DOI:10.32604/cmc.2022.030447

| Computers, Materials & Continua DOI:10.32604/cmc.2022.030447 |  |
| Article |
Transfer Learning for Chest X-rays Diagnosis Using Dipper Throated Algorithm
1Department of Computer Sciences, College of Computer and Information Sciences, Princess Nourah Bint Abdulrahman University, Riyadh, 11671, Saudi Arabia
2Department of Communications and Electronics, Delta Higher Institute of Engineering and Technology, Mansoura, 35111, Egypt
3Faculty of Artificial Intelligence, Delta University for Science and Technology, Mansoura, 35712, Egypt
4Electronics and Communications Engineering Department, Faculty of Engineering, Delta University for Science and Technology, Mansoura, 35712, Egypt
5Department of Computer Science, Faculty of Computer and Information Sciences, Ain Shams University, Cairo, 11566, Egypt
6Department of Computer Science, College of Computing and Information Technology, Shaqra University, 11961, Saudi Arabia
*Corresponding Author: Amel Ali Alhussan. Email: aaalhussan@pnu.edu.sa
Received: 26 March 2022; Accepted: 26 April 2022
Abstract: Most children and elderly people worldwide die from pneumonia, which is a contagious illness that causes lung ulcers. For diagnosing pneumonia from chest X-ray images, many deep learning models have been put forth. The goal of this research is to develop an effective and strong approach for detecting and categorizing pneumonia cases. By varying the deep learning approach, three pre-trained models, GoogLeNet, ResNet18, and DenseNet121, are employed in this research to extract the main features of pneumonia and normal cases. In addition, the binary dipper throated optimization (DTO) algorithm is utilized to select the most significant features, which are then fed to the K-nearest neighbor (KNN) classifier for getting the final classification decision. To guarantee the best performance of KNN, its main parameter (K) is optimized using the continuous DTO algorithm. To test the proposed approach, six evaluation metrics were employed namely, positive and negative predictive values, accuracy, specificity, sensitivity, and F1-score. Moreover, the proposed approach is compared with other traditional approaches, and the findings confirmed the superiority of the proposed approach in terms of all the evaluation metrics. The minimum accuracy achieved by the proposed approach is (98.5%), and the maximum accuracy is (99.8%) when different test cases are included in the evaluation experiments.
Keywords: Metaheuristic; pneumonia; dipper throated optimization; KNN
In children under the age of five, pneumonia damages the lungs and is responsible for 18% of all fatalities [1]. In addition, two billion people throughout the globe suffer yearly from pneumonia infections, and death might ensue if it is not detected and treated early. Pneumonia should be diagnosed as soon as possible. Rapid diagnosis using chest X-rays performed by a skilled radiologist is thus necessary to prevent misdiagnosis. An X-ray of the chest is the most frequent and cost-effective approach to diagnose pneumonia [2,3]. The lack of radiologist professionals, particularly in low-resource nations and rural areas, leads to lengthy delays for diagnosis, which in turn raises the mortality rate. It is difficult to make a definitive diagnosis of pneumonia using chest X-ray pictures since several conditions with similar symptoms and appearances, such as opacity, cavities, and pleural effusions might be misdiagnosed as pneumococcal illness. The detection of illnesses by using chest X-rays is thus hampered. Researchers have proposed a variety of technologies and computer-aided diagnostic (CAD) tools for X-ray image processing that enable radiologists to identify distinct types of chest X-ray pneumonia [4,5]. Many biological problems have been addressed using a combination of handcrafted and machine learning methods, as well as deep learning and machine learning. Recent advances in the approach of artificial intelligence (AI) I and machine learning, deep learning exploit multi-layered artificial neural networks in a wide range of applications, including voice recognition and language translation. Deep learning approaches for machine learning may learn representations from data such as videos, photos, and text without explicitly coding rules or depending on direct human intervention. With their versatility and capacity to draw inferences from data, they are able to increase their forecast accuracy by supplying more information [6,7].
In this paper, a novel approach is proposed for classifying the pneumonia cases robustly. The proposed approach is based on extracting a set of features from the given X-ray images using transfer learning of three strong deep learning architectures, namely, GoogLeNet, ResNet18, and DenseNet121. The extracted features are then processed to select only the most significant features that enable the K-nearest neighbor (KNN) classifier to achieve the best performance. To guarantee the best performance of KNN, its parameter is optimized using the dipper throated optimization algorithm. To check the superiority of the proposed approach, several experiments were conducted to compare the performance of the proposed approach with the other approaches. The achieved findings confirm the superiority of the proposed approach.
The remainder of this paper is structured as follows. A review of the recently published research is included in Section 2. Information on the algorithms employed to develop the proposed approach is provided in Section 3. Section 4 illustrates the structure of the suggested methodology. The findings and achieved results are given in Section 5. The conclusions and plans for future research are discussed in Section 6.
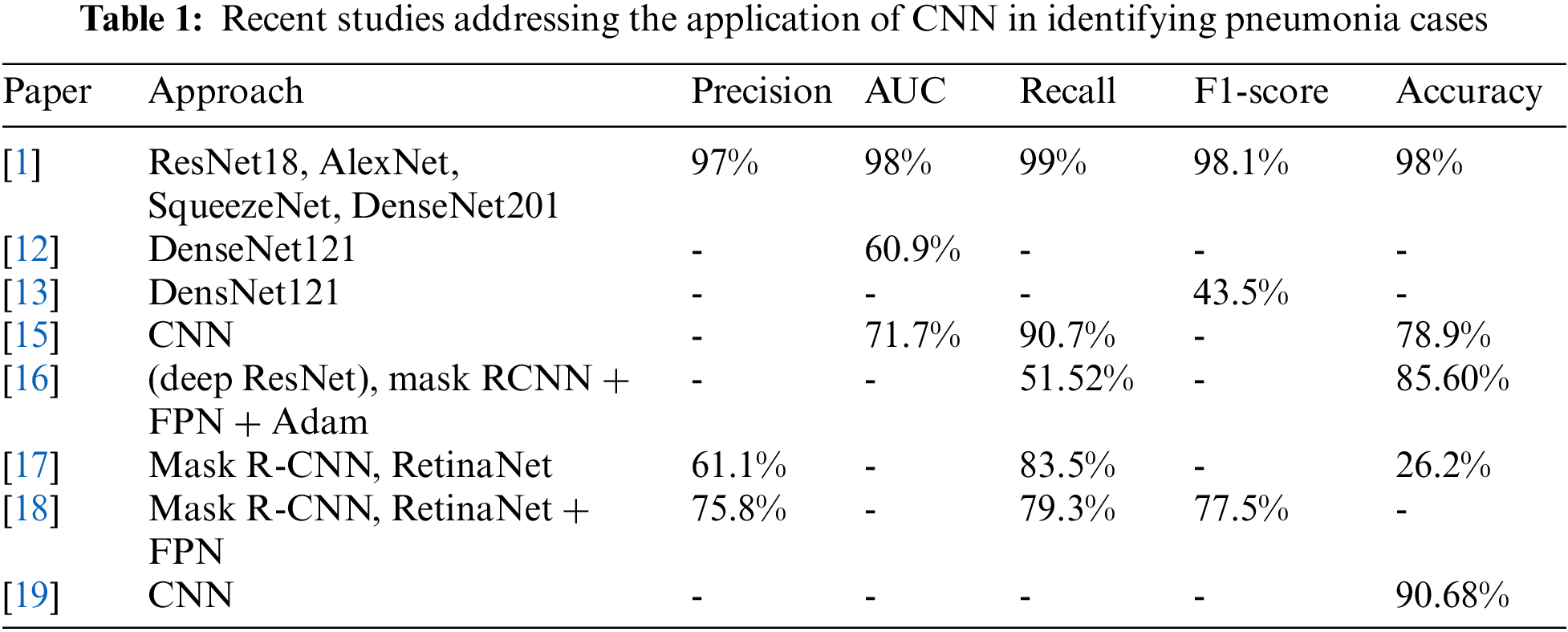
Pneumonia may be detected on chest X-ray pictures utilizing a number of approaches that have been published in the scientific literature. Some of these systems rely on handmade feature extraction techniques, while others depend on deep learning algorithms for extracting robust features and categorization [8–10]. The parameters of deep layered convolutional neural networks (CNNs) for pneumonia diagnosis have been altered by these approaches, allowing for higher illness detection accuracy via the usage of these models. As a baseline model, the researchers in [11–14] utilized logistic regression to identify patients with pneumonia by analyzing X-ray images. Using logistic regression, the area under the curve (AUC) yields unsatisfactory results. They were able to improve their results by using a set of dense layers in a convolutional network (DenseNet). The network was honed with the aid of Adam optimizer. According to the model’s AUC, it is possible to diagnose pneumonia with an accuracy of (60.9%), which is slightly higher than that of logistic regression (60%).
An accurate model for diagnosing pneumonia was developed by the researchers in [15] and is referred to as ChexNet. One of the most important features of ChexNet is the 121-layer CNN that analyzes and classifies the X-ray images then locates them using a thermal map. Pneumonia may be difficult to be diagnosed; thus, the model’s findings were compared against those of four radiologists using F1. In comparison to the typical radiologist, the ChexNet model achieved an F1-score of (0.435). On the other hand, mask-RCNN and a residual network were implemented by the authors in [16–20]. In order to identify pneumonia, it was employed to construct a computer-aided detection system. Overfitting was remedied by using residual mapping in the residual network. Batch normalization was utilized after each activation and convolution. Binary cross-entropy for the loss function and Intersection over Union were then combined (IoU). A pooling block was utilized to reduce the number of arguments. A bounding box was utilized to locate items in the Mask Regional CNN. The RPN and RoI-align were the two steps of the process. Extracting features was accomplished by using a bottom-up and top-down extraction strategy based on the feature pyramid network (FPN). Mask-RCNN had an accuracy of (78.06%) with a confidence level of (98%), whereas a confidence level of (70%) is achieved by the residual network.
A dropout layer-based and a dropout-free CNN architecture were introduced in [21–25]. Convolution, maximum pooling, and classification layers were all included in both CNNs. The feature extractor was separated into two portions by a sequence of convolution and maximum pooling layers. Each of the 32 × 32 convolution layers has a maximum pooling layer of 3 × 3 in size, and an activation function of type rectified linear unit (ReLU) in the first section. As well as the ReLU activator and the maximum pooling layer of size 2 × 2, the second section contains two convolution layers of 64 and 128 units [26–31]. ReLU is a well-known activation function in neural networks, particularly in CNNs. Nonlinearity was introduced into the model using the ReLU layer. It was discovered that three standard deep learning approaches based on CNNs could successfully categorize pneumonia and normal cases based on chest X-ray pictures [32–38]. These models are GoogLeNet, ResNet18, and DenseNet121. We found that ResNet-18 performed better than the other two. Tab. 1 summarizes the literature models and their findings.
In this section, the main algorithms employed in this research are introduced. These algorithms include deep learning for extracting the main features from the input images of the pneumonia cases. In addition, they employed a feature selection algorithm for selecting the significant features. Moreover, the classification of the pneumonia cases is performed in terms of the KNN algorithm, which is optimized using one of the recent parameters optimization algorithms in the literature. The following section briefly introduces each one of these algorithms.
An increasing number of clinical diagnostic and medical image applications are relying on deep learning, and one such application is that of CNNs, which are types of multi-layer neural networks specifically designed for the identification of visual patterns in pixel images with little or no prior processing. Many advantages come from using CNNs, including the capacity to extract more relevant information from pictures than can be done manually. Several CNN-based deep networks have been suggested by computer vision researchers to segment images, classify images, recognize objects, and pinpoint their locations inside images. Along with tackling natural computer vision issues, CNNs have shown to be very effective at identifying diseases including Alzheimer’s disease and breast cancer, as well as detecting and classifying skin blemishes.
Deep learning in medical image analysis has also been discussed in depth. In this research, we adopted a set of deep learning models for feature extraction; these models are GoogLeNet, ResNet18, and DenseNet121. In specific, we selected the features from the most promising model. Based on our findings that ResNet18 is the best performing model, the features are extracted mainly in terms of this model. The basic building block of ResNet18 is depicted in Fig. 1. The models consist of multiple units of this building block. The building block consists of two chunks of 3 × 3 convolutional layers with a relu activation function.
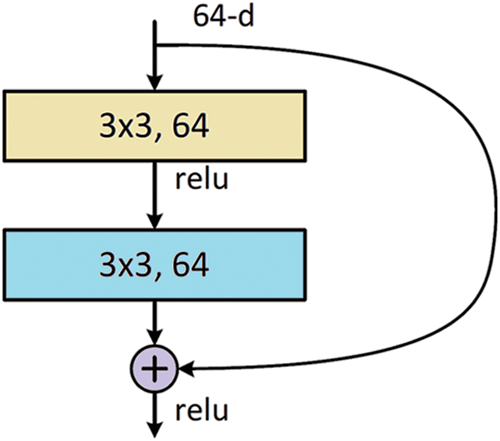
Figure 1: The building block for ResNet18
Pattern categorization, machine learning, information retrieval, data analysis, and data mining all depend mainly on feature selection. While the approach works well for a dataset with few characteristics, classification operations employing multiple features were not included in this method’s assessment. There may be the need for additional approaches like those provided by the feature selection if the dataset has a large number of characteristics, such as thousands. Fig. 2 illustrates this. There are two variables involved in classification using FS: an input variable that represents all of the data and a final output that represents the classification pattern based on features that were previously picked.
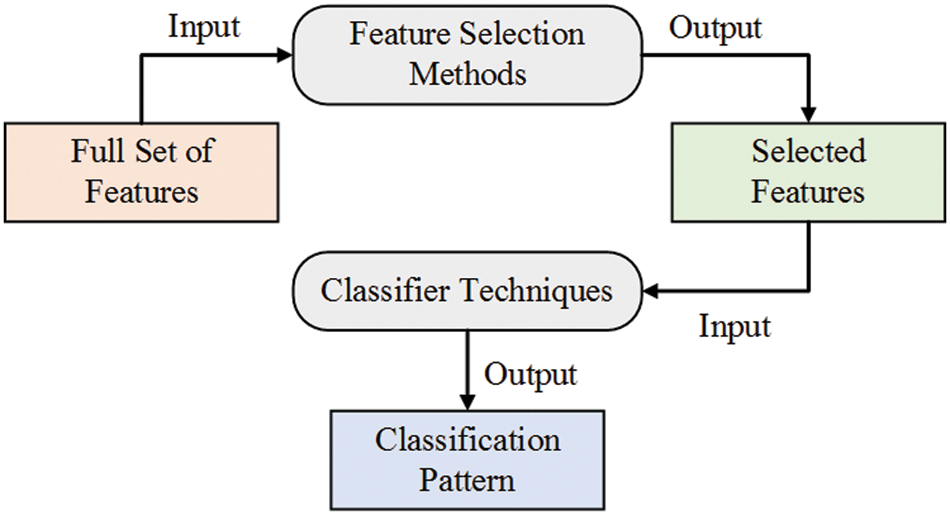
Figure 2: Feature selection for classification tasks
The most straightforward technique for pattern recognition is the KNN algorithm, which has been frequently employed in the literature as an easy-to-understand supervised classifier. By taking into account their K closest neighbor samples in train data, the KNN classifies any sample of test data. In a K-neighbor network, the samples belong to whichever cluster has the most significant number of fellow members. The KNN classifier has two parameters: the distance calculation technique and the number of K. SEN, SPE, and ACC are all metrics used to gauge the classifier’s effectiveness. PPV and NPV are used to estimate the classifier’s ability to predict outcomes accurately. Assuming that two features are retrieved from an input test sample, the binary KNN classification method is shown in Fig. 3.
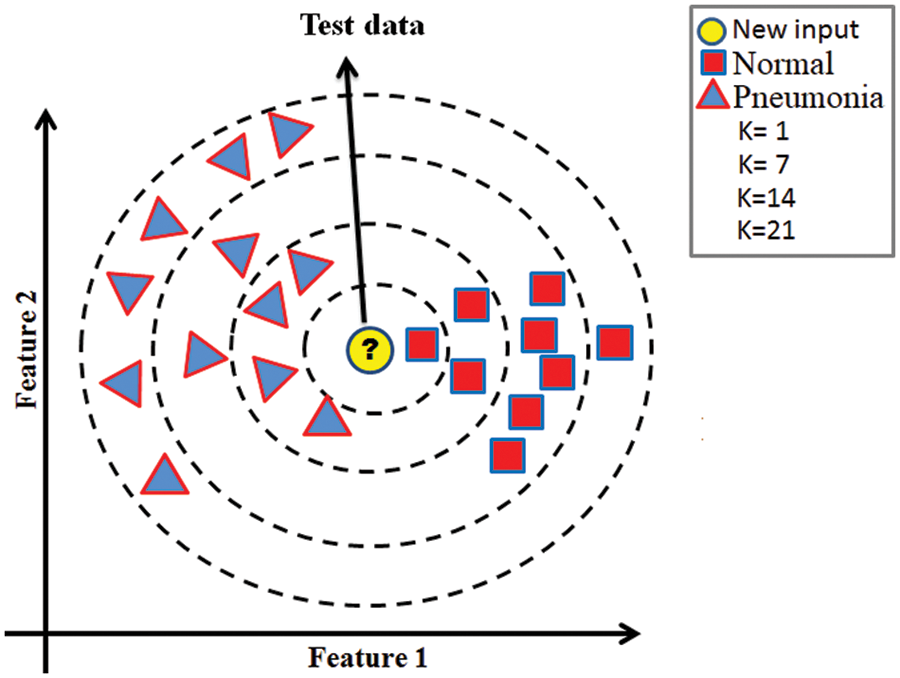
Figure 3: Illustration of the KNN classifier
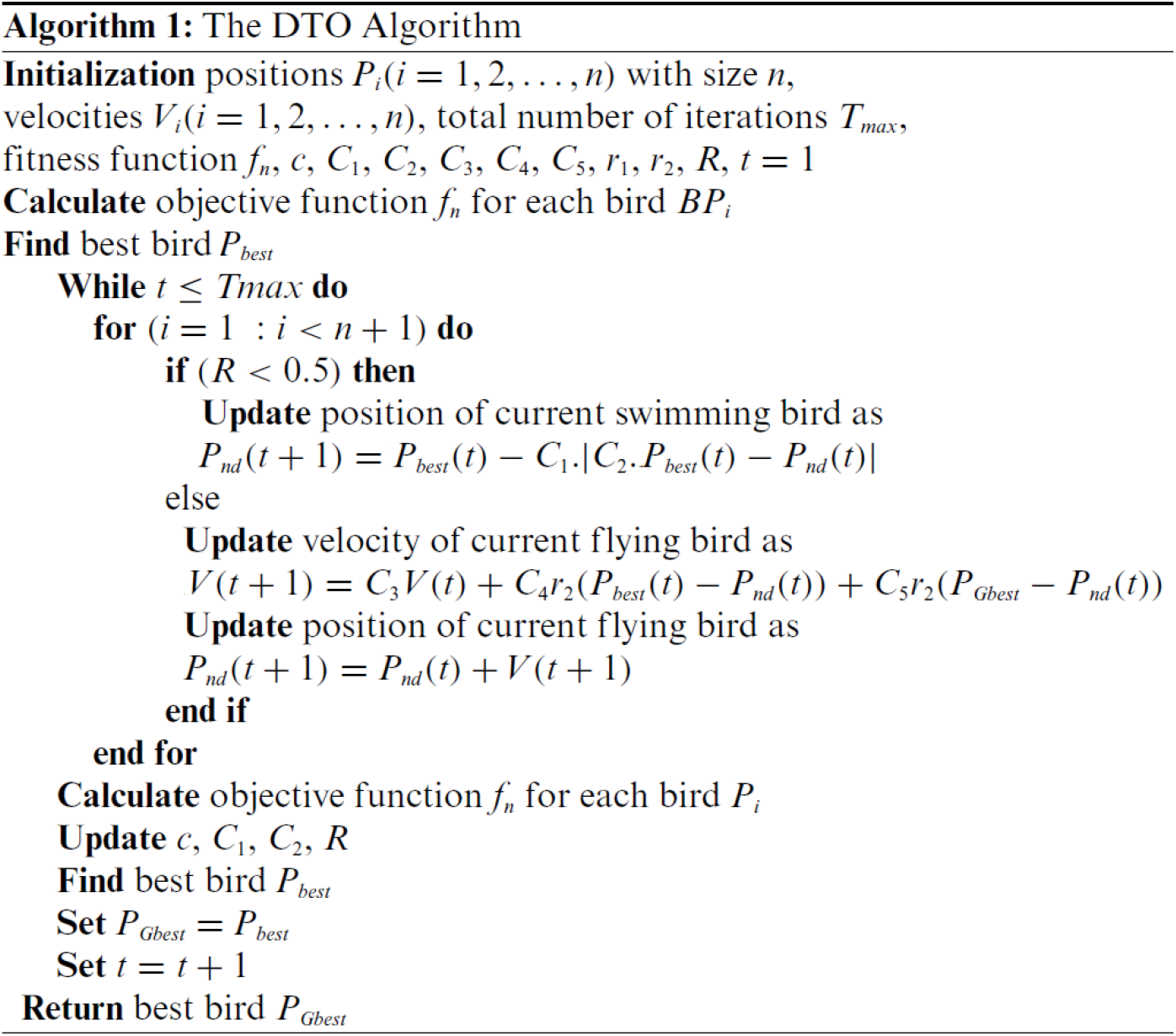
To achieve better performance of the classification models in machine learning, careful choice of the model parameters is an essential step, as the model performance depends mainly on the values of these parameters. The manual or random selection of these parameters cannot guarantee better performance. In addition, the trial-and-error method is usually time-consuming to reach the best parameters. In this case, the optimization algorithms are the best alternative to help researchers find the best set of parameters necessary for a machine learning model to achieve better performance. In the literature, many optimizers can perform this task. In this research, we adopted the recently published dripper throated optimization (DTO) algorithm for optimizing the K parameter of the KNN classification algorithm. The detailed steps of the DTO algorithm are presented in Algorithm 1. This algorithm is based on three matrices, namely positions (P), Velocities (V), and fitness function f, defined in the following.
where the position and velocity of the
The structure of the proposed system is depicted in Fig. 4. As shown in the figure, the process starts with collecting X-ray images from X-ray machines and then sending them to the machine learning blocks, based on a set of algorithms implemented to classify the input X-ray images as normal for pneumonia cases. The processing included in the machine learning block starts with preprocessing and augmentation, followed by the proposed approach. The details of the proposed approach are depicted in Fig. 5. More information on these steps is presented and discussed in the following sections.
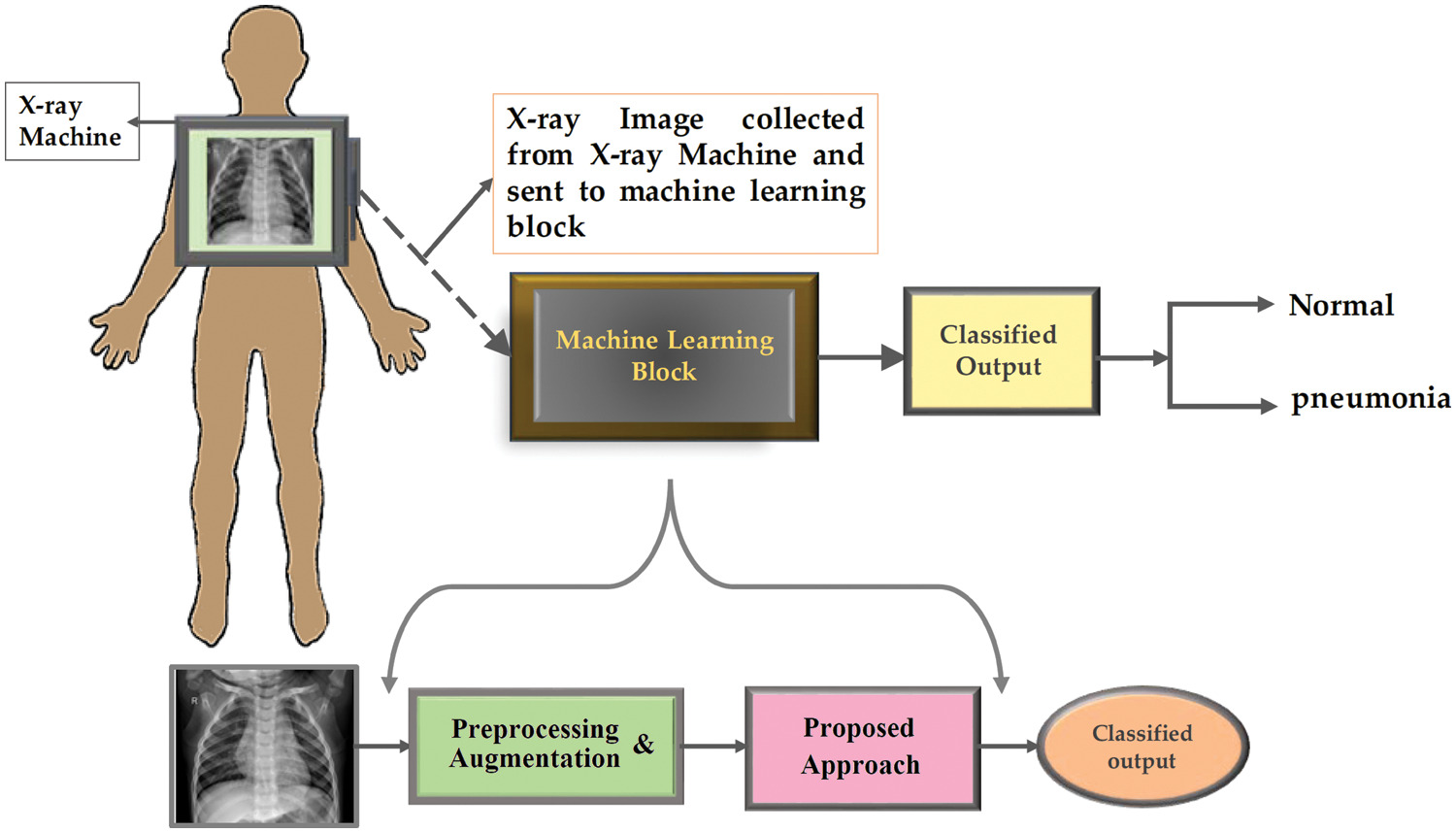
Figure 4: The structure of the proposed methodology
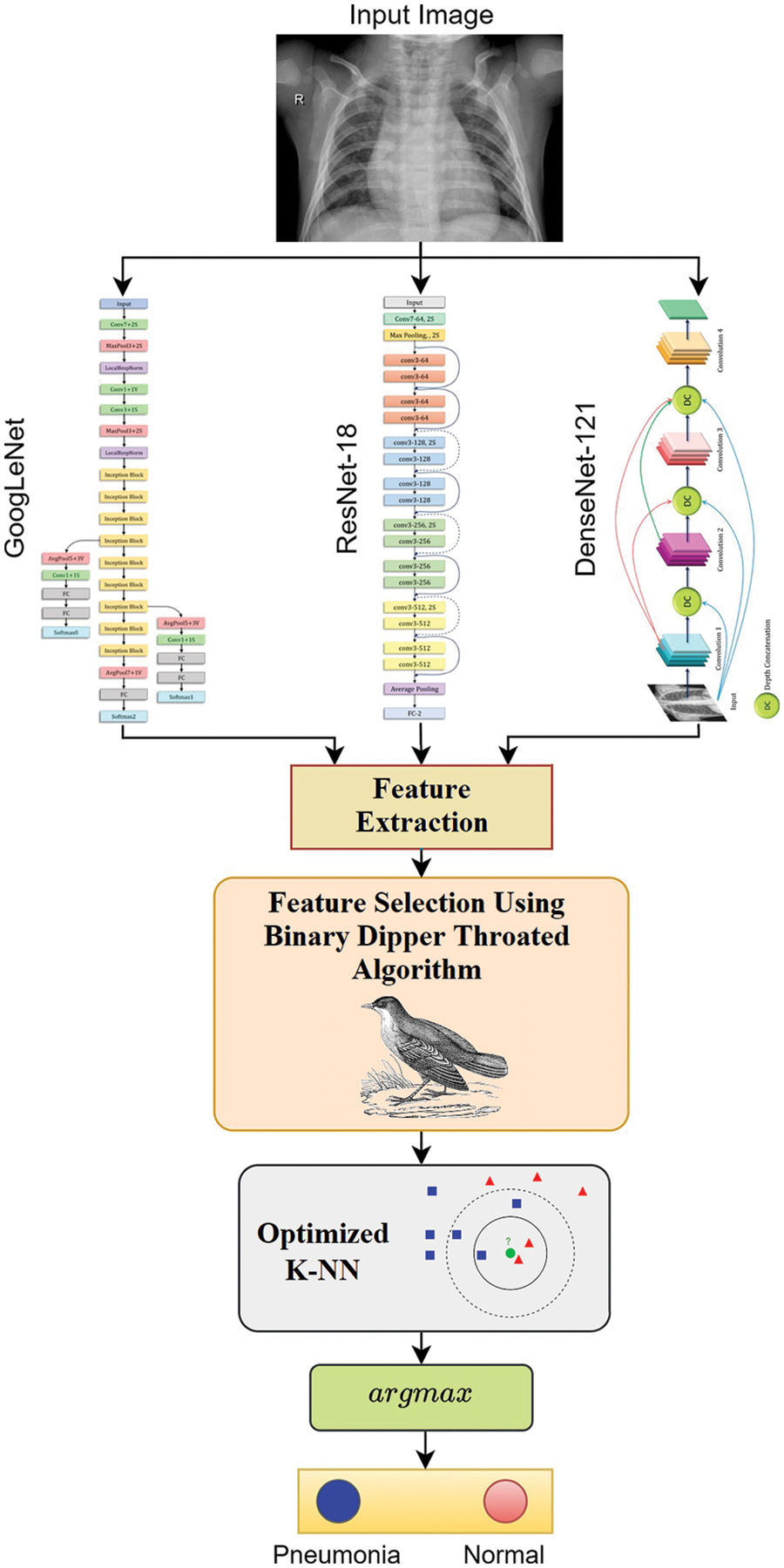
Figure 5: The detailed steps of the proposed methodology
The pneumonia images utilized in this research were taken from Kaggle’s pneumonia dataset, which contains 5247 images with resolutions between 400 and 2000 pixels [36]. Tab. 2 shows that 3906 of the 5247 chest X-ray pictures (2561 bacterial and 1345 viral) were taken from patients with pneumonia, while 1341 images were taken from healthy people. Some cases of pneumonia are caused by a combination of bacterial and viral infections. Even though viral and bacterial co-infections are not included in the dataset utilized in this research. In this research, we considered viral and bacterial pneumonia as one set referred to as pneumonia. Therefore, we have only two classes in our classification task, namely, normal case and pneumonia case. Fig. 6 presents samples of the images in the employed dataset.
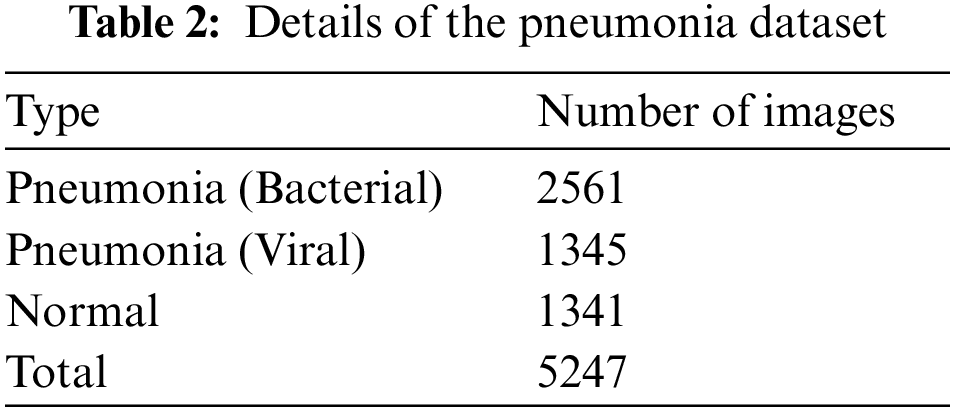
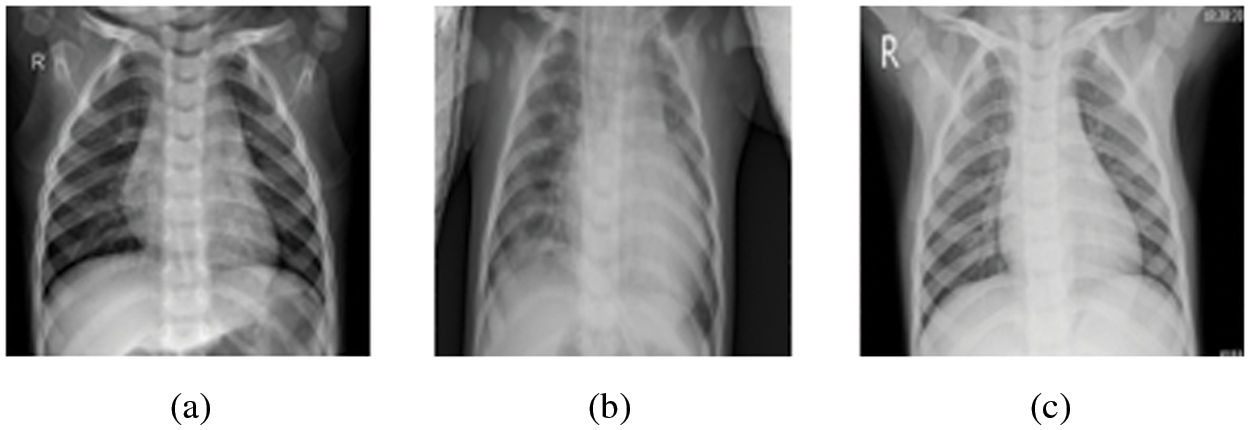
Figure 6: Sample images from the Kaggle dataset (a) Normal case, (b) Pneumonia (Bacteria) case, and (c) Pneumonia (Viral) case
4.2 Preprocessing & Augmentation
The dataset images are preprocessed to improve the results of the later steps of the proposed approach. Resizing the input images in the dataset is the first step that is applied to make the images fit the deep networks used in feature extraction. In addition, a data augmentation operation is applied to increase the number of images in the dataset. The operations utilized in the augmentation process are presented in Tab. 3. The sample output of these operations is depicted in Fig. 7.
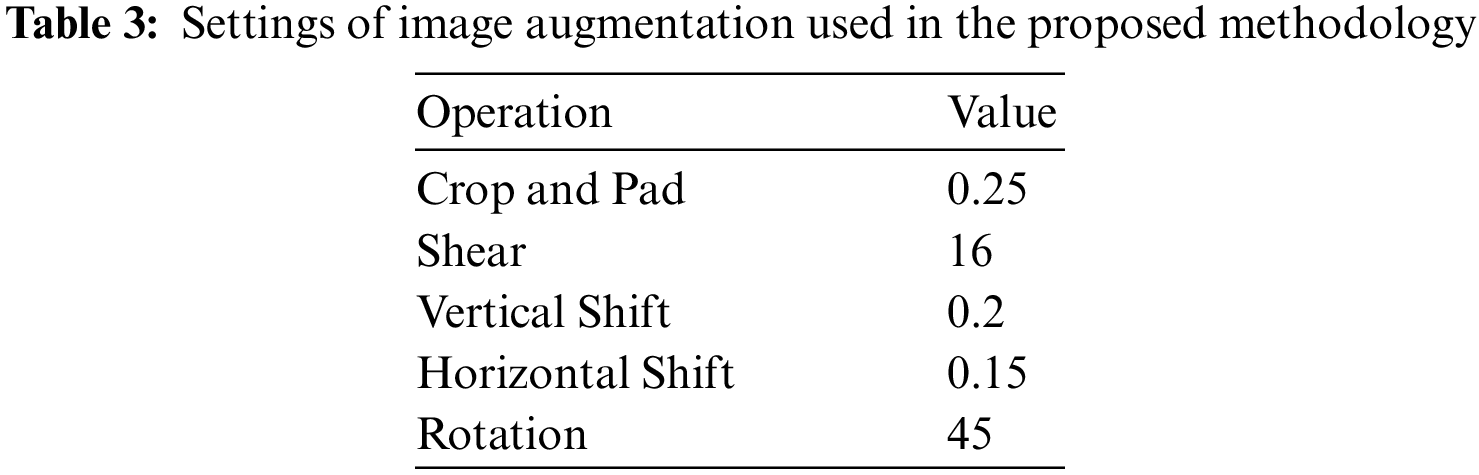
Figure 7: Image augmentation results based on five transformations
The effectiveness of the proposed approach is validated in this section using a set of experiments based on the adopted dataset. The dataset images are split into training and testing sets. The training set is employed in terms of five-fold cross-validation to achieve better generalization and proper performance. Fig. 8 shows the cross-validation process. Each split in the five-fold cross-validation is used to train the proposed approach using four-folds, and the fifth fold is used for testing.
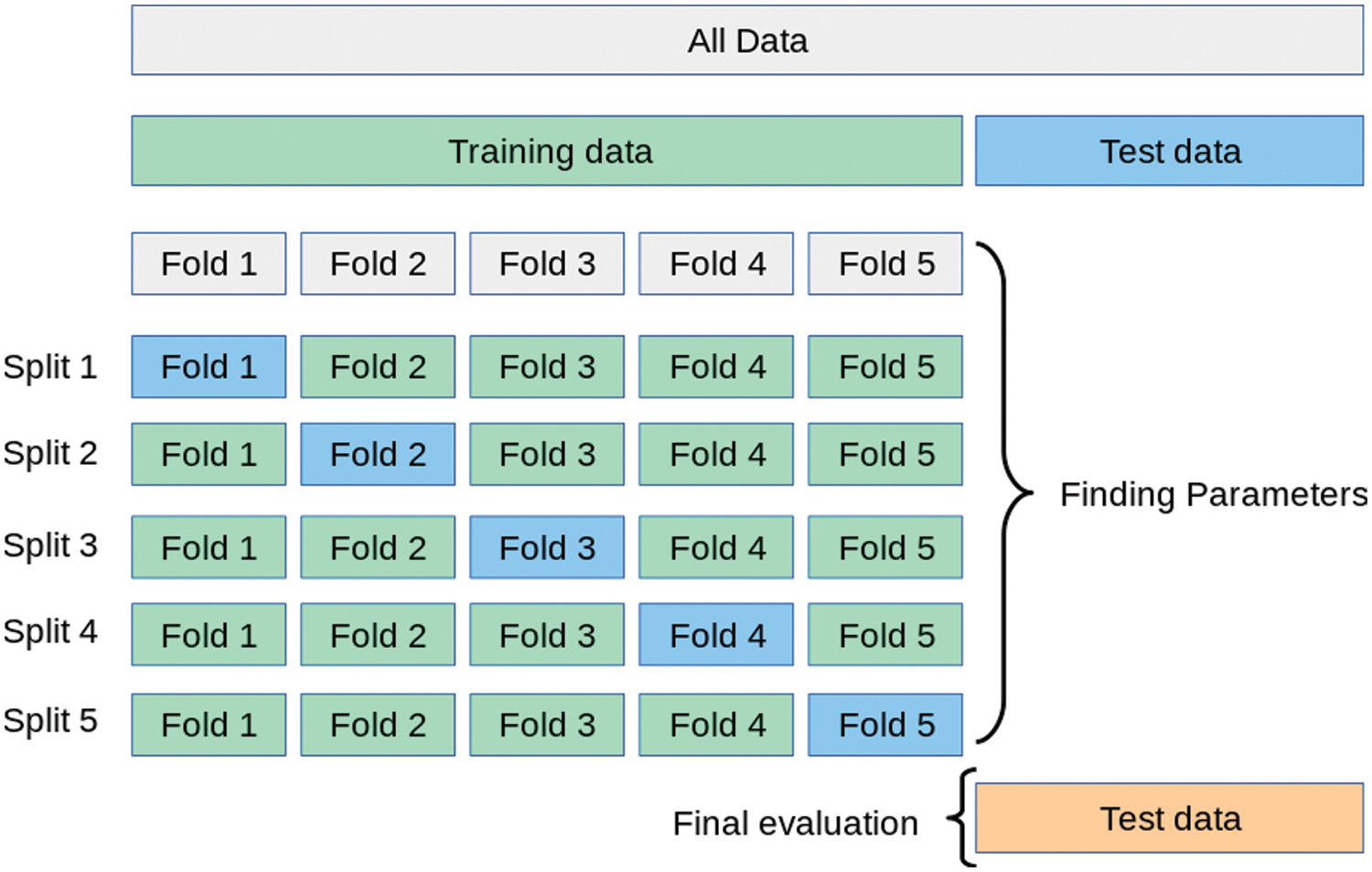
Figure 8: The five-fold cross-validation operation
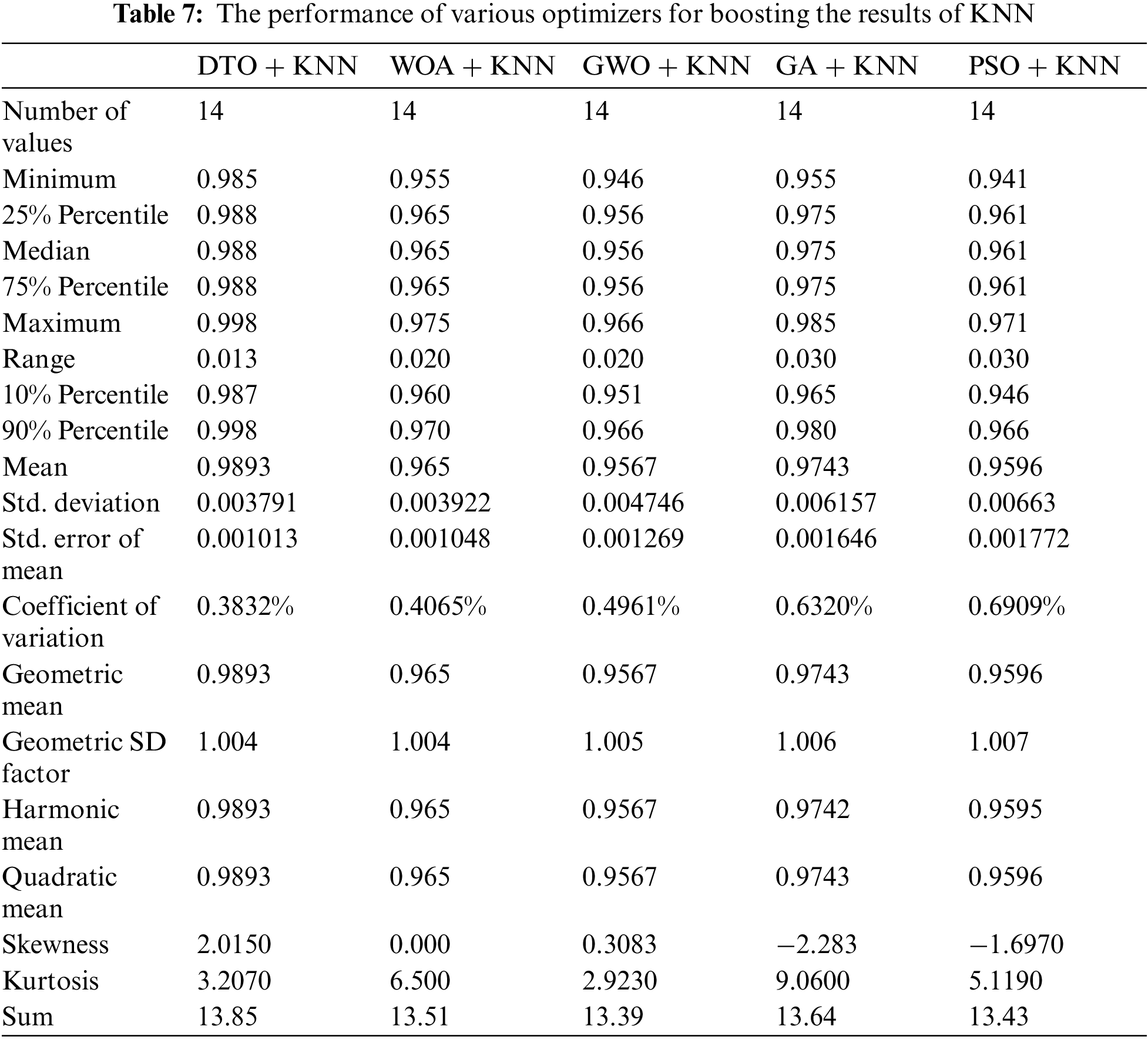
A set of evaluation metrics were employed to measure the performance of the proposed approach. These metrics are positive predictive value, negative predictive value, accuracy, specificity, sensitivity, and F1-score. The measurement of these metrics is presented in Tab. 4.
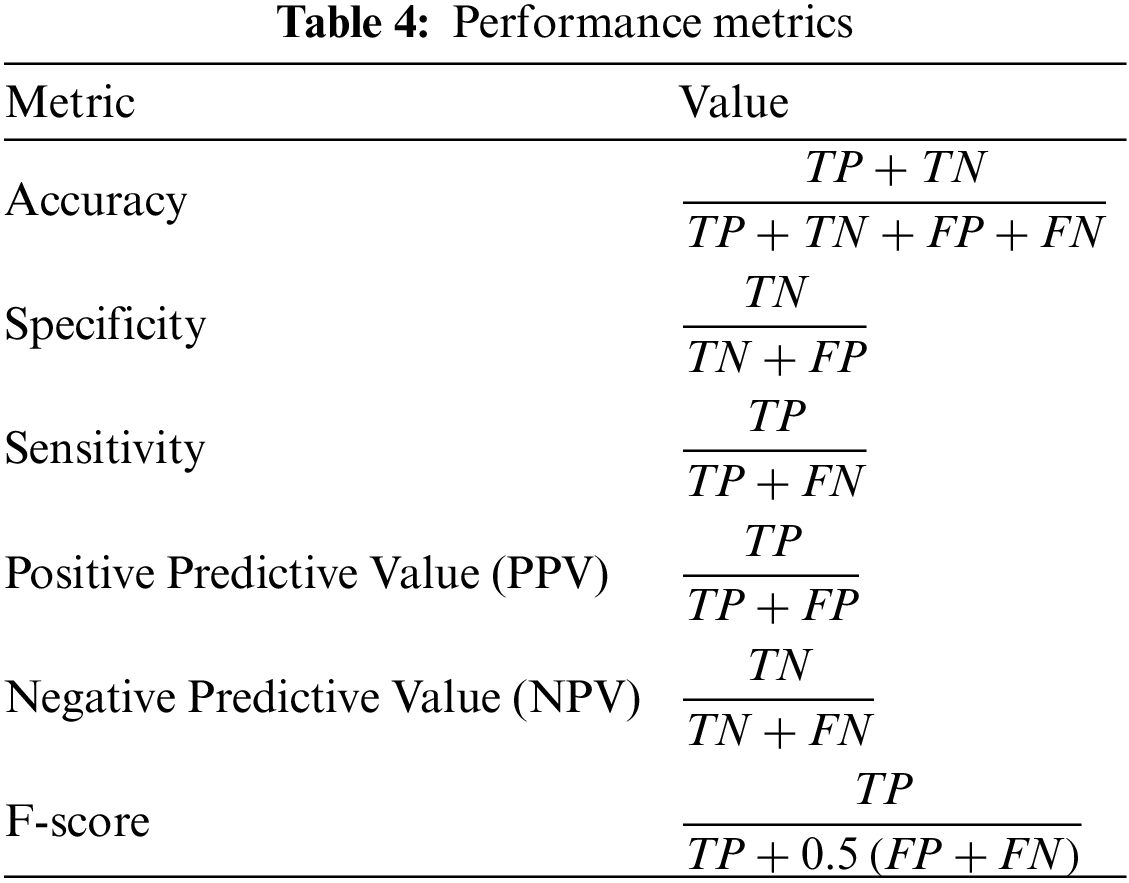
After extracting the main features using the three deep learning networks, namely, GoogLeNet, ResNet18, and DenseNet121, a set of experiments were conducted to find the best algorithm to be used for feature selection. Tab. 5 presents the results recorded by each of the binary optimization algorithms. From this table, it can be noted that the binary DTO achieves the best performance. Therefore, we adopted this algorithm for the feature selection in the proposed approach.
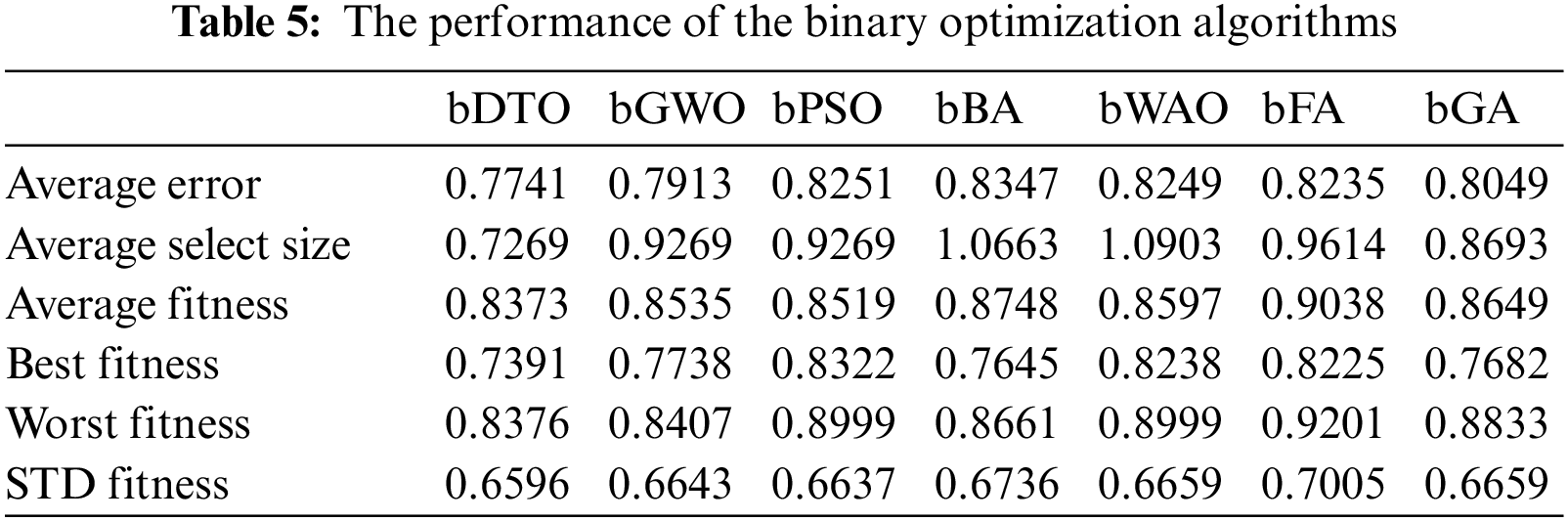
On the other hand, another set of experiments was implemented to measure the efficiency of three classifiers, namely, KNN, support vector machines (SVM), and random forest, using the selected features. Tab. 6 shows the results of the classification process in terms of the six evaluation criteria presented earlier. As shown in this table, the results achieved by the KNN classifier are the best among the three classifiers. Therefore, we adopted this classifier to classify the pneumonia cases in the proposed approach.
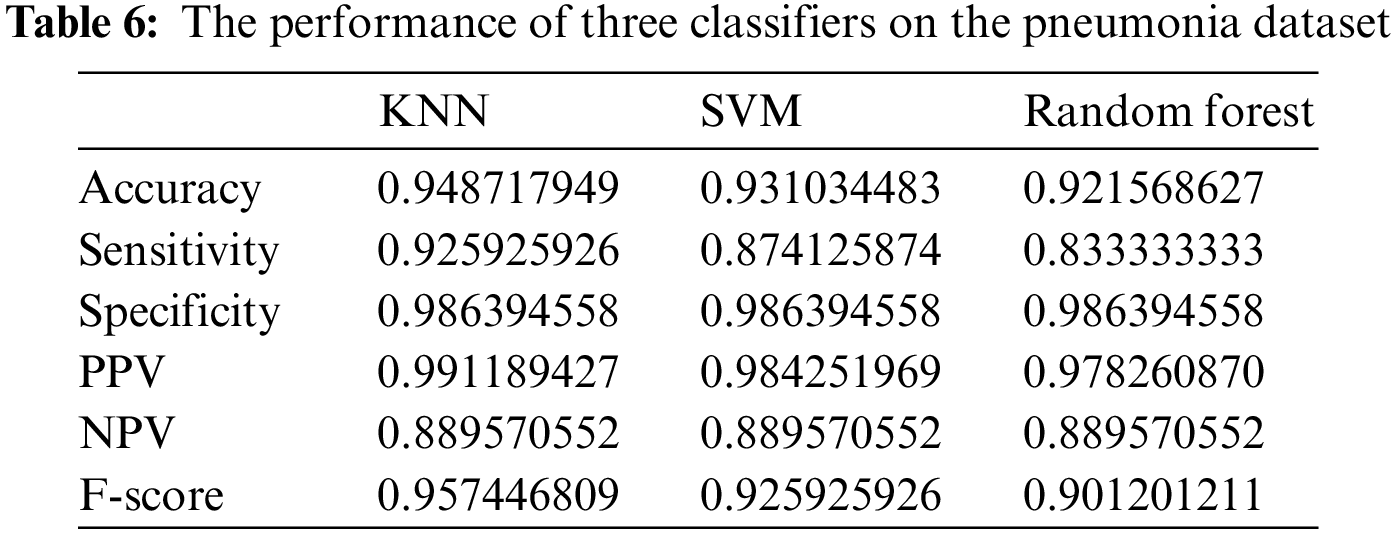
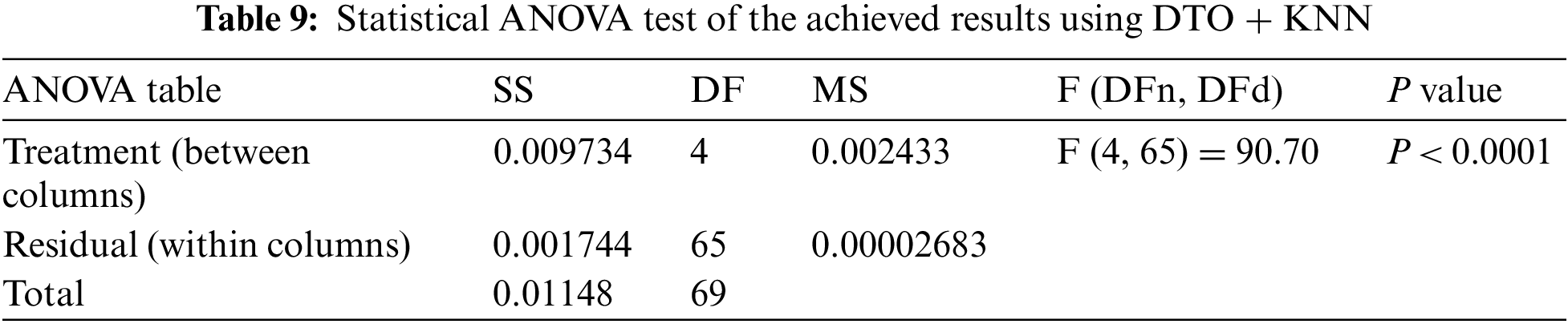
To achieve better classification results, a set of experiments were implemented to optimize the K parameter of the adopted KNN classification algorithm. Tab. 7 presents the performance of the optimized KNN using the continuous version of the optimizers used in feature selection. It can be noted from these results that KNN optimized using DTO (DTO + KNN) are better than the other optimizers. Therefore, this optimizer is recommended in the proposed approach. In addition, a more in-depth statistical analysis of the achieved results using the proposed approach with comparison to the other approaches is presented in terms of t-test and analysis of variance (ANOVA) test are presented in Tabs. 8 and 9.
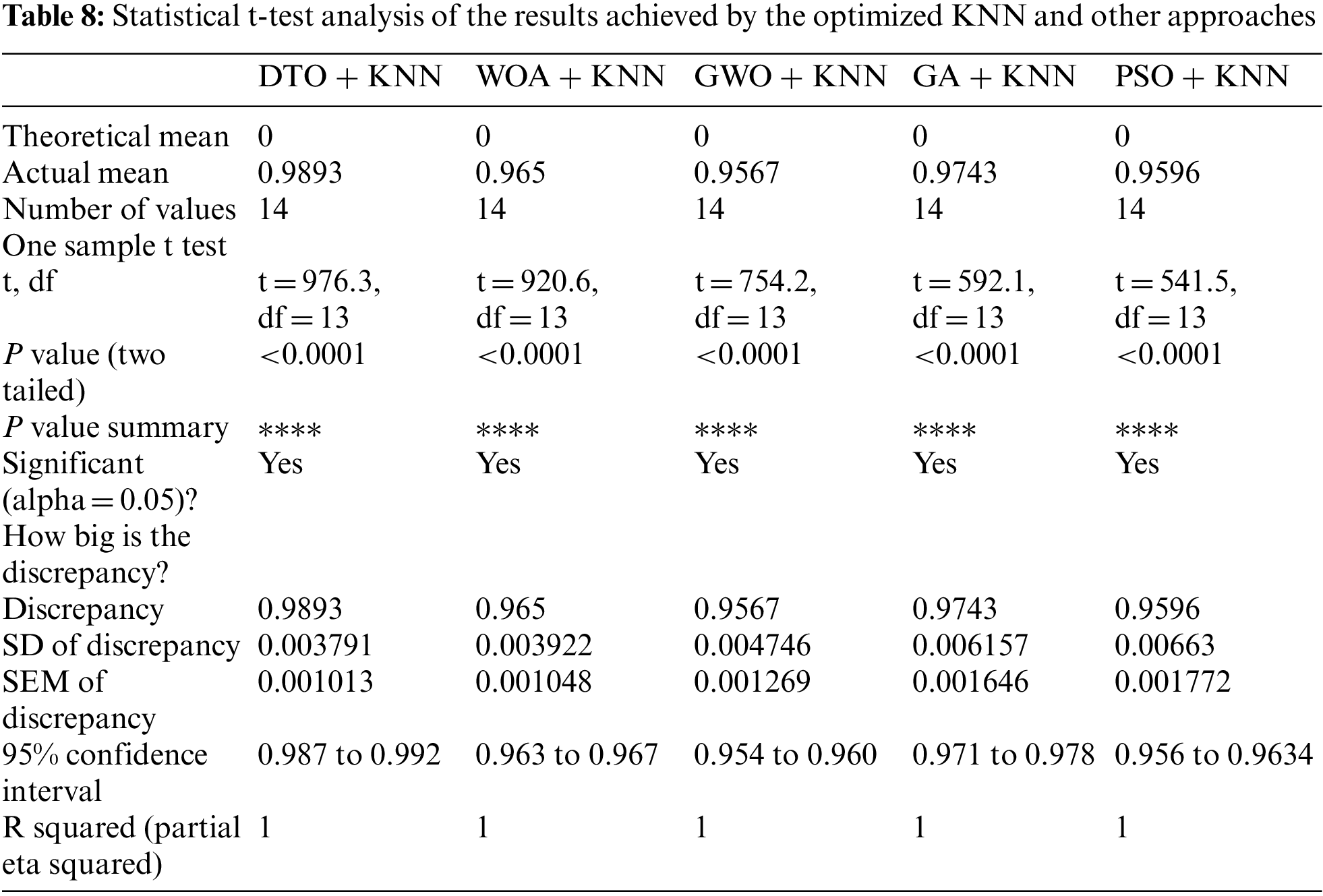
Moreover, Fig. 9 depicts the histogram of the results achieved by the proposed approach and other competing approaches. These results show the stability of the proposed approach in classifying the pneumonia cases in the images of the test sets. On the other hand, the plots presented in Fig. 10 analyze the performance of the adapted DTO + KNN approach. As shown in these plots, the proposed approach could achieve promising classification results with reduced error rates that outperform the other approaches incorporated in the conducted experiments.
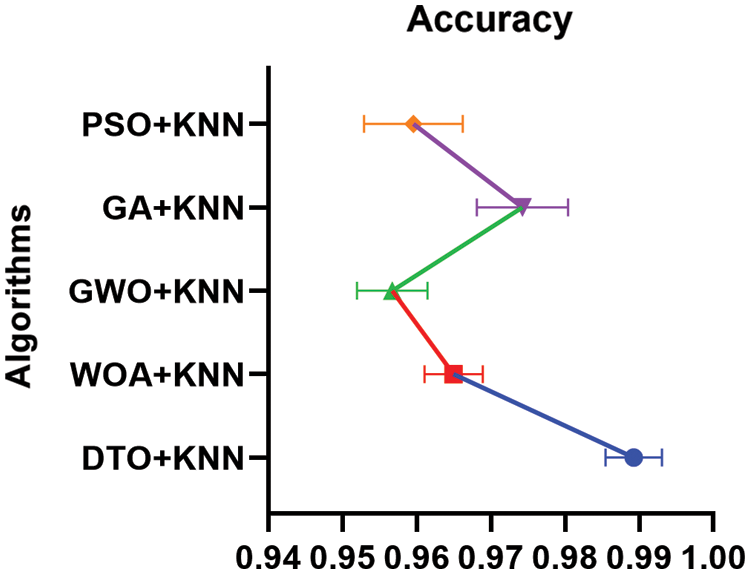
Figure 9: Histogram of the accuracy achieved by the proposed DTO + KNN and other approaches
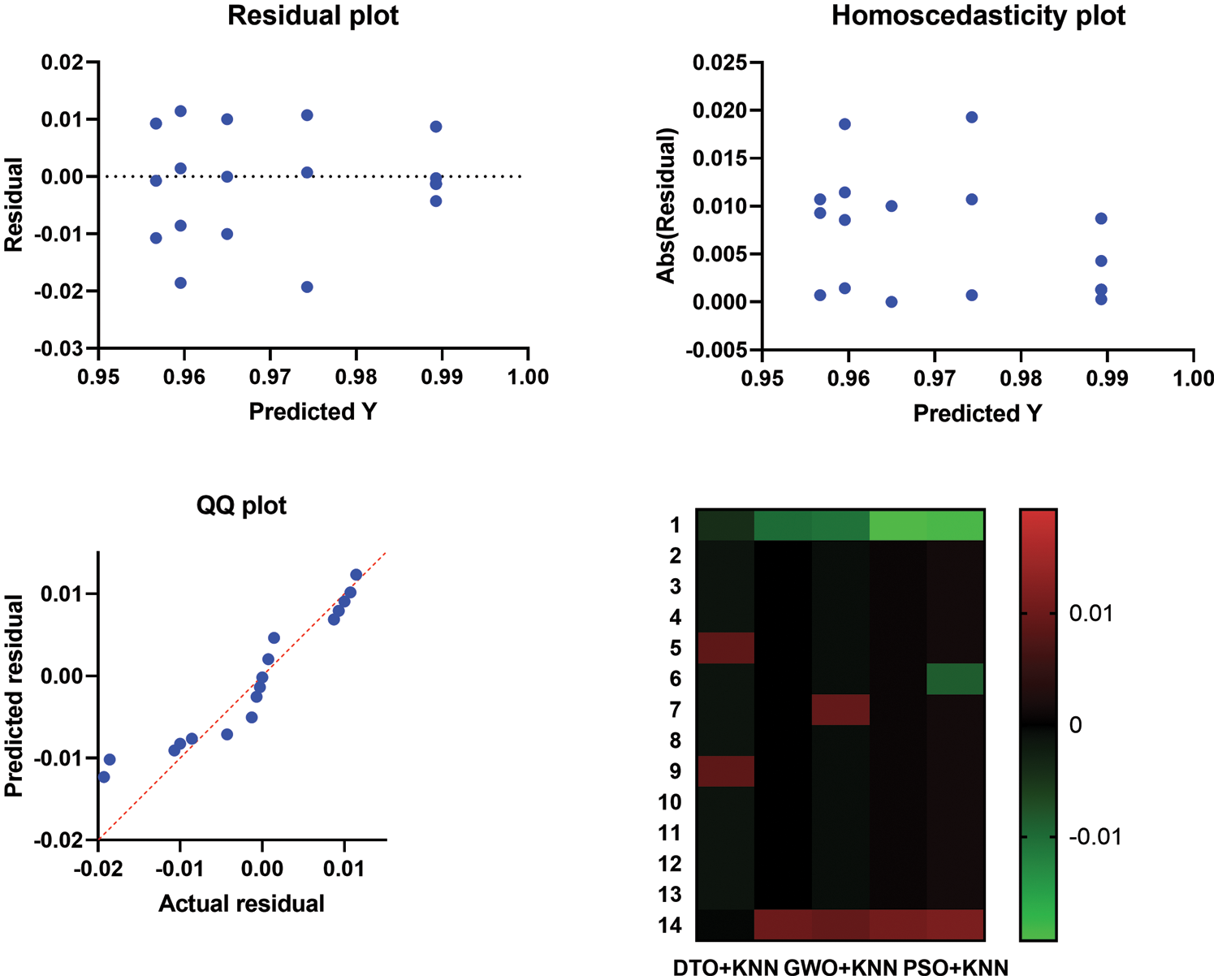
Figure 10: Homoscedasticity, residual, heat map, and QQ plots of the proposed approach
6 Conclusions and Future Perspectives
A novel approach is proposed in this paper for classifying the pneumonia cases based on deep learning for feature extraction and binary DTO for feature selection after preprocessing and augmenting the freely available Kaggle dataset. The selected features are used to train the KNN classifier using five-fold cross-validation. The proposed approach is evaluated in terms of several metrics and compared with other competing approaches in the literature. The recorded results emphasize the effectiveness of the proposed approach and its superiority. In addition, a set of experiments were conducted to analyze the achieved results statistically, and a set of plots were presented and discussed. The future perspectives of this work include the application of the proposed approach for classifying other types of lung diseases. In addition, the utilization of ensemble models can be considered a future work of the proposed approach.
Acknowledgement: Princess Nourah bint Abdulrahman University Researchers Supporting Project Number (PNURSP2022R308), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Funding Statement: Princess Nourah bint Abdulrahman University Researchers Supporting Project Number (PNURSP2022R308), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. T. Rahman, M. Chowdhury, A. Khandakar, K. Islam, K. Islam et al., “Transfer learning with deep convolutional neural network (CNN) for pneumonia detection using chest X-ray,” Applied Sciences, vol. 10, no. 1, pp. 3233, 2020. [Google Scholar]
2. E. Ayan and H. Unver, “Diagnosis of pneumonia from chest X-ray images using deep learning,” in Proc. of the Scientific Meeting on Electrical-Electronics Biomedical Engineering and Computer Science (EBBT), Istanbul, Turkey, pp. 1–5, 2019. [Google Scholar]
3. K. Nguyen, T. Son, T. Le, L. Tuan and T. Nguyen, “Applying multi-CNNs model for detecting abnormal problem on chest X-ray images,” in Proc. of the Int. Conf. on Knowledge and Systems Engineering (KSE), Ho Chi Minh City, Vietnam, pp. 300–305, 2018. [Google Scholar]
4. G. Labhane, R. Pansare, S. Maheshwari, R. Tiwari and A. Shukla, “Detection of pediatric pneumonia from chest X-ray images using CNN and transfer learning,” in Proc. of the Int. Conf. on Emerging Technologies in Computer Engineering: Machine Learning and Internet of Things (ICETCE), Jaipur, India, pp. 85–92, 2020. [Google Scholar]
5. O. Stephen, M. Sain, U. Maduh and D. Jeong, “An efficient deep learning approach to pneumonia classification in healthcare,” Journal of Healthcare Engineering, vol. 2019, no. 1, pp. 4180949, 2019. [Google Scholar]
6. A. Tahir, M. Chowdhury, A. Khandakar, S. Al-Hamouz, M. Abdalla et al., “A systematic approach to the design and characterization of a smart insole for detecting vertical ground reaction force (vGRF) in gait analysis,” Sensors, vol. 20, no. 1, pp. 957, 2020. [Google Scholar]
7. M. Chowdhury, K. Alzoubi, A. Khandakar, R. Khallifa, R. Abouhasera et al., “Wearable real-time heart attack detection and warning system to reduce road accidents,” Sensors, vol. 19, no. 1, pp. 2780, 2019. [Google Scholar]
8. M. Chowdhury, A. Khandakar, K. Alzoubi, S. Mansoor, A. Tahir et al., “Real-time smart-digital stethoscope system for heart diseases monitoring,” Sensors, vol. 19, no. 1, pp. 2781, 2019. [Google Scholar]
9. K. Kallianos, J. Mongan, S. Antani, T. Henry, A. Taylor et al., “How far have we come? Artificial intelligence for chest radiograph interpretation,” Clinical Radiology, vol. 74, no. 1, pp. 338–345, 2019. [Google Scholar]
10. V. Chouhan, S. Singh, A. Khamparia, D. Gupta, P. Tiwari et al., “A novel transfer learning based approach for pneumonia detection in chest X-ray images,” Applied Sciences, vol. 10, no. 1, pp. 559, 2020. [Google Scholar]
11. J. Lujan-Garcia, C. Yanez-Marquez, Y. Villuendas-Rey and O. Camacho-Nieto, “A transfer learning method for pneumonia classification and visualization,” Applied Sciences, vol. 10, no. 1, pp. 2908, 2020. [Google Scholar]
12. A. Ibrahim, H. A. Ali, M. M. Eid and E. -S. M. El-Kenawy, “Chaotic harris hawks optimization for unconstrained function optimization,” in 2020 16th Int. Computer Engineering Conf. (ICENCO), Cairo, Egypt, IEEE, pp. 153–158, 2020. [Google Scholar]
13. S. S. M. Ghoneim, T. A. Farrag, A. A. Rashed, E. -S. M. El-Kenawy and A. Ibrahim, “Adaptive dynamic meta-heuristics for feature selection and classification in diagnostic accuracy of transformer faults,” IEEE Access, vol. 9, pp. 78324–78340, 2021. [Google Scholar]
14. M. M. Eid, E. -S. M. El-Kenawy and A. Ibrahim, “A binary sine cosine-modified whale optimization algorithm for feature selection,” in 4th National Computing Colleges Conf. (NCCC 2021), Taif, Saudi Arabia, IEEE, pp. 1–6, 2021. [Google Scholar]
15. A. A. Salamai, E. -S. M. El-kenawy, and A. Ibrahim, “Dynamic voting classifier for risk identification in supply chain 4.0,” Computers, Materials & Continua, vol. 69, no. 3, pp. 3749–3766, 2021. [Google Scholar]
16. E. -S. M. El-Kenawy, S. Mirjalili, S. S. M. Ghoneim, M. M. Eid, M. El-Said et al., “Advanced ensemble model for solar radiation forecasting using sine cosine algorithm and newton’s laws,” IEEE Access, vol. 9, pp. 115750–115765, 2021. [Google Scholar]
17. E. -S. M. El-kenawy, H. F. Abutarboush, A. W. Mohamed and A. Ibrahim, “Advance artificial intelligence technique for designing double T-shaped monopole antenna,” Computers, Materials & Continua, vol. 69, no. 3, pp. 2983–2995, 2021. [Google Scholar]
18. A. Ibrahim, S. Mirjalili, M. El-Said, S. S. M. Ghoneim, M. Al-Harthi et al., “Wind speed ensemble forecasting based on deep learning using adaptive dynamic optimization algorithm,” IEEE Access, vol. 9, pp. 125787–125804, 2021. [Google Scholar]
19. E. -S. M. El-kenawy, A. Ibrahim, N. Bailek, K. Bouchouicha, M. A. Hassan et al., “Sunshine duration measurements and predictions in Saharan Algeria region: An improved ensemble learning approach,” Theoretical and Applied Climatology, vol. 2021, pp. 1–17, 2021. [Google Scholar]
20. E. -S. M. El-kenawy, A. Ibrahim, N. Bailek, K. Bouchouicha, M. A. Hassan et al., “Hybrid ensemble-learning approach for renewable energy resources evaluation in Algeria,” Computers, Materials & Continua, vol. 71, no. 3, pp. 5837–5854, 2022. [Google Scholar]
21. E. El-kenawy, A. Ibrahim, S. Mirjalili, Y. Zhang, S. Elnazer et al., “Optimized ensemble algorithm for predicting metamaterial antenna parameters,” Computers, Materials & Continua, vol. 71, no. 3, pp. 4989–5003, 2022. [Google Scholar]
22. A. Ibrahim, H. Abutarboush, A. Mohamed, M. Fouad and E. El-kenawy, “An optimized ensemble model for prediction the bandwidth of metamaterial antenna,” Computers, Materials & Continua, vol. 71, no. 1, pp. 199–213, 2022. [Google Scholar]
23. E. -S. M. El-Kenawy, S. Mirjalili, A. Ibrahim, M. Alrahmawy, M. El-Said et al., “Advanced meta-heuristics, convolutional neural networks, and feature selectors for efficient COVID-19 X-ray chest image classification,” IEEE Access, vol. 9, pp. 36019–36037, 2021. [Google Scholar]
24. E. -S. M. El-Kenawy, M. M. Eid, M. Saber and A. Ibrahim, “MbGWO-SFS: Modified binary grey wolf optimizer based on stochastic fractal search for feature selection,” IEEE Access, vol. 8, pp. 107 635–107 649, 2020. [Google Scholar]
25. E. S. M. El-Kenawy, A. Ibrahim, S. Mirjalili, M. M. Eid and S. E. Hussein, “Novel feature selection and voting classifier algorithms for COVID-19 classification in CT images,” IEEE Access, vol. 8, pp. 179317–179335, 2020. [Google Scholar]
26. Z. Xue, D. You, S. Candemir, S. Jaeger, S. Antani et al., “Chest X-ray image view classification,” in Proc. of the IEEE 28th Int. Symp. on Computer-Based Medical Systems, Sao Carlos, Brazil, pp. 66–71, 2015. [Google Scholar]
27. D. Ragab, M. Sharkas, S. Marshall and J. Ren, “Breast cancer detection using deep convolutional neural networks and support vector machines,” PeerJ, vol. 7, no. 1, pp. e6201, 2019. [Google Scholar]
28. S. Pereira, A. Pinto, V. Alves and C. Silva, “Brain tumor segmentation using convolutional neural networks in MRI images,” IEEE Transactions on Medical Imaging, vol. 35, no. 1, pp. 1240–1251, 2016. [Google Scholar]
29. A. Esteva, B. Kuprel, R. Novoa, J. Ko, S. Swetter et al., “Dermatologist-level classification of skin cancer with deep neural networks,” Nature, vol. 542, no. 1, pp. 115–118, 2017. [Google Scholar]
30. E. Ayan and H. Unver, “Data augmentation importance for classification of skin lesions via deep learning,” in Proc. of the 2018 Electric Electronics, Computer Science, Biomedical Engineering’s Meeting (EBBT), Istanbul, Turkey, pp. 1–4, 2018. [Google Scholar]
31. J. Ker, L. Wang, J. Rao and T. Lim, “Deep learning applications in medical image analysis,” IEEE Access, vol. 6, pp. 9375–9389, 2017. [Google Scholar]
32. G. Litjens, T. Kooi, B. Bejnordi, A. Setio, F. Ciompi et al., “A survey on deep learning in medical image analysis,” Medical Image Analysis, vol. 42, no. 1, pp. 60–88, 2017. [Google Scholar]
33. H. Sak, A. Senior and F. Beaufays, “Long short-term memory recurrent neural network architectures for large scale acoustic modeling,” in Proc. of the Annual Conf. of the Int. Speech Communication Association, INTERSPEECH, Singapore, pp. 338–342, 2014. [Google Scholar]
34. A. Gulli and S. Pal, Deep Learning with Keras, 1st ed., vol. 1, Birmingham, UK: Packt Publishing Ltd., 2017. [Google Scholar]
35. E. Tsironi, P. Barros, C. Weber and S. Wermter, “An analysis of convolutional long short-term memory recurrent neural networks for gesture recognition,” Neurocomputing, vol. 268, pp. 76–86, 2017. [Google Scholar]
36. P. Mooney, “Chest X-ray images (Pneumonia),” [Online]. Available https://www.kaggle.com/paultimothymooney/chest-xray-pneumonia (accessed on 10 March 2022). 2018. [Google Scholar]
37. E. Bisong, Building Machine Learning and Deep Learning Models on Google Cloud Platform, New York, USA, Springer, Apress, pp. 59–64, 2019. [Online]. Available https://link.springer.com/book/10.1007/978-1-4842-4470-8. [Google Scholar]
38. D. Ibrahim, “Deep-pneumonia framework using deep learning models based on chest X-ray images,” [Online]. Available https://github.com/Dr-Dina-M-Ibrahim/Pneumonia-Detection-using-Deep-Learning, 2018 (accessed on 10 February 2022). [Google Scholar]
39. A. Takieldeen, E., El-kenawy, E. Hadwan and M. Zaki, “Dipper throated optimization algorithm for unconstrained function and feature selection,” Computers, Materials & Continua, vol. 72, no. 1, pp. 1465–1481, 2022. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |