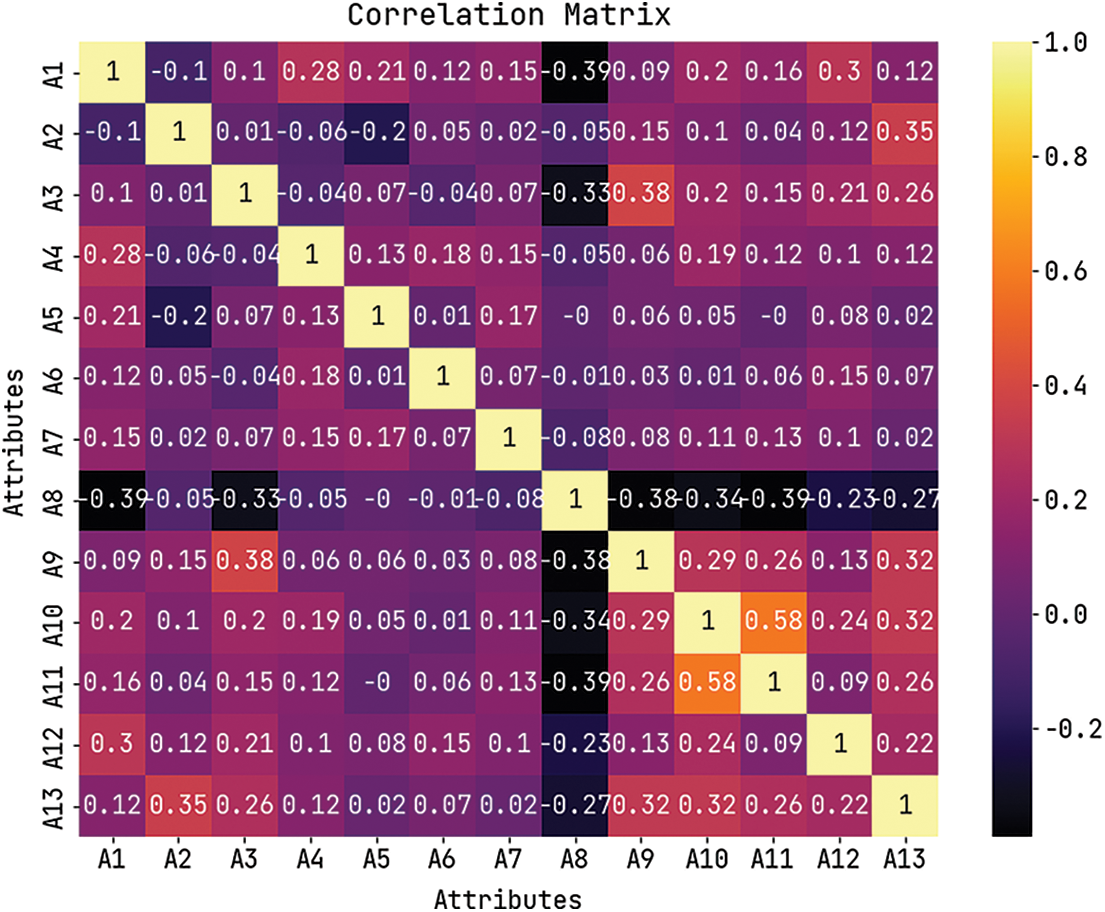
Figure 3: Correlation matrix of AIDSS-CDDC algorithm
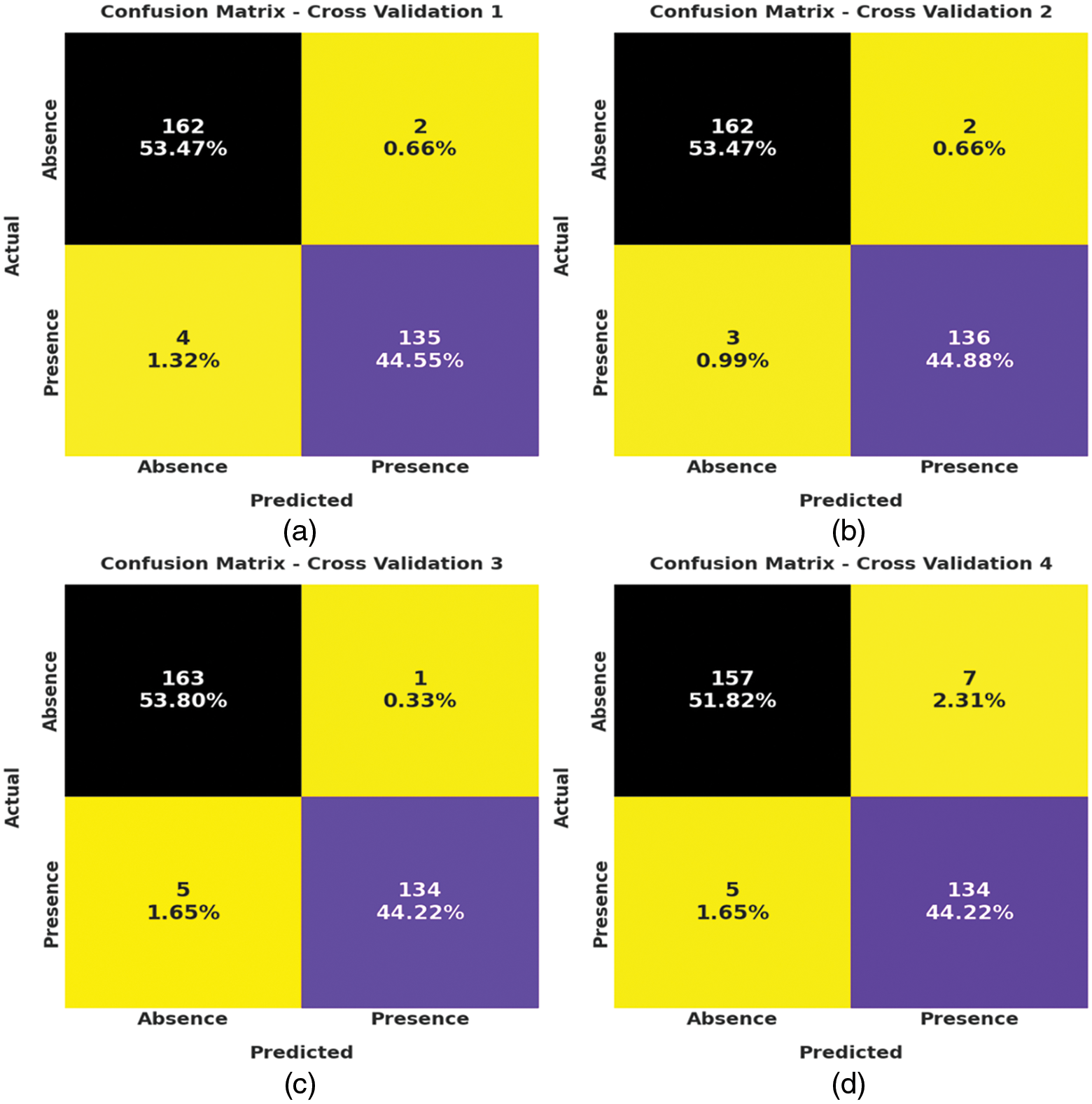
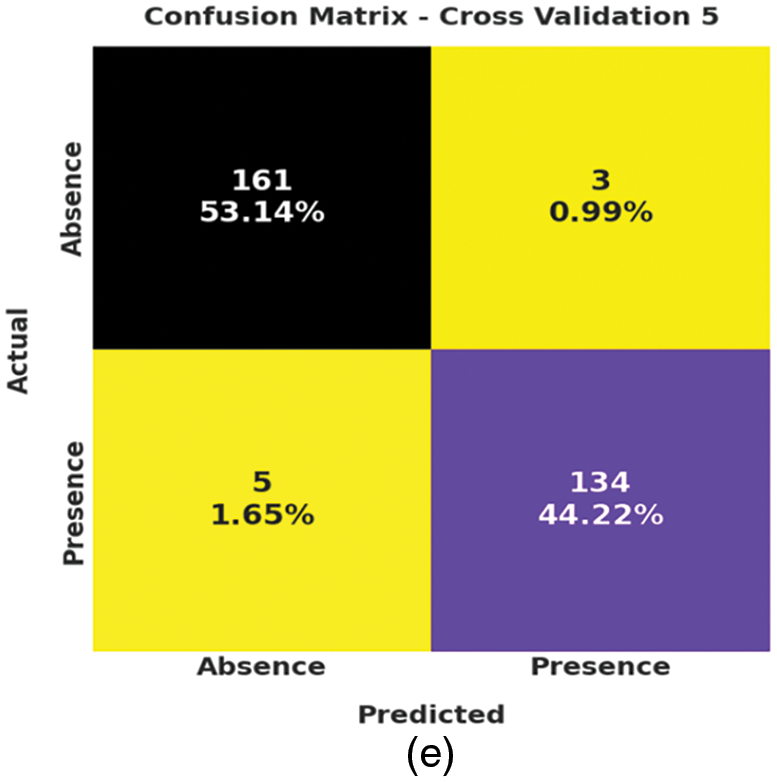
Figure 4: Confusion matrices of AIDSS-CDDC technique (a) CV-1, (b) CV-2, (c) CV-3, (d) CV-4, and (e) CV-5
Moreover, on CV-4, the proposed AIDSS-CDDC approach categorized 157 samples under absence class and 134 samples under presence class. At last, on CV-5, the proposed AIDSS-CDDC system recognized 161 samples as absence class and 134 samples as presence class.
Table 2 and Fig. 5 provide a detailed overview on the classification outcomes achieved by AIDSS-CDDC model under distinct Cross Validation (CV). The results imply that the proposed AIDSS-CDDC model gained effectual outcomes under every CV. For instance, on CV-1, AIDSS-CDDC model offered average
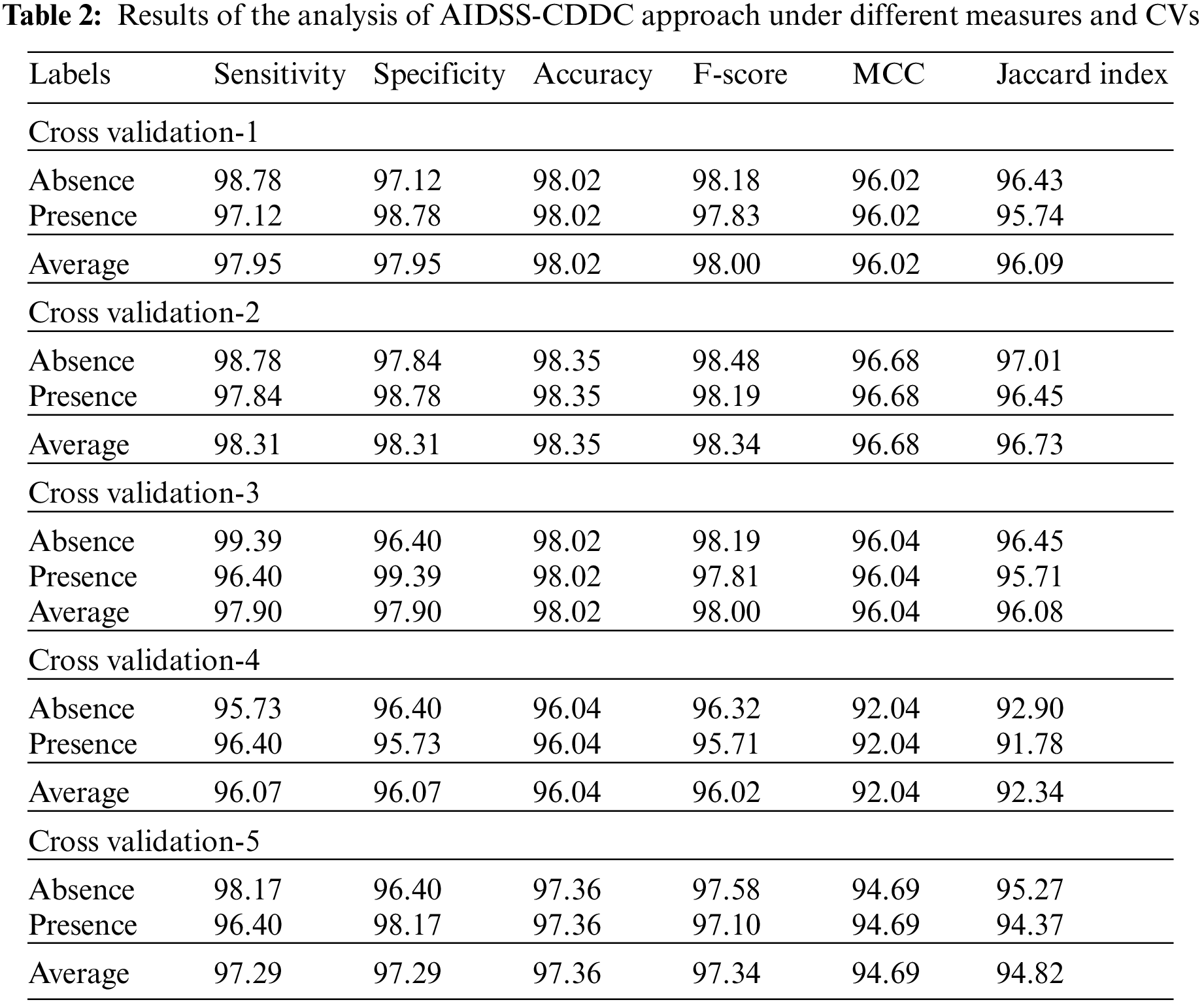
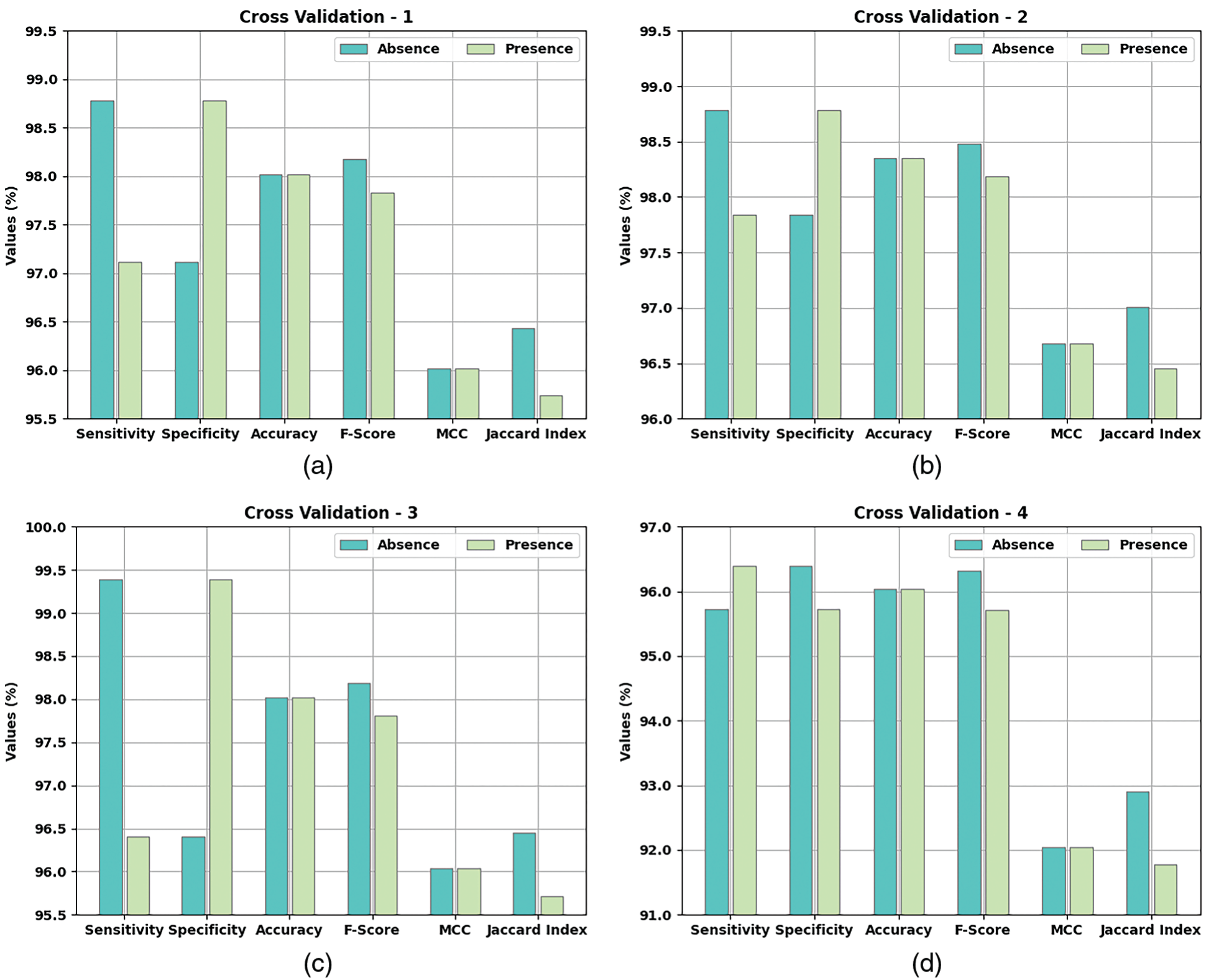
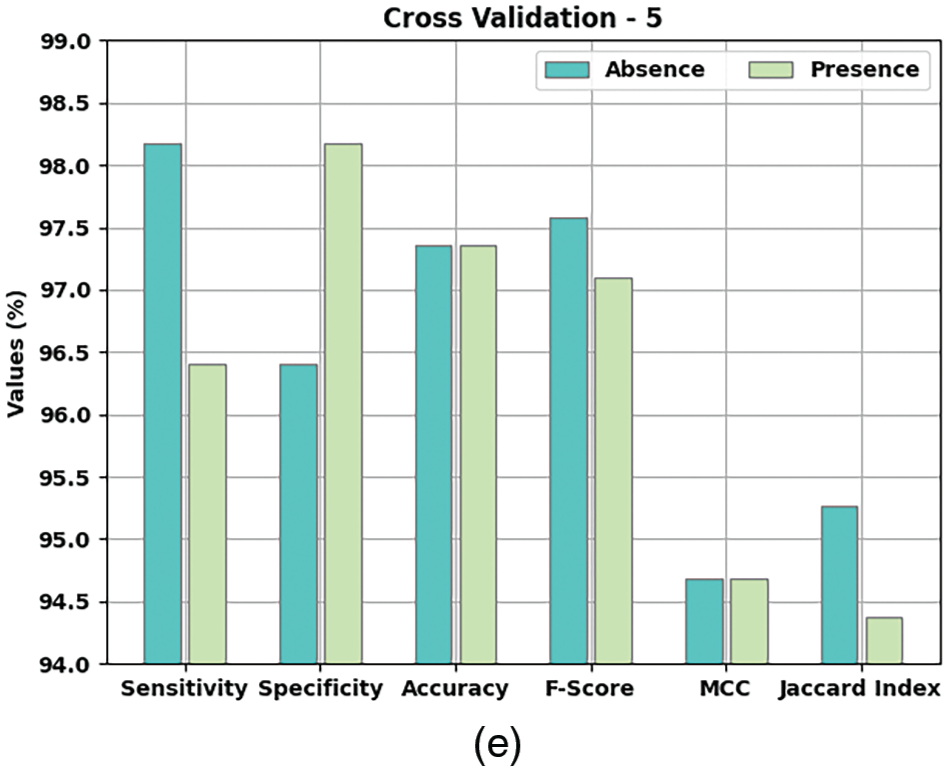
Figure 5: Results of the analysis of AIDSS-CDDC technique (a) CV-1, (b) CV-2, (c) CV-3, (d) CV-4, and (e) CV-5
Meanwhile, on CV-2, the proposed AIDSS-CDDC model achieved average
Both Training Accuracy (TA) and Validation Accuracy (VA) values, attained by the proposed AIDSS-CDDC algorithm on test dataset, are portrayed in Fig. 6. The experimental outcomes imply that the proposed AIDSS-CDDC system gained the maximum TA and VA values. To be specific, VA seemed to be higher than TA.
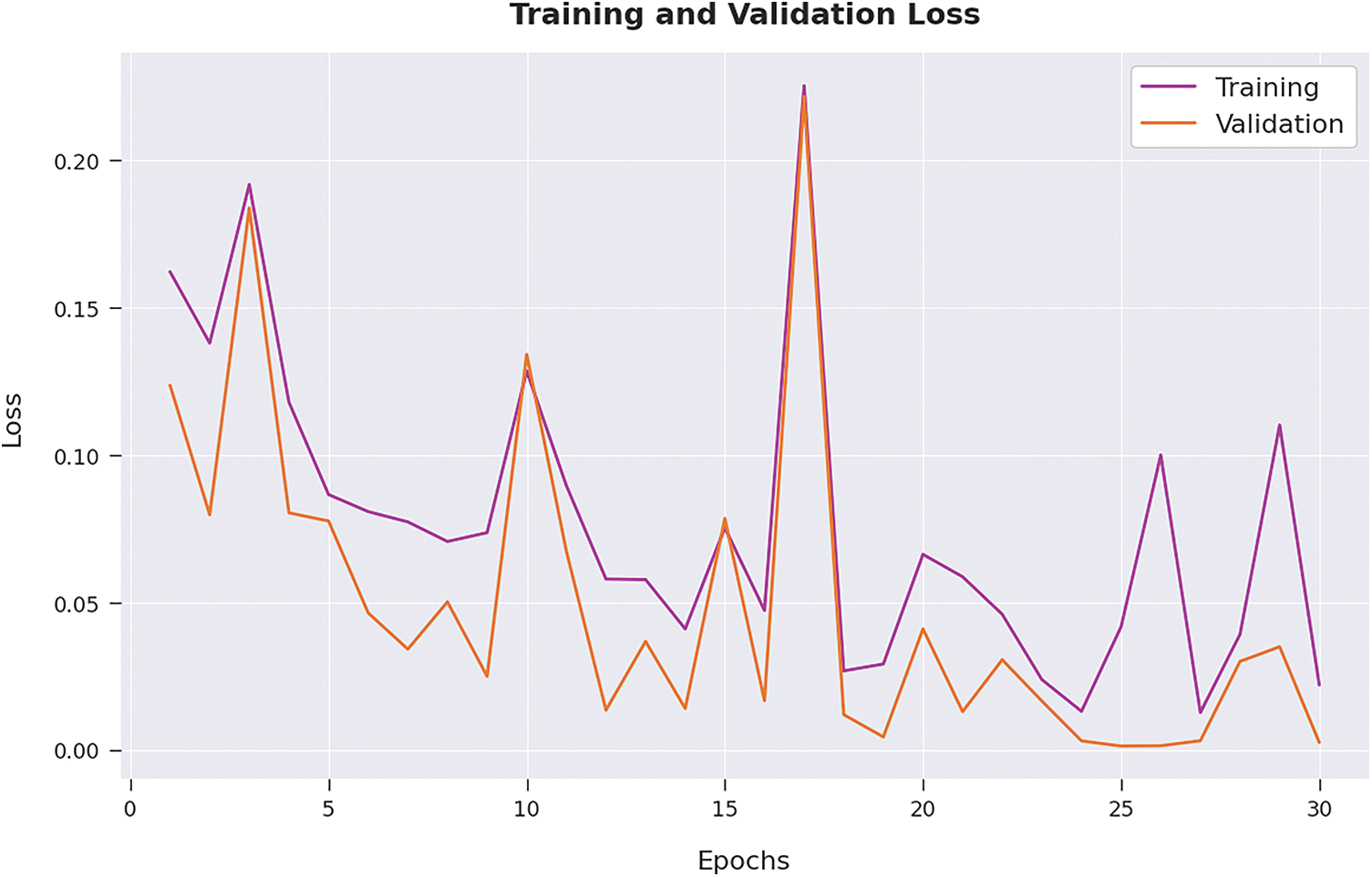
Figure 6: TA and VA analysis results of AIDSS-CDDC approach
Both Training Loss (TL) and Validation Loss (VL) values, achieved by the proposed AIDSS-CDDC model on test dataset, are shown in Fig. 7. The experimental outcomes infer that the proposed AIDSS-CDDC approach accomplished the least TL and VL values. To be specific, VL seemed to be lower than TL.
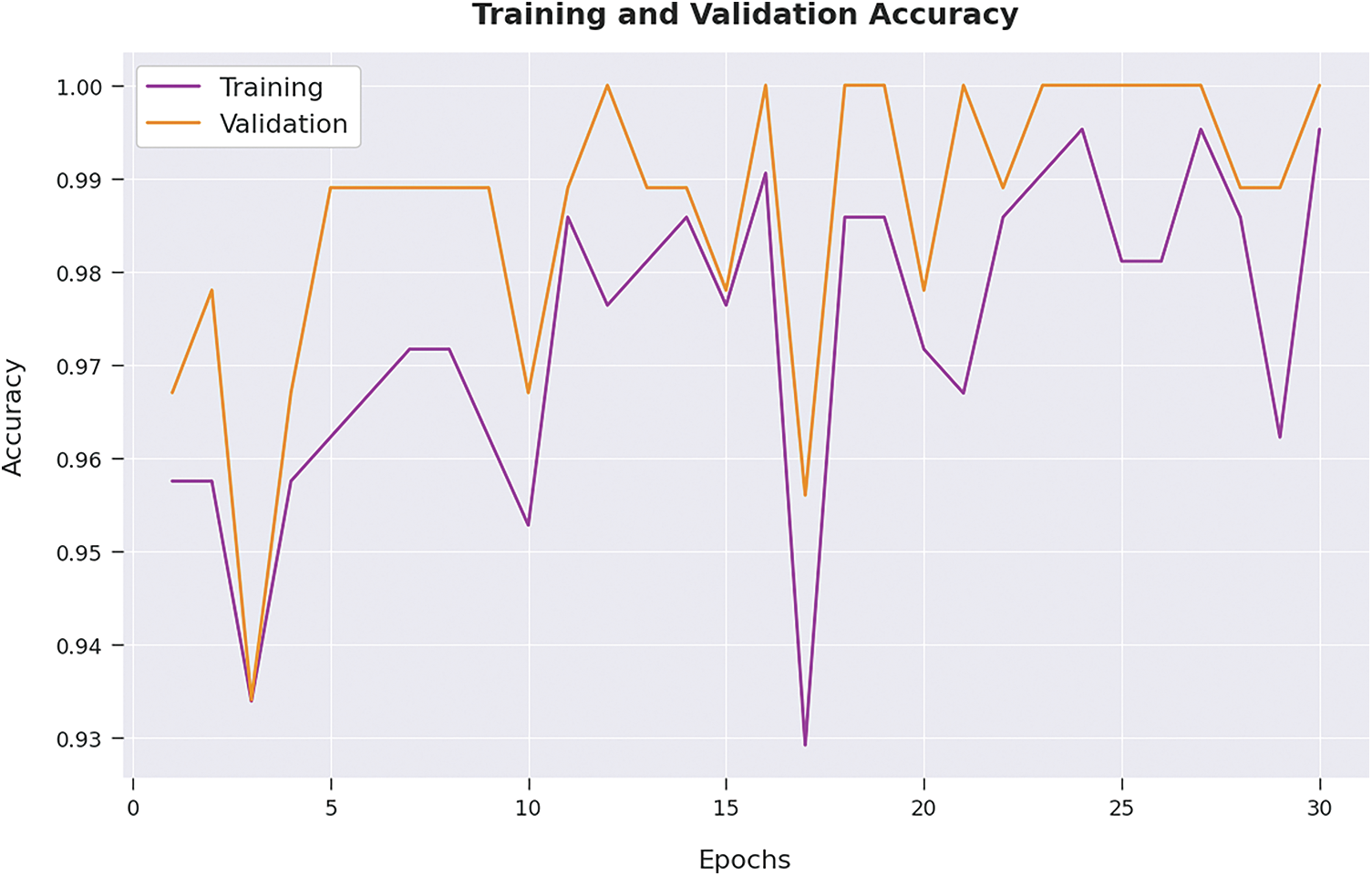
Figure 7: TL and VL analysis results of AIDSS-CDDC approach
Table 3 provides a comprehensive comparative CKD classification performance of the proposed AIDSS-CDDC model and other recent models such as Logistic Regression (LR), k-Nearest Neighbor (K-NN), Artificial Neural Network (ANN), Support Vector Machine with Radial Basis Function (SVM-RBF), SVM-Linear, Naive Bayes (NB), and Decision Tree (DT).
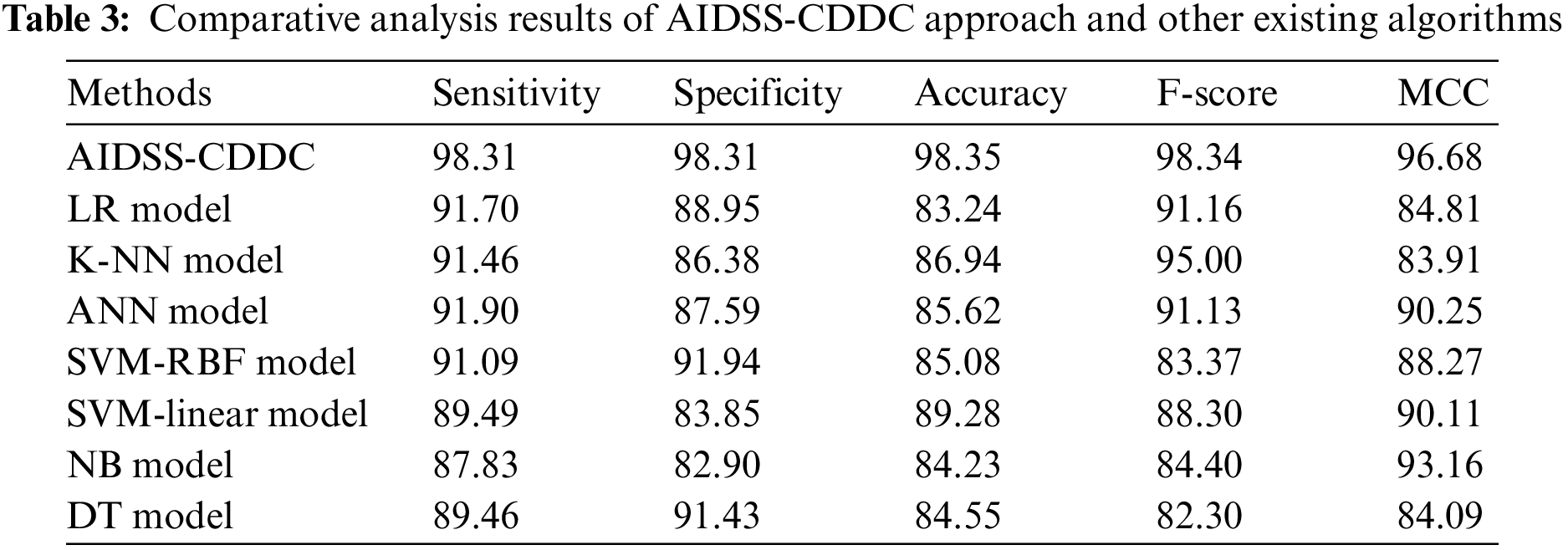
Fig. 8 shows the comparative
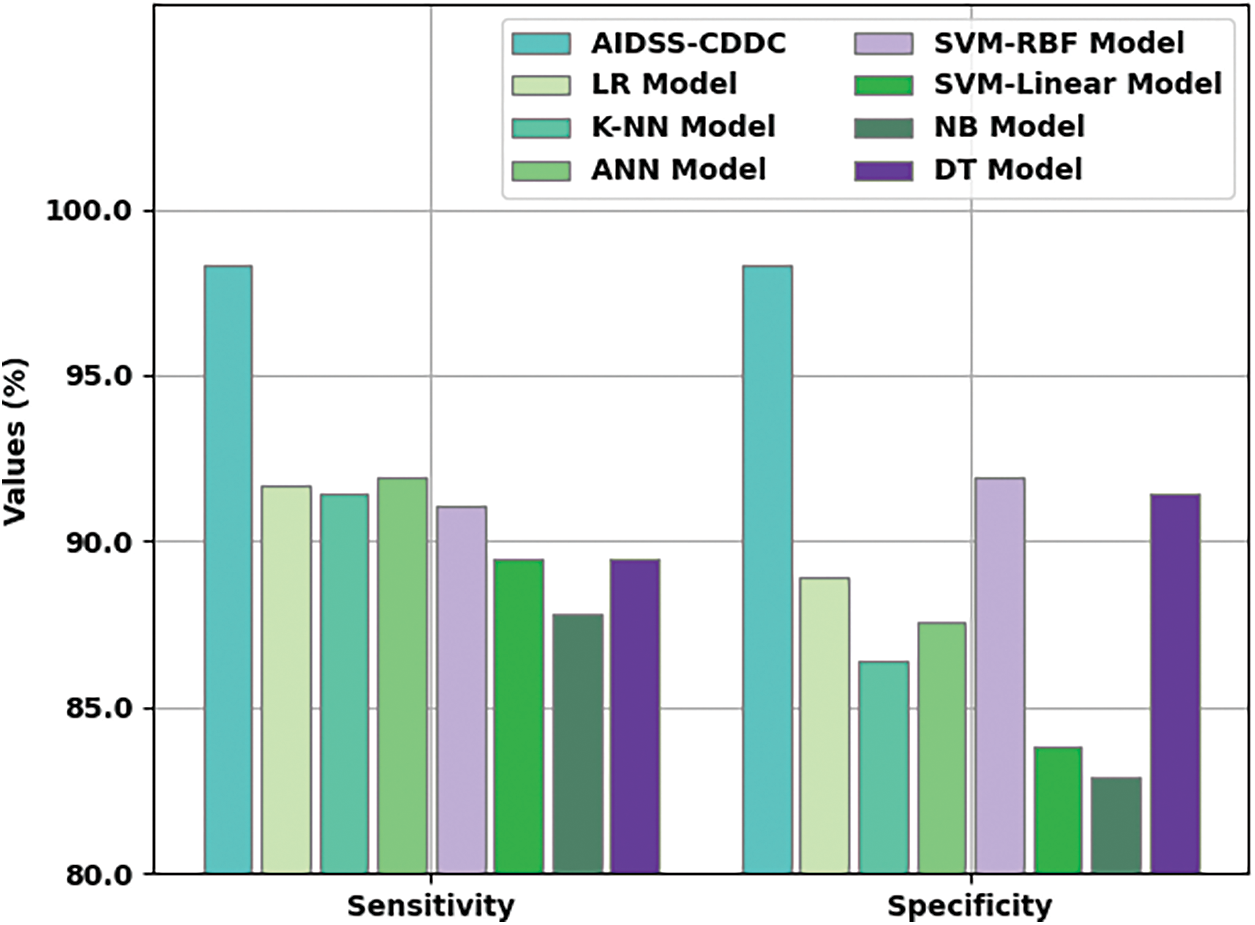
Figure 8:
Fig. 9 shows the comparative
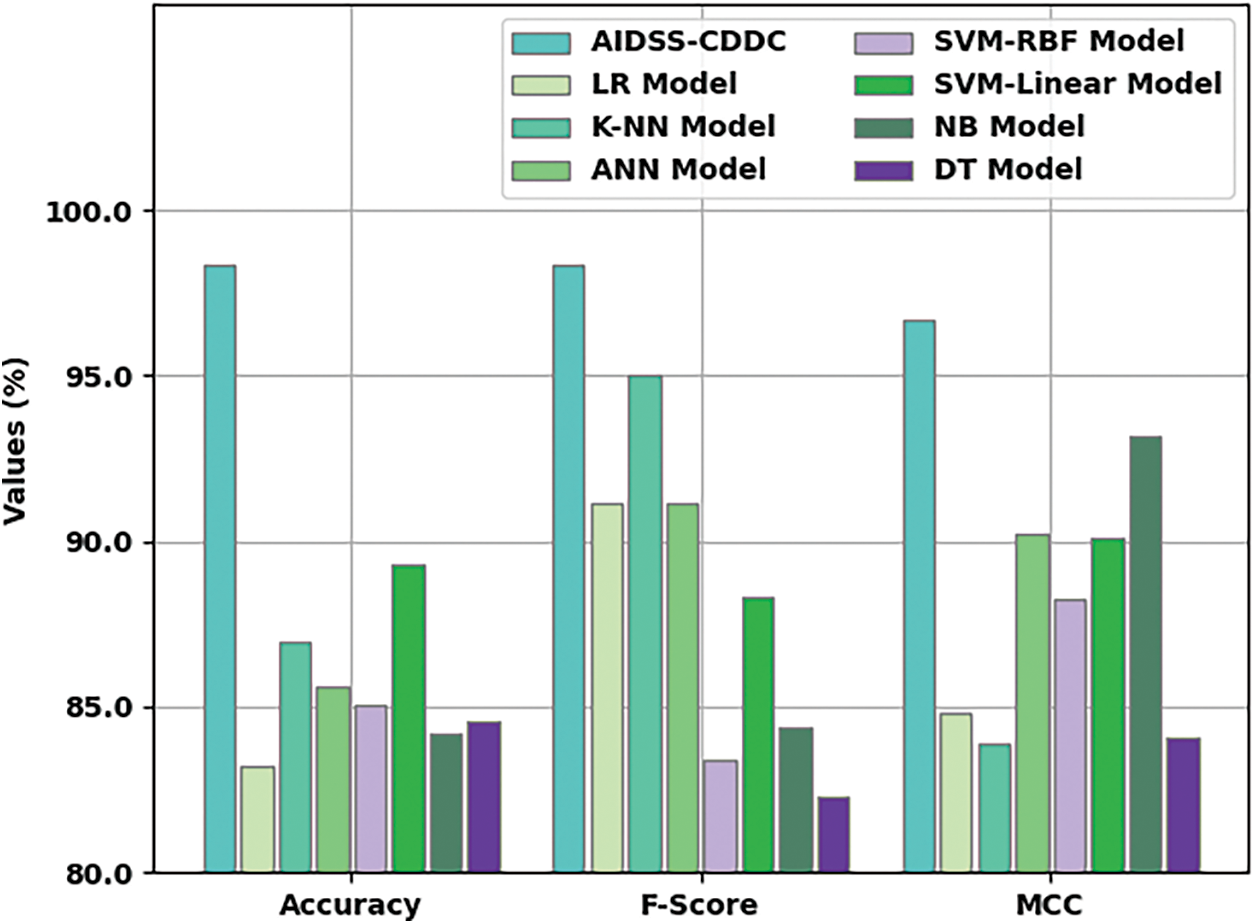
Figure 9: Comparative analysis results of AIDSS-CDDC approach and other existing algorithms
Finally, a detailed execution time analysis was conducted between the proposed the AIDSS-CDDC model and other recent models and the results are shown in Table 4 and Fig. 10. The results imply that SVM-linear model gained ineffectual outcomes with a maximum execution time of 16.860 s. At the same time, ANN and NB models shown slightly reduced execution times such as 9.620 and 9.150 s respectively. In addition, LR, KNN, SVM-RBF, and DT models produced moderately closer execution times such as 4.610, 4.770, 5.730, and 5.100 s respectively. However, the presented AIDSS-CDDC model accomplished the least and effectual execution time of 0.002 s. Therefore, the proposed AIDSS-CDDC model can be employed as an effectual tool for detection and classification of CVD.
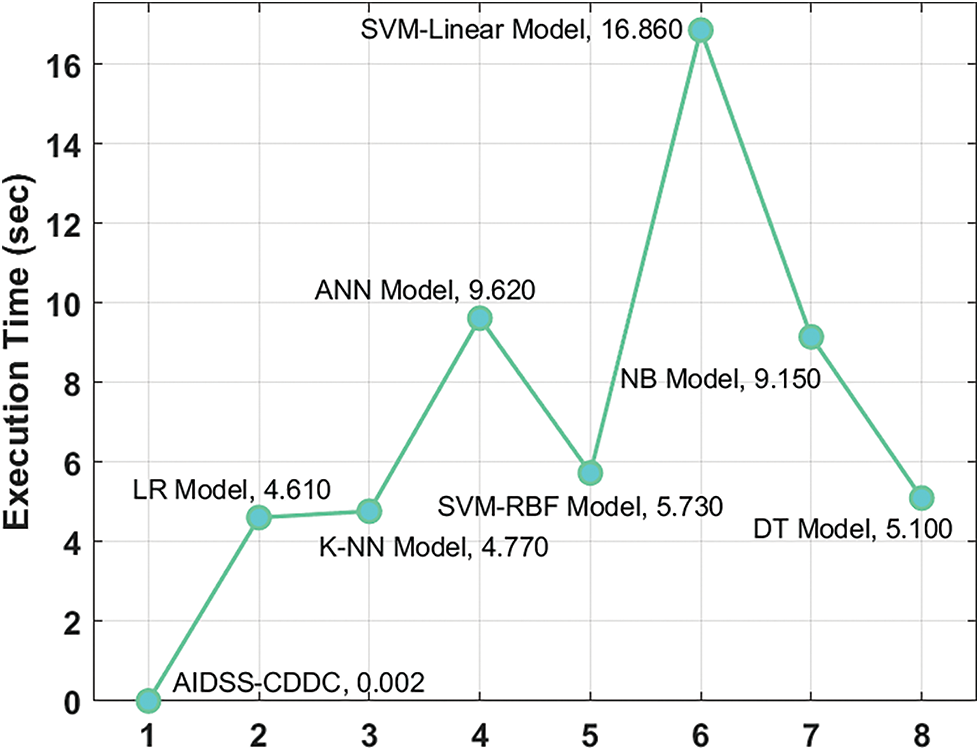
Figure 10: Execution time analysis results of AIDSS-CDDC technique and other existing methodologies
In current study, a novel AIDSS-CDDC model has been developed for CVD disease detection and classification in smart city environment. The proposed AIDSS-CDDC model enables the IoT devices to collect healthcare data which is then stored in cloud server for examination. Followed by, training and testing processes are executed to determine the patient’s health condition. In addition, the presented AIDSS-CDDC model employs data pre-processing and ISCO-FS technique to elect the feature subsets. Moreover, Adam optimizer with AE-GRU model is also employed for detection and classification of CVD. The experimental results highlight that the proposed AIDSS-CDDC model is a promising performer over other models. Thus, the presented AIDSS-CDDC model can be exploited for effectual detection of CVD in e-healthcare environment. In future, ensemble voting-based DL models can be utilized to enhance the detection efficacy of AIDSS-CDDC model.
Funding Statement: The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through Large Groups Project under Grant Number (71/43). Princess Nourah bint Abdulrahman University Researchers Supporting Project Number (PNURSP2022R114), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. The authors would like to thank the Deanship of Scientific Research at Umm Al-Qura University for supporting this work by Grant Code: (22UQU4210118DSR26).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. A. Kishor and W. Jeberson, “Diagnosis of heart disease using internet of things and machine learning algorithms,” in Proc. of Second Int. Conf. on Computing, Communications, and Cyber-Security, Lecture Notes in Networks and Systems book series, Singapore, Springer, vol. 203, pp. 691–702, 2021. [Google Scholar]
2. K. Shankar, E. Perumal, V. G. Díaz, P. Tiwari, D. Gupta et al., “An optimal cascaded recurrent neural network for intelligent COVID-19 detection using chest X-ray images,” Applied Soft Computing, vol. 113, no. Part A, pp. 1–13, 2021. [Google Scholar]
3. R. Gopi, P. Muthusamy, P. Suresh, C. G. G. S. Kumar, I. V. Pustokhina et al., “Optimal confidential mechanisms in smart city healthcare,” Computers, Materials & Continua, vol. 70, no. 3, pp. 4883–4896, 2022. [Google Scholar]
4. E. K. Hashi and M. S. U. Zaman, “Developing a hyperparameter tuning based machine learning approach of heart disease prediction,” Journal of Applied Science & Process Engineering, vol. 7, no. 2, pp. 631–647, 2020. [Google Scholar]
5. R. Beri, M. K. Dubey, A. Gehlot, R. Singh, M. A. Elnaby et al., “A novel fog-computing-assisted architecture of E-healthcare system for pregnant women,” The Journal of Supercomputing, vol. 78, no. 6, pp. 7591–7615, 2022. [Google Scholar]
6. S. Nandy, M. Adhikari, V. Balasubramanian, V. G. Menon, X. Li et al., “An intelligent heart disease prediction system based on swarm-artificial neural network,” Neural Computing and Applications, 2021. https://doi.org/10.1007/s00521-021-06124-1. [Google Scholar]
7. A. Al-Qarafi, F. Alrowais, S. Alotaibi, N. Nemri, F. N. Al-Wesabi et al., “Optimal machine learning based privacy preserving blockchain assisted internet of things with smart cities environment,” Applied Sciences, vol. 12, no. 12, pp. 5893, 2022. [Google Scholar]
8. A. M. Hilal, B. S. Alfurhood, F. N. Al-Wesabi, M. A. Hamza, M. A. Duhayyim et al., “Artificial intelligence based sentiment analysis for health crisis management in smart cities,” Computers, Materials & Continua, vol. 71, no. 1, pp. 143–157, 2022. [Google Scholar]
9. S. Pandu, A. Francis, P. Sekhar, P. Vijayarajan, A. A. Albraikan et al., “Artificial intelligence based solar radiation predictive model using weather forecasts,” Computers, Materials & Continua, vol. 71, no. 1, pp. 109–124, 2022. [Google Scholar]
10. P. Kaur, R. Kumar and M. Kumar, “A healthcare monitoring system using random forest and internet of things (IoT),” Multimedia Tools and Applications, vol. 78, no. 14, pp. 19905–19916, 2019. [Google Scholar]
11. T. Munirathinam, S. Ganapathy and A. Kannan, “Cloud and IoT based privacy preserved e-healthcare system using secured storage algorithm and deep learning,” Journal of Intelligent & Fuzzy Systems, vol. 39, no. 3, pp. 3011–3023, 2020. [Google Scholar]
12. I. M. E. Hasnony, O. M. Elzeki, A. Alshehri and H. Salem, “Multi-label active learning-based machine learning model for heart disease prediction,” Sensors, vol. 22, no. 3, pp. 1184, 2022. [Google Scholar]
13. A. Saboor, M. Usman, S. Ali, A. Samad, M. Abrar et al., “A method for improving prediction of human heart disease using machine learning algorithms,” Mobile Information Systems, vol. 2022, pp. 1–9, 2022. [Google Scholar]
14. J. P. Li, A. U. Haq, S. U. Din, J. Khan, A. Khan et al., “Heart disease identification method using machine learning classification in e-healthcare,” IEEE Access, vol. 8, pp. 107562–107582, 2020. [Google Scholar]
15. T. Vivekanandan and N. S. N. Iyengar, “Optimal feature selection using a modified differential evolution algorithm and its effectiveness for prediction of heart disease,” Computers in Biology and Medicine, vol. 90, pp. 125–136, 2017. [Google Scholar]
16. C. Zhang, L. Zhu, C. Xu and R. Lu, “PPDP: An efficient and privacy-preserving disease prediction scheme in cloud-based e-healthcare system,” Future Generation Computer Systems, vol. 79, pp. 16–25, 2018. [Google Scholar]
17. S. R. Kumar, N. Gayathri, S. Muthuramalingam, B. Balamurugan, C. Ramesh et al., “Medical big data mining and processing in e-healthcare,” in Internet of Things in Biomedical Engineering, pp. 323–339, 2019. https://doi.org/10.1016/B978-0-12-817356-5.00016-4. [Google Scholar]
18. S. Tuli, N. Basumatary, S. S. Gill, M. Kahani, R. C. Arya et al., “HealthFog: An ensemble deep learning based smart healthcare system for automatic diagnosis of heart diseases in integrated iot and fog computing environments,” Future Generation Computer Systems, vol. 104, pp. 187–200, 2020. [Google Scholar]
19. A. U. Haq, J. P. Li, J. Khan, M. H. Memon, S. Nazir et al., “Intelligent machine learning approach for effective recognition of diabetes in e-healthcare using clinical data,” Sensors, vol. 20, no. 9, pp. 2649, 2020. [Google Scholar]
20. M. A. Khan and F. Algarni, “A healthcare monitoring system for the diagnosis of heart disease in the iomt cloud environment using msso-anfis,” IEEE Access, vol. 8, pp. 122259–122269, 2020. [Google Scholar]
21. L. Abualigah and A. Diabat, “Advances in sine cosine algorithm: A comprehensive survey,” Artificial Intelligence Review, vol. 54, no. 4, pp. 2567–2608, 2021. [Google Scholar]
22. J. Xia, D. Yang, H. Zhou, Y. Chen, H. Zhang et al., “Evolving kernel extreme learning machine for medical diagnosis via a disperse foraging sine cosine algorithm,” Computers in Biology and Medicine, vol. 141, pp. 105137, 2022. [Google Scholar]
23. M. AlKandari and I. Ahmad, “Solar power generation forecasting using ensemble approach based on deep learning and statistical methods,” Applied Computing and Informatics, 2020. https://doi.org/10.1016/j.aci.2019.11.002. [Google Scholar]
24. Dataset: https://archive.ics.uci.edu/ml/datasets.php. [Google Scholar]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools