DOI:10.32604/cmc.2022.023693

| Computers, Materials & Continua DOI:10.32604/cmc.2022.023693 |  |
| Article |
Unstructured Oncological Image Cluster Identification Using Improved Unsupervised Clustering Techniques
1Dr. T. Thimmaiah Institute of Technology, VTU, KGF, Karnataka, India
2School of Computing & Information Technology, REVA University, Bengaluru, India
3Faculty of Science and Technology, University of the Faroe Islands, Faroe Islands, Denmark
4K S School of Engineering, Bengaluru, India
5Muthayammal Engineering College, Rasipuram, Tamil Nadu, India
*Corresponding Author: Syed Thouheed Ahmed. Email: syed.edu.in@gmail.com
Received: 17 September 2021; Accepted: 10 December 2021
Abstract: This paper presents, a new approach of Medical Image Pixels Clustering (MIPC), aims to trace the dissimilar patterns over the Magnetic Resonance (MR) image through the process of automatically identify the appropriate number of distinct clusters based on different improved unsupervised clustering schemes for enrichment, pattern predication and deeper investigation. The proposed MIPC consists of two stages: clustering and validation. In the clustering stage, the MIPC automatically identifies the distinct number of dissimilar clusters over the gray scale MR image based on three different improved unsupervised clustering schemes likely improved Limited Agglomerative Clustering (iLIAC), Dynamic Automatic Agglomerative Clustering (DAAC) and Optimum N-Means (ONM). In the second stage, the performance of MIPC approach is estimated by measuring Intra intimacy and Intra contrast of each individual cluster in the result of MR image based on proposed validation method namely Shreekum Intra Cluster Measure (SICM). Experimental results show that the MIPC approach is better suited for automatic identification of highly relative dissimilar clusters over the MR cancer images with higher Intra closeness and lower Intra contrast based on improved unsupervised clustering schemes.
Keywords: Magnetic resonance image; unsupervised clustering scheme; intra intimacy; intra contrast; iLIAC; shreekum intra cluster measure; medical image clustering
Cluster based image segmentation is a significant and mathematical process in the MR image analysis system for deeper investigation, enhancement, tumor predication and pattern identification. Generally, it is defined as a process of dividing MR image pixels into different numbers of dissimilar sub regions based on pixel intensity similarity [1]. The goal of cluster based image separation is to simplify or change the representation of an image into a version that is more meaningful and easier to investigate and identify. Recently, many of the researchers have been reported in [2], the cluster based segmentation process is applied in many medicine related application likely medical image segmentation, tumor or cancer predication, medical image enhancement, medical image compression, pattern identification, medical image classification and medical image retrieval. The result of the cluster based medical image separation is a finite number of dissimilar groups that jointly concealments the complete medical image and the quality of the clustering result depend on the superiority of the medical image quality. The major problem in the existing clustering schemes such as semi-supervised and unsupervised methods [3] is that to predetermine the appropriate number of clusters in the unstructured MR image pixel set and respectively the clustering quality is based on predetermined number of clusters. To overcome these issues, in this paper a new clustering technique called Medical Image Pixels Clustering, it intentions to automatically separate finite number of dissimilar patterns in the MR image based on different improved unsupervised clustering schemes without predetermined knowledge for deeper investigation, enhancement, pattern predication and analysis.
Several methods are available for cluster based MR image segmentation process including k-means, fuzzy C-means, neural network, fuzzy clustering and hierarchical clustering methods reported in [4–7]. The k-means technique is a semi-supervised partitioned clustering technique and is an iterative procedure that directly decomposes the MR image pixel set into many dissimilar clusters or regions by minimizing the criterion function (e.g., sum-of-square-error) [8]. Many of the authors suggested problem in the K-Means technique is that the entire segmentation result quality of MR image is based on predetermined k number of centroid pixel values. In [9], the authors Jianwei et al. have reported an improved K-Means technique MR brain image segmentation. The improved K-Means scheme is used to identify K distinct clusters over the disordered MR brain image with higher accuracy compared to existing scheme.
Another popular method called fuzzy c-means clustering (FCM) technique was reported in [10,11]. This method is suited to partition the noise-free image into a finest number of groups. Many researchers suggested that the drawback with this method is that it failed to segment images corrupted by noise or inaccurate edges. In [12] the authors Yogita et al. have reported a detail survey of fuzzy C-means (FCM) with intensity inhomogeneity correction and noise robustness. They are discussed how the FCM schemes is better suitable to identify distinct tissues such as cerebrospinal fluid, gray matter and white matter over the MR brain image. The authors Senthilkumar et al. [13] have presented a modified fuzzy C-means clustering scheme to identify the normal and abnormal tissues likely white matter, gray matter, cerebrospinal and tumor part respectively over the MRI brain image. The clustering scheme consists of pre-processing and segmentation stages. In the pre-processing stage, the authors are applied wrapping based curvelet transform over the MR brain image and removed the noise. Similarly, they are applied improved fuzzy C-Means technique [14,15] and segmented the normal and abnormal tumor cells over the MR brain image based on spatial information. In [16], the authors Jinn et al. have reported a hierarchical genetic algorithm with fuzzy learning vector quantization network to partition a multi-spectral MR brain image. The evaluation of this approach was based on a real case of a MR brain image of an individual suffering from meningioma.
The author's Chong et al. [17] have presented hybrid clustering scheme combined with morphological operations to improve the performance of MR image segmentation and reduced the non-brain tissue in the brain image. Firstly, the authors applied wiener filter and morphological operations over the MR image due to remove the non-brain tissue. Next, they are used combination of K-Means++ and kernel-based fuzzy C-Means algorithm to identify distinct tumor regions in the MR image without noise. In [18,19], the authors Kalyanapu et al. have presented a clustering scheme namely unified iterative partitioned fuzzy clustering (U-IPFC). The U-IPFC scheme uses to identify distinct tissues over the MR brain image with good accuracy. The authors in [18,19] have claimed that the U-IPFC has produced higher accuracy result compared to FCM and K-means schemes. The authors Arul et al. in [20] presented a hierarchical clustering based segmentation (HCS) scheme to identify the distinct groups in hierarchy manner over the dynamic contrast enhanced magnetic resonance (DCSMR) image pixel set. The authors claimed that the HCS scheme is acted a semi-quantitative analytical tool to discover the DCEMR images. Next, the same authors Arul et al. in [21] have extended the detailed research of MR image segmentation based on hierarchical clustering scheme. The authors have experimented HCS scheme over the Multi-parametric Magnetic Resonance Imaging (MPMRI) and identified finite number of dissimilar tissue patterns by sequence of merging process. Another author Filipovych et al. in [22] reported hierarchical clustering scheme based image segmentation and it uses to identify predetermined number of dissimilar clusters in the tree manner over the gray scale image.
3 Proposed Image Pixel Clustering Approach
This section describes detailed study of the MIPC approach of image pixels classification. The MIPC scheme consists of two stages clustering and validation. The first stage automatically identifies the distinct number of highly relative clusters over the gray scale image dataset based on three different improved unsupervised clustering schemes iLIAC, DAAC and ONM in distinct manner. The second stage, it estimates the intra cluster intimacy and intra cluster contrast over the result of clustering stage based on the proposed SICM scheme. The stages involved in the MIPC approach are illustrated in the Fig. 1 and the different stages are described in below subsections.
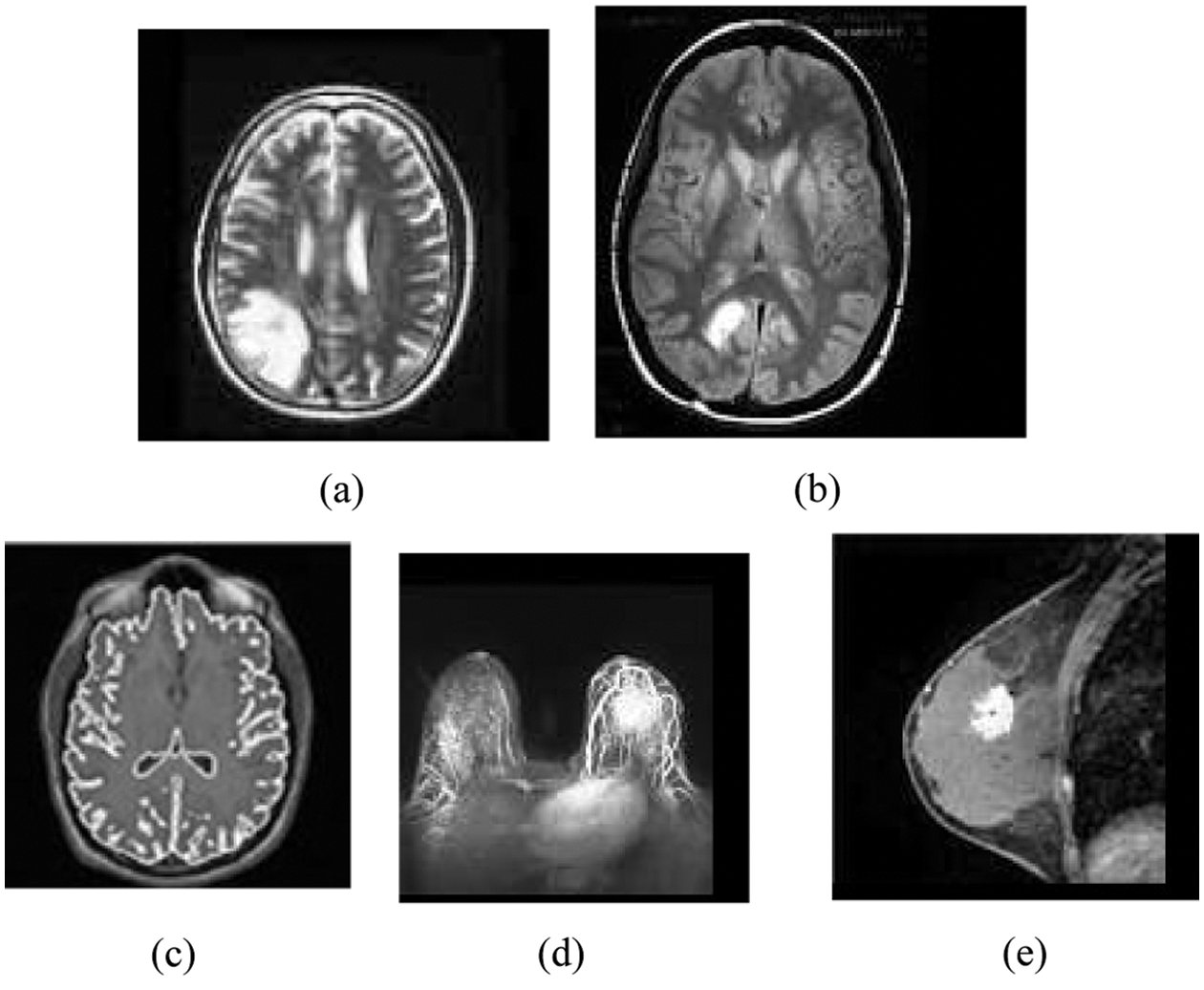
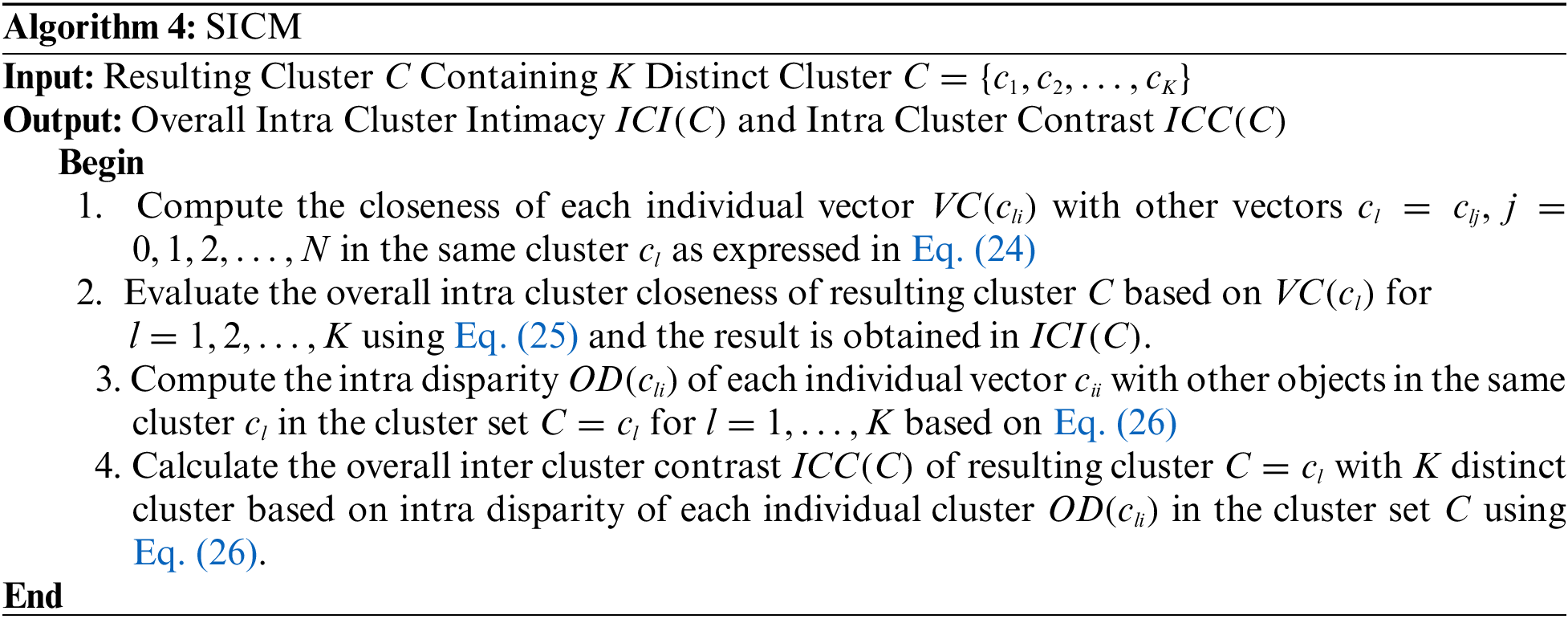
Figure 1: Original MR images: (a) Brain_1, (b) Brain_2, (c) Brain_3 (d) Breast_1 (e) Breast_2
This stage automatically identifies the distinct number of dissimilar clusters on the gray scale image based on three different improved clustering schemes iLIAC [23,24], DAAC [25] and ONM [26] in separate manner. Initially, the digital gray-scale image divides into (2 * 2) sizes of non-overlapping blocks and the image contains n objects plus is defined as
The MIPC approach identifies distinct number of dissimilar clusters over the MRI image dataset
where
where,
Here
where
where,
Next, the identified closest clusters pair
Then, updates the merged cluster
where,
Similarly, the MIPC approach is tested the same MRI image dataset
where
where,
Here,
where, n denotes the number of clusters in the input cluster set
In this,
where,
Next, updates the combined cluster
Similarly, in this subsection, the MIPC approach is partitioned the MRI image dataset into distinct number of different clusters based on improved partitioned clustering ONM scheme [25,26]. It consists of two stages likely dissimilar spatial centroid vector (DSCV) and partitioning respectively. In the DSCV stage, the ONM approach identifies the distinct number of centroid vectors over input MRI image vector set
where,
In this,
where,
Here,
In the last step, it modifies the centroid of each individual cluster in cluster set
In this,
This stage presents, the MIPC scheme estimates the closeness and separation among the data objects in each individual cluster in the cluster set of MR image vector set based on proposed cluster validation scheme (SICM). The proposed (SICM) is an improved version of existing validation techniques as reported in [27–29] and it aims to validate the quality of each individual cluster in the cluster set of MR image that identified by MIPC scheme based on probability concept. The SICM consists of two measures Intra Intimacy (II) and Intra Contrast (IC). The II measure uses to estimate the closeness of each individual vector with other vectors in the same cluster
where,
Similarly, the intra contrast measure aims to estimate the intra disparity among the vectors within the same cluster in the cluster set. First, it measures the intra disparity
Subsequently, the IC measure estimates the overall intra cluster contrast
This section discovers the computational complexity of MIPC approach has tested over MR image dataset by three different improved unsupervised clustering schemes namely iLIAC, DAAC and ONM. The MIPC system consumes time
First, it requires time
In the first stage, the (DAAC) clustering scheme needs time
Initially, the (ONM) clustering scheme consumptions
This section presents the MIPC approach, experimented on MR gray scale medical images based on three different improved unsupervised clustering schemes iLIAC, DAAC and ONM respectively. For the experimental purpose, we have taken 100 natural 100 2-D gray scale MR medical images with different sizes such as (120 * 120), (124 * 124) and (130 * 130) respectively and the grey values in the range 0–255.
A subset of this dataset containing ten sample standard MR brain and breast images via, Brain_1, Brain_2, Brain_3, Breast_1 and Breast_2 are reported as representative in this subsection. The sample MRI images are used in many research experiments as reported in (Lai & Huang 2011; Qi et al. 2015; Yong & Shuying 2007). Fig. 1 shows the five standard MRI gray scale images Brain_1, Brain_2, Brain_3, Breast_1 and Breast_2 as illustrated in Figs. 2a–2e respectively. In this experiment, each block of size (2 * 2) is considered as a vector and hence each sample image contains 3844, 4225, 3600, 3844 and 4225 vectors respectively.
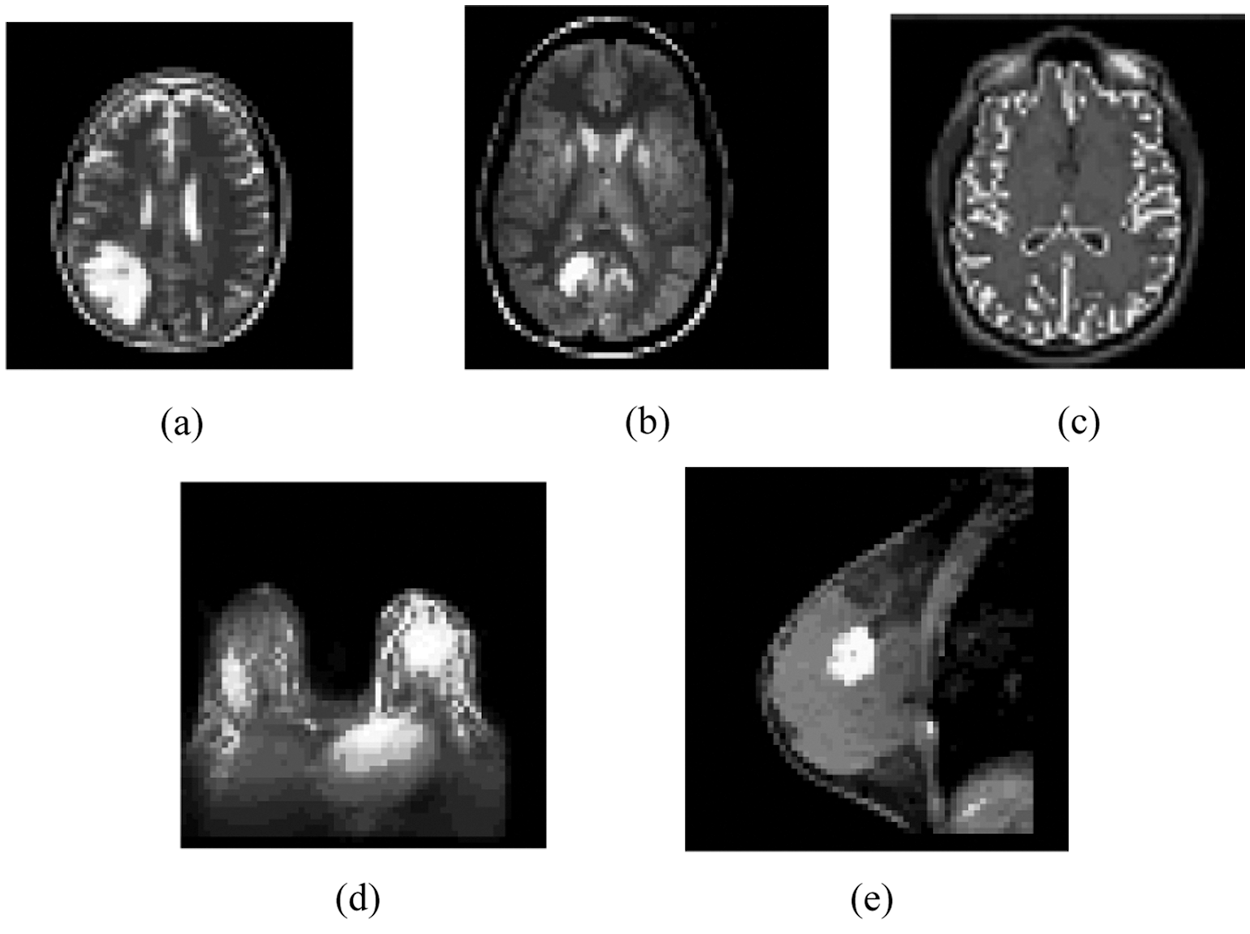
Figure 2: Result of the MIPC scheme tested on the ten gray scale images using iLIAC approach indicated in Fig. 1: (a) Result of brain_1 (b) Result of brain_2 (c) Result of brain_3 (d) Result of breast_1 (e) Result of breast_2
Firstly, the MIPC approach identifies distinct number of dissimilar clusters over the seven gray scale medical image datasets based on iLIAC scheme. Initially, it computes the control merge cost over seven gray scale MR images and the results are obtained in Tab. 1 as 7.87, 7.51, 7.71, 7.85, 7.44 respectively. Then it followed by computation of upper triangular distance matrix and in the case of sample gray scale MRI image datasets are presented in Fig. 2. The clustering scheme could identify 24, 25, 24, 25 and 25 distinct clusters over the MRI images in the Fig. 2. The results are incorporated in the Tab. 1. Fig. 3 demonstrates the clustering result of the iLIAC scheme has tested the MRI images likely Brain_1, Brain_2, Brain_3, Breast_1 and Breast_2 as obtained in Figs. 2a–2e respectively.

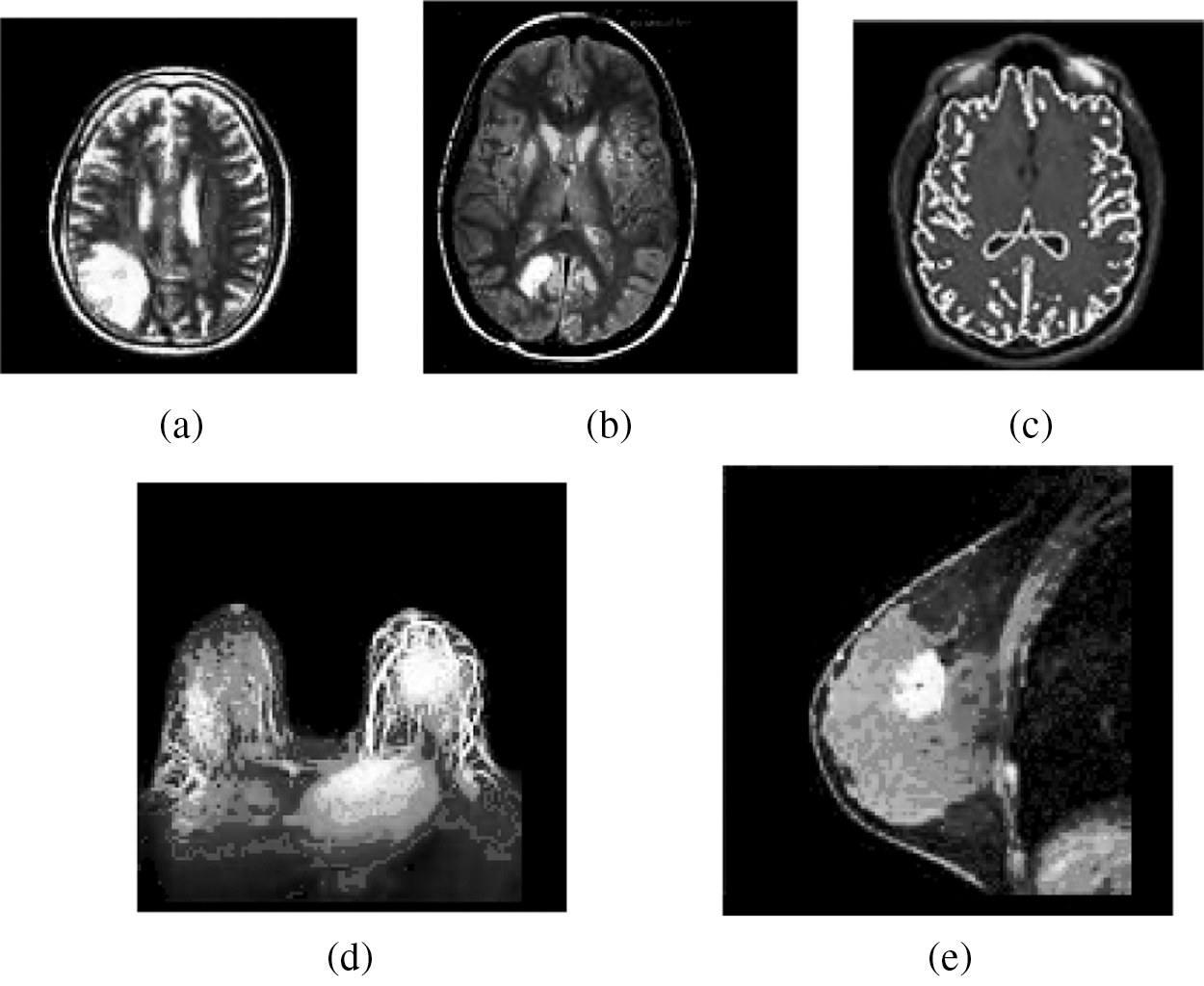
Figure 3: Result of the MIPC scheme tested on the ten gray scale MR images using DAAC approach indicated in Fig. 2: (a) Result of brain_1 (b) Result of brain_2 (c) Result of brain_3 (d) Result of breast_1 (e) Result of breast_2
Similarly, the MIPC approach detects distinct number of unrelated clusters on same five MR image datasets based on DAAC scheme. Primarily, it automatically traces the distinct representative objects over the five MR images as illustrated in Fig. 2 based on frequency of maximum occurrence (MO = 15) and the count of distinct representative objects are obtained in Tab. 2 as 33, 27, 33, 39, 27 respectively. The Maximum Occurrence is a predetermined threshold which used to dynamically find the appropriate number of distinct representative objects in dataset. Then it followed by sequence of merging process and divides the each individual image dataset into distinct number of dissimilar clusters based on count of representative objects as presented in Tab. 3. In the case of sample gray scale image datasets presented in Fig. 3, the clustering scheme could identify 33, 27, 33, 39 and 27 distinct clusters. The resulting clusters of the clustering scheme are incorporated in the Tab. 2. Fig. 3 demonstrates the clustering result of the MIPC (DAAC) on five gray scale MR images Brain_1, Brain_2, Brain_3, Breast_1 and Breast_2 as obtained in Figs. 3a–3e, 3 respectively.


In the same way, the MIPC approach divides the MR image dataset into distinct number of discrete clusters based on ONM scheme. In the beginning, it robotically traces the distinct number spatial centroid objects on each individual gray scale MR image dataset based on control centroid (CC = 15) and the results are incorporated in Tab. 3. The Control Centroid (CC) is a user defined threshold that is used to generate the spatial centroid objects in dataset dynamically. Then it followed by iterative process and divides the each individual image dataset into distinct number of dissimilar clusters based on spatial centroid objects as presented in Tab. 3. The resulting clusters of the five gray scale MR images are incorporated in the Tab. 3. Fig. 4 demonstrates the clustering result of the MIPC (ONM) on five gray scale medical images Brain_1, Brain_2, Brain_3, Breast_1 and Breast_2 as obtained in Figs. 4a–4e respectively.
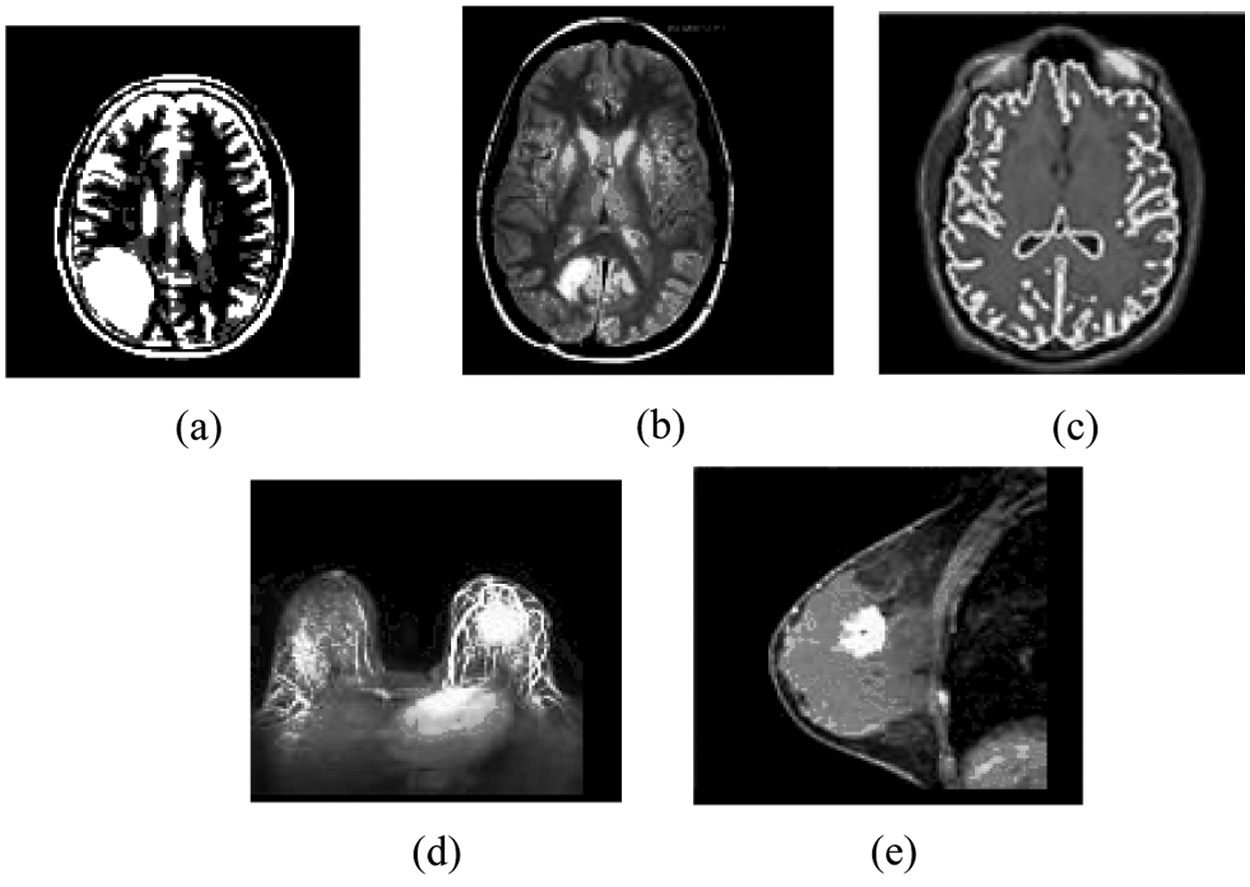
Figure 4: Result of the MIPC scheme tested on the ten MR images using DAAC approach indicated in Fig. 2: (a) Result of brain_1 (b) Result of brain_2 (c) Result of brain_3 (d) Result of breast_1 (e) Result of breast_2
The performance of the MIPC approach with three improved clustering schemes has been validated based on improved SICM schemes. It calculates the intra intimacy and intra cluster contrast over the each individual cluster in cluster set of MR images which tested by MIPC approach and the clustering results as shown in Tabs. 1–3 respectively. Initially, it measures the size of each individual cluster over the results of the five gray scale medical images Brain_1, Brain_2, Brain_3, Breast_1 and Breast_2 respectively. Next, it estimates the intra closeness
Then, it followed to calculate the overall intra intimacy
The validation results of MR images which tested by iLIAC, DAAC and ONM clustering schemes are obtained in Tab. 5 as 39.93, 43.56, 46.62, 26.60, 22.075; 22.71, 11.72, 22.72, 17.48, 14.60 and 27.85, 26.41, 29.78, 20.82, 16.60 respectively. It is clearly shown in the performance measurement results as illustrated in Figs. 4, 5, and 6 that the proposed SICM has flawlessly estimated intra cluster intimacy and intra cluster contrast over the result of MR cancer image. Accordingly to the performance measurement results, that the DAAC clustering schemes has identified appropriate number of dissimilar groups (Normal & Abnormal regions) over the MR cancer images with good accuracy compared to ONM and iLIAC schemes without predetermined input. Similarly, the ONM scheme has produced better clustering results with higher intra closeness and lower intra contrast compared to iLIAC scheme.

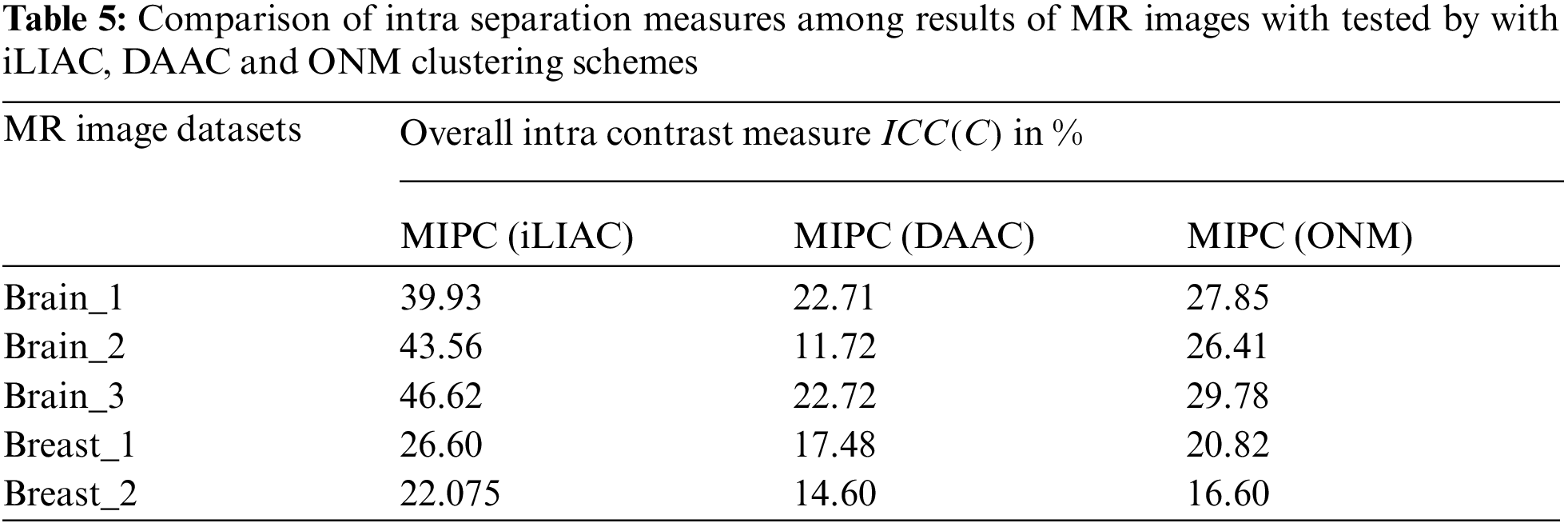
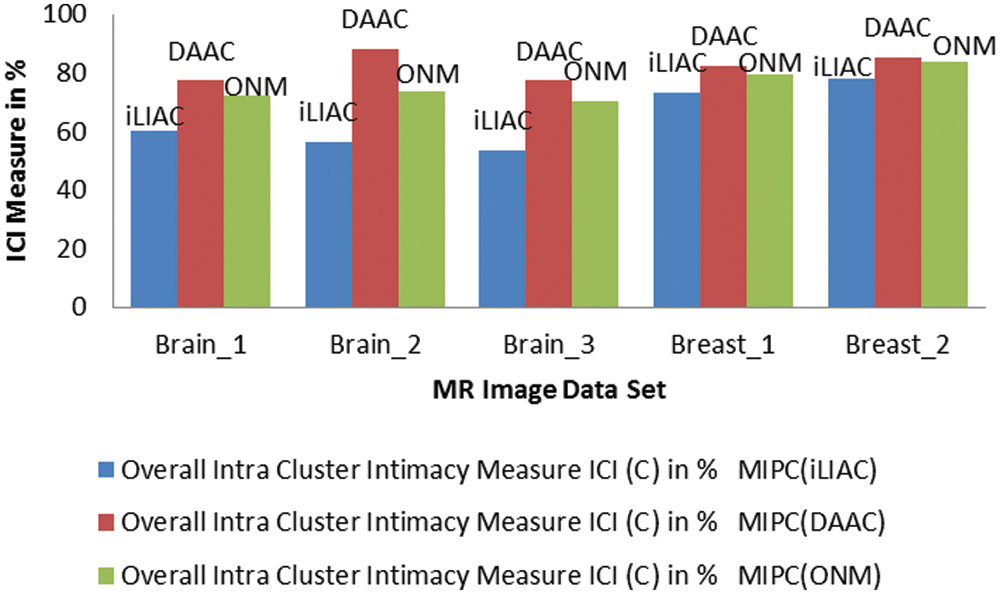
Figure 5: Comparisons of (ICI) performance measure over clustering results of MR images tested by improved unsupervised clustering schemes iLIAC, DAAC and ONM
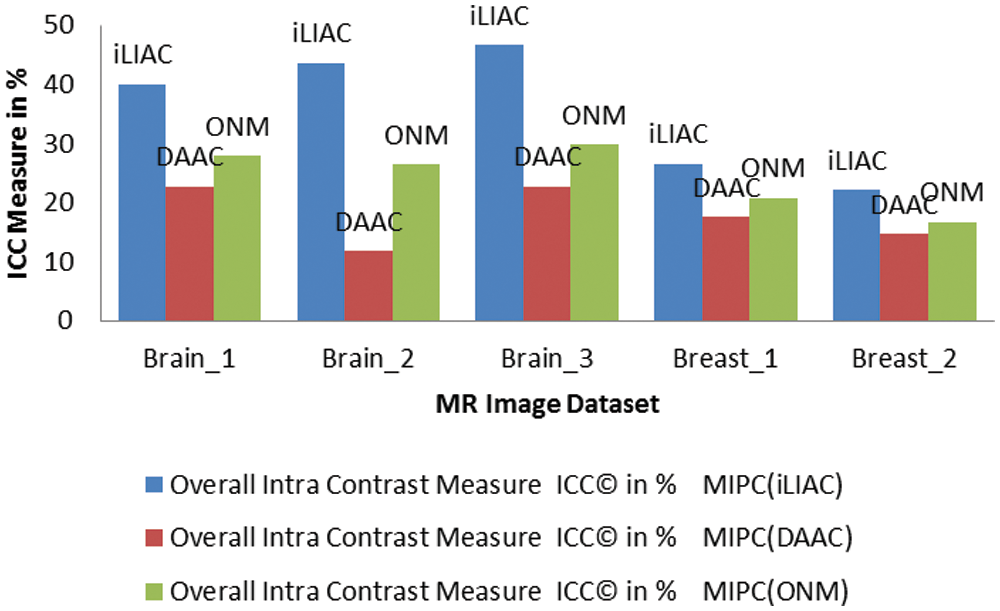
Figure 6: Evaluations of (ICC) performance measure over clustering results of MR images tested by improved unsupervised clustering schemes iLIAC, DAAC and ONM
This article presents Inherent Image Pixels Classification using three different improved unsupervised clustering schemes iLIAC, DAAC and ONM. The MIPC approach is aimed to trace the dissimilar pattern over the gray scale medical image through automatic identification of the distinct number of highly relative clusters in the medical image dataset based on improved unsupervised cluster schemes for deeper investigation and analysis. First, the MIPC approach automatically identifies the distinct number of dissimilar clusters over the medical image dataset based on three different clustering schemes iLIAC, DAAC and ONM in the separate manner. Next, the results of the MR images are validated based on proposed SICM scheme. We tested the MIPC approach with three improved unsupervised clustering schemes on five gray scale cancer MR images likely Brain_1, Brain_2, Brain_3, Breast_1 and Breast_2. According to the experimental results, the MIPC approach is more efficient and effective for automatic identification of the maximum number of highly relative clusters including normal and abnormal regions over the gray-scale MR cancer image with higher intra intimacy and lower intra contrast. After conducting various experiments, we concluded that the MIPC approach is better suitable to identify appropriate number of dissimilar regions (normal & abnormal), improving clusters quality and validate the clustering result for plateful to investigate (normal & abnormal regions) the dissimilar patterns in the MR cancer images.
Acknowledgement: This work is supported by Faculty of Science and Technology, University of the Faroe Islands, Faroe Islands, Denmark and REVA University, Bengaluru. The authors like to extend thanks to reviewers and experimental continuation of experts in this research.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. https://en.wikipedia.org/wiki/Medical_image_computing#Clustering, 2021. [Google Scholar]
2. https://en.wikipedia.org/wiki/Image_segmentation, 2021. [Google Scholar]
3. S. Sreedhar Kumar and M. Madheswaran, “A brief survey of unsupervised agglomerative hierarchical clustering schemes,” International Journal of Engineering & Technology (UAE), vol. 8, no. 1, pp. 29–37, 2019. [Google Scholar]
4. S. Sreedhar Kumar, M. Deepak and P. Karthik, “Reconstruction of MR image using sparse signal sequences in frequency domain,” International Journal of Innovating Technology and Exploring Engineering (IJITEE), vol. 9, no. 3, pp. 895–902, 2020. [Google Scholar]
5. K. Dhawan, Medical Image Analysis. Wiley Inter-Science Publications, 2003. [Google Scholar]
6. J. Alfredo, F. Costa, C. Jackson and G. De Souza, “Image segmentation through clustering based on natural computing techniques,” in Image Segmentation, Dr. Pei-Gee Ho (Edition), In Tech, 2011. [Google Scholar]
7. A. Jain, N. Murty and J. Flynn, “Data clustering: A review,” ACM Computer Surveys, vol. 31, no. 3, pp. 264–323, 1999. [Google Scholar]
8. L. Davies and W. Bouldin, “Cluster separation measure,” IEEE Transaction on Pattern Analysis and Machine Intelligence, vol. 1, no. 2, pp. 95–105, 1979. [Google Scholar]
9. L. Jianwei and L. Guo, “An improved k-means algorithm for brain image segmentation,” in 3rd Int. Conf. on Mechatroincs, Robotics and Automation (ICMRA 2015), Atlantis Press, Netherland, pp. 1087–1090, 2015. [Google Scholar]
10. J. Bezdek, Pattern Recognition with Fuzzy Objective Function Algorithms. New York: Plenum Press, 1981. [Google Scholar]
11. J. Bezdek, R. Ehrlich and F. William, “FCM: The fuzzy c-means clustering algorithm,” Computers and Geosciences, vol. 10, pp. 191–203, 1984. [Google Scholar]
12. K. Yogita, M. Milind and M. Mushrif, “FCM clustering algorithm for segmentation of brain MR images,” Advances in Fuzzy Systems, vol. 12, no. 3, pp. 1–14, 2016. [Google Scholar]
13. C. Senthilkumar and R. Gnanamurthy, “A fuzzy clustering based MRI brain image segmentation using back propagation neural networks,” Cluster Computing, vol. 22, no. 5, pp. 12305–12312, 2019. [Google Scholar]
14. J. Liu, M. Li, J. Wang, F. Wu and T. Liu, “A survey of MRI-based brain tumor segmentation methods,” Tsinghua Science Technology, vol. 19, no. 6, pp. 578–595, 2014. [Google Scholar]
15. N. Kwak and H. Choi, “Input feature selection for classification problems,” IEEE Transaction on Neural Networking, vol. 13, no. 1, pp. 143–159, 2014. [Google Scholar]
16. Y. Jinn-Yi and C. Fu, “A hierarchical genetic algorithm for segmentation of multi-spectral human-brain MRI,” Expert System with Application, Wiley, vol. 34, pp. 1285–1295, 2008. [Google Scholar]
17. Z. Chong, S. Xuanjing, H. Cheng and Q. Qingji, “Brain tumor segmentation based on hybrid clustering and morphological operations,” International Journal of Biomedical Imaging, vol. 2019, pp. 1–12, 2019. [Google Scholar]
18. S. Kalyanapu and K. Bhaskar, “Segmentation of MR brain images using unified iterative partitioned fuzzy clustering,” International Journal of Recent Technology and Engineering, vol. 8, no. 1, pp. 2755–2758, 2019. [Google Scholar]
19. N. Arul, S. Pettitt and C. Wright, “Hierarchical clustering based segmentation (HCS) aided interpretation of the DCE MR images of the prostate,” Conference on Medical Image Understanding Analysis, vol. 17, pp. 1–6, 2015. [Google Scholar]
20. N. Arul, M. Laura, S. Lynne, N. Sarah and L. Wright, “Hierarchical cluster analysis to aid diagnostic image data visualization of MR and other medical imaging modalities,” in Imaging Mass Sepctrometry, Humana Press, US, pp. 95–123, 2017. [Google Scholar]
21. G. Jorge, A. Hector and B. Carlos, “Dynamic image segmentation method using hierarchical clustering,” Progress in Pattern Recognition, Image Analysis, Computer Vision and Application, US, pp. 177–184, 2009. [Google Scholar]
22. S. Filipovych, M. Resnick and C. Davatzikos, “Semi-supervised cluster analysis of imaging data,” NeuroImage, vol. 54, no. 3, pp. 2185–2197, 2011. [Google Scholar]
23. M. Gunashree, S. T. Ahmed, M. Sindhuja, P. Bhumika and B. Anusha, “A new approach of multilevel unsupervised clustering for detecting replication level in large image set,” Procedia Computer Science, vol. 171, pp. 1624–1633, 2020. [Google Scholar]
24. S. T. Ahmed, M. Sandhya and S. Sharmila, “A dynamic MooM dataset processing under TelMED protocol design for QoS improvisation of telemedicine environment,” Journal of Medical Systems, vol. 43, no. 8, pp. 1–12, 2019. [Google Scholar]
25. G. Reddy, M. Reddy, K. Lakshmanna and D. S. Rajput, “Hybrid genetic algorithm and a fuzzy logic classifier for heart disease diagnosis,” Evolutionary Intelligence, vol. 13, no. 2, pp. 185–196, 2020. [Google Scholar]
26. V. Kolisetty and D. S. Rajput, “A review on the significance of machine learning for data analysis in big data,” Jordanian Journal of Computers and Information Technology, vol. 6, no. 1, pp. 56–578, 2020. [Google Scholar]
27. S. M. Basha and D. S. Rajput, “Aspects of deep learning: Hyper-parameter tuning, regularization, and normalization,” in Intelligent Systems, Apple Academic Press, Singapore, pp. 171–186, 2019. [Google Scholar]
28. S. M. Basha and D. S. Rajput, “A roadmap towards implementing parallel aspect level sentiment analysis,” Multimedia Tools and Applications, vol. 78, no. 20, pp. 29463–29492, 2019. [Google Scholar]
29. S. M. Basha and D. S. Rajput, “Survey on evaluating the performance of machine learning algorithms: Past contributions and future roadmap,” in Deep Learning and Parallel Computing Environment for Bioengineering systems, Academic Press,India, pp. 153–164, 2019. [Google Scholar]
30. S. T. Ahmed, S. Sreedhar Kumar, B. Anusha, P. Bhumika and M. Gunashree, “A generalized study on data mining and clustering algorithms,” in Int. Conf. on Computational Vision and Bio Inspired Computing, Cham, Springer, pp. 1121–1129, 2018. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |