 Open Access
Open Access
ARTICLE
Lung Cancer Detection Using Modified AlexNet Architecture and Support Vector Machine
1 Faculty of Computer Science and Information Technology, The Superior University, Lahore, 54000, Pakistan
2 Department of Computer Science, National University of Technology, Islamabad, 45000, Pakistan
3 Faculty of IT, University of Central Punjab, Lahore, 54100, Pakistan
* Corresponding Author: Iftikhar Naseer. Email:
Computers, Materials & Continua 2023, 74(1), 2039-2054. https://doi.org/10.32604/cmc.2023.032927
Received 02 June 2022; Accepted 05 July 2022; Issue published 22 September 2022
Abstract
Lung cancer is the most dangerous and death-causing disease indicated by the presence of pulmonary nodules in the lung. It is mostly caused by the instinctive growth of cells in the lung. Lung nodule detection has a significant role in detecting and screening lung cancer in Computed tomography (CT) scan images. Early detection plays an important role in the survival rate and treatment of lung cancer patients. Moreover, pulmonary nodule classification techniques based on the convolutional neural network can be used for the accurate and efficient detection of lung cancer. This work proposed an automatic nodule detection method in CT images based on modified AlexNet architecture and Support vector machine (SVM) algorithm namely LungNet-SVM. The proposed model consists of seven convolutional layers, three pooling layers, and two fully connected layers used to extract features. Support vector machine classifier is applied for the binary classification of nodules into benign and malignant. The experimental analysis is performed by using the publicly available benchmark dataset Lung nodule analysis 2016 (LUNA16). The proposed model has achieved 97.64% of accuracy, 96.37% of sensitivity, and 99.08% of specificity. A comparative analysis has been carried out between the proposed LungNet-SVM model and existing state-of-the-art approaches for the classification of lung cancer. The experimental results indicate that the proposed LungNet-SVM model achieved remarkable performance on a LUNA16 dataset in terms of accuracy.Keywords
Lung cancer is one of the most dangerous cancer types with the highest mortality rate in the world. In the early stage, the symptoms of lung cancer are not obvious [1]. Therefore, early and accurate detection of pulmonary nodules is extremely significant to increase the survival rate of lung cancer [2]. At present, chest imaging examination is the main method to detect pulmonary nodules, specifically includes magnetic resonance imaging, X-ray, Computed tomography (CT) scan, and other methods. CT scan is the most common diagnostic technique due to its high resolution and low price [3]. The sensitivity of CT scan has made it an exceedingly adopted method for lung cancer screening however, the classification of pulmonary nodules is still a time-consuming process. Moreover, the size of CT scan is rapidly increasing which has increased the workload of radiologists in their routine diagnostic process [4].
A Computer-aided diagnosis (CAD) system in this context help to decrease the workload of radiologists and effectively increase the accuracy in diagnostic process of lung cancer [5]. Zheng et al. [6] presented an revelatory CAD lung cancer diagnosis system in which Channel-dependent activation mapping (CDAM) technique applied to envision the features and emphasized on decision process. Prevailing studies on pulmonary nodules detection indicate that CAD systems based on Deep learning (DL) have attained remarkable performance [7].
Machine learning (ML) technique is widely used to classify the image. In ML algorithms, features are manually extracted from images for the detection purpose. A Support vector machine (SVM) classifier is a ML algorithm that can be mostly applied for classification. SVM algorithm finds hyper-plane that segregates into binary and multiple classes. Halder et al. [8] introduced an adaptive morphology-based technique with SVM for lung nodules detection. A 10-fold cross-validation method adopted Lung image database consortium-image database resource initiative (LIDC/IDRI) dataset to evaluate the suggested system. Deep learning is renowned ML technique that has outperformed in every domain of life such as robotics, natural language processing, agriculture, and medical among many others. Ren et al. [9] applied Manifold regularized classification deep neural network (MRC-DNN) technique to classify lung nodules in CT images. Convolutional neural network (CNN) architecture is used to extract features automatically that are very valuable for the object detection [10]. Manickavasagam et al. [11] suggested to use CNN based on five convolutional layers (CNN-5CL) to make CAD system robust to classifying pulmonary nodules. Onishi et al. [12] generated multiplaner images to train CNN model by using generative adversarial networks (GAN) to classify pulmonary nodules in CT scan images.
Various public and private lung cancer datasets are available that help to develop methods for the early detection of lung cancer. For example, Zheng et al. [13] explored maximum intensity projection image to detect lung nodule using CNN in CT scans images and achieved 94.2% of sensitivity on the Lung nodule analysis 2016 (LUNA16) dataset. Liu et al. [14] presented a novel multi-model ensemble framework consisted of 3D convolutional neural network to detect malignancy suspiciousness in lung nodules. They have used image enhancement technique to transform low contrast nodules into high contrast nodules. Multi-model network architecture is applied for different sizes of nodules that provides less training time of the framework. Furthermore, ensemble learning is applied to enhance the efficiency of the lung nodule classification system and multi-model achieved satisfactory classification results with 90.6% of accuracy.
In another study, Xu et al. [15] presented a method based on CNN for the evaluation of lung nodule malignancy and simultaneously addressed two challenges: small dataset, and imbalance in the dataset. They have used a multiple lighter network ensemble method to solve small dataset problem and a new loss function with cross-entropy to provide the solution of the imbalance problem. Al-Shabi et al. [16] applied attention operation on 3D axial attention technique using CNN for the classification of lung nodule and achieved 92.81% of accuracy. Various techniques have been proposed for the lung nodule detection in diagnosing lung cancer using state-of-the-art ML and DL approaches. However, performance related issues of early detection of lung cancer still remains an open question. Therefore, a holistic approach is proposed for efficient and accurate lung cancer diagnosis.
The following are the main contributions:
a. We preprocessed public available LUNA16 dataset to reduce the computational time.
b. Modified AlexNet Architecture is proposed for the feature extractions from large volume of CT scan images to support efficient classification.
c. For efficient model training, three optimizers are applied hence reducing the computational time and efficient resource utilization.
d. In order to go for early detection of lung cancer and prevent death rate, machine learning approach is implemented for efficient detection.
The rest of the paper is organized as follows: Section 2 investigates the recent works on Lung Cancer detection. Section 3 represents the different steps of the proposed approach. The results of proposed model are illustrated in Section 4. The key conclusions are presented in Section 5.
In this section, we analyses existing relevant research works that majorly focus on deep learning techniques. The review is carried out using Elsevier, Springer, Scopus and Web of science with different research criteria such as lung cancer diagnosis, lung nodule detection and classification. We select 20 relevant research papers from year 2020 to 2022. The review is explained below:
Jiang et al. [17] designed a pulmonary nodules classification system employed an attentive and ensemble 3D dual path networks. Authors applied contextual attention to improve deep features and spatial attention was used to locate Region of interest (ROIs) and an ensemble was applied to enhance the robustness of detection method. Jena et al. [18] designed a Kernel based non-gaussian CNN (KNG-CNN) model for proficient lung cancer classification. The model consists of three convolutions with three pooling and two fully connected layers. In preprocessing phase, Contrast limited adaptive histogram equalization (CLAHE) technique has been applied to segment ROI. Subsequently, morphological features were extracted and finally, KNG-CNN model was applied to classify lung nodule and obtained 87.3% of accuracy on LIDC-IDRI dataset.
Dodia et al. [19] introduced Receptive field regularized V-net (RFR V-Net) deep learning network to classify lung nodules. Further, RFR technique is applied on convolution encode block and deconvolution decoder block for feature extraction. Moreover, SqueezeNet and ResNet are executed together for the classification of lung nodule. Vijh et al. [20] introduced the hybrid bio-inspired Whale optimization algorithm with adaptive particle swarm optimization (WOA-APSO) for feature selection with convolutional neural network for classification of lung cancer nodules. The Wiener filter has been applied to eliminate noise from CT image in preprocessing. The global thresholding method was used to segment the cancer and non-cancer images. Different feature extraction methods were performed to extract information from the segmented CT images and proposed system achieved 97.18% of accuracy. Rani et al. [21] introduced a method for identification of lung cancer nodules from CT images. Histogram equalization and tophat technique was applied to remove noise from CT images. Advance target map superpixel-based region segmentation technique was presented to segment the cancer regions. Boosted deep convolutional neural network (BDCNN) was presented for classification of lung cancer.
Ananth et al. [22] presented an optimized DCNN for segmentation and classification of CT lung images. The model consisted of four phases: Wighted mean histogram analysis (WMHA) was applied for removal noise and increase the quality of image. Hybrid Dual tree-complex wavelet transform (DT-CWT) with gabor filter was used to extract features. Furthermore, DCNN was suggested to classify CT images into benign and malignant. Finally, Fuzzy c-means with equilibrium optimizer (FCM-EO) was introduced to remove the outliers and recognize the cancer regions from the malignant images. The experimentation was conducted on public available LIDC-IDRI dataset. Guo et al. [23], developed a sequential method for the detection of lung cancer from CT lung images. The method comprised of two classifiers: CNN-based and feature-based classifier. Harris Hawks optimizer was used in CNN-based classifier and renowned haralick and local binary pattern were applied for results. First, CNN-based classifier was applied and then feature-based classifier on dataset for the detection of lung cancer.
Hesamian et al. [24] discussed that irregular nodule shapes, precisely segmenting nodules, low intensity contrast among the nodules from lung CT images is still a very thought-provoking task. The researchers addressed this problem and developed a deep learning based system. The system created a synthetic image from slices in unique color patterns. Additionally, a DL based segmentation technique was adopted on synthetic images and developed a modified U-Net model for distinctive color patterns that provided more accurate and effective results to solve the segmentation and classification of lung nodule tasks. Guo et al. [25] developed an automated lung cancer detection system based on CNN with hybrid attention mechanism and residual connection. The system provided end-to-end convolutional neural network based classification network that extracted features automatically, and converted into high level classification and aggregate features into classes. Clinical scintigraphy images were used to classify the multi-class classification network. Shah et al. [26] presented a NoduleNet model for the classification of lung nodule based on DL. NoduleNet model employed with customized Visual geometry group (VGG16) and VGG19 architecture.
Chen et al. [27] introduced robust Lung nodule classification and cancer detection system (LDNNET) consisted of dense neural network. LDNNET system is an adaptive architecture and comprised of dense block, batch normalization and dropout. This network was evaluated on two datasets LUNA16 and Kaggle DSB 2017 and achieved satisfactory performance. Another study based on LUNA16 dataset was conducted by Bansal et al. [28], for lung cancer segmentation and classification. Xu et al. [29] presented a model based on ensemble learning to classify lung nodules from CT images. YOLOv3 network was trained on a publicly available dataset to identify lung nodules, meanwhile, CNN was trained on dataset to diagnose benign and malignant lung nodules. Subsequently, confidence probability consisted of an ensemble learning were taken as data features from YOLOv3 and CNN models. Public and private datasets were tested and output of confidence probability forwarded into the logistic regression model to identify lung nodules as benign and malignant.
Naik et al. [30] elaborated pulmonary nodule classification system based on fractal network to classify the pulmonary nodules. Fractalnet model was applied on LUNA16 dataset to train and validate the performance of the system and achieved 94.7% of accuracy. Muzammil et al. [31] applied feature and ensemble learning based fusion methods for pulmonary nodule classification. Linear discriminant analysis (LDA), SVM and AdaBoostM2 algorithms were trained and tested on LUNA16 for pulmonary nodules classification and achieved 96.89% of accuracy. Similarly, another study [32] developed a segmentation method for the detection of lung cancer based on deep learning. Many other state-of-the-art existing studies [33–36] addressed various field of life and discussed other real world problems. In this study, we have focused on the detection of lung cancer using modify AlexNet architecture.
Summary of state-of-the-art existing studies is shown in Tab. 1 which have adopted CNN architectures to detect pulmonary nodules.
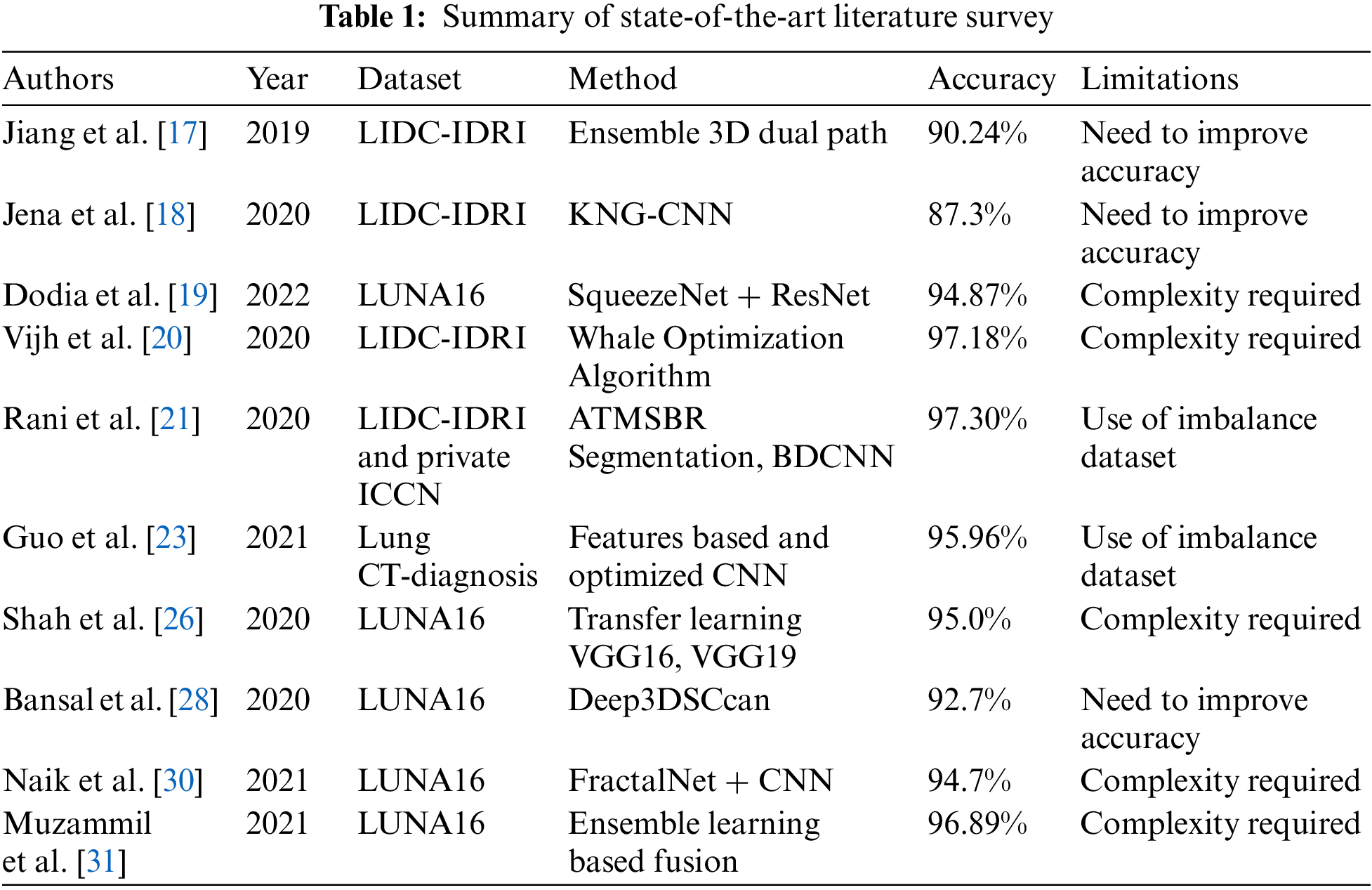
From Tab. 1, we have analyzed that existing methods of CNN-based pulmonary nodule detection from CT scan lack the efficiency and accuracy in terms of feature extraction. Moreover, the methods have achieved an accuracy range from 87.3% to 97.3% with greater time and resource consumption. The research work consisted of [17,18,28] requires to improve the detection accuracy. The study [21,23] was based on an imbalanced dataset while other research works [19,20,26,30,31] are based on a complex model.
In this study, we are aiming to develop a diagnostic system that delivers an efficient solution for the detection of lung cancer. Therefore, we propose a LungNet-SVM model that accurately detects lung cancer nodules with less time and efficient resource consumption. The proposed LungNet-SVM model is presented below in Section 3.
The proposed LungNet-SVM model is divided into two phases, one is training and the second is the validation phase as shown in Fig. 1. Firstly, CT scan images are acquired from the publicly available lung cancer namely LUNA16 dataset. These images are provided as input to the data preprocessing phase to tune the data for the modeling phase. In data preprocessing phase, the input images are reshaped while noise and outliers are removed. Furthermore, the input patch size is divided into three sizes such as 16 × 16, 32 × 32 and 48 × 48. Moreover, this preprocessed data is forwarded to data splitting phase, where preprocessed data is split into training and validation sets where the training set consists of 80% while 20% reserved for validation purpose.
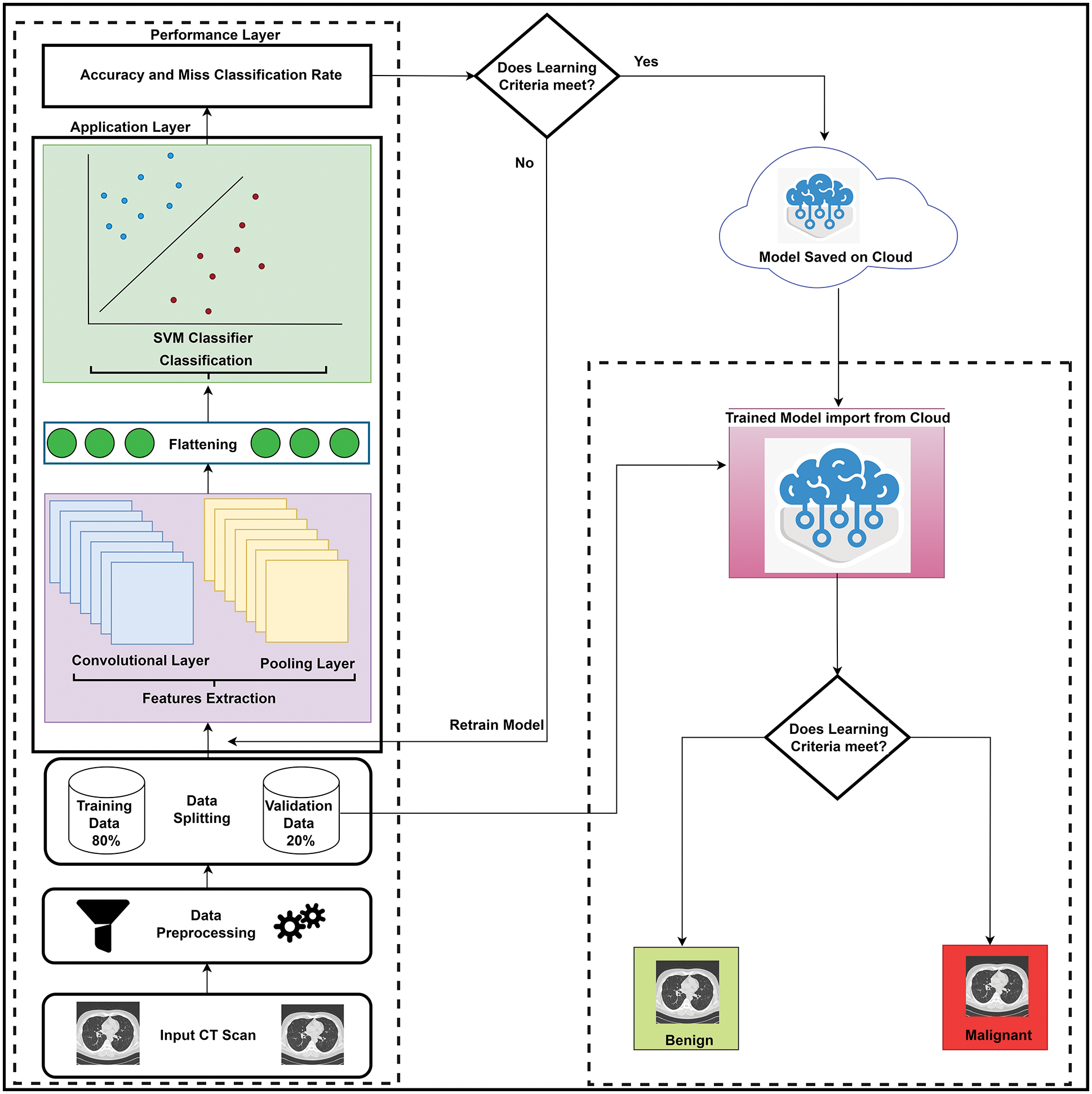
Figure 1: The proposed LungNet-SVM model
Training data comprises two layers such as application and performance layers. We have extracted features by using the proposed LungNet-SVM modified convolutional neural network in the application layer. In features extraction process, important information obtained from the input images and forwarded to the next step. Three optimizers Adaptive moment estimation (Adam), Root mean square propagation (RMSprop), and Stochastic gradient descent (SGD) is applied with hyper parameters including 0.0001 learning rate, 50 batch size, and 200 epochs.
The proposed LungNet-SVM model was evaluated in the performance layer in terms of accuracy and miss classification rate. The convolution layer is the core part of a CNN and it is responsible to extract important features from the input data. Convolution operations (represented by *) are performed in convolutional layers and a filter is required to apply the convolutional operation to an image. The output of the convolutional operator is called a feature map or activation map. The convolutional operation [32] is shown in Eq. (1).
where U is denoted as the input matrix (image), V is the filter size of p × q and Z denotes the output feature map. Input U is convolved with the filter V and produces the feature map Z. This Convolutional operation represents by U × V. The output of the convolutional layer forward to a nonlinear activation function (AF). An activation function adds non-linearity to a network.
Several nonlinear activation functions such as Rectified linear unit (ReLU), Sigmoid, Hyperbolic tangent (Tanh), and softmax can be applied on the activation map to remove linearity/to normalize the network values. In this work, the ReLU activation function is applied to compute activation. If the input is zero or less than zero then the ReLU output is 0. ReLU [37] represents mathematically given in Eq. (2).
In a convolutional neural network, the convolution layer is followed by the Pooling layer and applied to reduce the dimension of the feature map but retains the important information and it is also called down sampling. Various types of operations are applied in the pooling layer such as average pooling, sum pooling max pooling, and, min pooling can be used to reduce the size of the activation map. In pooling layer, the important information retains but size of the activation map is reduced. Before passing to the fully connected layer, the feature map is flattened. In flattening, the feature map matrix converts into a long vector. In the convolutional layer, convolutional operations are performed on 80% of preprocessed CT image data. The proposed LungNet-SVM adopts twelve layers including seven convolutional layers, three pooling, and two fully connected layers.
The proposed LungNet-SVM CNN network architecture for the lung nodule detection represents in Fig. 2. The proposed network architecture takes 16 × 16, 32 × 32 and 48 × 48 grayscale image input sizes, and the image size 32 × 32 × 1 demonstrates in Fig. 2. In the first two convolutional layers total 32 number of filters are applied with kernel size 3 × 3, with the same padding.
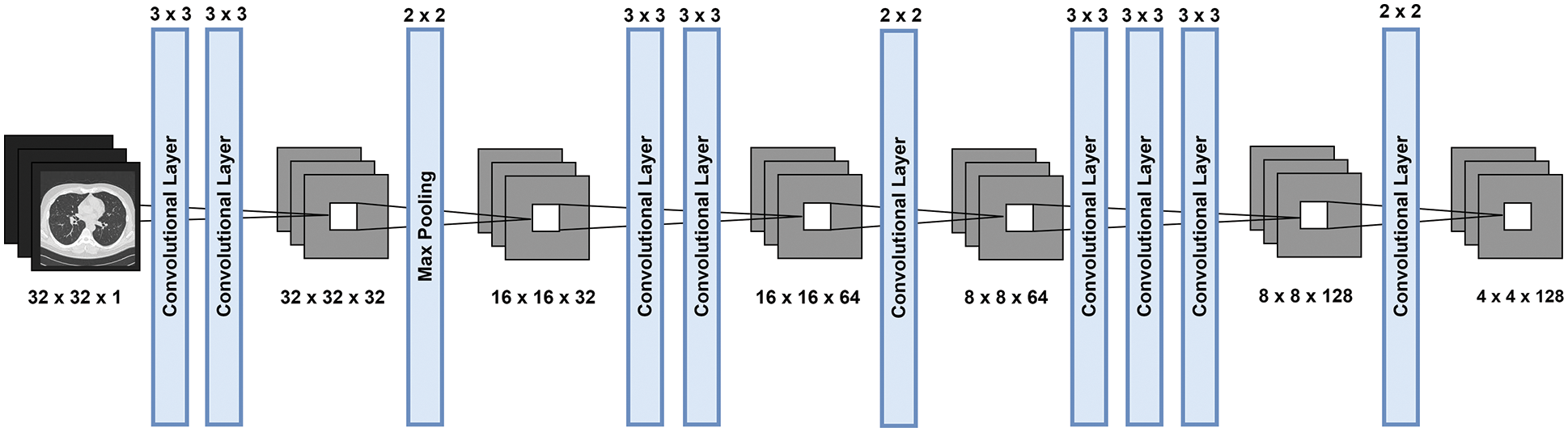
Figure 2: The diagrammatic representation of modified AlexNet architecture
ReLU activation function is applied to remove non-linearity from the proposed model. Convolutional layers are followed by a max-pooling layer. Filter size 2 × 2 with stride 2 is adopted in the max-pooling layer and the size of the image reduces and becomes 16 × 16 × 32.
Subsequently, two convolutional layers applied 64 filters, kernel size 3 × 3 and the same padding with ReLU activation function. Next, the second max-pooling layer, 2 × 2 kernel size and stride applied and image size becomes 8 × 8 × 64. The last three convolutional layers are applied with 128 filters, kernel size 3 × 3 and ReLU activation function. In the third max-pooling layers, kernel size 2 × 2 and 2 strides applied and size becomes 4 × 4 × 128. Subsequently applied third max-pooling layer, flatten converts multidimensional input to 1D vector and becomes a vector size of 2048 × 1.
After features extraction, the convolutional neural network classification operations are performed by using a Fully connected (FC) layer [38]. FC layer is a conventional neural network where each neuron of the previous layer is connected to other neurons of the next layer. The output of the fully connected layer is forwarded to AF which produces class scores. The support vector machine and softmax are most commonly used to compute the classification purpose. In the LungNet-SVM model, the SVM classifier is applied to achieve the best accuracy in the classification of lung cancer into benign and malignant. Consequently, these results are forwarded to the performance layer.
Deep learning needs computational resources and substantial time to train the model. For this purpose, various optimizers play a significant role to achieve less computational resources and time. In this work, three optimizers Adam, RMSprop, and SGD are applied to achieve better performance from the proposed LungNet-SVM model. Adam optimizer adopts less resources and computation time which leads to the model boost in the learning process. RMSprop is an optimization method that is adopted in neural networks and uses an adaptive learning rate. Stochastic gradient descent is an optimization technique that uses to achieve the best fit using model parameters, momentum and it is widely adopted in ML.
In the performance layer, statistical parameters such as accuracy and the miss classification rate of the LungNet-SVM measures. If learning criteria does not meet the requirements, then there is a need to retrain the proposed LungNet-SVM model and if learning criteria meets the requirements, then results and model are saved on the Cloud for further use. When proposed LungNet-SVM training completes then the validation phase starts.
In the validation phase, the trained LungNet-SVM model is imported from the cloud and 20% of validation data is forwarded to the trained LungNet-SVM model for the evaluation of the proposed model. If lung cancer does not detect cancer nodule, then the model shows benign and if the trained model detects cancer nodule, then it shows malignant.
In this work, the proposed LungNet-SVM model has been trained and validated on a benchmark of the publicly available LUNA16 dataset [39]. This dataset comprises a total number of 1018 CT scan images. The dataset is further divided into 80% for training and 20% for validation of the LungNet-SVM model.
The statistical performance evaluation [40] metrics such as Accuracy [41], Miss classification rate [42], Sensitivity [43], and Specificity [44] as metrics are adopted to validate the overall performance of the LungNet-SVM model. Accuracy and miss classification rate illustrates the correct and wrong prediction respectively by the proposed LungNet-SVM model. These criteria are as follows:
In the present work, the LungNet-SVM model is proposed to detect lung cancer, and the proposed approach is implemented in Python using the Keras tool. Firstly, in the proposed LungNet-SVM model, three optimizers Adam, RMSprop, and SGD applied to the LUNA16 dataset to achieve effective results and compared with each other. Secondly, the performance is evaluated with other state-of-the-art methods to detect lung cancer. The comparative analysis of the proposed LungNet-SVM model for training is shown in Tab. 2. The three input image sizes such as 16 × 16, 32 × 32, and 48 × 48 are considered with Adam, RMSprop, and SGD optimizers in the training phase of the proposed LungNet-SVM model.
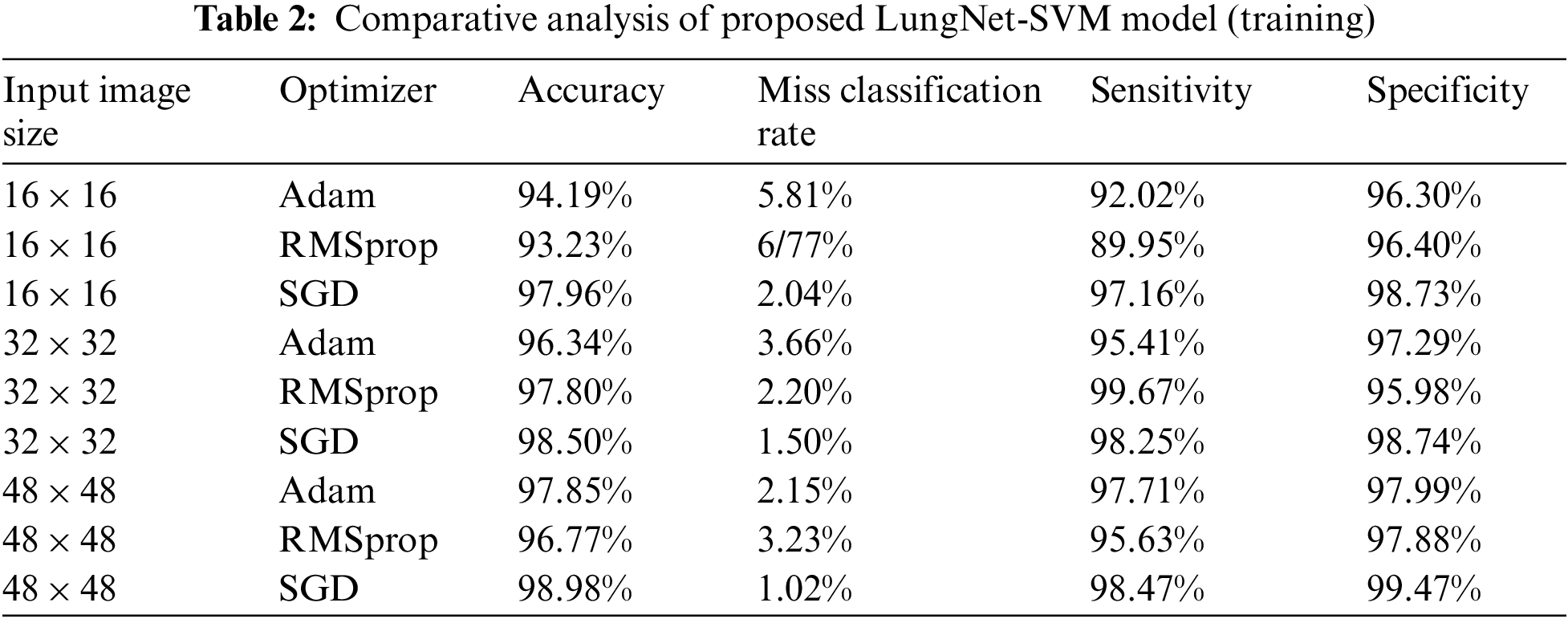
In the training phase, input image size 16 × 16, the proposed LungNet-SVM model employs SGD optimizer achieves the highest accuracy of 97.96% while miss classification rate, sensitivity, and specificity of 2.04%, 97.16%, and 98.73% respectively. The proposed model with optimizers Adam achieves accuracy, miss classification rate, sensitivity, and specificity of 94.19%, 5.81%, 92.02%, and 96.30% respectively. The proposed LungNet-SVM model with RMSprop achieves accuracy, miss classification rate, sensitivity, and specificity of 93.23%, 2.04%, 97.16%, and 98.73% respectively.
It is found that when input image size 32 × 32 the proposed LungNet-SVM model employs SGD optimizer achieves the highest accuracy of 98.50%, while the miss classification rate, sensitivity, and specificity of 1.50%, 98.25%, and 98.74% respectively. The proposed model with optimizers Adam achieves accuracy, miss classification rate, sensitivity, and specificity of 96.34%%, 3.66%, 95.41%, and 97.29% respectively. The proposed LungNet-SVM model with RMSprop achieves accuracy, the miss classification rate, sensitivity, and specificity of 97.80%, 2.20%, 99.67%, and 95.98% respectively.
When input image size 48 × 48, the proposed LungNet-SVM model employs SGD optimizer achieves the highest accuracy of 98.98% while miss classification rate, sensitivity, and specificity of 1.02%, 98.47%, and 99.47% respectively. The proposed model with optimizers Adam achieves accuracy, miss classification rate, sensitivity, and specificity of 97.85%%, 2.15%, 97.71%, and 97.99%% respectively. The proposed LungNet-SVM model with RMSprop achieves accuracy, miss classification rate, sensitivity, and specificity of 96.77%, 3.23%, 95.63%, and 97.88% respectively.
The comparative analysis of the proposed LungNet-SVM model for the validation phase is shown in Tab. 3. The three input image sizes such as 16 × 16, 32 × 32, and 48 × 48 are considered with Adam, RMSprop, and SGD optimizers in the validation phase of the proposed LungNet-SVM model.
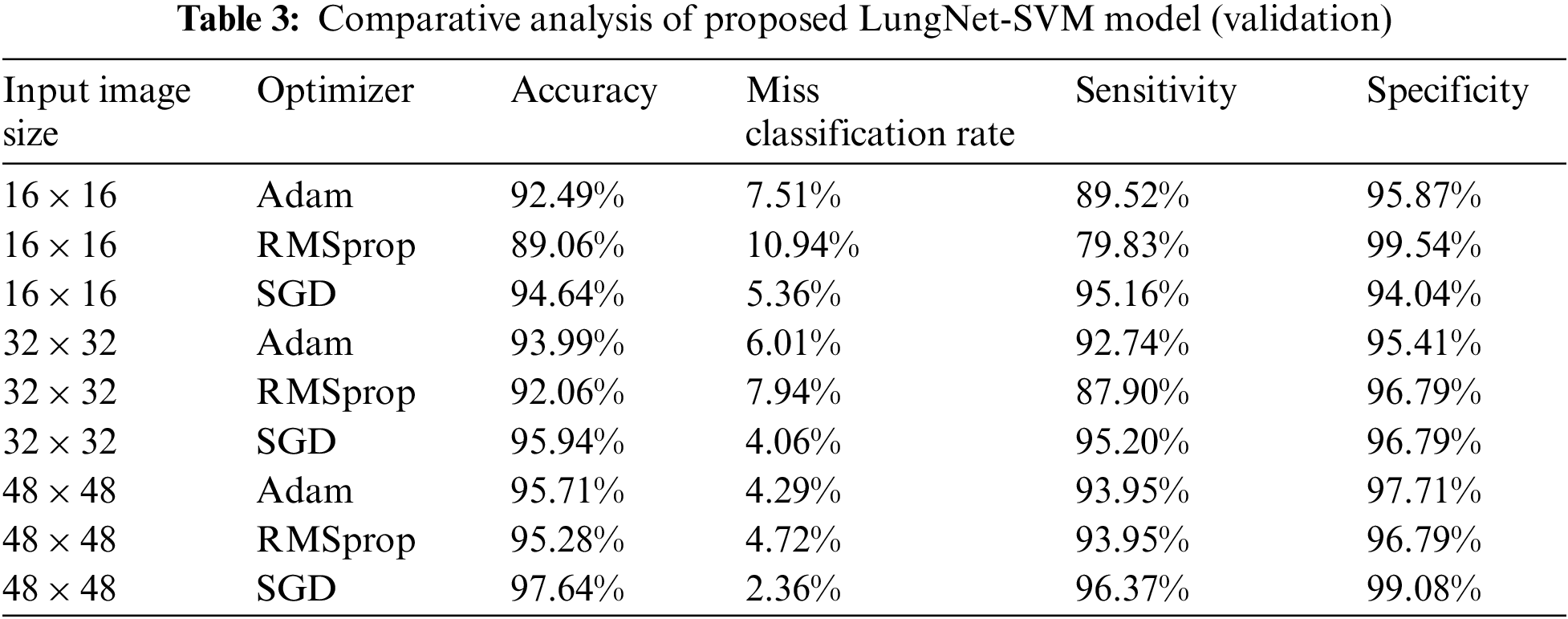
In the validation phase, input image size 16 × 16, the proposed LungNet-SVM model employs SGD optimizer achieves the highest accuracy of 94.64% while the miss classification rate, sensitivity and specificity of 5.36%, 95.16%, and 94.04% respectively. The proposed model with optimizers Adam achieves accuracy, miss classification rate, sensitivity and specificity of 92.49%, 7.51%, 89.52% and 95.87% respectively. The proposed LungNet-SVM model with RMSprop achieves accuracy, miss classification rate, sensitivity, and specificity of 89.06%, 10.94%, 79.83% and 99.54% respectively.
It is observer that when input image size 32 × 32 the proposed LungNet-SVM model employs SGD optimizer achieves the highest accuracy of 95.94%, while miss classification rate, sensitivity and specificity of 4.06%, 98.25%, and 95.20% respectively. The proposed model with optimizers Adam achieves accuracy, miss classification rate, sensitivity and specificity of 93.99%, 6.01%, 92.74% and 95.41% respectively. The proposed LungNet-SVM model with RMSprop achieves accuracy, miss classification rate, sensitivity, and specificity of 92.06%, 7.94%, 87.90% and 96.79% respectively.
When input image size 48 × 48, the proposed Lung Net-SVM model employs SGD optimizer achieves the highest accuracy of 97.64% while miss classification rate, sensitivity, and specificity of 2.36%, 96.37%, and 99.08% respectively. The proposed model with optimizers Adam achieves accuracy, miss classification rate, sensitivity, and specificity of 95.71%, 4.29%, 93.95%, and 97.99% respectively. The proposed LungNet-SVM model with RMSprop achieves accuracy, miss classification rate, sensitivity, and specificity of 95.28%, 4.72%, 93.95%, and 96.79% respectively.
The proposed LungNet-SVM model performed on three input image sizes with various optimizers such as Adam, RMSprop and SGD. The training and validation results of the proposed LungNet-SVM model are shown that when input image size is 48 × 48, the proposed LungNet-SVM model with SGD optimizer achieves the highest accuracy while compared with other image sizes such as 16 × 16, 32 × 32 and optimizers such as Adam and RMSprop.
Tab. 4 represents the confusion matrix of the LungNet-SVM model for the training phase with SGD optimizer which achieves the highest accuracy. A total number of 1860 samples are obtained to train the model with the SGD optimizer. A total number of 1860 samples was further divided into benign and malignant.
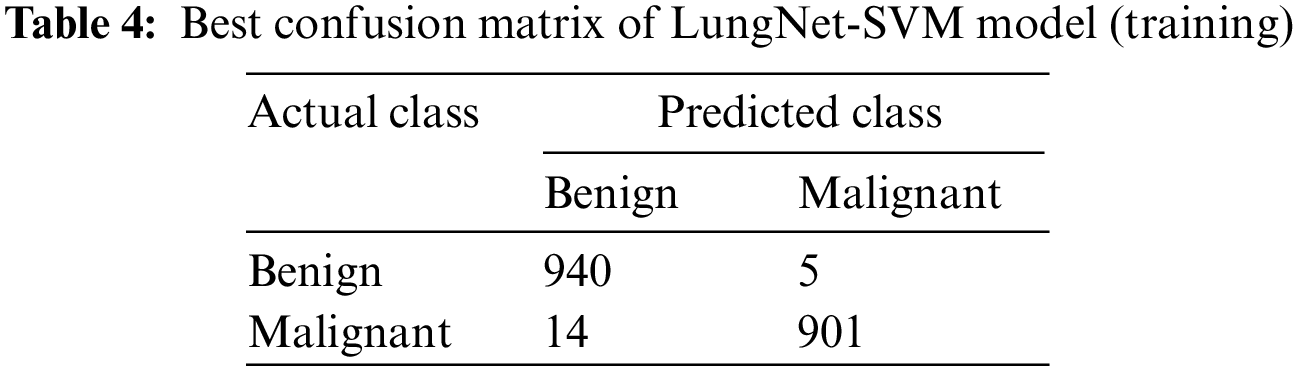
In the benign case, a total number of 945 samples are used to train the model, the proposed LungNet-SVM model correctly predicts 940 samples, and 5 samples are wrongly predicted. In the malignant case, a total number of 915 samples are used to train the proposed model, LungNet-SVM model correctly predicts 901 number of sample and wrongly predicts 14 samples.
Tab. 5 represents the confusion matrix of the LungNet-SVM model for the validation phase with the SGD optimizer that has obtained the highest accuracy. A total number of 466 samples are obtained to validate the proposed model. A total number of 466 samples was further divided into benign and malignant.
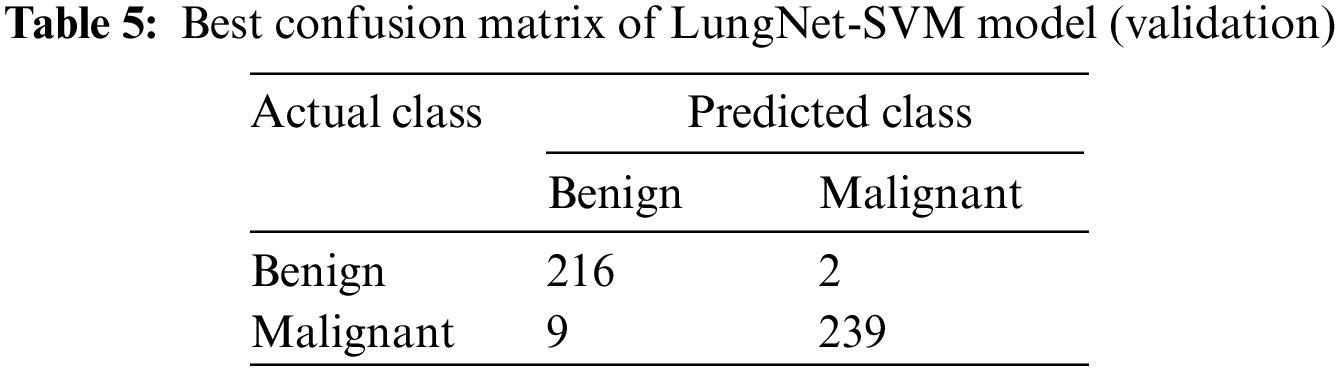
In the benign case, a total number of 218 samples was used to validate the model, the proposed LungNet-SVM model correctly predicts 215 samples and wrongly predicts 3 number of sample. In the malignant case, a total number of 248 samples to validate the proposed model. The proposed LungNet-SVM model correctly predicts 239 samples and wrongly predicts 9 samples.
Fig. 3 illustrates the results of benign and malignant proposed obtained from the proposed modified AlexNet model for the detection of lung cancer. First three images shown as true negative because the proposed LungNet-SVM model correctly predicted benign as these are actual benign. Next three images are shown as false positive, the LungNet-SVM model wrongly predicted as malignant but these are actual benign. For malignant, first three images are shown as false negative and the proposed LungNet-SVM model are predicted as benign but these are actual as malignant. Last three images are shown as true positive, the proposed LungNet-SVM model are predicted as malignant as these are actual malignant.
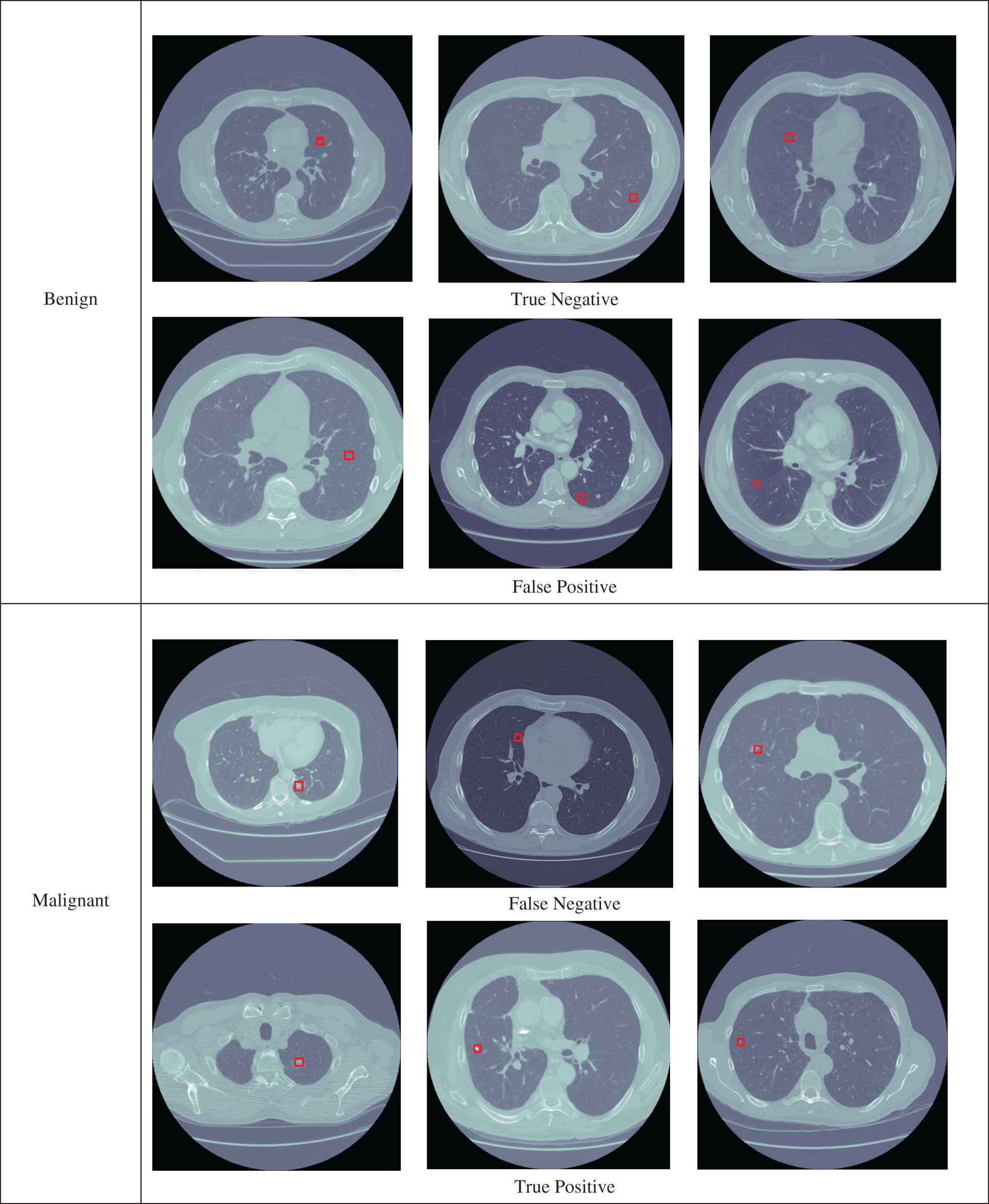
Figure 3: The benign and malignant lung nodules detection by the proposed LungNet-SVM model
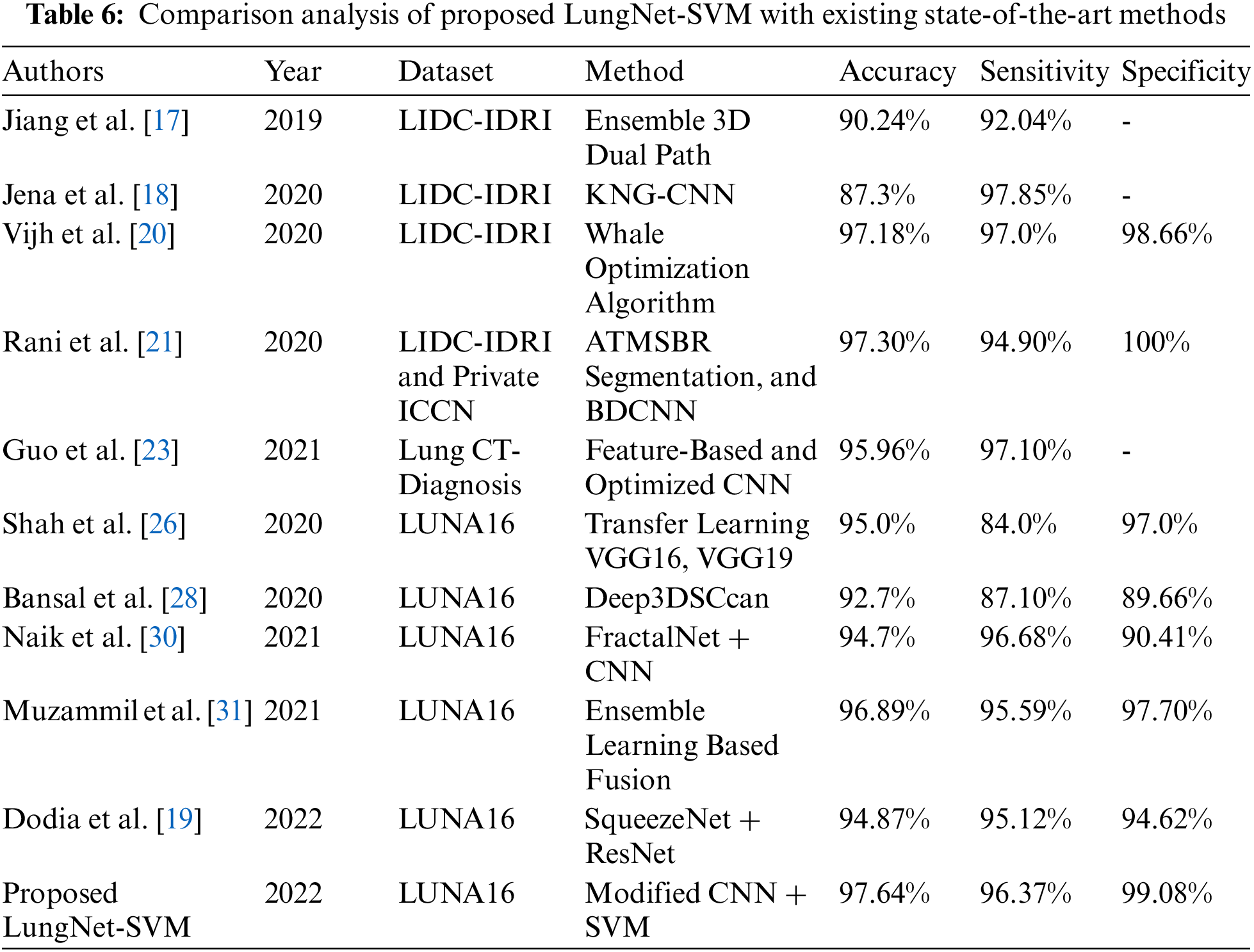
Tab. 6 demonstrates the comparison analysis of the proposed LungNet-SVM model with existing state-of-the-art methods. Previously, several methods based on various CNN architectures were applied to publically available LIDC-IDRI, LUNA16, and private ICCN, Lung CT-Diagnosis datasets for the detection of lung cancer. The results in terms of accuracy, sensitivity, and specificity proved that the proposed LungNet-SVM model is more accurate and efficient as compared to previous approaches.
This work introduces a LungNet-SVM for effectual segmentation and classification of pulmonary nodules from CT images. The proposed LungNet-SVM model is the modified version of AlexNet architecture. The proposed model comprises seven convolutional layers, three pooling layers, and two fully connected layers followed by a support vector machine algorithm used as a classifier. While the training and validation phase of the proposed model, three input image sizes such as 16 × 16, 32 × 32, and 48 × 48 are considered and three optimizers Adam, RMSprop, and SGD are applied to tune the proposed model for the highest accuracy. It is found that the proposed LungNet-SVM with SGD optimizer achieved the highest accuracy in 48 × 48 input image sizes. The experimental analysis is carried out by using the publically available LUNA16 dataset and implemented in Python. The proposed model has achieved 97.42% of accuracy, 2.58% of miss classification rate, 96.37% of sensitivity, and 98.62% of specificity. The proposed LungNet-SVM model achieves the highest accuracy when compared with other state-of-the-art studies.
The proposed LungNet-SVM classifies lung cancer into two categories benign and malignant. The proposed LungNet-SVM based on modified AlexNet architecture is used to increase the performance of the early detection system in terms of cancer and non-cancerous. The work establishes that the proposed model has some limitations such as it is trained and validated on a single dataset, three CT image sizes as well as three optimizers. The proposed model can be implemented on other publicly available datasets with various convolutional neural network architectures and optimizers to achieve better performance in the detection of lung cancer.
Acknowledgement: We thank our families and colleagues who provided us with moral support.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. S. Luo, J. Zhang, N. Xiao and Y. Qiang, “Das-net: A lung nodule segmentation method based on adaptive dual-branch attention and shadow mapping,” Applied Intelligence, vol. 52, no. 4, pp. 1–15, 2022. [Google Scholar]
2. M. Toğaçar, B. Ergen and Z. Cömert, “Detection of lung cancer on chest ct images using minimum redundancy maximum relevance feature selection method with convolutional neural networks,” Biocybernetics and Biomedical Engineering, vol. 40, no. 1, pp. 23–39, 2020. [Google Scholar]
3. A. Pezeshk, S. Hamidian, N. Petrick and B. Sahiner, “3-D convolutional neural networks for automatic detection of pulmonary nodules in chest CT,” IEEE Journal of Biomedical and Health Informatics, vol. 23, no. 5, pp. 2080–2090, 2018. [Google Scholar]
4. S. P. Pawar and S. N. Talbar, “Lungseg-net: Lung field segmentation using generative adversarial network,” Biomedical Signal Processing and Control, vol. 64, pp. 102296–102307, 2021. [Google Scholar]
5. D. Liu, F. Liu, Y. Tie, L. Qi and F. Wang, “Res-trans networks for lung nodule classification,” International Journal Computer Assisted Radiology and Surgery, vol. 17, pp. 1059–1068, 2022. [Google Scholar]
6. S. Zheng, Z. Shen, C. Pei, W. Ding, H. Lin et al., “Interpretative computer-aided lung cancer diagnosis: From radiology analysis to malignancy evaluation,” Computer Methods and Programs in Biomedicine, vol. 210, pp. 106363–106374, 2021. [Google Scholar]
7. H. Jiang, F. Shen, F. Gao and W. Han, “Learning efficient, explainable and discriminative representations for pulmonary nodules classification,” Pattern Recognition, vol. 113, pp. 107825–107834, 2021. [Google Scholar]
8. A. Halder, S. Chatterjee, D. Dey, S. Kole and S. Munshi, “An adaptive morphology based segmentation technique for lung nodule detection in thoracic CT image,” Computer Methods Programs in Biomedicine, vol. 197, pp. 105720–105736, 2020. [Google Scholar]
9. Y. Ren, M. Y. Tsai, L. Chen, J. Wang, S. Li et al., “A manifold learning regularization approach to enhance 3D CT image-based lung nodule classification,” International Journal Computer Assisted Radiology and Surgery, vol. 15, no. 2, pp. 287–295, 2020. [Google Scholar]
10. L. Alzubaidi, J. Zhang, A. J. Humaidi, A. Dujaili, Y. Duan et al., “Review of deep learning: Concepts, CNN architectures, challenges, applications, future directions,” Journal of Big Data, vol. 8, no. 1, pp. 1–74, 2021. [Google Scholar]
11. R. Manickavasagam, S. Selvan and M. Selvan, “Cad system for lung nodule detection using deep learning with CNN,” Medical and Biological Engineering Computing, vol. 60, no. 1, pp. 221–228, 2022. [Google Scholar]
12. Y. Onishi, A. Teramoto, M. Tsukamoto, K. Saito, H. Imaizumi et al., “Multiplanar analysis for pulmonary nodule classification in CT images using deep convolutional neural network and generative adversarial networks,” International Journal Computer Assististed Radiolgy and Surgery, vol. 15, no. 1, pp. 173–178, 2020. [Google Scholar]
13. S. Zheng, J. Guo, X. Cui, R. N. J. Veldhuis, M. Oudkerk et al., “Automatic pulmonary nodule detection in CT scans using convolutional neural networks based on maximum intensity projection,” IEEE Transaction on Medical Imaging, vol. 39, no. 3, pp. 797–805, 2020. [Google Scholar]
14. H. Liu, H. Cao, E. Song, G. Ma, X. Xu et al., “Multi-model ensemble learning architecture based on 3D CNN for lung nodule malignancy suspiciousness classification,” Journal of Digital Imaging, vol. 33, no. 5, pp. 1242–1256, 2020. [Google Scholar]
15. X. Xu, C. Wang, J. Guo, Y. Gan, J. Want et al., “Mscs-deepln: Evaluating lung nodule malignancy using multi-scale cost-sensitive neural networks,” Medical Image Analysis, vol. 65, pp. 101772–101787, 2020. [Google Scholar]
16. M. Al-Shabi, K. Shak and M. Tan, “3D Axial-attention for lung nodule classification,” International Journal of Computer Assisted Radiology Surgery, vol. 16, no. 3, pp. 1319–1324, 2021. [Google Scholar]
17. H. Jiang, F. Gao, X. Xu, F. Huang and S. Zhu, “Attentive and ensemble 3D dual path networks for pulmonary nodules classification,” Neurocomputing, vol. 398, pp. 422–430, 2019. [Google Scholar]
18. S. R. Jena and S. T. George, “Morphological feature extraction and KNG-CNN classification of CT images for early lung cancer detection,” International Journal of Imaging System and Technology, vol. 30, no. 4, pp. 1324–1337, 2020. [Google Scholar]
19. S. Dodia, A. Basava and M. A. Padukudru, “A novel receptive field-regularized v-net and nodule classification network for lung nodule detection,” International Journal of Imaging System and Technology, vol. 32, no. 1, pp. 88–101, 2022. [Google Scholar]
20. S. Vijh, P. Gaurav and H. M. Pandey, “Hybrid bio-inspired algorithm and convolutional neural network for automatic lung tumor detection,” Neural Computing Applications, vol. 32, no. 18, pp. 1–14, 2020. [Google Scholar]
21. K. V. Rani and S. J. Jawhar, “Superpixel with nanoscale imaging and boosted deep convolutional neural network concept for lung tumor classification,” International Journal of Imaging System and Technology, vol. 30, no. 4, pp. 899–915, 2020. [Google Scholar]
22. A. D. Ananth and C. Palanisamy, “Extended and optimized deep convolutional neural network-based lung tumor identification in big data,” International Journal of Imaging System and Technology, vol. 32, no. 3, pp. 918–934, 2021. [Google Scholar]
23. Z. Guo, L. Xu, Y. Si and N. Razmjooy, “Novel computer-aided lung cancer detection based on convolutional neural network-based and feature-based classifiers using metaheuristics,” International Journal of Imaging System and Technology, vol. 31, no. 4, pp. 1954–1969, 2021. [Google Scholar]
24. M. H. Hesamian, W. Jia, X. He, Q. Wang and P. J. Kennedy, “Synthetic CT images for semi-sequential detection and segmentation of lung nodules,” Applied Intelligence, vol. 51, no. 3, pp. 1616–1628, 2021. [Google Scholar]
25. Y. Guo, Q. Lin, S. Zhao, T. Li, Y. Cao et al., “Automated detection of lung cancer-caused metastasis by classifying scintigraphic images using convolutional neural network with residual connection and hybrid attention mechanism,” Insights into Imaging, vol. 13, no. 1, pp. 1–13, 2022. [Google Scholar]
26. G. Shah, R. Thammasudjarit, A. Thakkinstian and T. Suwatanapongched, “Nodulenet: A lung nodule classification using deep learning,” Ramathibodi Medical Journal, vol. 43, no. 4, pp. 11–19, 2020. [Google Scholar]
27. Y. Chen, Y. Wang, F. Hu, L. Feng, T. Zhou et al., “Ldnnet: Towards robust classification of lung nodule and cancer using lung dense neural network,” IEEE Access, vol. 9, pp. 50301–50320, 2021. [Google Scholar]
28. G. Bansal, V. Chamola, P. Narang, S. Kumar and S. Raman, “Deep3Dscan: Deep residual network and morphological descriptor based framework for lung cancer classification and 3D segmentation,” IET Image Process, vol. 14, no. 7, pp. 1316–1326, 2020. [Google Scholar]
29. Y. Xu, S. Wang, X. Sun, Y. Yang, J. Fan et al., “Identification of benign and malignant lung nodules in CT images based on ensemble learning method,” Interdisciplinary Science: Computational Life Science, vol. 14, no. 1, pp. 130–140, 2022. [Google Scholar]
30. A. Naik, D. R. Edla and V. Kuppili, “Lung nodule classification on computed tomography images using fractalnet,” Wireless Personal Communications, vol. 119, no. 2, pp. 1209–1229, 2021. [Google Scholar]
31. M. Muzammil, I. Ali, I. U. Haq, A. A. Khaliq and S. Abdullah, “Pulmonary nodule classification using feature and ensemble learning-based fusion techniques,” IEEE Access, vol. 9, pp. 113415–113427, 2021. [Google Scholar]
32. A. Shimazaki, D. Ueda, A. Choppin, A. Yamamoto, T. Honjo et al., “Deep learning-based algorithm for lung cancer detection on chest radiographs using the segmentation method,” Scientific Reports, vol. 12, no. 1, pp. 1–10, 2022. [Google Scholar]
33. W. Sun, G. C. Zhang, X. R. Zhang, X. Zhang and N. N. Ge, “Fine-grained vehicle type classification using lightweight convolutional neural network with feature optimization and joint learning strategy,” Multimedia Tools and Applications, vol. 80, pp. 30803–30816, 2021. [Google Scholar]
34. H. Sun and R. Grishman, “Lexicalized dependency paths based supervised learning for relation extraction,” Computer Systems Science and Engineering, vol. 43, no. 3, pp. 861–870, 2022. [Google Scholar]
35. W. Sun, Y. T. Du, X. Zhang and G. C. Zhang, “Detection and recognition of text traffic signs above the road,” International Journal of Sensor Networks, vol. 35, no. 2, pp. 69–77, 2021. [Google Scholar]
36. H. Sun and R. Grishman, “Employing lexicalized dependency paths for active learning of relation extraction,” Intelligent Automation & Soft Computing, vol. 34, no. 3, pp. 1415–1423, 2022. [Google Scholar]
37. J. Qu, W. Cai and Y. Zhao, “Learning time-dependent pdes with a linear and nonlinear separate convolutional neural network,” Journal of Computational Physics, vol. 453, no. 3, pp. 110928–110965, 2022. [Google Scholar]
38. Z. Cai, J. Chen and M. Liu, “Least-squares relu neural network (LSNN) method for linear advection-reaction equation,” Journal of Computational Physics, vol. 443, no. 10, pp. 110514–110543, 2021. [Google Scholar]
39. S. Y. Siddiqui, A. Haider, T. M. Ghazal, M. A. Khan, I. Naseer et al., “IOMT cloud-based intelligent prediction of breast cancer stages empowered with deep learning,” IEEE Access, vol. 9, pp. 146478–146491, 2021. [Google Scholar]
40. A. A. A. Setio, A. Traverso, T. D. Bel, M. S. Berens, P. Cerello et al., “Validation, comparison, and combination of algorithms for automatic detection of pulmonary nodules in computed tomography images: The luna16 challenge,” Medical Image Analysis, vol. 42, pp. 1–13, 2017. [Google Scholar]
41. I. Naseer, B. S. Khan, S. Saqib, S. N. Tahir, S. Tariq et al., “Diagnosis heart disease using mamdani fuzzy inference expert system,” EAI Endorsed Transactions on Scalable Information Systems, vol. 7, no. 26, pp. 1–9, 2020. [Google Scholar]
42. S. Y. Siddiqui, I. Naseer, M. A. Khan, M. F. Mushtaq, R. A. Naqvi et al., “Intelligent breast cancer prediction empowered with fusion and deep learning,” Computers, Materials & Continua, vol. 67, no. 1, pp. 1033–1049, 2021. [Google Scholar]
43. S. Y. Siddiqui, S. Abbas, M. A. Khan, I. Naseer, T. Masood et al., “Intelligent decision support system for COVID-19 empowered with deep learning,” Computers, Materials & Continua, vol. 66, no. 2, pp. 1719–1732, 2020. [Google Scholar]
44. A. Elnakib, H. M. Amer and F. E. Z. A. Chadi, “Early lung cancer detection using deep learning optimization,” International Journal Online Biomedical Engineering, vol. 16, no. 6, pp. 82–94, 2020. [Google Scholar]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools